Abstract
OBJECTIVE
MRI is currently the imaging modality of choice for the detection, characterization, and staging of rectal cancer. A variety of examinations have been used for preoperative staging of rectal cancer, including digital rectal examination, endorectal (endoscopic) ultrasound, CT, and MRI. Endoscopic ultrasound is the imaging modality of choice for small and small superficial tumors. MRI is superior to CT for assessing invasion to adjacent organs and structures, especially low tumors that carry a high risk of recurrence.
CONCLUSION
High-resolution MRI is an accurate and sensitive imaging method delineating tumoral margins, mesorectal involvement, nodes, and distant metastasis. In this article, we will review the utility of rectal MRI in local staging, preoperative evaluation, and surgical planning. MRI at 3 T can accurately delineate the mesorectal fascia involvement, which is one of the main decision points in planning treatment.
Keywords: 3-T MRI, rectal cancer, tumor staging
Colorectal cancer is the third most common cause of cancer and cancer-related deaths in the United States [1]. Sixty-five percent of all colorectal cancers are located in the rectum, and 98% of these are adenocarcinoma.
Rectal Anatomy
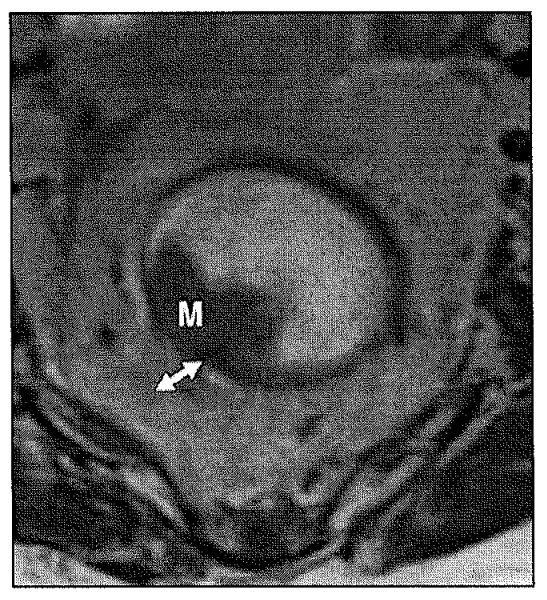
The rectum varies in length from 10 to 15 cm from the upper end of the anal canal to the recto-sigmoid junction. The rectum can be divided into three parts. These three parts are defined from the anal verge as lower rectum (0–6 cm), middle rectum (7–11 cm), and upper rectum (12–15 cm). The rectosigmoid junction is considered to be at the level of S3 by anatomists and at the level of sacral promontory by surgeons. The distal ring is regarded as the muscular anorectal ring by surgeons and as the dentate line by the anatomists. The rectal wall is composed of three layers: the mucosa, which is a fine low-signal line; the submucosa, which is represented as high-signal layers; and the muscularis propria, which has two low-signal layers (outer longitudinal and inner circular) at T2-weighted images (Fig. 1).The rectum is surrounded by mesorectal fat containing lymph nodes, superior hemorrhoidal vessels, and fibrous tissue, which are represented as high signal intensity surrounding the muscularis propria. The mesorectal fascia is an important barrier to the radial spread of tumors, which also forms the plane of dissection in total mesorectal excision (Fig. 2) and appears as thin layer of low signal surrounding the mesorectal fat [2].
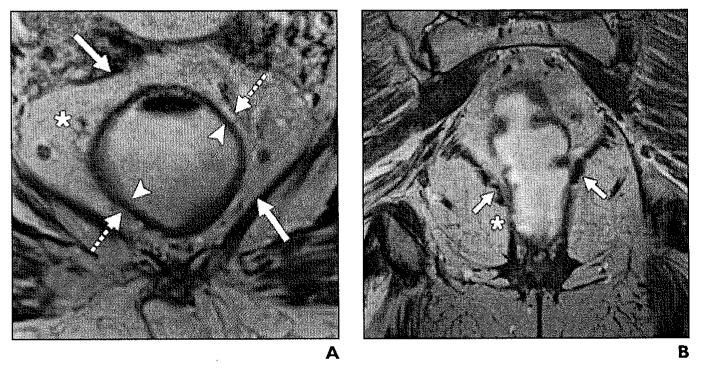
Fig. 1.
