Abstract
Background
Duodenal adenocarcinoma is a rare cancer usually studied as a group with periampullary or small bowel adenocarcinoma; therefore, its natural history is poorly understood.
Methods
Patients with duodenal adenocarcinoma were identified from a single-institution pancreaticoduodenectomy database. Patients with adenocarcinoma arising from the ampulla of Vater were excluded. Univariate and multivariate analyses were performed to identify clinicopathologic variables associated with survival and recurrence after resection.
Results
From 1984 to 2006, a total of 122 patients with duodenal adenocarcinoma underwent pancreaticoduodenectomy. Overall survival after resection was 48% at 5 years and 41% at 10 years. Five-year survival decreased as the number of lymph nodes involved by metastasis increased from 0 to 1–3 to ≥4 (68%, 58%, 17%, respectively, P < 0.01) and as the lymph node ratio increased from 0 to >0–0.2 to >0.2–0.4 to >0.4 (68%, 57%, 14%, 14%, respectively, P < 0.01). Lymph node metastasis was the only independent predictor of decreased survival in multivariate analysis. Recurrence after resection was predominantly distant (81%). Adjuvant chemoradiation did not decrease local recurrence or prolong overall survival; however, patients who received chemoradiation more commonly had nodal metastasis (P = 0.03).
Conclusions
The prognostic significance of both the absolute number and ratio of involved lymph nodes emphasizes the need for adequate lymphadenectomy to accurately stage duodenal adenocarcinoma. The mostly distant pattern of recurrence underscores the need for the development of effective systemic therapies.
Evidence to guide treatment decisions in duodenal adenocarcinoma is limited as a result of the rare nature of the disease. Among periampullary adenocarcinomas (pancreatic, ampullary, distal bile duct, and duodenal), the duodenum is the primary site for only 7% of cases.1 Though most small bowel adenocarcinomas (56%) arise in the duodenum, small bowel cancers account for only 2% of all gastrointestinal cancers in the United States.2,5 Many previous studies have grouped duodenal adenocarcinoma with periampullary or small bowel adenocarcinoma.1,4–6 Those that have considered duodenal adenocarcinoma alone have been limited by small numbers (Table 1).
TABLE 1.
Published series on surgical resection of duodenal adenocarcinoma
| Study | Study period | Total no. patients resected | PD, n (%) | 5-year survival (%) | Predictors of survival
|
||
|---|---|---|---|---|---|---|---|
| Nonpredictors | Univariate | Multivariate | |||||
| Alwmark et al.11 | 1958–1973 | 19 | 12 (63) | 25 | – | – | – |
| Ouriel et al.6 | 1950–1981 | 19 | 11 (58) | 30/65a | – | Histologic grade, nodal metastasis | – |
| Lowell etal.12 | 1970–1991 | 17 | 8 (47) | 45 | – | First/second portion of duodenum | – |
| Barnes et al.7 | 1967–1991 | 36 | 27 (75) | 54 | Nodal metastasis | Stage | – |
| Delcore et al.13 | 1960–1990 | 28 | 21 (75) | 60 | – | GI bleeding, symptomatic >4 months, nodal metastasis | – |
| Rotman et al.14 | 1978–1988 | 46 | 38 (83) | 45 | Gender, age, weight loss, jaundice, T stage, tumor size, pancreatic invasion nodal metastasis, location of metastatic nodes | – | – |
| Sexe et al.20 | 1987–1991 | 34 | 31 (91) | 23 | AJCC stage | – | – |
| Sohn et al.21 | 1984–1996 | 48 | 35 (73) | 53 | Nodal metastasis, adjuvant chemoradiation, tumor size, histologic grade | Positive margins, segmental resection, tumor in Third/fourth portion of duodenum | – |
| Bakaeen et al.15 | 1976–1996 | 68 | 50 (74) | 54 | Histologic grade, tumor size, location in duodenum, adjuvant chemoradiation | Age, weight loss, T stage, nodal metastasis, AJCC stage | Weight loss, positive margins, nodal metastasis, AJCC stage |
| Kaklamanos et al.8 | 1978–1998 | 37 | 26 (70) | 30/60a | Age, gender, grade, T stage | Nodal metastasis | Nodal metastasis |
| Ryder et al.9 | 1957–1998 | 31 | 27 (87) | 43 | Nodal metastases, location in duodenum, type of resection, adjuvant chemoradiation | Tumor size, histologic grade, transmural invasion | |
| Sarela etal.10 | 1983–2000 | 72 | 56 (78) | 71 | Gender, grade, T stage | Age, nodal metastasis | Age, nodal metastasis |
| Hurtuk et al.16 | 1984–2005 | 35 | 24 (69) | 45 | Grade, positive margins, nodal metastasis, venous or perineural invasion | T4, tumor ≤ 3.5 cm | – |
| Lee et al.19 | 1995–2007 | 28 | 27 (96) | 44 | Age, gender, weight loss, CA19-9, grade, tumor size | T stage, nodal metastasis, AJCC stage | Nodal metastasis |
