Abstract
A systematic review and meta-analysis were performed examining the efficacy of aripiprazole for the treatment of irritability associated with autistic disorder in children and adolescents. Aripiprazole was found to be more effective in reducing irritability compared with placebo at 8 weeks, SMD −0.64 [−0.90 to −0.39, P < 0.00001] as determined by the Aberrant Behaviour Checklist irritability subscale (ABC-I). Pooled data from two eight week trials show that sedation is the most commonly reported adverse event. Statistically significant weight gain was also associated with aripiprazole, but there was a decrease in serum prolactin. Most adverse effects were deemed to be mild to moderate in severity. Four open trials and three case series all show support for aripiprazole in reducing the behavioural symptoms of autism. Long-term studies are required to determine the efficacy and safety of aripiprazole in autistic disorder in children.
Keywords: aripiprazole, autism, children
Introduction
Autism or autistic disorder is a lifelong condition characterised by a triad of impaired social interaction, communication difficulties and restricted repetitive behaviours. Manifestation is typically before the age of three.1,2 The term Autistic Spectrum Disorder (ASD) is usually taken to include the conditions autism, Aspergers syndrome and Pervasive Developmental Disorders not otherwise specified (PDD NOS). These three categories can be found within the Pervasive Developmental Disorder group of disorders in DSMIV1 along with the extremely rare Retts syndrome and childhood disintegrative disorder. In ICD-102, the categories atypical autism and PDD other, together comprise the DSM IV category of PDD-NOS. The prevalence of ASD has been estimated to be 1% of the child population3 with boys being on average 4 times more affected than girls.4,5 The majority of individuals suffer with lifetime morbidity,6 co-morbid disorders include intellectual impairment which has been estimated to occur in 30%7 of individuals and epilepsy in 5% to 44%.8
Behavioural and educational programmes remain the cornerstone of treatment for ASD. However, despite these interventions, challenging behaviours such as aggression, self-injurious behaviour, tantrums and quickly changing moods (irritability) can remain problematic. Psychopharmacology in autism is not primarily directed at the core social and communication components and for this reason it is considered relatively non-specific.9 The pharmacological approach is used to ameliorate the associated behavioural symptoms.10 Antipsychotics may have a role in alleviating these symptoms.
Before the introduction of atypical antipsychotics, haloperidol was frequently employed in the treatment of ASD although its use was limited by the high rate of extrapyramidal side effects (EPSEs). Haloperidol has been superseded in the past decade by the atypical antipsychotic risperidone. Three randomised controlled trials, several open label studies including a 6 month follow up study from a double-blind randomised controlled trial have demonstrated risperidone’s efficacy in reducing the severe behavioural problems associated with autism.11–13 The FDA in the USA licensed risperidone for the treatment of irritability associated with autism in 2006. Adverse effects of risperidone included increased appetite, weight gain, GI disturbances, sedation, hyperprolactinaemia, tremor and tachycardia.14
Lately interest has grown in the use of aripiprazole for reducing the behavioural symptoms of autism largely because it rarely causes hyperprolactinaemia and because it is believed to have a favourable metabolic profile than other atypical antipsychotics. This review examines the therapeutic efficacy and safety of aripiprazole in autism and also the pharmacodynamics and pharmacokinetics of the drug in children and adolescents.
Pharmacological Properties
Mechanism of action
Aripiprazole is 7-[4-[4-(2,3-dichlorophenyl)-1-piperazinyl] butoxy]-3,4-dihydrocarbostyril.
Aripiprazole is a partial agonist at dopamine D2 receptors.15 This means that aripiprazole binds to D2 receptors and prevents attachment of endogenous dopamine but at the same time stimulates D2 receptors (but to a lesser degree than by dopamine itself). When aripiprazole occupies 100% of D2 receptors the overall effect is to reduce receptor-mediated activity by around 70%.15
The action of partial agonists is to some extent dependent upon prevailing intensity of neurotransmitter function. For example, where there is a high level of dopamine production, aripiprazole will act so as to reduce net dopaminergic transmission. Where dopamine activity is low, aripiprazole is likely to act so as to increase dopaminergic transmission. This ability to act both as an antagonist and agonist has theoretical importance in schizophrenia where positive symptoms are thought to be related to excess dopamine and negative symptoms to dopaminergic hypofunction.
Both animal models16 and human studies17 suggest that dopamine hypofunction underlies some of the features of autism. Aripiprazole a partial dopamine agonist as described above acts essentially as an agonist in areas of low dopaminergic function and so may in theory improve symptoms of autism.
Aripiprazole’s observed effects on D2 receptors predict an atypical profile. This is because typical adverse effects tend only to occur when substantially more than 70% of receptors are blocked by dopamine antagonists.18 Aripiprazole seems unable to exert an effect functionally equivalent to this level of dopamine receptor antagonism and so typical adverse effects would not be expected. In fact, a Positron Emission Tomography (PET) study has confirmed that aripiprazole does not cause EPSE even when 95% of D2 and D3 receptors are occupied by the drug.19 In addition, animal experiments with aripiprazole support the prediction of atypicality in humans.20,21
Other receptor activities help predict adverse and side effects likely to be associated with aripiprazole. Aripiprazole is a partial agonist at 5HT1A receptors, an action which may protect against dopamine-mediated adverse effects and provide anxiolytic activity.22 It is also a potent antagonist at 5HT2A receptors and so may, in theory, offer protection against EPSE. Aripiprazole has only moderate activity at alpha-1 adrenergic receptors, histamine H1, receptors and serotonin 5HT2C receptors. So, a low incidence of (respectively) postural hypotension, sedation and weight gain might be predicted.23
Pharmacokinetics
Two studies (n = 9 and n = 11) have examined acute and chronic pharmacokinetics of aripiprazole in healthy males.24 Peak plasma levels were obtained 3.4–6.8 hours after single oral administration (2.8–3.8 hours after 14 days) and plasma half-life ranged from 47.4 hours to 68.1 hours (mean 60 hours). Steady state plasma levels were obtained after 14 days of continual dosing and plasma levels obtained were linearly related to dose given (5 mg to 30 mg a day). No clinically significant changes in physical examinations, clinical chemistry or ECG results were observed.
