SUMMARY
In vertebrates, activation of innate immunity is an early response to injury, implicating it in the regenerative process. However, the mechanisms by which innate signals might regulate stem cell functionality are unknown. Here we demonstrate that type 2 innate immunity is required for regeneration of skeletal muscle after injury. Muscle damage results in rapid recruitment of eosinophils, which secrete IL-4 to activate the regenerative actions of muscle resident fibro/adipocyte progenitors (FAPs). In FAPs, IL-4/IL-13 signaling serves as a key switch to control their fate and functions. Activation of IL-4/IL-13 signaling promotes proliferation of FAPs to support myogenesis, while inhibiting their differentiation into adipocytes. Surprisingly, type 2 cytokine signaling is also required in FAPs, but not myeloid cells, for rapid clearance of necrotic debris, a process that is necessary for timely and complete regeneration of tissues.
INTRODUCTION
The regenerative response of skeletal muscle to injury is dependent on the quiescent population of skeletal muscle stem cells, termed the satellite cells, which reside beneath the basal lamina of each myofiber (Brack and Rando, 2012; Wang and Rudnicki, 2012). Upon injury, these quiescent satellite cells become activated and undergo proliferation, giving rise to myogenic progenitors (MPs) that ultimately differentiate into mature myofibers. In this context of injury and repair, a number of factors have been identified that promote proliferation and differentiation of MPs (Kuang et al., 2008). For instance, autocrine Notch signaling regulates the activation and proliferation of satellite cells (Bjornson et al., 2012; Conboy et al., 2003; Conboy and Rando, 2002; Mourikis et al., 2012), whereas paracrine actions of IL-6 and insulin-like growth factors (IGFs) have been implicated in the differentiation of MPs into mature myotubes (Bodine et al., 2001; Rommel et al., 2001; Serrano et al., 2008).
In addition to satellite cells, recent studies have identified an important role for fibro/adipogenic progenitors (FAPs) in muscle regeneration and its fatty degeneration (Joe et al., 2010; Uezumi et al., 2010). FAPs, which do not arise from the myogenic lineage, are bipotential cells capable of giving rise to fibroblasts and adipocytes. The close association of FAPs with regenerating muscle fibers, along with their expression of factors that influence myogenic differentiation, such as IL-6 and IGF-1, suggests that these stromal cells may play a supportive role in myogenic differentiation (Joe et al., 2010). However, reflecting their adipogenic potential, FAPs can also give rise to ectopic adipocytes that accumulate in degenerating muscles (Uezumi et al., 2010). Based on these findings, it has been postulated that factors that modulate the proliferation or differentiation of FAPs could potentially influence the muscle’s regenerative response to injury; however, none have been identified to date.
Muscle injury results in rapid activation of the innate immune system, which exerts pleiotropic effects on regenerating muscle (Brunelli and Rovere-Querini, 2008; Tidball and Villalta, 2010). Within minutes of injury, neutrophils infiltrate injured skeletal muscle and release tissue-damaging reactive molecules, which exacerbate muscle damage (Tidball, 1995). This initial burst of collateral damage caused by the innate immune system is followed by a wave of reparative macrophages. For instance, it has been proposed that classically activated (M1) macrophages infiltrate early to facilitate the clearance of necrotic debris, whereas alternatively activated (M2) macrophages infiltrate later to assist with muscle growth (Arnold et al., 2007). In support of this idea, impairment in transcriptional programming of M2 macrophages, as in mice with reduced expression of C/EBPβ, results in smaller regenerated myofibers (Ruffell et al., 2009), potentially reflecting the reduced secretion of myogenic growth factor IGF-1 by these cells (Wynes and Riches, 2003). While these studies demonstrate a facilitative role of innate immune cells in muscle regrowth after injury, a direct molecular link between the innate immune system and muscle progenitor biology remains to be established.
In a number of species, tissue regeneration is associated with the presence of the molecular signature for type 2 innate immune response, such as alternatively activated (M2) macrophages and eosinophils (Allen and Wynn, 2011; Palm et al., 2012). This observation led us to postulate that signals, such as IL-4 and IL-13, that orchestrate type 2 innate immune responses might be good candidates for mediating the crosstalk between the immune system and skeletal muscle stem cells. Here, we report that muscle injury leads to the recruitment of IL-4 secreting eosinophils, which form an adaptive niche for proliferating stem cells in regenerating muscles. Loss of IL-4/IL-13 signaling or genetic absence of eosinophils severely compromises the ability of injured muscles to regenerate. Unexpectedly, we find that the regenerative effects of IL-4/IL-13 are not mediated by its signaling in myeloid cells but rather in FAPs. In FAPs, IL-4 acts as a molecular switch to control their fate between fibroblasts and adipocytes, and to promote the clearance of necrotic debris. Consequently, global or cell-specific loss of IL-4/IL-13 signaling in FAPs severely impairs their functionality, resulting in persistence of necrotic debris and impairment in muscle regeneration.
RESULTS
IL-4/IL-13 signaling is required for muscle regeneration
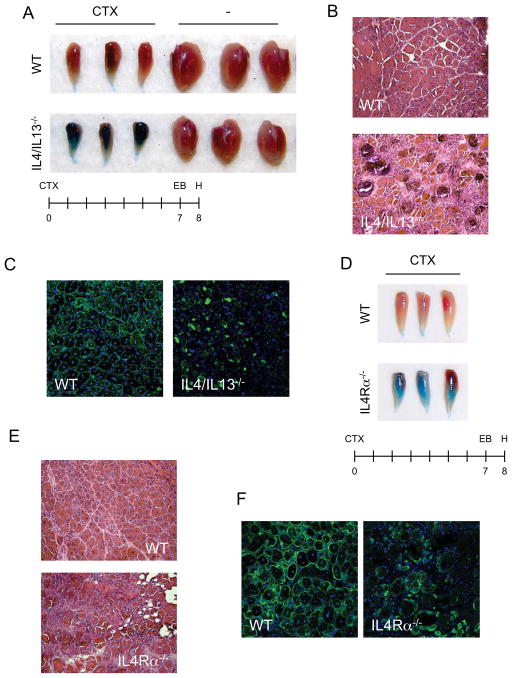
To investigate the functions of IL-4/IL-13 in muscle regeneration, we used cardiotoxin (CTX) to acutely injure the tibialis anterior (TA) muscles of mice. CTX administration induces local muscle necrosis, which is rapidly followed by recruitment of inflammatory cells, clearance of cellular debris, and regeneration of injured muscle (Uezumi et al., 2010). To evaluate the overall efficacy of the regenerative response, we gave mice an intraperitoneal injection of Evans blue dye, which accumulates in damaged muscle fibers (Figure 1). Wild type (WT) mice exhibited a robust regenerative response resulting in the restoration of intact, regenerating fibers, as evidenced by the absence of Evans blue staining of TA muscles (Figure 1A). In striking contrast, Evans blue dye uptake was markedly higher in TA muscles of IL-4/IL-13−/− mice, indicative of a failure of the regenerative response to restore mature, intact myofibers (Figure 1A). In agreement with this interpretation, histological examination revealed that TA muscles of WT mice contained centrally nucleated regenerative myofibers, which were largely absent from those of IL-4/IL-13−/− mice (Figure 1B). Instead, cellular debris and inflammatory infiltrate persisted in the injured TA muscles of IL-4/IL-13−/− mice (Figure 1B). Moreover, immunostaining for desmin, a marker of mature myofibers, showed a near complete absence of regenerated muscle fibers in IL-4/IL-13−/− mice (Figure 1C). This impairment in muscle regeneration did not result from altered trafficking of immune cells into injured muscles of IL-4/IL-13−/− mice (Table S1).
Figure 1. IL-4/IL-13 signaling is required for muscle regeneration.
(A, D) Tibialis anterior (TA) muscles of WT, IL-4/IL-13−/− (A) and IL-4Rα−/− (D) mice 8 days after injury by cardiotoxin (CTX). (B, D) Representative TA muscle sections of WT, IL-4/IL-13−/− (B) and IL-4Rα−/− (D) were stained with hematoxylin and eosin 8 days after injury, n=6 per genotype (magnification, X200). (C, F) Fluorescent microscopy of WT, IL-4/IL-13−/− (C) and IL-4Rα−/− (F) TA muscles 8 days after injury (magnification, X200). Desmin (green), and DAPI (blue). See also Table S1.