| Han et al.17 | 1990–2006 | 22 | 18 (82) | 30 | – | Positive margins | – |
| Struck et al.18 | 1989–2006 | 30 | 25 (83) | 33 (3 y) | Positive margins, T stage, adjuvant therapy | – | Nodal metastasis, stage |
| Current study | 1984–2006 | 112 | 112 (100) | 48 | T stage, tumor size | Grade, positive margins, perineural invasion, nodal metastasis, vascular invasion | Nodal metastasis, |
PD pancreaticoduodenectomy, GI gastrointestinal
After segmental resection/pancreaticoduodenectomy
The surgical approach to adenocarcinoma of the duodenum can be variable and largely depends on the location of the tumor. Tumors arising in the first, second or third portion of the duodenum typically require pancreaticoduodenectomy, whereas tumors occurring in the fourth portion may be more amenable to segmental duodenal resection. Unlike pancreatic adenocarcinoma, the majority of patients diagnosed with duodenal adenocarcinoma will be candidates for curative resection.7–10 Small retrospective case series suggest that 5-year survival rates after curative resection are relatively favorable compared with other periampullary malignancies, ranging 45–71%.1,6–18
Studies examining prognostic factors after resection of duodenal adenocarcinoma have shown contradictory results (Table 1).6–21 Most studies recognize the importance of regional lymph node metastasis.6,8,10,13,15,18,19 However, other factors such as T stage, location, and size of the tumor have not been consistently associated with outcome.8,9,14,15,18,21 Furthermore, the role of adjuvant therapy and the patterns of failure after resection have been poorly characterized.7,15,18,19,22
The purpose of this study, therefore, was to review a large cohort of patients who underwent pancreaticoduodenectomy for duodenal adenocarcinoma at a single institution in an attempt to describe the clinical and pathologic factors predictive of survival after resection of duodenal adenocarcinoma and the patterns of disease recurrence after resection.
METHODS
Patients
The study population was drawn from the Johns Hopkins Hospital pancreaticoduodenectomy database. Patients who underwent pancreaticoduodenectomy for duodenal adenocarcinoma between 1984 and 2006 were included in the analysis. Patients with adenocarcinoma arising from the ampulla of Vater have been reported previously and were excluded.23 Clinical data such as patient demographics, technical aspects of the operation, history of familial adenomatous polyposis, presenting symptoms, laboratory values, and the use of adjuvant chemoradiation were reviewed. Pathologic data including association with adenoma, T stage, nodal metastasis, number of nodes involved and examined, resection margin status, tumor size, vascular invasion, perineural invasion, and histologic grade were also reviewed. The study was approved by the Johns Hopkins Hospital Institutional Review Board.
Statistical Analysis
Clinical and pathologic data were compared by the Fisher’s exact and Wilcoxon rank sum tests for categorical and continuous variables, respectively. Log rank tests and multivariate Cox proportional hazard models were used to identify variables associated with overall survival. Patients were excluded from survival analysis if they died in the 30-day postoperative period, underwent palliative (R2) resection, or underwent resection of synchronous liver metastases with potentially curative intent. The validity of the proportional hazard assumptions was checked visually by complementary log–log plots and verified by Schoenfeld residuals. Statistical analyses were performed by Stata software, version 10 (StataCorp, College Station, TX) and R, version 2.5.1.24
RESULTS
Clinical Characteristics
A retrospective review of the Johns Hopkins Hospital pancreaticoduodenectomy database identified 122 patients with duodenal adenocarcinoma who underwent surgical resection between 1984 and 2006. The demographic, clinical, and procedural characteristics of the study cohort are shown in Table 2. Common presenting symptoms were abdominal pain (39%), weight loss (35%), jaundice (25%), duodenal obstruction (25%), and upper gastrointestinal bleeding (24%), with the latter likely leading to a high intraoperative transfusion rate of 48 %. Serum tumor markers were elevated in only a third of duodenal adenocarcinoma patients: carcinoembryonic antigen was >3 ng/ml in 33% (12 of 36), and CA19-9 was >36 U/ml in 32% (12 of 37) of patients.