A third study25 examined the pharmacokinetics of aripiprazole in patients aged 10 to 17 years. Peak plasma levels were seen after two hours after 14 days of administration of aripiprazole at fixed doses of 20 mg, 25 mg and 30 mg. Thus it appears peak plasma levels are attained quicker in children and adolescents than with adults. Similarly it is reported that peak plasma concentrations are higher in 10–17 year olds than in healthy adults (435 to 653 ng/ml vs. 393 to 452 ng/ml respectively), perhaps because children and adolescents have a lower body weight and therefore a lower volume of distribution than adults.
Metabolism
Aripiprazole is metabolised by multiple enzymatic pathways involving the cytochromes CYP2D6 and CYP3A4.26 Inhibitors of either of these enzymes (quinidine and ketoconazole, respectively) are known to decrease clearance of aripiprazole and to increase aripiprazole plasma levels. Aripiprazole is not metabolised by CYP1A1, CYP1A2, CYP2C9, or CYP2C19 in vitro and so interactions with inducers or inhibitors of these enzymes are not expected. Aripiprazole seems not to affect the metabolism of drugs metabolised by CYP2D6, CYP2C9, CYP2C19 and CYP3A4. Aripiprazole appears not to interact with warfarin or omeprazole. In addition, aripiprazole appears not to interact in any way with lithium or valproate.26
Hepatic impairment seems not to have clinically important effects on aripiprazole metabolism27 and aripiprazole pharmacokinetics appear not to be influenced by age or gender.28,29 Dose adjustment is not necessary in renal impairment: pharmacokinetics do not differ between healthy volunteers and subjects with severe renal failure. Absorption of aripiprazole appears not to be influenced by food or gastric pH.
Methodology
Method of review
A systematic review and meta-analysis were performed. A literature search was conducted using the terms aripiprazole and autism using Embase, Medline, PsychInfo, Google Scholar and Pubmed search engines on 28 and 29 June 2010. There were no language restrictions and search engines from inception to date of search were employed. All Clinical trials and any case studies involving children or adolescents using aripiprazole for the treatment of autism or autistic disorder were selected. The reference lists of relevant publications were scrutinised then appropriate articles obtained in full.
Meta-analysis
Randomised controlled trials were included in the meta-analysis. Data was extracted on an intention to treat basis to calculate standardised mean difference (SMD) Hedges’ adjusted g and a random effects model was used to pool the studies. Two researchers (first author and an independent researcher) independently extracted and analysed the data for analysis. Discrepancies were resolved by consensus.
Details of selected studies
In all two randomised double-blind placebo-controlled trials, four open trials and three case series were identified. The two randomized double blind placebo-controlled trials were funded by the manufacturer of aripiprazole. See Tables 1, 2 and 3 for details of the studies.
Table 1.
Randomised double-blind placebo-controlled trials using aripiprazole to alleviate the behavioural symptoms of ASD in children and adolescents.
| Author (Year) | Study design | Study duration | Age Years Diagnosis |
Number of patients Recruited (completed) | Main efficacy measure | Outcome |
|---|---|---|---|---|---|---|
| Marcus et al 200930 | R db pc fixed dose 5 mg, 10 mg, 15 mg | 8 weeks | 6 to 17 Autistic disorder |
218 (178) | ABC-I CGI-I |
Significantly greater reduction in ABC-I scores for all doses compared with placebo |
| Owen et al 200931 | r db pc flexible dosing |
8 weeks | 6 to 17 Autistic disorder |
98 (75) | ABC-I CGI-I |
Significantly greater reduction in ABC-I scores for the aripiprazole group compared with placebo |
Abbreviations: r db pc, randomised double-blind placebo controlled trial; ABC-I, Aberrant Behaviour Checklist irritability subscale; CGI-I, Clinical Global Impression improvement score.
Table 2.
Non-randomised open trials using aripiprazole to alleviate the behavioural symptoms of ASD in children and adolescents.
| Author (Year) | Study design | Study duration | Age Years Diagnosis |
Number of patients Recruited (completed) |
Main efficacy measure | Outcome |
|---|---|---|---|---|---|---|
| Masi et al 200953 | Retrospective naturalistic follow up | All patients had aripiprazole for at least 12 weeks and were followed for 4–12 months | 4 to 15 autistic disorder PDD NOS | 34 10 had autistic disorder |
CGI-I | 4 of the autism group were deemed responders to treatment. |
| Valicenti-McDermott et al 200654 | Retrospective chart review | Range of aripiprazole usage was 6 to 15 months | 5 to 19 any developmental disability | 32 16 had autism +/− other co-morbid conditions |
CGI-I | Aripiprazole found to be effective in 5 of the 16 children with autism. |
| Gibson et al 200755 | Naturalistic retrospective | Aripiprazole for at least two weeks | 11 to 18 Any mental disorder |
45 3 had autism or PDD |
CGI-I CGI-S (chart extracted) |
No specific results given for autism. |
| Rugino et al 200556 | Retrospective chart review & Pros OL | Range of 14 to 210 days | 5 to 17 Any mental disorder |
17 6 had autism |
CGI-I & ABI-I CGI-S |
One autism patient reported as responding to treatment (reduction in aggression). |
Abbreviations: PROS OL, prospective open label study; CGI-I, Clinical Global Impression improvement score; CGI-S, Clinical Global Impression severity of illness score.