Two distinct receptors (IL-4Rα/γc and IL-4Rα/ IL-13Rα1) transduce the biologic effects of IL-4 and IL-13 in cells (Kelly-Welch et al., 2003). Using IL-4Rα−/− mice, we next investigated whether the canonical IL-4/IL-13 signaling pathways mediate the regenerative responses in injured muscles. Both at a gross and microscopic level, IL-4Rα−/− mice exhibited similar defects in skeletal muscle regeneration as IL-4/IL-13−/− mice (Figure 1D and E). Moreover, immunofluorescence staining for desmin demonstrated an absence of centrally nucleated fibers in the regenerating TA muscles of IL-4Rα−/− mice (Figure 1F). These findings provide strong genetic evidence that the IL-4/IL-13 immune signals play a central role in skeletal muscle regeneration, and raise three important questions: what is the cellular source of IL-4 during muscle regeneration, what cells respond to IL-4/IL-13 in regenerating muscle, and how do IL-4/IL-13 promote muscle regeneration?
Eosinophils are required for muscle regeneration
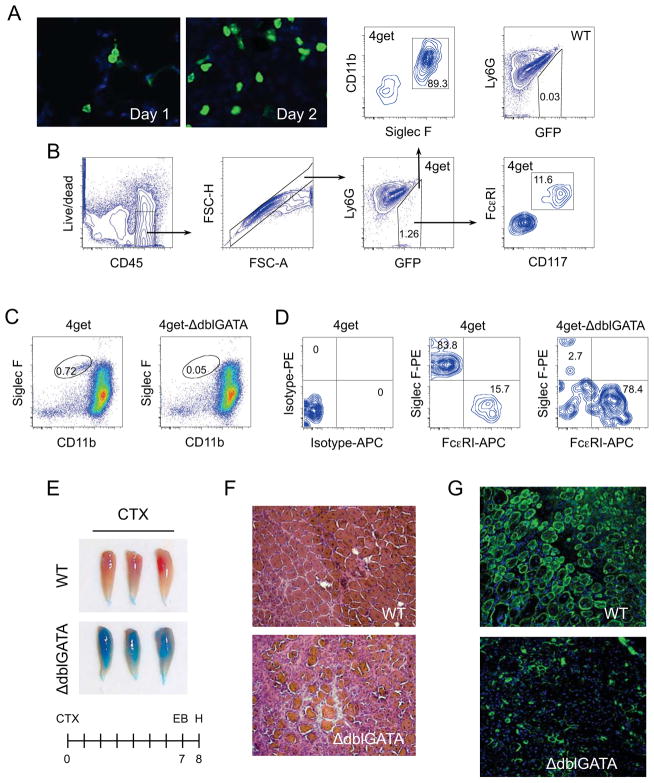
To prospectively identify the cellular source of IL-4 in regenerating muscles, we utilized 4get reporter mice, which express GFP from the 3′UTR of the endogenous IL-4 gene (Mohrs et al., 2001). GFP+ cells, which were readily identified thorough out the time course of muscle regeneration (Figure 2A, S1A), were primarily of hematopoietic origin, as they co-expressed CD45 (Figure S1B). To characterize the identity of these IL-4 expressing cells, we analyzed the expression of markers for eosinophils (Siglec F+CD11b+), basophils (FceRI+CD117−), mast cells (FceRI+CD117+), and Th2 cells (CD3+CD4+) in the GFP+ population that infiltrated injured muscles of 4get mice. Notably, ~85–90% of GFP+ cells were eosinophils, whereas the remainder were mast cells (Figure 2B). Consistent with this, expression of chemokines and cytokines involved in chemotaxis of eosinophils was induced in CTX-injured muscles (Figure S1C).
Figure 2. IL-4 expressing eosinophils are required for muscle regeneration after injury.
(A) IL-4 expressing cells infiltrate injured muscles. TA muscles from 4get mice were stained for GFP 1 or 2 days after injury with CTX (magnification, X400). (B) Flow cytometric gating strategy for identification of IL-4 expressing cells in injured muscles. Eosinophils were defined as being CD11b+ and Siglec F+, whereas mast cells were CD117+ and FcεRI+. (C) IL-4 expressing cells in 4get and 4get-ΔdblGATA mice 36 hours after injury with CTX. (D) Eosinophils in 4get and 4get-ΔdblGATA mice 36 hours after muscle injury. (E) TA muscles from WT and ΔdblGATA mice 8 days after injury. (F) Hematoxylin and eosin staining of representative muscle sections taken from WT and 4get-ΔdblGATA mice 8 days after injury, n=6 per genotype (magnification, X200). (G) Immunostaining of TA muscle sections of WT and ΔdblGATA mice 8 days after CTX injury (magnification, X200). Desmin (green) and DAPI (blue). See also Figure S1.
Because eosinophils were the dominant cell type secreting IL-4 in injured muscles, we next investigated their involvement in muscle’s regenerative response using eosinophil-deficient ΔdblGATA mice (Yu et al., 2002). Flow cytometric analyses of 4get-ΔdblGATA mice revealed a near complete absence (>93% reduction) of infiltrating eosinophils in injured TA muscles (Figure 2C). Moreover, the absence of eosinophils reduced the total number of GFP+ cells by ~75% (Figure S1D), decreased the number of IL-4-expressing eosinophils by ~30-fold (Figure 2D), and completely abolished the expression of IL-4 in mononuclear cells derived from ΔdblGATA mice (Figure S1E). Consequently, 4get-ΔdblGATA mice were unable to regenerate their injured muscles, as assessed by Evans blue staining of their TA muscles (Figure 2E). Paralleling the changes in the gross phenotype, hematoxylin and eosin staining of tissue sections revealed a paucity of centrally nucleated regenerating myofibers (Figure 2F), a finding that was confirmed by immunostaining for desmin (Figure 2G). These results suggest that eosinophil-derived factors, such as IL-4, are essential for orchestrating skeletal muscle regeneration.
Myeloid cells and FAPs exhibit competence for IL-4/IL-13 signaling
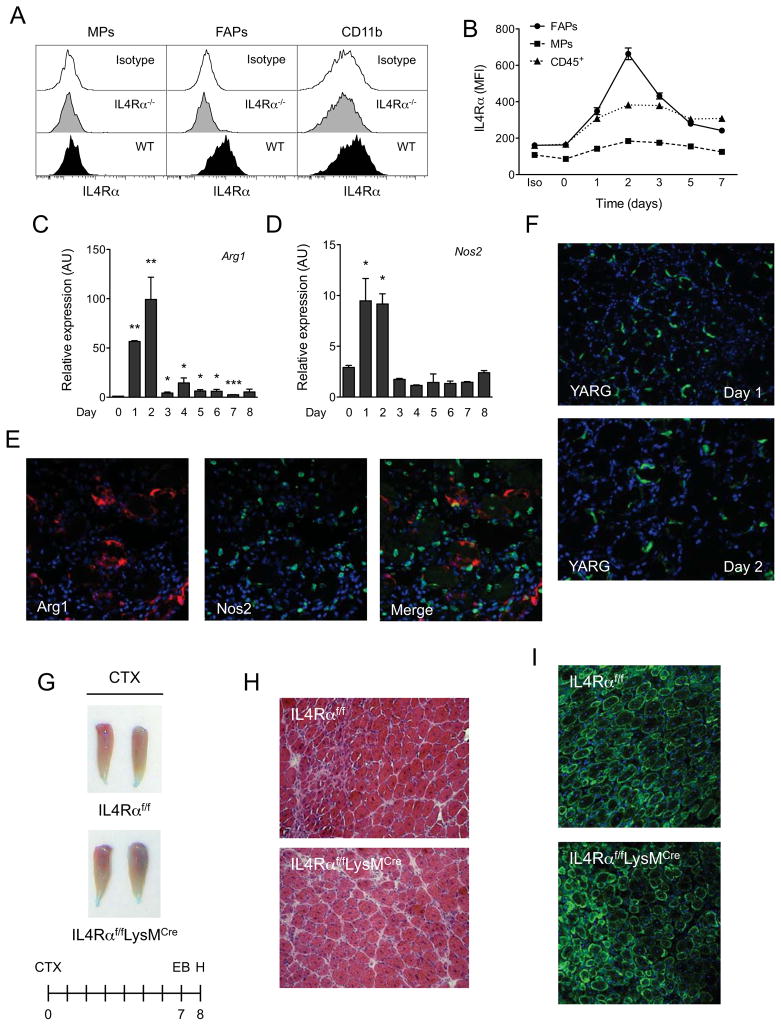
To elucidate the target cells that respond to IL-4/IL-13 in injured muscles, we profiled the expression of IL-4Rα on various cell types that participate in the repair of damaged skeletal muscle. For these studies, we utilized flow cytometry and established gating strategies to identify myogenic progenitors (MPs), fibro/adipocyte progenitors (FAPs), and myeloid cells (Figure S2A), and then evaluated the expression of IL-4Rα on each of these populations. Figure 3A shows that IL-4Rα is specifically expressed on wild type FAPs and myeloid cells, and is largely absent from the cell surface of MPs. Because stimulation with IL-4/IL-13 induces the expression of IL-4Rα (Kelly-Welch et al., 2003), the relative change in its expression serves as a good indicator of target cell responsiveness to these cytokines. Indeed, temporal profiling revealed that IL-4Rα expression was rapidly induced on the surface of FAPs and CD45+ cells, but not MPs, after injury (Figure 3B). This observed increase in IL-4Rα expression coincided with the presence of IL-4-expressing eosinophils in injured muscle (Figure 2A), implying that the primary regenerative actions of these cytokines might be restricted to this early time frame after injury.