TABLE 2.
Demographic, clinical, and procedural characteristics of the entire study population
| Characteristic | Value |
|---|---|
| Duodenal adenocarcinoma, n | 122 |
| Age, y, median (range) | 66 (33–103) |
| Male gender | 66 (54%) |
| Familial adenomatous polyposis | 3 (2%) |
| Abdominal pain | 48 (39%) |
| Weight loss | 43 (35%) |
| Jaundice | 31 (25% |
| Duodenal obstruction | 31 (25%) |
| Upper gastrointestinal bleeding | 29 (24%) |
| Pylorus preservation | 88 (72%) |
| Intraoperative transfusion | 59 (48%) |
| Portomesenteric vein resection | 3 (2.4%) |
| 30-d mortality | 3 (2.4%) |
| Delayed gastric emptying | 19 (16%) |
| Postoperative pancreatic fistula | 16 (13%) |
| Intra-abdominal abscess | 8 (7%) |
Pathologic Characteristics
On pathologic examination, 4 patients (3%) had T1, 17 (16%) T2, 55 (51)% T3, and 32 (30%) had T4 tumors. A median of 15 nodes (range, 2–37) were retrieved and evaluated from each specimen. Nodal metastasis was noted in 79 patients (65%), with 24 patients (22%) having 4 or more positive nodes. The risk of lymph node metastasis increased with T stage (T1, 25%; T2, 53%; T3, 71%; T4, 76%; P < 0.01). Positive margins were encountered in 10 patients (8%); five patients underwent microscopic margin positive (R1) resection, either involving the retroperitoneal (n = 2), proximal duodenal (n = 1), or vascular groove margin (n = 2), and five patients underwent macroscopic margin positive (R2) resection, leaving gross residual tumor on the superior mesenteric artery (SMA, n = 3) or the superior mesenteric vein (SMV, n = 2). The median tumor size was 4 cm (range, 0.5–13 cm). Microscopic vascular invasion was identified in 30 patients (39%), while 30 patients (37%) had perineural invasion. Tumors were well differentiated in 4 cases (4%), moderately in 68 (60%), and poorly differentiated in 41 (36%).
Survival after Resection
Survival analysis was performed on 112 of 122 patients with invasive duodenal adenocarcinoma. Ten patients were excluded from survival analysis; three patients died in the perioperative period, five underwent palliative (R2) resection, and two underwent resection of synchronous liver metastases. The overall survival probability after pancreaticoduodenectomy for invasive duodenal adenocarcinoma was 48% at 5 years and 41% at 10 years, after a median follow-up of 33 months (range, 1–303 months). In univariate analysis, the presence of lymph node metastasis, poor tumor differentiation, perineural invasion, vascular invasion, and positive margins were all associated with decreased survival (Table 3). Surprisingly, tumor size and T stage (invasion into the duodenal wall) were not associated with survival (Table 3); nor were age (P = 0.63), gender (P = 0.82), race (P = 0.89), preoperative weight loss (P = 0.88), or intraoperative transfusion (P = 0.51). In multivariate analysis, only lymph node metastasis was independently associated with a reduction in overall survival (Table 3). The choice of the final multivariate model was limited by the fact that many of the variables were highly interrelated. For example, we could not include Vascular and Perineural Invasion in the model together, as the two variables were too closely linked. Vascular Invasion was consistently more informative, so we elected to include this over Perineural Invasion. Tumor Differentiation was also excluded from the final model because it did not provide information beyond Lymph Node Metastasis, Margin Status, and Perineural Invasion, and seriously attenuated the parameter estimates for the other variables. Finally, we were specifically interested in the effect of adjuvant chemoradiation on survival (independent of other pathologic predictors) and elected to include this variable in the final multivariate model, despite the lack of statistical significance in univariate analysis.
TABLE 3.