Table 3.
Case studies using aripiprazole to alleviate the behavioural symptoms of ASD in children and adolescents.
| Author (Year) | Number of patients | Age Years Diagnosis |
Main efficacy measure | Duration of treatment | Dose per day | Outcome |
|---|---|---|---|---|---|---|
| Huang et al 201057 | 3 | 1 = Male 9 yrs PDD-NOS |
CBCL | 5 mg | Improvements in behaviour | |
| 2 = Male 7 yrs autistic disorder | 5 mg | Improvement in behaviour | ||||
| 3 = Male 11 yrs PDD-NOS |
20 mg | Improvement in behaviour | ||||
| Stigler et al 200458 | 5 | 1 = male 11 yrs autistic disorder | CGI-I | 1 = 15 mg | 1 = Much improved | |
| 2 = male 18 yrs, autistic disorder and mod MR | 2 = 15 mg | 2 = Very much improved | ||||
| 3 = male 16 yrs Asperger’s |
3 = 10 mg | 3 = Much improved | ||||
| 4 = male 5 yrs autistic disorder | 4 = 10 mg | 4 = Very much improved | ||||
| 5 = male 11 yrs Autism |
5 = 10 mg | 5 = Much improved |
Abbreviations: CBCL, child behaviour checklist; CGI-I, Clinical Global Impression improvement.
Therapeutic Efficacy
At the time of writing only two short-term randomized, double-blind placebo controlled trials had been published.30,31 Both trials evaluated the efficacy of oral aripiprazole in reducing the irritability associated with autism. Both were conducted over 8 weeks and participants were 6 to 17 years of age. All patients had a diagnosis of autistic disorder as determined by the (DSM-IV-TR)1 and the Autism Diagnostic Interview-Revised (ADI-R).32 Patients also had severe behaviour disturbances such as aggression, self-injurious behaviour, tantrums and agitation either alone or two or more together. The primary outcome measure was the change in baseline in the Aberrant Behaviour Checklist irritability subscale (ABC-I)33 and patients had to have a baseline score of 18 or greater to be included in either study. Patients also had to have a Clinical Global Impressions-Severity (CGI-S)34 score of 4. The main secondary outcome measure was the Clinical Global Impression-Improvement (CGI-I) scale.35 The exclusion criteria were PDD, PDD-NOS, Rett syndrome and childhood disintegrative disorder as well as schizophrenia, psychosis, bipolar disorder and major depression.
The ABC-I scale is a 58 item scale used to measure an assortment of behaviours in individuals with a learning disability both in community and in institutions.33,36–38 It is an observer rating scale and is not dependent on underlying diagnoses. It is administered by parents or carers. The irritability subscale of the ABC consists of 15 items and rates behaviours such as self injury, aggressiveness to others, inappropriate screaming, temper tantrums, irritability, depressed mood and quickly changing moods. Each item is rated 0 to 3 with 0 being “not at all a problem” and 3 “the problem is severe in degree”. The ABC-I has been used in other intervention studies to assess the effectiveness of drugs on behaviour in ASD.11,12,39 18 is taken to be above the norm.
For both studies aripiprazole produced significantly greater improvement compared with placebo in the two rating scales at week 8.
The Marcus et al study30 was a fixed dose trial comparing aripiprazole 5 mg/day, 10 mg/day, 15 mg/day and placebo. At week one the aripiprazole 15 mg/day group produced significantly greater improvements than placebo on the ABC-I scale. From week 2 all other strengths of aripiprazole showed significant improvements over placebo.
The Owen study31 used a flexible dosing regime for aripiprazole. At week one it was reported that there was a significant improvement in the ABC-I scale for patients in the active arm when aripiprazole was given at 2 mg/day. At week 8 aripiprazole produced significant reductions in the ABC-I scores compared with placebo (−12.9, −5.0 respectively, [95% Cl: −11.7 to −4.1]; P < 0.001, effect size −0.87). The CGI scale indicated from week one up to week 8 significant improvements with aripiprazole compared with placebo. At week eight the reported mean CGI scores for aripiprazole and placebo were 2.2 vs. 3.6 respectively [95% Cl: −1.9 to −1.0]; P < 0.001. Response to treatment was regarded as a 25% or greater reduction in the ABC-I scores and a score of 2 or less on the CGI-I scale. Response to treatment as judged both by the ABC-I and CGI-I scales became statistically significant from week 2 for the aripiprazole group through to week 8 (30.4% vs. 4.1% at week 2 and 52.2% vs. 14.3% at week 8). At the end of the study 5% of patients were receiving 2 mg/day, 33% 5 mg/day, 41% 10 mg/day and 21% 15 mg/day.
Meta-analysis of the randomised controlled trials
Using a random effect model aripiprazole was shown to be significantly more effective than placebo in reducing irritability as judged by the ABC-I rating scale. See Figure 1. A moderate effect size was observed.
Figure 1.