Figure 3. IL-4/IL-13 signaling in myeloid cells is dispensable for muscle regeneration.
(A) Expression of IL-4Rα in muscle progenitors (MPs), fibro/adipogenic progenitors (FAPs) and myeloid cells (CD11b+). (B) Time course of IL-4Rα expression in FAPs, MPs and CD45+ hematopoietic cells. (C, D) Expression of arginase 1 (Arg1) and iNOS (Nos2) mRNAs in regenerating TA muscles of wild type mice, n=4–6 per time point. (E) Fluorescent microscopy for Arg1 (red), Nos2 (green) and DAPI (blue) 2 days after muscle injury of WT mice (magnification, X400). (F) GFP staining of TA muscles of YARG mice after injury (magnification, X200). (G) TA muscles 8 days after injury in IL-4Rαf/f and IL-4Rαf/fLysMCre mice. (H) Representative day 8 muscle sections from IL-4Rαf/f and IL-4Rαf/fLysMCre mice stained with hematoxylin and eosin, n=6 per genotype. (I) Fluorescent microscopy of IL-4Rαf/f and IL-4Rαf/fLysMCre TA muscles 8 days after injury. Desmin in green and DAPI in blue. Error bars represent SEM. See also Figure S2.
IL-4/IL-13 signaling in myeloid cells is dispensable for muscle regeneration
An early event that occurs after muscle injury is the recruitment of macrophages, which exert pleiotropic effects on muscle regeneration (Tidball and Villalta, 2010). Classically activated (also referred to as M1) macrophages infiltrate damaged muscle within the first 2 days, and have been implicated in the clearance of cellular debris and stimulation of myogenesis. In contrast, alternatively activated (also referred to as M2) macrophages, whose numbers increase during later stages of muscle regeneration (days 4–10), are implicated in the promotion of muscle growth via secretion of paracrine factors. Because IL-4, the dominant regulator of alternative macrophage activation (Martinez et al., 2009; Odegaard and Chawla, 2011), is expressed during the early phase of muscle injury (Figure 2A, S2B), we postulated that the observed defects in muscle regeneration might stem from impairment in alternative macrophage activation.
First, we examined the time course of macrophage recruitment into injured muscle. In contrast to the previous report (Arnold et al., 2007), quantitative RT-PCR analysis of whole TA muscles revealed that expression of mRNAs encoding classical (Nos2, Il6 and Tnfa) and alternative activation (Arg1 and Ym1) genes was rapidly induced during the first two days of muscle injury (Figures 3C, D, and S2C–F). To exclude the possibility that markers of classical and alternative activation might be concurrently expressed in the same cells, we analyzed expression of arginase 1 (Arg1) and inducible nitric oxide synthase (Nos2) by immunostaining. Arg1 expression was primarily restricted to cells that express the macrophage marker Cd68 (Figure S2G). Furthermore, alternative (Arg1) and classical (Nos2) activation markers were expressed in distinct macrophages (Figure 3E), indicative of concurrent infiltration of damaged muscle by both types of macrophages. This observation was independently verified using YARG mice (Reese et al., 2007), which have a fluorescent reporter introduced into the Arg1 gene (Figure 3F). In aggregate, these data demonstrate that muscle injury leads to rapid recruitment of both alternatively (M2) and classically (M1) activated macrophages.
We tested whether alternatively activated macrophages are required for muscle regeneration using control (IL-4Rαf/f) and myeloid cell specific knockouts of the IL-4Rα (IL-4Rαf/fLysMCre). To our surprise, unlike the IL-4/IL-13−/− and IL-4Rα−/− mice, myeloid cell deletion of IL-4Rα (Figure S2H) did not result in a strong defect in skeletal muscle regeneration (Figure 3G–I). These findings suggest that myeloid cells are not the primary targets for the regenerative effects IL-4/IL-13 during muscle injury.
IL-4/IL-13 stimulate proliferation of FAPs in vivo
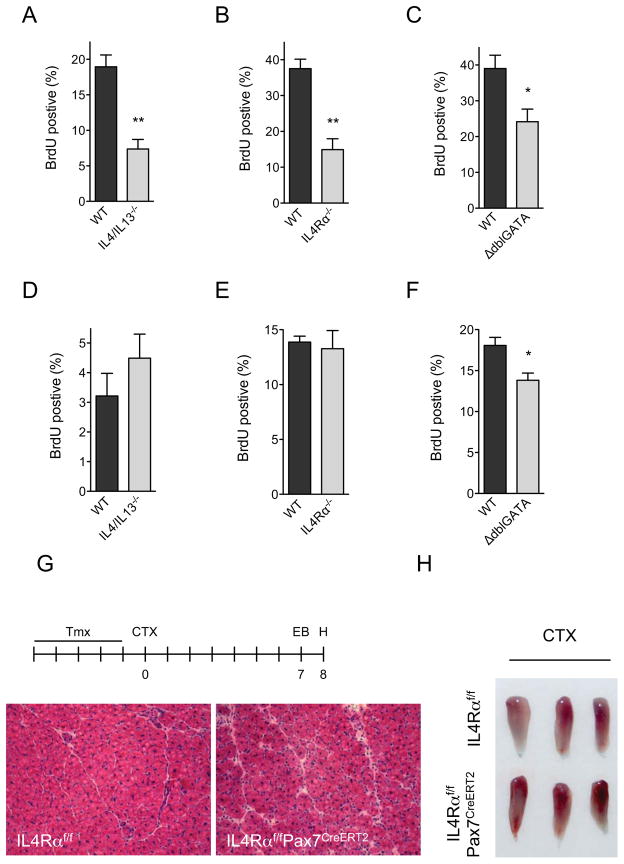
FAPs, which are present in healthy muscle, undergo rapid proliferation after muscle injury to support myogenic differentiation (Joe et al., 2010). Because IL-4Rα is highly expressed in FAPs (Figure 3B), we hypothesized that IL-4/IL-13 might regulate proliferation or differentiation of FAPs. To investigate this postulate, we utilized flow cytometry to identify FAPs and MPs on day 1 after injury, and followed their proliferation using BrdU (5-bromodeoxyuridine) incorporation. Strikingly, absence of IL-4/IL-13 or IL-4Rα decreased BrdU incorporation in FAPs by ~60% (Figure 4A, B, and S3A), and reduced the FAP content of regenerating muscles on day 3 by ~40–45% (Figure S3C). Albeit to a slightly lower degree, BrdU incorporation was also reduced in FAPs of ΔdblGATA mice (~40%), which lack IL-4 expressing eosinophils in their regenerating muscles (Figure 4C).
Figure 4. IL-4/IL-13 signaling controls proliferation of FAPs but not satellite cells during muscle regeneration.