Univariate and multivariate proportional hazard model for overall survival after curative resection of duodenal adenocarcinoma (n = 112)
| Characteristic | No. of patients | 5-year overall survival | Univariate analysis
|
Multivariate analysis
|
||
|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | |||
| T stage | ||||||
| 1–2 | 19 | 58% | ||||
| 3–4 | 80 | 46% | 1.4 (0.7–2.8) | 0.29 | – | – |
| Missing | 13 | – | – | – | – | – |
| Tumor size | ||||||
| <2 cm | 14 | 44% | ||||
| ≥2 cm | 91 | 49% | 1.2 (0.5–2.6) | 0.64 | – | – |
| Missing | 7 | – | – | – | – | – |
| Lymph node metastasis | ||||||
| N0 | 41 | 68% | Reference | Reference | ||
| N1 | 47 | 58% | 2.1 (1.3–3.8) | 0.03 | 1.9 (1.2–3.3) | 0.02 |
| N2 | 24 | 17% | 4.3 (2.1–9.8) | <0.01 | 3.1 (1.6–5.7) | <0.01 |
| Missing | 0 | – | – | – | – | – |
| Tumor differentiation | ||||||
| Well/moderate | 69 | 58% | ||||
| Poor | 36 | 39% | 2.0 (1.2–3.2) | <0.01 | – | – |
| Missing | 7 | – | – | – | – | – |
| Perineural invasion | ||||||
| No | 49 | 59% | ||||
| Yes | 25 | 44% | 2.2 (1.2–4.0) | <0.01 | – | – |
| Missing | 38 | – | – | – | – | – |
| Vascular invasion | ||||||
| No | 44 | 65% | ||||
| Yes | 25 | 34% | 3.7 (1.9–7.0) | <0.01 | 1.4 (0.9–52.3) | 0.11 |
| Missing | 43 | – | – | – | – | – |
| Margin status | ||||||
| R0 | 107 | 55% | ||||
| R1 | 5 | 0% | 3.3 (1.5–7.2) | <0.01 | 1.7 (0.2–5.5) | 0.59 |
| Missing | 0 | – | – | – | – | – |
| Adjuvant chemoradiation | ||||||
| No | 78 | 48% | ||||
| Yes | 34 | 47% | 1.1 (0.6–1.9) | 0.82 | 1.26 (0.8–2.0) | 0.96 |
| Missing | 0 | – | – | – | – | – |
CI confidence interval
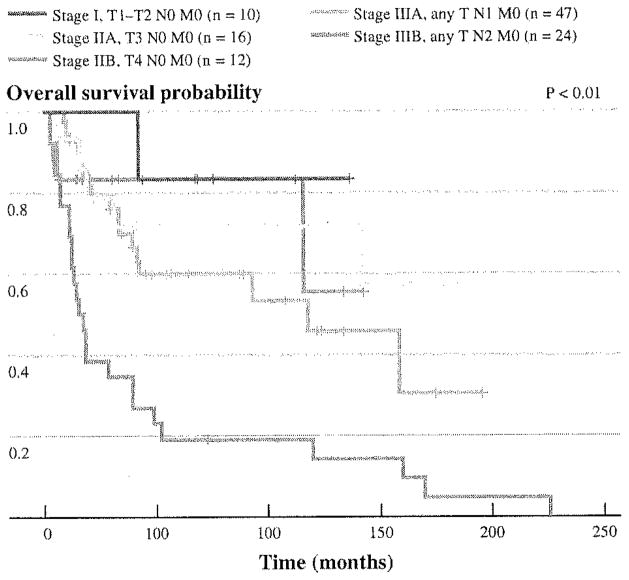
Figure 1 shows the Kaplan–Meier curves of 109 patients who had adequate pathologic data to be categorized according to the 7th edition (2010) of the American Joint Committee on Cancer (AJCC) Staging System.25 Consistent with the results of our univariate and multivariate analyses, T status did not appear to offer a powerful discriminatory prediction among node negative patients (stages I, IIA, and IIB), whereas the difference in survival probabilities between N0, N1 (1–3 positive nodes, stage IIIA), and N2 (4 or more involved nodes, stage IIB) patients appeared robust.
FIG. 1.
Kaplan-Meier plots of overall survival after pancreaticoduodenectomy for duodenal adenocarcinoma based on the 7th edition (2010) of the AJCC staging system. N1 1–3 positive lymph nodes, N2 ≥ 4 positive lymph nodes
We performed further analysis to evaluate the prognostic significance of Lymph Node Ratio (LNR, ratio of positive to total number of examined lymph nodes) after resection of duodenal adenocarcinoma. As the LNR increased from 0 (n = 41) to >0–0.2 (n = 40) to >0.2–0.4 (n = 22) to >0.4 (n = 9), there was a significant decrease in overall survival (5-year, 68%, 57%, 14%, and 14%, respectively, P < 0.01).