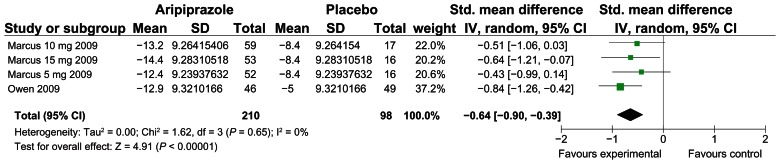
Forest plot of aripiprazole vs. placebo, improvement in ABC-I.
Safety and Tolerability
No participants died during the period of the two randomized double-blind controlled trials. No significant changes in the ECG measurements were observed compared with placebo. No seizures were reported in the active treatment arms of the two trials.
Marcus and colleagues30 reported two serious adverse events: presyncope with unsteady gait and eyes rolling back on day 17 of treatment with aripiprazole 5 mg (which was judged as being mild in intensity) and aggression occurring 1 day after discontinuing aripiprazole 10 mg apparently because of increased agitation. This incident of aggression was believed to be unrelated to study medication. Owen and co-workers reported that there were no serious adverse events during the study or 30 days after discontinuation of study treatment.31
Pooling the adverse event data from the two trials, sedation is the most commonly reported event. Please see Table 4. Table 4 records the pooled results from tables available in both the RCT papers. However the text in the Marcus and co-workers study states that with regards to treatment-emergent EPS this occurred in 6 (11.8%) of the placebo group, and 12 (23.1%), 13 (22.0%) and 12 (22.2%) of the aripiprazole 5 mg/day, 10 mg/day and 15 mg/ day groups respectively.
Table 4.
Pooled top 10 treatment-emergent adverse-events occurring in 5% or more of patients.
| AE | Placebo (n = 101) n (%) | Aripiprazole (n = 212) n (%) |
|---|---|---|
| Sedation | 4 (3.96) | 44 (20.75) |
| Fatigue | 2 (1.98) | 35 (16.51) |
| Vomiting | 6 (5.94) | 29 (13.68) |
| Increased appetite | 7 (6.93) | 27 (12.74) |
| Somnolence | 4 (3.96) | 22 (10.38) |
| Tremor | 0 (0) | 21 (9.91) |
| Pyrexia | 1 (0.99) | 19 (8.96) |
| Drooling | 0 (0) | 19 (8.96) |
| Headache | 10 (9.90) | 16 (7.55) |
| Extrapyramidal disorder | 0 (0) | 13 (6.13) |
Marcus and collegues30 reported that 21 participants withdrew from their trial because of adverse events: 4 (7.7%) in the placebo group, 5 (9.4%) in the aripiprazole 5 mg/day group, 8 (13.6%) in the 10 mg/day group and 4 (7.4%) in 15 mg/day group. Owen et al31 recorded that 5 (10.6%) in the aripiprazole group and 3 (6.0%) of patients in the placebo group withdrew due to AE. Marcus and colleagues reported the top three reasons for discontinuing study treatments were sedation (placebo n = 0, aripiprazole 5 mg n = 1, 10 mg n = 4, 15 mg n = 2) drooling and tremor.30
In the Owen trial31 there were 7(14.9%) cases of EPS in the aripiprazole group and 4 (8.0%) in the placebo group. One patient in the aripiprazole group received benzatropine. Three scales were used to measure EPS, Simpson-Angus Scale40 (SAS), Abnormal Involuntary Movement Scale41 (AIMS) and Barnes Akathisia Rating Scale42 (BAS). All scales revealed no statistical difference between aripiprazole and placebo (SAS −0.6 vs. −0.5 P = 0.763, AIMS −1.1 vs. −0.5 P = 0.115, BAS −0.1 vs. −0.1 P = 0.699).
In the Marcus et al30 trial there were 37 (22.4%) cases of EPS in the aripiprazole groups and 6 cases (11.8%) in the placebo group. 10 patients received benzatropine in the aripiprazole group and one in the placebo group. [Placebo, propranolol n = 1, EPS n = 6 (11.8%); aripiprazole 5 mg, propranolol n = 2, benzatropine n = 2, EPS = 12 (23.1%); aripiprazole 10 mg, benzatropine n = 1, EPS n = 13 (22.0%), aripiprazole 15 mg, benzatropine n = 5, EPS = 12 (22.2%)]. Aripiprazole was reported as improving AIMS score from baseline to endpoint last observation carried forward (LOCF) compared with placebo (placebo +0.2; aripiprazole 5 mg daily −0.2; 10 mg daily −0.1; 15 mg daily −0.2 all P < 0.05). SAS total scores were significantly different for aripiprazole 10 mg daily (+0.7) and placebo (−0.4) P = 0.006 but not 5 mg daily or 15 mg daily and placebo. No statistically significant differences were discovered in the BAS between any of the aripiprazole groups and placebo.
All aripiprazole groups in the Marcus et al study30 showed significant weight gain. However, no participants withdrew from the study due to increases in weight. Mean weight changes reported at week 8 were placebo +0.3 kg, aripiprazole 15 mg/day +1.5 kg, aripiprazole 5 mg/day and 10 mg/day + 1.3 kg. Similarly, Owen and co-workers found clinically significant increases in weight of 7% or more in the aripiprazole group compared with placebo (LOCF 28.9% vs. 6.1% of patients P < 0.01). Mean weight changes reported were +2.0 kg for the aripiprazole group compared with 0.8 kg for the placebo arm. One patient withdrew because of weight and appetite increases.31
Owen and collegues reported no statistically significant differences in mean change from baseline to endpoint in the median values for fasting triglycerides, low-density lipoprotein, high density lipoprotein, and total cholesterol.31 Marcus et al30 indicated that all patients had normal low-density lipoprotein and fasting total cholesterol throughout the study. However, with regards to fasting triglycerides 3.6% of patients in the placebo group, 11.5% in the aripiprazole 5 mg daily group, 3.1% in the aripiprazole 10mg daily group and 10% in the aripiprazole 15 mg daily group had greater than reference range of 120 mg/dL for females or 160 mg/dL for males. It was also noted at baseline that two patients had lower than 30 mg/dL of high-density lipoprotein, at the end of the study two participants in the aripiprazole 15 mg daily and one in the 5 mg daily group had lower than normal values. Both studies found that aripiprazole, at all doses, caused a statistically significant fall in serum prolactin levels compared with placebo at the end of the trials.