(A–C) Quantification of BrdU incorporation in FAPs of WT, IL-4/IL-13−/− and ΔdblGATA mice 24 hours after muscle injury, n=4 per genotype. (D–F) Quantification of BrdU incorporation in MPs of WT, IL-4/IL-13−/− and ΔdblGATA mice 24 hours after muscle injury, n=4 per genotype. (G, H) Signaling via IL-4Rα in satellite cells is dispensable for muscle regrowth after injury. (G) Representative day 8 muscle sections from IL-4Rαf/f and IL-4Rαf/fPax7CreERT2 mice stained with hematoxylin and eosin, n=6 per genotype (magnification, X200). (H) Gross appearance of IL-4Rαf/f and IL-4Rαf/fPax7CreERT2 TA muscles 8 days after injury with CTX. Error bars represent SEM. See also Figure S3.
Muscle injury also leads to proliferation of MPs, which are required for myogenesis (Brack and Rando, 2012; Wang and Rudnicki, 2012). We tested whether absence of eosinophils or loss of IL-4/IL-13 signaling decreased the proliferation of MPs. In contrast to FAPs, the rate of BrdU incorporation in MPs was not significantly different amongst WT, IL-4/IL-13−/−, and IL-4Rα−/− mice (Figure 4D, E, and S3B). However, we did observe a modest decrease in the proliferation rate of MPs in eosinophil-deficient ΔdblGATA mice (Figure 4F). These data are consistent with the observation that IL-4Rα is not expressed on MPs at any stage of muscle regeneration (Figure 3B), thereby suggesting that FAPs are likely the primary target of IL-4/IL-13 signaling in regenerating muscle. Congruent with this idea, we failed to detect significant differences in the proliferation rates of FAPs or MPs in IL-4Rαf/f and IL-4Rαf/fLysMCre mice (Figure S3D, E).
Previous studies have implicated IL-4 signaling in myoblast fusion and muscle fiber growth (Horsley et al., 2003), prompting us to examine the role of IL-4Rα in regenerating muscle fibers. Western blot analysis of purified FAPs and MPs revealed robust expression of IL-4Rα and PDGFRα in FAPs but not MPs (Figure S3F). In contrast to MPs, IL-4Rα protein was detectable in MP-derived myotubes, which displayed fused morphology and higher expression of desmin (Figure S3F). This observation prompted us to generate mice (designated IL-4Rαf/fPax7CreERT2) in which IL-4Rα could be deleted in Pax7+ satellite cells in a tamoxifen-inducible manner (Lepper et al., 2009). As quantified by qPCR, administration of tamoxifen for 5 days induced >90% recombination at the IL-4Rα locus (Figure S3G). Since Pax7+ satellite cells give rise to all regenerating myofibers (Lepper et al., 2009), we investigated the requirement for IL-4Rα in satellite cell-derived myofiber regrowth. Notably, deletion of IL-4Rα in satellite cells did not impair the muscle’s regenerative response to injury by CTX, as assessed by gross and microscopic histology (Figure 4G, H). In agreement with these observations, we failed to detect expression of IL-4Rα in regenerating myofibers in vivo (Figure S3H).
IL-4 signaling via IL-4Rα and STAT6 stimulates proliferation of FAPs in vitro
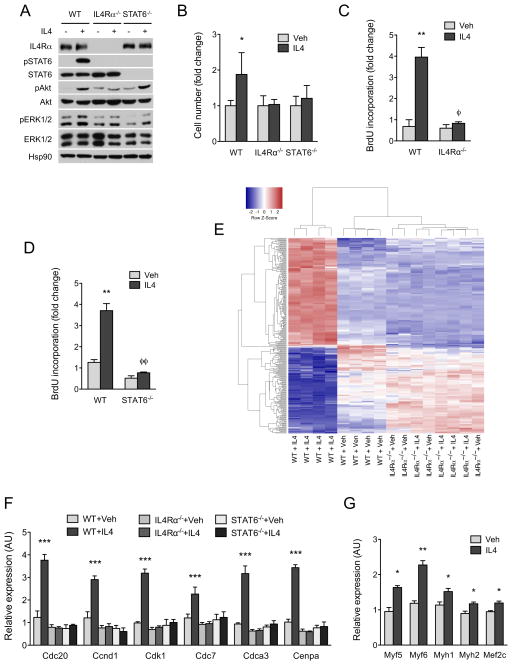
We purified FAPs from CTX-injured muscles (day 1) using the Miltenyi MACS purification system in order to study the signaling pathways activated by IL-4 in these cells. This method of negative selection allowed us to isolate FAPs that were ≥98% pure; i.e. CD31−CD45−α7-integrin−SCA-1+ (Figure S4A). Similar to what was observed in vivo, stimulation of wild type FAPs with IL-4 induced cell proliferation, as evinced by the increase in cell number (~2-fold at 48 hours, and ~4-fold at 72 hours, Figure S4B). To elucidate the pathways that mediate the mitogenic effects of IL-4, we investigated the downstream signaling pathways that are activated by IL-4 in FAPs. Stimulation with IL-4 activated three major signaling pathways in FAPs: signal transducer of transcription 6 (STAT6), the protein kinase Akt, and weakly the extracellular signal–regulated protein kinases (ERKs) (Figure 5A). As expected, inhibition of PI3K by LY294002 prevented serine phosphorylation of Akt, whereas the MEK1 inhibitor PD98059 potently inhibited phosphorylation of ERK1/2 (Figure S4C). Moreover, consistent with the direct tyrosine phosphorylation of STAT6 proteins by Janus kinases (Shuai and Liu, 2003), treatment with PI3K or MEK1 inhibitors did not alter the phosphorylation status of STAT6 (Figure S4C). Lastly, activation of these three signaling pathways in FAPs showed an absolute dependence on IL-4Rα, as their phosphorylation and activation was abolished in IL-4Rα−/− FAPs (Figure 5A).
Figure 5. IL-4 promotes proliferation of FAPs in vitro.
(A) Activation of signaling pathways by IL-4 in WT, IL-4Rα−/− and STAT6−/− FAPs. (B) Quantification of cell number after stimulation of WT, IL-4Rα−/− and STAT6−/− FAPs with vehicle or IL-4 for 48 hours, n=4 per genotype and time point. For each genotype, cell number is normalized to its vehicle control. (C, D) BrdU incorporation in WT, IL-4Rα−/− and STAT6−/− FAPs after stimulation vehicle or IL-4 (10nM) for 24 hours. (E) Heat map of differentially expressed genes in WT and IL-4Rα−/− FAPs treated with vehicle or IL-4 for 24 hours; red-induced; blue-repressed. GO terms associated with DNA replication, cell cycle, and mitosis are enriched in the upregulated gene set, whereas those associated with triglyceride metabolism and lipid biogenesis are enriched in the downregulated gene set. (F) Quantitative RT-PCR analysis of cell cycle genes in WT, IL-4Rα−/− and STAT6−/− FAPs after stimulation with vehicle or IL-4 for 24 hours, n=4 per genotype and treatment. (G) Expression of myogenic genes in wild type MPs cultured with FAPs conditioned media. FAPs were stimulated with vehicle or IL-4 for 72 hours prior to collection of conditioned media, n=3 per treatment. Error bars represent SEM. See also Figure S4.
We next examined which pathway was required for IL-4-induced proliferation of FAPs. Consistent with the loss of IL-4 signaling, IL-4 failed to increase cell number or BrdU incorporation in IL-4Rα−/− FAPs (Figure 5B, C). Likewise, STAT6−/− FAPs failed to proliferate in response to IL-4, as quantified by the change in cell number and the rate of BrdU incorporation (Figure 5B, D). In contrast, treatment of FAPs with PI3K or MEK1 inhibitors did not significantly alter the stimulatory effects of IL-4 on BrdU incorporation (Figure S4D). Together, these findings suggest that STAT6 predominantly mediates the mitogenic effects of IL-4 in FAPs.