Adjuvant Chemoradiotherapy
In addition to curative surgical resection, 34 (30%) of 112 patients received adjuvant radiotherapy with fluorouracil-based concurrent chemotherapy. There was no difference in T-stage, tumor size, margin status, histologic grade, perineural, or vascular invasion between patients who received adjuvant chemoradiation and those who did not; however, 27 patients (79%) who received adjuvant chemoradiotherapy had nodal metastasis, compared with 44 (56%) of those who did not (P = 0.03). Despite this difference, survival between patients who did and did not receive adjuvant chemoradiation was comparable (5 year, 47% vs. 48%, P = 0.82). This also held true on multivariate analysis (P = 0.96, Table 3), where we adjusted for nodal metastasis.
Patterns of Failure after Resection
Site of first recurrence after resection of duodenal adenocarcinoma was documented in 27 patients. The site of first recurrence was locoregional (tumor bed or regional lymph nodes) in 5 patients (19%), distant (liver, peritoneum, lung, supraclavicular lymph nodes) in 16 (59%), and both locoregional and distant in 6 (22%), Therefore, 81% of patients had at least a component of distant failure. The most common site of first recurrence was the liver (n = 9), followed by the peritoneum (n = 7). Adjuvant chemoradiotherapy was administered to 14 (52%) of the 27 patients with documented site of first recurrence. Of these 14 patients, recurrence was locoregional in 4 patients (29%), distant in 8 (57%), and locoregional and distant in 2 (14%). There was no difference in the distribution of recurrence between patients who received adjuvant chemoradiotherapy and those who did not (P = 0.37).
DISCUSSION
This study represents the largest reported series of surgical resection for duodenal adenocarcinoma to date (Table 1). Ten-year overall survival after radical resection of invasive duodenal adenocarcinoma was 41%. In multivariate analysis, nodal metastasis was the only factor independently associated with worse overall survival. An increased number of involved lymph nodes was also associated with decreased overall survival, with 5-year survival ranging from 68% for node-negative patients to 17% when 4 or more lymph nodes were involved. Similarly, 5-year survival after resection was poor (14%) when the ratio of involved to examined lymph nodes was >0.2. Recurrence after resection occurred most commonly at distant sites. Adjuvant chemoradiotherapy, although used more commonly for patients with nodal metastasis, did not appear to prolong overall survival or affect the distribution of recurrence.
The importance of adequate lymphadenectomy for accurate staging of duodenal adenocarcinoma was first demonstrated by Sarela et al., who reported that the prognostic discrimination by N stage was improved with pathologic examination of at least 15 regional lymph nodes.10 Similarly, a recent population study on small bowel adenocarcinoma showed that retrieval of at least 10 lymph nodes enhanced the prognostic ability of the AJCC staging system.26 Our study is the first to demonstrate that the actual number of involved lymph nodes with metastasis is associated with survival, validating the current AJCC staging system, which stratifies N stage as N0 (no lymph node metastasis), N1 (1–3 positive lymph nodes), and N2 (4 or more positive lymph nodes). However, the AJCC guidelines set as the minimum number of regional lymph nodes that need to be assessed pathologically for duodenal or small bowel adenocarcinoma to 6 only. There appears to exist a convincing argument to revise this threshold to a higher one, although additional studies are needed to optimally define this number.27
Seventy-two percent of patients with duodenal adenocarcinoma in this series underwent pylorus-preserving pancreaticoduodenectomy. One could argue that the performance of a classic pancreaticoduodenectomy (with antrectomy) in these patients can increase the number of retrieved lymph nodes thereby enhancing staging. Two recent randomized trials from Europe of patients with periampullary tumors showed pylorus-preserving and classic pancreaticoduodenectomy to be associated with equal functional or oncologic outcomes.28,29 Similarly, a previous trial from our institution which randomized 294 patients with resectable periampullary adenocarcinoma (9 patients had duodenal adenocarcinoma) to pylorus-preserving or radical pancreaticoduodenectomy (with antrectomy and extended retroperitoneal lymphadenectomy) showed no difference in survival (median 30 vs. 28 months, P = 0.79), despite a significantly higher number of resected lymph nodes in the radical group (median 16 vs. 26, P = 0.001). More important, of the 148 patients in the radical group, only one (0.6%) had a perigastric lymph node as the only positive node that would have not been resected as part of a pylorus-preserving procedure and would have resulted in understaging.30 On the basis of the above, it is the authors’ opinion that both pylorus-preserving and classic pancreaticoduodenectomy are equivalent surgical options for patients with cancer arising in the second or third portion of the duodenum.