Naturalistic open studies and case studies showed support for aripiprazole in reducing behavioural symptoms of autism but in a minority of patients. Response rates varied from 16.7% to 40.0% in the open studies.43–45 All the studies included patients having other PDD diagnoses as well as those having autistic disorder. Where possible data were extracted that pertains to patients who have a diagnosis of autistic disorder. See Tables 2 and 3 for further details.
Place in Therapy of Aripiprazole in the Management of Autistic Disorder
The mainstay of treatment and management of autistic disorder in children and adolescents are behavioural and educational therapies. These interventions may include speech and language therapy, occupational therapy (e.g. for sensory difficulties such as sensitivity to touch and light), social skills training and psychological behavioural programmes. The educational programme should be offered in a structured environment and be adaptable according to the needs of the child. The behavioural difficulties that can occur in autistic disorder are varied both in presentation and severity of symptoms. This dictates that at times medication is needed to treat the challenging behaviour that this heterogeneous condition elicits. Clinical experience (of the authors) has shown that pharmacological intervention can enable patients to access more meaningful behavioural and educational therapies. What can complicate the presentation of autistic disorder is the high prevalence of co-morbid conditions such as Attention Deficit Hyperactivity Disorder (ADHD), epilepsy, anxiety, Obsessive-Compulsive Disorder (OCD) and mood disorders.46 Where these disorders occur it is important that they are treated appropriately. Autistic disorder is also associated with a high prevalence of a learning disability which can further complicate the picture.47
The restricted repetitive behaviours associated with autistic disorder have usually been targeted for treatment with selective serotonin reuptake inhibitors (SSRIs). The reason for this is more than likely due to the similarities between the repetitive behaviours seen in autistic disorder and OCD where SSRIs have known efficacy. Case reports and open label trials suggests that SSRIs maybe beneficial in reducing repetitive behaviours. The few RCT trials that have been conducted in children have involved small numbers and have produced mixed results. The trial by Hollander et al 200559 showed liquid fluoxetine to be more effective than placebo in reducing repetitive behaviours in children. The RCT by King et al 200948 showed citalopram to be no better than placebo but was associated with more side effects when compared with placebo such as impulsiveness, hyperactivity and increased energy levels. The Cochrane Collaboration have carried out a systematic review on SSRIs in ASD49 and they concluded that there is no evidence that SSRIs are effective in childhood autism and that the emerging evidence is that they are not effective and may cause harm.
Risperidone was the first drug to be given a licence for the treatment of irritability associated with autistic disorder in children and adolescents. Two 8 week double-blind trials11,50 and an open label 6 month extension of the RUPP trial43 demonstrated the efficacy of risperidone in this population. The ABC-I rating scale was used as the main primary measure in all these trials. To the authors’ knowledge there are no direct studies comparing risperidone and aripiprazole in the treatment of autism. However, the two 8 week trials of risperidone reported effect sizes of 1.211 and 0.7.50 In the aripiprazole study by Owen and colleagues31 they report an effect size of 0.87. Our meta-analysis suggested an effect size of 0.64. Aripiprazole and risperidone each has a moderate to large effect size indirectly suggesting similar efficacy.
The prescribing information for risperidone indicates somnolence and increase in appetite are the most frequently occurring treatment-emergent adverse effects. (Risperidone N = 76, placebo N = 80, somnolence 88.2% vs 28.8%, increased appetite 64.5% vs. 23.8%) Weight increases occur in 6.6% of patients compared to none in the placebo group. Martin et al reported that the weight changes that risperidone produces is over what one would expect in the normal development in children and adolescents.51 Actual weight changes reported in the RUPP were 2.7 kg for the risperidone group and 0.8 kg for placebo (P < 0.001). Not surprisingly it was reported that the increase in weight in the risperidone group was associated with increased appetite. The RUPP 6 month trial43 showed a mean increase of 5.1 kg in weight from baseline.
The PI of risperidone also indicates tachycardia occurred in 9.2% of patients and none in placebo, dystonia 15.8% and 7.5%, parkinsonism 10.5% and none in placebo.14
Of the atypical antipsychotics, risperidone has one of the highest risks of causing increases in prolactin. The consequences of chronically raised prolactin in adults is well established with adverse events such as amenorrhoea, decrease in fertility, galactorrhoea and gynaecomastia.44 There are also concerns about decreases in bone mineral density and osteoporosis.44 With regards to children and hyperprolactinaemia the picture is far less clear however concerns do remain. In a separate publication of the RUPP trial looking at prolactin levels, a reported two to four fold increase in mean serum levels was associated with risperidone treatment.52 Patients were followed over 22 months. Anderson et al52 state that if clinical signs of raised prolactin are evident reducing the dose or switching to an antipsychotic that has a lower risk of hyperprolactinaemia are options.
Aripiprazole was found not to be associated with increases in serum prolactin levels, but actually decreased prolactin. This is one of the advantages that aripiprazole has over risperidone in the treatment of irritability associated with autism.