In order to understand how IL-4/STAT6 transcriptional axis regulates proliferation of FAPs, we performed microarray analyses from WT and IL-4Rα−/− FAPs stimulated with IL-4 for 24 hours. Pathway analysis of differentially expressed genes revealed that Gene Ontology (GO) terms associated with DNA replication, cell cycle, and mitosis were highly enriched in the upregulated gene set, whereas those associated with triglyceride metabolism and lipid biogenesis were significantly down regulated in WT FAPs treated with IL-4 (Table S2, 3, and 4). Quantitative RT-PCR analyses further verified that IL-4 induced the expression of a number of cell cycle genes, including Cdc20, Ccnd1, Cdk1, Cdc7, Cdca3, and Cenpa, in WT but not IL-4Rα−/− or STAT6−/− FAPs (Figure 5E). In aggregate, these data demonstrate that mitogenic actions of IL-4 enhance the proliferation of FAPs by promoting their entry and progression through the cell cycle.
In co-culture systems, FAPs can promote differentiation of myogenic progenitors (MPs) into myofibers (Joe et al., 2010). Therefore, we tested whether stimulation of FAPs with IL-4 might enhance their capacity to support myogenic differentiation. Wild type MPs were cultured with FAPs conditioned medium, and myogenic gene expression was measured 5 days later. Treatment of MPs with conditioned medium taken from IL-4 stimulated WT but not IL-4Rα−/− FAPs enhanced myogenic differentiation, as evidenced by increased expression of myogenic transcription factors and structural proteins (Figure 5G and S4E). Since conditioned medium taken from IL-4 stimulated FAPs did not stimulate proliferation of MPs in single myofiber preparations (Figure S4F), it suggests that factors derived from FAPs likely work in trans to enhance terminal differentiation of myoblasts.
IL-4 inhibits adipogenic differentiation of FAPs
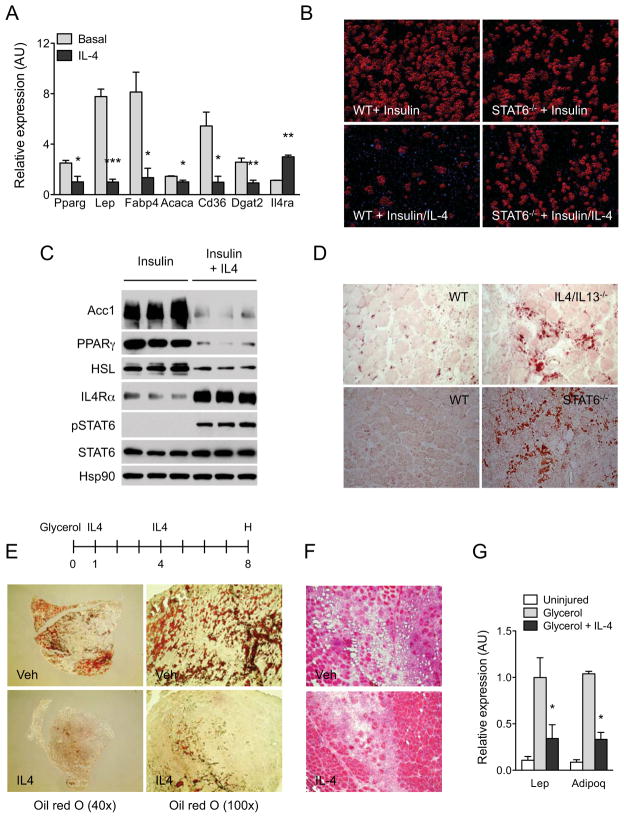
FAPs isolated from injured muscle can be induced to differentiate into mature adipocytes in culture (Joe et al., 2010; Uezumi et al., 2010). Since a large number of genes involved in triglyceride synthesis and adipogenesis were repressed by IL-4 in FAPs (Table S2, 4), we tested whether IL-4 could prevent differentiation of FAPs into adipocytes. As shown in Figure S5A, FAPs cultured in growth medium spontaneously differentiated into lipid-laden adipocytes, a process that was completely inhibited by IL-4. Moreover, quantitative RT-PCR analysis revealed that IL-4 suppressed the expression of a number of genes that are normally induced during adipogenesis (Rosen et al., 2000), including PPARγ, Lep, Fabp4, Acaca, Cd36, and Dgat2 (Figure 6A).
Figure 6. IL-4 inhibits adipogenic differentiation of FAPs in vitro and in vivo.
(A) Quantitative RT-PCR analysis of adipogenic genes in WT FAPs cultured for 7 days in the presence or absence of IL-4, n=3 per treatment. (B) Oil Red O staining of WT and STAT6−/− FAPs differentiated with insulin in the absence or presence of IL-4 (magnification, X290). (C) Immunoblot analysis for adipogenic proteins in WT FAPs treated with insulin or insulin plus IL-4. (D) Oil red O staining of regenerating TA muscles was performed on day 5 (magnification, X200). (E) TA muscles were analyzed on day 8 after glycerol injection by Oil Red O staining. After initiating glycerol-induced muscle damage, mice were injected with vehicle or IL-4 complex on days 1 and 4, n=4–6 per treatment. Representative sections stained for Oil Red O are shown. (F) Hematoxylin and eosin staining of TA muscle sections on day 8 after glycerol-induced injury (magnification, X200). (G) Quantitative RT-PCR analysis of adipocyte-specific genes in uninjured and glycerol injured TA muscles, n=4–6 per treatment. Error bars represent SEM. See also Figure S5.
Because IL-4 suppressed spontaneous differentiation of FAPs into adipocytes, we tested whether it could also inhibit adipogenic conversion of FAPs by insulin. As assessed by microscopy and staining for Oil Red O, treatment with IL-4 potently suppressed insulin-induced differentiation of FAPs into adipocytes (Figure 6B and S5B). This was further affirmed by expression analysis, which revealed a strong repressive effect of IL-4 on expression of adipogenic genes in WT FAPs (Figure S5C). Importantly, signaling via IL-4Rα and STAT6 was required because no significant inhibition of adipogenesis was observed in IL-4Rα−/− or STAT6−/− FAPs (Figure 6B and S5C). Congruent with these observations, treatment with IL-4 repressed expression of adipogenic proteins, such as PPARγ, Acc1, and HSL, by insulin (Figure 6C).
Since IL-4 signaling inhibits adipogenic differentiation of FAPs, we tested whether genetic disruption of IL-4/IL-13 signaling results in fatty infiltration of regenerating muscle. In agreement with published reports (Uezumi et al., 2010), we failed to detect a significant number of adipocytes in regenerating muscles of WT mice (Figure 6D). In contrast, clusters of Oil Red O positive adipocytes were readily detected in regenerating muscles of IL-4/IL-13−/− and STAT6−/− mice (Figure 6D).
IL-4 prevents fatty degeneration of muscle
FAPs are bipotential cells capable of facilitating skeletal muscle regeneration or contributing to its fatty degeneration (Joe et al., 2010; Uezumi et al., 2010). Since IL-4 serves as a molecular switch for controlling the fate of FAPs in regenerating muscle, we postulated that administration of IL-4 might prevent fatty degeneration of injured skeletal muscle. To test this hypothesis, we injected glycerol into the TA muscles of WT mice to induce fatty degeneration of skeletal muscle (Arsic et al., 2004). As reported, glycerol-injected TA muscles got infiltrated by Oil Red O positive cells (Uezumi et al., 2010), which stained positive for the adipocyte lipid droplet protein perilipin (Figure 6E and S5D). In contrast, fatty infiltration and perilipin staining was dramatically reduced in TA muscles of all mice (n=6) that received intraperitoneal injections of IL-4 (Figure 6E, F and S5D). Paralleling the decrease in fatty infiltration, expression of adipocyte-specific genes, such as leptin (Lep) and adiponectin (Adipoq), was reduced by ~65% in TA muscles of mice treated with IL-4 (Figure 6G).