Two studies have specifically examined the results of adjuvant radiotherapy after resection of duodenal adenocarcinoma.22,31 Our institution has previously published a pilot study on 14 patients with node-positive duodenal adenocarcinoma who underwent pancreaticoduodenectomy followed by adjuvant chemoradiotherapy with concurrent fluorouracil-based chemotherapy.31 The study suggested that adjuvant chemoradiation provided improved local control compared with historical controls treated with surgery alone (93% vs. 67%), but did not prolong overall survival (5 year, 44% vs. 43%). Conversely, a retrospective study of 32 patients with duodenal adenocarcinoma from Duke University Medical Center compared patients who underwent resection alone with those who received resection and adjuvant chemoradiotherapy and found no marked improvement in overall survival (44% vs. 57%), disease-free survival (44% vs. 54%) or local control (49% vs. 70%).22 Our study failed to show a beneficial effect of adjuvant chemoradiotherapy both in terms of overall survival (Table 3) or locoregional failure. However, adjuvant chemoradiation was able to confer similar survival to patients who received it despite a higher prevalence of lymph node metastasis, and our institutional practice still reserves its use in cases of margin-positive resection or multiple lymph node involvement after a course of adjuvant chemotherapy if no systemic progression of disease is noted at restaging.
Most patients (81%) in this study for whom site of first recurrence after resection of duodenal adenocarcinoma was documented (n = 27) did so at a distant site, consistent with previous studies reporting distant disease failure in 52–92% of cases (Table 4) 7,15,18,19,22 Given the tendency of the disease to recur in a systemic fashion, the role of adjuvant systemic chemotherapy certainly deserves further investigation. Following the successful paradigm of colorectal cancer and initial encouraging results from a retrospective study of 80 patients with metastatic small bowel adenocarcinoma, investigators from the MD Anderson Cancer Center recently reported the results of a phase II prospective trial of 30 patients with metastatic or unresectable small bowel or ampullary adenocarcinoma receiving capecitabine and oxaliplatin (CAPOX) doublet.32 Overall response rate was 50% with a complete response rate of 10%. Median time to progression was 11 months and median overall survival was 20 months.33 These results compare favorably to the only other phase II prospective study of patients with advanced small bowel adenocarcinoma (conducted by the Eastern Cooperative Oncology Group), in which the combination of fluorouracil, doxorubicin, and mitomycin C was associated with a response rate of only 18% and a median survival of 8 months.34 On the basis of the above, our current institutional practice is to offer duodenal adenocarcinoma patients with high-risk pathologic features adjuvant chemotherapy with a fluoropyrimidine–oxaliplatin combination first, followed by adjuvant chemoradiation if no systemic progression of disease is noted at restaging, realizing that the efficacy of this approach certainly warrants further prospective evaluation.
TABLE 4.
Published series on recurrence patterns after resection of duodenal adenocarcinoma
An inherent limitation of our retrospective study is that it only included patients with duodenal adenocarcinoma who were selected to undergo pancreaticoduodenectomy and not segmental resection. Therefore, it cannot answer the question of which approach is oncologically sounder. We do not advocate pancreaticoduodenectomy for adenocarcinoma located in the fourth portion of the duodenum for which a segmental margin-negative resection can be performed safely with the understanding that this approach may compromise the extent of lymphadenectomy and staging information.21 Nonetheless, the fact that our entire study cohort underwent pancreaticoduodenectomy (with a median number of 15 examined lymph nodes) only strengthens our conclusion on the prognostic significance of lymphadenectomy for the accurate staging of these patients.
In summary, pancreaticoduodenectomy can be performed safely for patients with duodenal adenocarcinoma and is associated with an approximately 40% 10-year survival. Lymph node metastasis is strongly associated with outcome, which appears to be particularly poor when ≥4 or >20% of examined lymph nodes are involved. Therefore, appropriate lymphadenectomy is important for accurate staging. The predominantly distant pattern of failure after surgical resection of duodenal adenocarcinoma underscores the need for effective adjuvant systemic therapy.