No long term trials of aripiprazole have as yet been published to the authors knowledge so the place of aripiprazole in the long term treatment of autism is yet to be determined. Progress in the treatment of autism is only likely to come about through rigorous, randomised, controlled trials of promising agents in short and long-term treatment. Direct comparisons of active drugs are also required to determine relative efficacy and tolerability.
Dosage Recommendations
In November 2009 aripiprazole was granted approval by the FDA in the USA to be used in the treatment of irritability associated with autistic disorder in paediatric patients aged 6 to 17 years.45 The American prescribing information states that aripiprazole should be started at 2 mg per day. Thereafter it maybe increased to 5 mg per day. Dose increments no higher than 5 mg per day over at least one week maybe made up to 15 mg per day. Aripiprazole is available as 1 mg/ml oral solution which can facilitate small dose increments tailored to the needs of individual patients.
Conclusion
Our meta-analysis found aripiprazole to be more effective in reducing irritability compared with placebo at 8 weeks in children and adolescents with an effect size of −0.64 [−0.90 to −0.39, P < 0.00001] as determined by the Aberrant Behaviour Checklist irritability subscale (ABC-I).
From two eight week randomised double-blind trials 84.3%30 (Marcus et al) and 91.5%31 (Owen et al) of patients receiving aripiprazole had reported at least one adverse effect. Most adverse effects were deemed to be mild to moderate in severity.
It is suggested that aripiprazole should be reserved for treating irritability associated with autism in children and adolescents where behavioural and educational therapies alone have failed to have an impact and it is felt that the behaviour is severe enough to warrant the use of an antipsychotic. Currently most evidence lies with risperidone however, if risperidone is not effective and/or is not tolerated aripiprazole should be considered. Certainly, if the avoidance of hyperprolactinaemia is important aripiprazole could be thought of first line.
The long term efficacy and safety of aripiprazole in autism is yet to be determined.
Footnotes
Disclosures
This manuscript has been read and approved by all authors. This paper is unique and not under consideration by any other publication and has not been published elsewhere. Professor Taylor has received consultancies fees, lecturing honoraria and/ or research funding from AstraZeneca, Janssen-Cilag, Servier, Sanofi-Aventis, Lundbeck, Bristol-Myers Squibb, Novartis, Eli Lilly and Wyeth. Mrs Douglas-Hall, Dr Curran and Miss Bird do not have any conflicts of interest. The peer reviewers report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th edition. Washington DC: American Psychiatric Association; 1994. pp. 124–7. [Google Scholar]
- 2.World Health Organisation. The ICD-10 Classification of Mental and Behavioural Disorders. Switzerland: World Health Organisation; 2003. [Google Scholar]
- 3.Baird G, Cass H, Slonims V. Diagnosis of autism. BMJ. 2003;327:488–93. doi: 10.1136/bmj.327.7413.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fombonne E. Epidemiological surveys of autism and other pervasive developmental disorders: an update. J Autism Dev Disord. 2003;33:365–82. doi: 10.1023/a:1025054610557. [DOI] [PubMed] [Google Scholar]
- 5.Fombonne E. Epidemiology of pervasive developmental disorders. Pediatr Res. 2009;65:591–8. doi: 10.1203/PDR.0b013e31819e7203. [DOI] [PubMed] [Google Scholar]
- 6.Howlin P, Goode S, Hutton J, et al. Adult outcome for children with autism. J Child Psychol Psychiatry. 2004;45:212–29. doi: 10.1111/j.1469-7610.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- 7.Fombonne E. Autism and newborn encephalopathy. Dev Med Child Neurol. 2006;48:84. doi: 10.1017/S0012162206000193. [DOI] [PubMed] [Google Scholar]
- 8.Tuchman R, Rapin I. Epilepsy in autism. Lancet Neurol. 2002;1:352–8. doi: 10.1016/s1474-4422(02)00160-6. [DOI] [PubMed] [Google Scholar]
- 9.West L, Waldrop J, Brunssen S. Pharmacologic treatment for the core deficits and associated symptoms of autism in children. J Pediatr Health Care. 2009;23:75–89. doi: 10.1016/j.pedhc.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Posey DJ, Stigler KA, Erickson CA, et al. Antipsychotics in the treatment of autism. J Clin Invest. 2008;118:6–14. doi: 10.1172/JCI32483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCracken JT, McGough J, Shah B, et al. Risperidone in children with autism and serious behavioral problems. N Engl J Med. 2002;347:314–21. doi: 10.1056/NEJMoa013171. [DOI] [PubMed] [Google Scholar]
- 12.Shea S, Turgay A, Carroll A, et al. Risperidone in the treatment of disruptive behavioral symptoms in children with autistic and other pervasive developmental disorders. Pediatrics. 2004;114:e634–41. doi: 10.1542/peds.2003-0264-F. [DOI] [PubMed] [Google Scholar]
- 13.Nagaraj R, Singhi P, Malhi P. Risperidone in children with autism: randomized, placebo-controlled, double-blind study. J Child Neurol. 2006;21:450–5. doi: 10.1177/08830738060210060801. [DOI] [PubMed] [Google Scholar]
- 14.Janssen-Cilag Ltd. Risperidal (Risperidone tablets/solution) Prescribing Information Sheet Revised. 2009. http://www.janssen.com/products.html.