FAPs clear necrotic debris in regenerating muscle
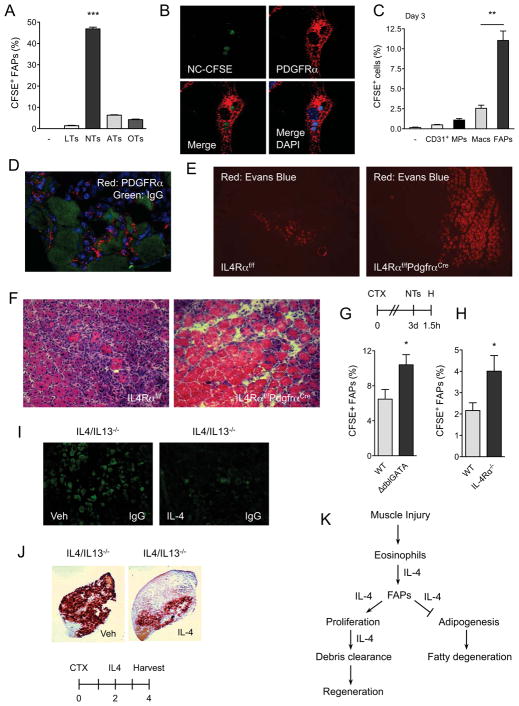
Although FAPs can support myogenic differentiation, this observation is unlikely to explain the histologic defects in muscle regeneration observed in mice lacking type 2 innate immunity, including ΔdblGATA, IL-4/IL-13−/− and IL-4Rα−/− mice. In these animals, a common histologic finding is the impaired clearance of necrotic muscle fibers, prompting us to examine whether FAPs might participate in the phagocytosis of necrotic debris. To investigate this idea, we purified FAPs from injured muscles of WT mice on day 3 and fed them CFSE-labeled live, necrotic, apoptotic, or opsonized thymocytes (Mukundan et al., 2009). Remarkably, FAPs were very efficient at phagocytizing necrotic thymocytes, as assessed by the percentage of FAPs that were positive for CFSE and their mean fluorescence intensity (Figure 7A, and S6A, B). This response was specific for necrotic thymocytes because live, apoptotic, and opsonized thymocytes were not phagocytized at any appreciable level by FAPs (Figure 7A and S6A, B). Moreover, confocal microscopy revealed that FAPs, which uniformly express PDGFRα (Uezumi et al., 2010), had indeed engulfed the necrotic thymocytes (Figure 7B and S6C).
Figure 7. FAPs phagocytize cellular debris in regenerating muscle.
(A) FAPs phagocytize necrotic cells in vitro. FAPs isolated from regenerating muscles were fed CFSE-labeled thymocytes for 1h, n=3. LTs: live thymocytes, NTs: necrotic thymocytes, ATs: apoptotic thymocytes, OTs: opsonized thymocytes. (B) Confocal microscopy of WT FAPs phagocytizing necrotic thymocytes (magnification, X1200). PDGFRα staining (red) identifies FAPs, whereas CFSE (green) represents necrotic thymocytes. Blue is DAPI. (C, D) FAPs are efficient at phagocytizing necrotic debris in vivo. (C) Three days after CTX-induced injury, TA muscles were injected with CFSE-labeled necrotic thymocytes and phagocytosis was enumerated 1h later. Data is plotted as percent uptake in the indicated cellular population, n=3. (D) Confocal microscopy of FAPs in regenerating muscle (magnification, X600). Red-PDGFRα, Green-IgG. (E, F) Representative images of IL-4Rαf/f and IL-4Rαf/fPdgfrαCre TA muscles 8 days after injury CTX, n=4–5 per genotype. (E) Evans Blue staining of CTX-injured muscles (magnification, X100). (F) Hematoxylin and eosin staining of representative section (magnification, X200). (G, H) Quantification of CFSE+ FAPs in WT, ΔdblGATA, and IL-4Rα−/− mice, n=6–8 per genotype. (I, J) IL-4 promotes clearance of necrotic debris in regenerating muscle. Representative images of IgG deposition (magnification, X580) (I) or calcified necrotic debris as visualized by Alizarin Red S stain (magnification, X40) (J), n=5 per treatment. (K) Model for functions of type 2 immunity in regenerating muscles. Error bars represent SEM. See also Figure S6 and S7.
We next asked whether FAPs were capable of phagocytizing necrotic cellular debris in the context of injured muscle, a setting where macrophages, the professional phagocytes implicated in clearance of necrotic debris (Arnold et al., 2007), are abundantly present. For these experiments, CFSE-labeled necrotic thymocytes were injected into injured TA muscles and uptake of necrotic debris by various cellular populations was quantified 1 hour later by flow cytometry. Surprisingly, compared to macrophages, FAPs were ~4-fold more efficient at phagocytizing necrotic thymocytes (Figure 7C and S6D-H). In contrast, no significant uptake of necrotic thymocytes was observed in MPs or CD31+ endothelial cells. Consistent with this observation, PDGFRα+ FAPs were observed circumscribing necrotic muscle fibers, which can be distinguished by non-specific deposition of IgG (Figure 7D). Together, these data demonstrate for the first time that FAPs are capable of phagocytizing necrotic debris in injured muscle, leading us to postulate that they might be the primary cell type responsible for clearance of necrotic fibers in injured muscle.
To test this hypothesis, we examined whether PDGFRα is selectively expressed in FAPs of injured muscle. Analysis of PdgfrαEGFP mice, which harbor a knock-in of H2B-eGFP fusion gene into the endogenous Pdgfra locus (Hamilton et al., 2003), showed that GFP was exclusively expressed in FAPs of regenerating muscles (Figure S7A). Based on these observations, we crossed IL-4Rαf/f with PdgfrαCre mice to selectively delete IL-4Rα in FAPs (designated IL-4Rαf/fPdgfrαCre) (Roesch et al., 2008). Flow cytometric analysis of mononuclear cells isolated from regenerating muscles confirmed the absence of IL-4Rα protein in FAPs but not CD45+ or CD31+ cells (Figure S7B). Notably, after CTX-mediated injury, skeletal muscles of IL-4Rαf/fPdgfrαCre mice recapitulated many of the histologic features observed in mice lacking type 2 immunity, including persistence of necrotic debris, larger areas of injury, and decreased formation of regenerative muscle fibers (Figure 7E–F). In aggregate, these findings suggest that regenerative actions of type 2 innate cytokines IL-4 and IL-13 are directly mediated via their actions in FAPs.
Finally, we examined the role of type 2 innate signals in clearance of necrotic debris in injured muscle. Three days after muscle injury with CTX, mice were injected with CFSE-labeled necrotic thymocytes and the presence of CFSE in FAPs was assessed 90 mins later. This time point was chosen because CFSE signal in WT FAPs peaked at 60 mins followed by a decline over the next hour (Figure S7C). In mice impaired in type 2 innate signaling, such as ΔdblGATA and IL-4Rα−/− mice, a higher percentage of FAPs were CFSE+, suggesting delayed clearance of necrotic debris in these cells (Figure 7G, H, and S7D). Consistent with this notion, administration of IL-4 into IL-4/IL-13−/− was sufficient to enhance clearance of necrotic muscle fibers, as assessed by deposition of IgG in the necrotic myofibers or staining for calcified myofibers (Figure 7I, J).
DISCUSSION
Although activation of the immune system is one of the earliest events that occur during tissue regeneration (Stoick-Cooper et al., 2007), the molecular mechanisms by which inflammatory signals regulate stem and progenitor cell biology remain poorly understood. The results presented here provide a clear mechanism by which type 2 innate signals, such as IL-4/IL-13, regulate the functions of the resident muscle progenitor cells, the fibro/adipocyte progenitors (FAPs), to facilitate muscle regeneration. This is the first demonstration of how cell extrinsic signals control the functionality of FAPs in regenerating muscle.
The functions performed by FAPs in injured muscles are context-dependent. In the experimental paradigm of CTX-mediated muscle regeneration, factors synthesized by FAPs support myogenic differentiation of MPs (Joe et al., 2010). In contrast, in the experimental paradigm of glycerol-induced muscle degeneration, FAPs contribute to adipogenic infiltration of injured muscle, resulting in its fatty degeneration (Uezumi et al., 2010). In both of these experimental models, cell non-autonomous signals control the ultimate fate adopted by FAPs in injured muscle. In this context, our current work demonstrates that injury leads to the formation of a dynamic niche that provides signals to regulate the functionality of FAPs. Specifically, muscle injury leads to the recruitment of eosinophils, which form the transitional niche for the proliferating FAPs via secretion of IL-4 (Figure 7K). In the presence of IL-4, FAPs proliferate as fibroblasts to support myogenesis by facilitating the clearance of necrotic debris. However, in its absence, FAPs fail to clear necrotic muscle fibers and differentiate into adipocytes, contributing to the persistence of cellular debris and fatty degeneration of skeletal muscle.