Footnotes
Presented at the Society of Surgical Oncology 63rd Annual Cancer Symposium, March 3–7, 2010, St. Louis, MO.
References
- 1.Yeo CJ, Sohn TA, Cameron JL, et al. Periampullary adenocarcinoma: analysis of 5-year survivors. Ann Surg. 1998;227:821–31. doi: 10.1097/00000658-199806000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bilimoria KY, Bentrem DJ, Wayne JD, et al. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg. 2009;249:63–71. doi: 10.1097/SLA.0b013e31818e4641. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 4.Agrawal S, McCarron EC, Gibbs JF, et al. Surgical management and outcome in primary adenocarcinoma of the small bowel. Ann Surg Oncol. 2007;14:2263–9. doi: 10.1245/s10434-007-9428-2. [DOI] [PubMed] [Google Scholar]
- 5.Hatzaras I, Palesty JA, Abir F, et al. Small-bowel tumors: epidemiologic and clinical characteristics of 1260 cases from the Connecticut tumor registry. Arch Surg. 2007;142:229–35. doi: 10.1001/archsurg.142.3.229. [DOI] [PubMed] [Google Scholar]
- 6.Ouriel K, Adams JT. Adenocarcinoma of the small intestine. Am J Surg. 1984;147:66–71. doi: 10.1016/0002-9610(84)90036-9. [DOI] [PubMed] [Google Scholar]
- 7.Barnes G, Romero L, Hess KR, et al. Primary adenocarcinoma of the duodenum: management and survival in 67 patients. Ann Surg Oncol. 1994;1:73–8. doi: 10.1007/BF02303544. [DOI] [PubMed] [Google Scholar]
- 8.Kaklamanos IG, Bathe OF, Franceschi D, et al. Extent of resection in the management of duodenal adenocarcinoma. Am J Surg. 2000;179:37–41. doi: 10.1016/s0002-9610(99)00269-x. [DOI] [PubMed] [Google Scholar]
- 9.Ryder NM, Ko CY, Hines OJ, et al. Primary duodenal adenocarcinoma: a 40-year experience. Arch Surg. 2000;135:1070–4. doi: 10.1001/archsurg.135.9.1070. [DOI] [PubMed] [Google Scholar]
- 10.Sarela AI, Brennan MF, Karpeh MS, et al. Adenocarcinoma of the duodenum: importance of accurate lymph node staging and similarity in outcome to gastric cancer. Ann Surg Oncol. 2004;11:380–6. doi: 10.1245/ASO.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 11.Alwmark A, Andersson A, Lasson A. Primary carcinoma of the duodenum. Ann Surg. 1980;191:13–8. doi: 10.1097/00000658-198001000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lowell JA, Rossi RL, Munson JL, et al. Primary adenocarcinoma of third and fourth portions of duodenum. Favorable prognosis after resection. Arch Surg. 1992;127:557–60. doi: 10.1001/archsurg.1992.01420050081010. [DOI] [PubMed] [Google Scholar]
- 13.Delcore R, Thomas JH, Forster J, et al. Improving resectability and survival in patients with primary duodenal carcinoma. Am J Surg. 1993;166:626–30. doi: 10.1016/s0002-9610(05)80668-3. [DOI] [PubMed] [Google Scholar]
- 14.Rotman N, Pezet D, Fagniez PL, et al. Adenocarcinoma of the duodenum: factors influencing survival. French Association for Surgical Research. Br J Surg. 1994;81:83–5. doi: 10.1002/bjs.1800810128. [DOI] [PubMed] [Google Scholar]
- 15.Bakaeen FG, Murr MM, Sarr MG, et al. What prognostic factors are important in duodenal adenocarcinoma? Arch Surg. 2000;135:635–41. doi: 10.1001/archsurg.135.6.635. [DOI] [PubMed] [Google Scholar]
- 16.Hurtuk MG, Devata S, Brown KM, et al. Should all patients with duodenal adenocarcinoma be considered for aggressive surgical resection? Am J Surg. 2007;193:319–24. doi: 10.1016/j.amjsurg.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Han SL, Cheng J, Zhou HZ, et al. The surgical treatment and outcome for primary duodenal adenocarcinoma. J Gastrointest Cancer. 2009;40:33–37. doi: 10.1007/s12029-009-9073-z. [DOI] [PubMed] [Google Scholar]