- 15.Burris KD, Molski TF, Xu C, et al. Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J Pharmacol Exp Ther. 2002;302:381–9. doi: 10.1124/jpet.102.033175. [DOI] [PubMed] [Google Scholar]
- 16.Mittleman G, Goldowitz D, Heck DH, et al. Cerebellar modulation of frontal cortex dopamine efflux in mice: relevance to autism and schizophrenia. Synapse. 2008;62:544–50. doi: 10.1002/syn.20525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ernst M, Zametkin AJ, Matochik JA, et al. Low medial prefrontal dopaminergic activity in autistic children. Lancet. 1997;350:638. doi: 10.1016/s0140-6736(05)63326-0. [DOI] [PubMed] [Google Scholar]
- 18.Tauscher J, Kufferle B, Asenbaum S, et al. Striatal dopamine-2 receptor occupancy as measured with [123I]iodobenzamide and SPECT predicted the occurrence of EPS in patients treated with atypical antipsychotics and haloperidol. Psychopharmacology. 2002;162:42–9. doi: 10.1007/s00213-002-1082-6. [DOI] [PubMed] [Google Scholar]
- 19.Yokoi F, Grunder G, Biziere K, et al. Dopamine D2 and D3 receptor occupancy in normal humans treated with the antipsychotic drug aripiprazole (OPC 14597): a study using positron emission tomography and [11C] raclopride. Neuropsychopharmacology. 2002;27:248–59. doi: 10.1016/S0893-133X(02)00304-4. [DOI] [PubMed] [Google Scholar]
- 20.Hirose T, Uwahodo Y, Yamada S. Efficacy and favourable side-effect profile of aripiprazole determined in rates with apomorphine-induced stereotypy, catalepsy, and ptosis induction. Int J Neuropsychopharmacol. 2000;3:S131. [Google Scholar]
- 21.Nakai S, Hirose T, Uwahodo Y, et al. Diminished catalepsy and dopamine metabolism distinguish aripiprazole from haloperidol or risperidone. Eur J Pharmacol. 2003;472:89–97. doi: 10.1016/s0014-2999(03)01857-0. [DOI] [PubMed] [Google Scholar]
- 22.Jordan S, Koprivica V, Chen R, et al. The antipsychotic aripiprazole is a potent, partial agonist at the human 5-HT1A receptor. Eur J Pharmacol. 2002;441:137–40. doi: 10.1016/s0014-2999(02)01532-7. [DOI] [PubMed] [Google Scholar]
- 23.McQuade RD, Burris KD, Jordan SKT, et al. Aripiprazole: a dopamine-serotonin system stabilizer. Int J Neuropsychopharmacol. 2002;5( Suppl 1):S176. [Google Scholar]
- 24.Mallikaarjun S, Salazar DE, Bramer SL. Pharmacokinetics, tolerability, and safety of aripiprazole following multiple oral dosing in normal healthy volunteers. J Clin Pharmacol. 2004;44:179–87. doi: 10.1177/0091270003261901. [DOI] [PubMed] [Google Scholar]
- 25.Findling RL, Kauffman RE, Sallee FR, et al. Tolerability and pharmacokinetics of aripiprazole in children and adolescents with psychiatric disorders: an open-label, dose-escalation study. J Clin Psychopharmacol. 2008;28:441–6. doi: 10.1097/JCP.0b013e31817dd520. [DOI] [PubMed] [Google Scholar]
- 26.Citrome L, Josiassen R, Bark N, et al. Pharmacokinetics of aripiprazole and concomitant lithium and valproate. J Clin Pharmacol. 2005;45:89–93. doi: 10.1177/0091270004269870. [DOI] [PubMed] [Google Scholar]
- 27.Mallikaarjun S, Tammara BK, Salazar DE. The effects of hepatic impairment on the pharmacokinetics of aripiprazole. Clin Pharmacol Ther. 2002;71 [Google Scholar]
- 28.Mallikaarjun S, Tammara BK, Salazar DE. The effects of age and gender on the pharmacokinetics of aripiprazole. Clin Pharmacol Ther. 2002;71 [Google Scholar]
- 29.Blumer JL, Findling R, Kauffman R. Pharmacokinetics, tolerability and safety of aripiprazole in children and adolescents with conduct disorder. Clin Pharmacol Ther. 2002;71 [Google Scholar]
- 30.Marcus RN, Owen R, Kamen L, et al. A placebo-controlled, fixed-dose study of aripiprazole in children and adolescents with irritability associated with autistic disorder. J Am Acad Child Adolesc Psychiatry. 2009;48:1110–9. doi: 10.1097/CHI.0b013e3181b76658. [DOI] [PubMed] [Google Scholar]
- 31.Owen R, Sikich L, Marcus RN, et al. Aripiprazole in the treatment of irritability in children and adolescents with autistic disorder. Pediatrics. 2009;124:1533–40. doi: 10.1542/peds.2008-3782. [DOI] [PubMed] [Google Scholar]
- 32.Lord C, Rutter M, Le CA. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 33.Aman MG, Singh NN, Stewart AW, et al. The aberrant behavior checklist: a behavior rating scale for the assessment of treatment effects. Am J Ment Defic. 1985;89:485–91. [PubMed] [Google Scholar]
- 34.Guy W. The Clinical Global Impressions Scale. In: Guy W, editor. ECDEU Assessment Manual for Psychopharmacology Rev Ed. Rockville, MD: National Institute of Mental Health; 1976. pp. 157–69. [Google Scholar]
- 35.Kadouri A, Corruble E, Falissard B. The improved Clinical Global Impression Scale (iCGI): development and validation in depression. BMC Psych. 2007;7:7. doi: 10.1186/1471-244X-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aman MG, Singh NN. Aberrant behavior checklist: ABC. East Aurora, NY: Slosson Educational Publications; 1986. [Google Scholar]
- 37.Marshburn EC, Aman MG. Factor validity and norms for the aberrant behavior checklist in a community sample of children with mental retardation. J Autism Dev Disord. 1992;22:357–73. doi: 10.1007/BF01048240. [DOI] [PubMed] [Google Scholar]