A surprising finding of our work was that IL-4/IL-13 signaling is not required in macrophages to mediate muscle regeneration. This result contrasts with previous reports that have suggested an important role for macrophages, in particular M2-type alternatively activated macrophages, in muscle repair after injury (Arnold et al., 2007; Ruffell et al., 2009). One of these studies employed CD11b-DTR mice to acutely deplete monocytes and macrophages after initiation of muscle injury. These authors observed that early depletion of CD11b+ cells resulted in a dramatic defect in muscle regeneration, whereas later depletion of CD11b+ cells had only a modest effect on muscle’s regenerative response (Arnold et al., 2007). Based on these findings, the authors concluded that early depletion of circulating monocytes, which give rise to recruited macrophages in injured muscles (Gordon and Taylor, 2005), prevents muscle regeneration. However, eosinophils, which share the myeloid developmental lineage with monocytes and macrophages (Rothenberg and Hogan, 2006), express both CD11b and F4/80 (encoded by Emr1) (McGarry and Stewart, 1991; Wu et al., 2011). In light of our genetic results with eosinophil-deficient ΔdblGATA mice, an alternative interpretation of these previous results would be that administration of diphtheria toxin to CD11b-DTR mice also depletes eosinophils, which we now know are required for myogenesis.
It is widely recognized that regeneration of injured tissues requires clearance of cellular debris (Stoick-Cooper et al., 2007). Previously, this function has been ascribed to macrophages, which rapidly infiltrate injured muscle (Arnold et al., 2007; Tidball and Villalta, 2010). Although persistence of necrotic debris was observed in mice lacking type 2 cytokines IL-4 and IL-13, deletion of its signaling receptor, IL-4Rα, in myeloid cells did not significantly impair debris clearance or muscle regeneration. These observations suggest the potential involvement of a non-myeloid cell in the removal of damaged muscle fibers. Indeed, multiple lines of evidence presented here demonstrate that FAPs, which rapidly proliferate after injury, are critically important for the clearance of injured muscle fibers. First, FAPs are capable of rapidly phagocytizing necrotic cellular debris in vitro, and can outcompete macrophages (4:1) for engulfment of necrotic debris in vivo. Second, the ability of FAPs to degrade necrotic debris is reduced in animals lacking type 2 innate signals. Third, deletion of IL-4Rα in FAPs is sufficient to impair the clearance of muscle debris, delaying muscle regeneration. Fourth, administration of IL-4 to injured IL-4/IL-13−/− mice, which normally harbor necrotic debris, enhances the clearance of necrotic muscle fibers. Taken together, these data delineate a novel function for FAPs during muscle’s regenerative response to injury, and demonstrate that type 2 cytokines (IL-4 and IL-13) control nearly all known aspects of FAPs’ functionality.
A previous report by Horsley et. al. had suggested that regenerating myofibers secrete IL-4 to stimulate myoblast fusion and muscle fiber growth (Horsley et al., 2003). However, the results reported here differ from those of Horsley et. al. in a number of important ways. First, using the highly sensitive and specific IL-4 reporter line, the 4get mice, we failed to detect expression of IL-4 in regenerating myofibers during the entire time course of muscle regeneration. Instead, we found that eosinophils were the dominant cell type competent for IL-4 secretion in regenerating muscles. Second, our results with IL-4Rαf/fPax7CreERT2 mice, which lack IL-4Rα in Pax7+ satellite cells, suggest that IL-4Rα signaling in Pax7-derived myoblasts and myofibers is not essential for regeneration of muscle after CTX injury. Rather, our data suggests a dominant role for IL-4/IL-4Rα signaling in the regulation of FAPs fate and actions in regenerating muscles.
From an evolutionary perspective, it is intriguing to speculate why type 2 innate immune responses are integrally involved in orchestrating tissue repair and regeneration. An insight into this issue is provided by the interactions between the mammalian host and helminth pathogens, the potent inducers of type 2 immunity (Anthony et al., 2007; Maizels and Yazdanbakhsh, 2003). Because helminth infections are ubiquitous (many vertebrate species are infected with tens to thousands of worms in the wild), chronic (lasting months to years), and tissue damaging (the migratory behavior of helminths causes severe tissue damage in the host), it has been postulated that anti-helminth immunity might have been grafted onto the host’s modules of tissue repair and remodeling (Allen and Wynn, 2011; Karp and Murray, 2012; Palm et al., 2012). Partial support for this hypothesis is provided by studies demonstrating the involvement of type 2 innate immunity in the repair of damaged epithelial surfaces, such as skin, colon or lung, via formation of scar tissue (Chen et al., 2012; Loke et al., 2007; Lucas et al., 2010; Seno et al., 2009). However, whether type 2 innate immune responses actively orchestrate complete regeneration of tissues is a question that previously had not been addressed. Our work provides the first example of how type 2 innate immune cells and their signals instruct the behavior of resident stem cells, such as FAPs in skeletal muscle, to orchestrate tissue regeneration. In aggregate, these findings suggest that activation of type 2 innate immunity confers tolerance to tissue damage by stimulating multiple cellular pathways of repair, ranging from the quick fix provided by scar formation to the complete regeneration of tissues, such as skeletal muscle. Finally, since venomous bites, toxin ingestion, and helminth infections cause injury to a variety of host tissues (Palm et al., 2012), it will be important to determine whether type 2 innate immune responses are universally employed to promote tolerogenic disposal of necrotic debris, thereby promoting the repair of damaged tissues. If so, it will suggest that activation of type 2 innate immunity is a general strategy employed by the host to defend tissues against damage caused by environmental insults and pathogens (Medzhitov et al., 2012; Palm et al., 2012).
EXPERIMENTAL PROCEDURES
Animals
All animal colonies were maintained in the CVRI’s pathogen-free barrier facility, and all experiments were performed in accordance with institutional guidelines. Male mice age 8–16 weeks old were used for the muscle regeneration studies. The following strains of mice were used in these studies: Balb/cJ (wild type), ΔdblGATA, IL-4/IL-13−/−, 4get, 4get ΔdblGATA, IL-4Rα−/−, STAT6−/−, IL-4Rαf/f, and IL-4Rαf/fLysMCre mice, all on the BALB/cJ background. YARG, IL-4Rαf/f, IL-4Rαf/fPdgfrαCre, IL-4Rαf/fPax7CreERT2, and Pdgfrα-GFP mice on C57Bl/6J background were also used in these studies. Muscle damage was induced by injection of 50 μl of 20 μM cardiotoxin Naja nigricollis (Calbiochem) directly into the mid-belly of TA muscle. For the muscle degeneration model, 50 μl of 50% glycerol solution in 1X HBSS was injected into the mid-belly of TA muscle. The contralateral side received PBS as vehicle control. IL-4 (2 μg, Peprotech) complexed to anti-IL-4 antibody (4 μg, 11B11, UCSF) was intraperitoneally injected on day 1 and 4 after initiation of muscle injury with glycerol. To assess muscle damage, mice were given an intraperitoneal injection of Evans Blue Dye (25 mg/kg) 24 hours prior to sacrifice. To induce Cre-mediated recombination, IL-4Rαf/fPax7CreERT2 mice were injected daily with tamoxifen (1.5 mg per 20g body weight) for 5 consecutive days. Mice were rested for two days prior to injection of CTX for muscle regeneration studies.
BrdU Labeling Studies
In vitro
FAPs were cultured in DMEM supplemented with 2% BSA for 24 hours in the presence or absence of IL-4 (10 nM). Cells were labeled with BrdU (10 μM, Sigma) during the last 30 minutes of stimulation.
In vivo
All BrdU labeling studies were performed 1 day after injection of CTX into the TA muscles. Mice were given an intraperitoneal injection of BrdU (100mg/kg) 3 hours prior to sacrifice.
Statistical analysis
All experiments were repeated at least 2–3 independent times, and the data is presented as mean ± s.e.m. Statistical significance was determined using the Student’s t-test. A P value of <0.05 was considered to be statistically significant, and is presented as * (P < 0.05), ** (P < 0.01), or *** (P < 0.001).
Supplementary Material
HIGHLIGHTS.
Type 2 cytokine signaling via IL-4Rα is required for muscle regeneration.
Eosinophils secrete IL-4 and are required for regeneration of injured muscles.
Myeloid or satellite cell IL-4Rα signaling is dispensable for muscle regeneration.
IL-4Rα signaling regulates the functions of fibro/adipogenic progenitors in muscle.