- 18.Struck A, Howard T, Chiorean EG, et al. Non-ampullary duodenal adenocarcinoma: factors important for relapse and survival. J Surg Oncol. 2009;100:144–8. doi: 10.1002/jso.21319. [DOI] [PubMed] [Google Scholar]
- 19.Lee HG, You DD, Paik KY, et al. Prognostic factors for primary duodenal adenocarcinoma. World J Surg. 2008;32:2246–52. doi: 10.1007/s00268-008-9678-6. [DOI] [PubMed] [Google Scholar]
- 20.Sexe RB, Wade TP, Virgo KS, et al. Incidence and treatment of periampullary duodenal cancer in the U.S. veteran patient population. Cancer. 1996;77:251–4. doi: 10.1002/(SICI)1097-0142(19960115)77:2<251::AID-CNCR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 21.Sohn TA, Lillemoe KD, Cameron JL, et al. Adenocarcinoma of the duodenum: factors influencing long-term survival. J Gastro-intest Surg. 1998;2:79–87. doi: 10.1016/s1091-255x(98)80107-8. [DOI] [PubMed] [Google Scholar]
- 22.Kelsey CR, Nelson JW, Willett CG, et al. Duodenal adenocarcinoma: patterns of failure after resection and the role of chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2007;69:1436–41. doi: 10.1016/j.ijrobp.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Winter JM, Cameron JL, Olino K, et al. Clinicopathologic analysis of ampullary neoplasms in 450 patients: implications for surgical strategy and long-term prognosis. J Gastrointest Surg. 2010;14:379–87. doi: 10.1007/s11605-009-1080-7. [DOI] [PubMed] [Google Scholar]
- 24.R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing Development Core Team; 2007. [Google Scholar]
- 25.Edge SB, Byrd DR, Compton CC, et al. American Joint Committee on Cancer (AJCC) staging manual. 7. New York: Springer-Verlag; 2010. [Google Scholar]
- 26.Nicholl MB, Ahuja V, Conway WC, et al. Small bowel adenocarcinoma: understaged and undertreated? Ann Surg Oncol. 2010;17:2728–32. doi: 10.1245/s10434-010-1109-x. [DOI] [PubMed] [Google Scholar]
- 27.Gibbs JF. Duodenal adenocarcinoma: is total lymph node sampling predictive of outcome? Ann Surg Oncol. 2004;11:354–5. doi: 10.1245/ASO.2004.02.914. [DOI] [PubMed] [Google Scholar]
- 28.Seiler CA, Wagner M, Bachmann T, et al. Randomized clinical trial of pylorus-preserving duodenopancreatectomy versus classical Whipple resection—long term results. Br J Surg. 2005;92:547–56. doi: 10.1002/bjs.4881. [DOI] [PubMed] [Google Scholar]
- 29.Tran KT, Smeenk HG, van Eijck CH, et al. Pylorus preserving pancreaticoduodenectomy versus standard Whipple procedure: a prospective, randomized, multicenter analysis of 170 patients with pancreatic and periampullary tumors. Ann Surg. 2004;240:738–45. doi: 10.1097/01.sla.0000143248.71964.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg. 2002;236:355–66. doi: 10.1097/00000658-200209000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swartz MJ, Hughes MA, Frassica DA, et al. Adjuvant concurrent chemoradiation for node-positive adenocarcinoma of the duodenum. Arch Surg. 2007;142:285–8. doi: 10.1001/archsurg.142.3.285. [DOI] [PubMed] [Google Scholar]
- 32.Overman MJ, Kopetz S, Wen S, et al. Chemotherapy with 5-fluorouracil and a platinum compound improves outcomes in metastatic small bowel adenocarcinoma. Cancer. 2008;113:2038–45. doi: 10.1002/cncr.23822. [DOI] [PubMed] [Google Scholar]
- 33.Overman MJ, Varadhachary GR, Kopetz S, et al. Phase II study of capecitabine and oxaliplatin for advanced adenocarcinoma of the small bowel and ampulla of Vater. J Clin Oncol. 2009;27:2598–603. doi: 10.1200/JCO.2008.19.7145. [DOI] [PubMed] [Google Scholar]
- 34.Gibson MK, Holcroft CA, Kvols LK, et al. Phase II study of 5-fluorouracil, doxorubicin, and mitomycin C for metastatic small bowel adenocarcinoma. Oncologist. 2005;10:132–7. doi: 10.1634/theoncologist.10-2-132. [DOI] [PubMed] [Google Scholar]