- 38.Brown EC, Aman MG, Havercamp SM. Factor analysis and norms for parent ratings on the Aberrant Behavior Checklist-Community for young people in special education. Res Dev Disabil. 2002;23:45–60. doi: 10.1016/s0891-4222(01)00091-9. [DOI] [PubMed] [Google Scholar]
- 39.Fido A, Al-Saad S. Olanzapine in the treatment of behavioral problems associated with autism: an open-label trial in Kuwait. Med Princ Pract. 2008;17:415–8. doi: 10.1159/000141508. [DOI] [PubMed] [Google Scholar]
- 40.Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand. 1970;212:11–9. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 41.National Institute of Mental Health. Abnormal Involuntary Movement Scale (AIMS). 1974. Washington, DC: US Government Printing Office; 1974. (US Public Health Service Publication No MH-9-17) [Google Scholar]
- 42.Barnes TRE. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–6. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- 43.Research Units on Pediatric Psychopharmacology Autism Network. Risperidone treatment of Autistic Disorder: longer-term benefits and blinded discontinuation after 6 months. Am J Psychiatry. 2005;162:1361–9. doi: 10.1176/appi.ajp.162.7.1361. [DOI] [PubMed] [Google Scholar]
- 44.Coker F, Taylor D. Antidepressant-induced hyperprolactinaemia: incidence, mechanisms and management. CNS Drugs. 2010;24:563–74. doi: 10.2165/11533140-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 45.Bristol-Myers Squibb. Abilify tablets and oral solution. Prescribing Information Revised. 2009. http://packageinserts.bms.com/pi/pi_abilify.pdf.
- 46.Simonoff E, Pickles A, Charman T, et al. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry. 2008;47:921–9. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- 47.O’Brien G, Pearson J. Autism and learning disability. Autism. 2004;8:125–40. doi: 10.1177/1362361304042718. [DOI] [PubMed] [Google Scholar]
- 48.King BH, Hollander E, Sikich L, et al. Lack of efficacy of citalopram in children with autism spectrum disorders and high levels of repetitive behavior: citalopram ineffective in children with autism. Arch Gen Psychiatry. 2009;66:583–90. doi: 10.1001/archgenpsychiatry.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williams K, Wheeler DM, Silove N, et al. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD) Cochrane Database Syst Rev. 2010;8:CD004677. doi: 10.1002/14651858.CD004677.pub2. [DOI] [PubMed] [Google Scholar]
- 50.Pandina GJ, Bossie CA, Youssef E, et al. Risperidone improves behavioral symptoms in children with autism in a randomized, double-blind, placebo-controlled trial. J Autism Dev Disord. 2007;37:367–73. doi: 10.1007/s10803-006-0234-7. [DOI] [PubMed] [Google Scholar]
- 51.Martin A, Scahill L, Anderson GM, et al. Weight and leptin changes among risperidone-treated youths with autism: 6-month prospective data. Am J Psychiatry. 2004;161:1125–7. doi: 10.1176/appi.ajp.161.6.1125. [DOI] [PubMed] [Google Scholar]
- 52.Anderson GM, Scahill L, McCracken JT, et al. Effects of short- and long-term risperidone treatment on prolactin levels in children with autism. Biol Psychiatry. 2007;61:545–50. doi: 10.1016/j.biopsych.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 53.Masi G, Cosenza A, Millepiedi S, et al. Aripiprazole monotherapy in children and young adolescents with pervasive developmental disorders: a retrospective study. CNS Drugs. 2009;23:511–21. doi: 10.2165/00023210-200923060-00005. [DOI] [PubMed] [Google Scholar]
- 54.Valicenti-McDermott MR, Demb H. Clinical effects and adverse reactions of off-label use of aripiprazole in children and adolescents with developmental disabilities. J Child Adolesc Psychopharmacol. 2006;16:549–60. doi: 10.1089/cap.2006.16.549. [DOI] [PubMed] [Google Scholar]
- 55.Gibson AP, Lynn CM, Mican LM, et al. Effectiveness and tolerability of aripiprazole in child and adolescent inpatients: a retrospective evaluation. Int Clin Psychopharmacol. 2007;22:101–5. doi: 10.1097/YIC.0b013e3280119e0c. [DOI] [PubMed] [Google Scholar]
- 56.Rugino TA, Janvier YM. Aripiprazole in children and adolescents: clinical experience. J Child Neurol. 2005;20:603–10. doi: 10.1177/08830738050200071301. [DOI] [PubMed] [Google Scholar]
- 57.Huang SC, Tsai SJ, Yang HJ. Aripiprazole improves social interaction in Taiwanese children with pervasive developmental disorder. Chang Gung Med J. 2010;33:211–5. [PubMed] [Google Scholar]
- 58.Stigler KA, Posey DJ, McDougle CJ. Aripiprazole for maladaptive behavior in pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2004;14:455–63. doi: 10.1089/cap.2004.14.455. [DOI] [PubMed] [Google Scholar]
- 59.Hollander E, Phillips A, Chaplin W, et al. A placebo controlled crossover trial of liquid fluoxetine on repetitive behaviours in childhood and adolescent autism. Neuropsycho pharmacology. 2005;30:582–589. doi: 10.1038/sj.npp.1300627. [DOI] [PubMed] [Google Scholar]