Acknowledgments
We thank members of the Chawla laboratory and B. Black for discussions, and A. Loh for comments on the manuscript. This work was supported by NIH grant DK081405, and an NIH Director’s Pioneer Award (DP1AR064158) to A.C, and by NIH grants R37 Merit Award (AG023806), P01 (AG036695), and NIH Director’s Pioneer Award (DP1 OD000392) to T.A.R., by California Institute for Regenerative Medicine Predoctoral Fellowship (TG2-01159) to AAM, and support from the NIH Training Grant (5T32HL007731) to J.E.M. All animal care and procedures were performed in accordance with Stanford University’s A-PLAC and UCSF’s IACUC guidelines. The authors declare that they have no competing financial interests.
Footnotes
Extended Experimental Procedures are included in the Supplemental Information.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen JE, Wynn TA. Evolution of Th2 immunity: a rapid repair response to tissue destructive pathogens. PLoS Pathog. 2011;7:e1002003. doi: 10.1371/journal.ppat.1002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony RM, Rutitzky LI, Urban JF, Jr, Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsic N, Zacchigna S, Zentilin L, Ramirez-Correa G, Pattarini L, Salvi A, Sinagra G, Giacca M. Vascular endothelial growth factor stimulates skeletal muscle regeneration in vivo. Mol Ther. 2004;10:844–854. doi: 10.1016/j.ymthe.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Bjornson CR, Cheung TH, Liu L, Tripathi PV, Steeper KM, Rando TA. Notch signaling is necessary to maintain quiescence in adult muscle stem cells. Stem Cells. 2012;30:232–242. doi: 10.1002/stem.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- Brack AS, Rando TA. Tissue-specific stem cells: lessons from the skeletal muscle satellite cell. Cell Stem Cell. 2012;10:504–514. doi: 10.1016/j.stem.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelli S, Rovere-Querini P. The immune system and the repair of skeletal muscle. Pharmacol Res. 2008;58:117–121. doi: 10.1016/j.phrs.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Chen F, Liu Z, Wu W, Rozo C, Bowdridge S, Millman A, Van Rooijen N, Urban JF, Jr, Wynn TA, Gause WC. An essential role for T(H)2-type responses in limiting acute tissue damage during experimental helminth infection. Nat Med. 2012;18:260–266. doi: 10.1038/nm.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302:1575–1577. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Rando TA. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell. 2002;3:397–409. doi: 10.1016/s1534-5807(02)00254-x. [DOI] [PubMed] [Google Scholar]
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- Hamilton TG, Klinghoffer RA, Corrin PD, Soriano P. Evolutionary divergence of platelet-derived growth factor alpha receptor signaling mechanisms. Mol Cell Biol. 2003;23:4013–4025. doi: 10.1128/MCB.23.11.4013-4025.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsley V, Jansen KM, Mills ST, Pavlath GK. IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell. 2003;113:483–494. doi: 10.1016/s0092-8674(03)00319-2. [DOI] [PubMed] [Google Scholar]
- Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp CL, Murray PJ. Non-canonical alternatives: what a macrophage is 4. J Exp Med. 2012;209:427–431. doi: 10.1084/jem.20120295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly-Welch AE, Hanson EM, Boothby MR, Keegan AD. Interleukin-4 and interleukin-13 signaling connections maps. Science. 2003;300:1527–1528. doi: 10.1126/science.1085458. [DOI] [PubMed] [Google Scholar]
- Kuang S, Gillespie MA, Rudnicki MA. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell. 2008;2:22–31. doi: 10.1016/j.stem.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Lepper C, Conway SJ, Fan CM. Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature. 2009;460:627–631. doi: 10.1038/nature08209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loke P, Gallagher I, Nair MG, Zang X, Brombacher F, Mohrs M, Allison JP, Allen JE. Alternative activation is an innate response to injury that requires CD4+ T cells to be sustained during chronic infection. J Immunol. 2007;179:3926–3936. doi: 10.4049/jimmunol.179.6.3926. [DOI] [PubMed] [Google Scholar]
- Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Muller W, Roers A, Eming SA. Differential roles of macrophages in diverse phases of skin repair. J Immunol. 2010;184:3964–3977. doi: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol. 2003;3:733–744. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- McGarry MP, Stewart CC. Murine eosinophil granulocytes bind the murine macrophage-monocyte specific monoclonal antibody F4/80. J Leukoc Biol. 1991;50:471–478. doi: 10.1002/jlb.50.5.471. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science. 2012;335:936–941. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- Mourikis P, Sambasivan R, Castel D, Rocheteau P, Bizzarro V, Tajbakhsh S. A critical requirement for notch signaling in maintenance of the quiescent skeletal muscle stem cell state. Stem Cells. 2012;30:243–252. doi: 10.1002/stem.775. [DOI] [PubMed] [Google Scholar]
- Mukundan L, Odegaard JI, Morel CR, Heredia JE, Mwangi JW, Ricardo-Gonzalez RR, Goh YP, Eagle AR, Dunn SE, Awakuni JU, et al. PPAR-delta senses and orchestrates clearance of apoptotic cells to promote tolerance. Nat Med. 2009 doi: 10.1038/nm.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI, Chawla A. Alternative macrophage activation and metabolism. Annu Rev Pathol. 2011;6:275–297. doi: 10.1146/annurev-pathol-011110-130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm NW, Rosenstein RK, Medzhitov R. Allergic host defences. Nature. 2012;484:465–472. doi: 10.1038/nature11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese TA, Liang HE, Tager AM, Luster AD, Van Rooijen N, Voehringer D, Locksley RM. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447:92–96. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch K, Jadhav AP, Trimarchi JM, Stadler MB, Roska B, Sun BB, Cepko CL. The transcriptome of retinal Muller glial cells. J Comp Neurol. 2008;509:225–238. doi: 10.1002/cne.21730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3:1009–1013. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes Dev. 2000;14:1293–1307. [PubMed] [Google Scholar]
- Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- Ruffell D, Mourkioti F, Gambardella A, Kirstetter P, Lopez RG, Rosenthal N, Nerlov C. A CREB-C/EBPbeta cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair. Proc Natl Acad Sci U S A. 2009;106:17475–17480. doi: 10.1073/pnas.0908641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seno H, Miyoshi H, Brown SL, Geske MJ, Colonna M, Stappenbeck TS. Efficient colonic mucosal wound repair requires Trem2 signaling. Proc Natl Acad Sci U S A. 2009;106:256–261. doi: 10.1073/pnas.0803343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano AL, Baeza-Raja B, Perdiguero E, Jardi M, Munoz-Canoves P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab. 2008;7:33–44. doi: 10.1016/j.cmet.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Shuai K, Liu B. Regulation of JAK-STAT signalling in the immune system. Nat Rev Immunol. 2003;3:900–911. doi: 10.1038/nri1226. [DOI] [PubMed] [Google Scholar]
- Stoick-Cooper CL, Moon RT, Weidinger G. Advances in signaling in vertebrate regeneration as a prelude to regenerative medicine. Genes Dev. 2007;21:1292–1315. doi: 10.1101/gad.1540507. [DOI] [PubMed] [Google Scholar]
- Tidball JG. Inflammatory cell response to acute muscle injury. Med Sci Sports Exerc. 1995;27:1022–1032. doi: 10.1249/00005768-199507000-00011. [DOI] [PubMed] [Google Scholar]
- Tidball JG, Villalta SA. Regulatory interactions between muscle and the immune system during muscle regeneration. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1173–1187. doi: 10.1152/ajpregu.00735.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- Wang YX, Rudnicki MA. Satellite cells, the engines of muscle repair. Nat Rev Mol Cell Biol. 2012;13:127–133. doi: 10.1038/nrm3265. [DOI] [PubMed] [Google Scholar]
- Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynes MW, Riches DWH. Induction of Macrophage Insulin-Like Growth Factor-I Expression by the Th2 Cytokines IL-4 and IL-13. J Immunol. 2003;171:3550–3559. doi: 10.4049/jimmunol.171.7.3550. [DOI] [PubMed] [Google Scholar]
- Yu C, Cantor AB, Yang H, Browne C, Wells RA, Fujiwara Y, Orkin SH. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J Exp Med. 2002;195:1387–1395. doi: 10.1084/jem.20020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.