Normal anatomy of mesorectum.
A, T2-weighted axial image shows hyperintense mesorectal fat (asterisk) surrounded by mesorectal fascia, which is represented by thin hypointense line (solid arrows). Submucosa is represented by hyperintense layer (arrowheads), whereas muscularis propria is hypointense layer (dashed arrows).
B, Coronal T2-weighted high-resolution MRI shows normal anatomy of levator ani muscle (arrows) and puborectal muscle (asterisk).
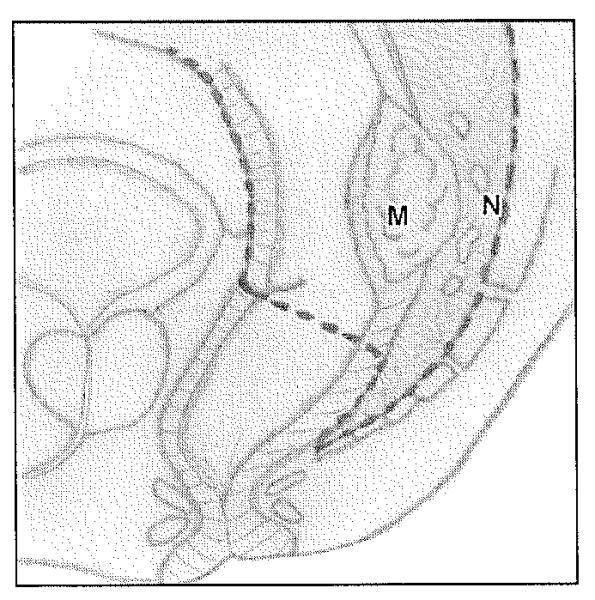
Fig. 2.
Diagram of total mesorectal excision, which involves en bloc resection of tumor (M) with mesorectal fat and lymph nodes (N) with intact mesorectal fascia. (Drawing by Corona Villalobos CP)
Once the diagnosis of rectal cancer has been established, treatment mainly depends on accurate staging that includes assessment of various factors, such as depth of tumor invasion, mesorectal fat and fascia involvement, status of circumferential resection margin, invasion to surrounding structures, and distant metastasis. Thmors confined to the rectal wall can be treated by local excision, whereas tumors that involve mesorectal fat require total mesorectal excision, with or without neoadjuvant therapy. Hence, the main goal of imaging modalities is to provide an accurate assessment for planning treatment and thereby guiding effective management in patients with rectal cancer [3-5]. A multidisciplinary team consisting of a trained pelvic MRI radiologist, colorectal surgeon, medical and radiation oncologists, and gastrointestinal pathologist play a crucial role in overall care in patients with rectal cancer.
Diagnostic Modalities
Endorectal ultrasound (i.e., endoscopic ultrasound) has been considered the imaging modality of choice for depth of tumor invasion and is accurate in assessing the tumor’s growth into the bowel wall. Studies have reported overall accuracy of 69–97% for assessing T stage with endoscopic ultrasound [6, 7]. The accuracy of endoscopic ultrasound for nodal staging (70–75%) is less than that for tumor staging [8]. Endoscopic ultrasound is accurate in discriminating stage T1 from T2 tumors. However, it is ditncult to perform in patients with stenosing and high rectal tumors and has limited diagnostic ability in evaluating tumor extension into mesorectal fat and fascia and invasion into adjacent organs [9, 10]. MRI is currently one of the most accurate noninvasive modalities for staging rectal carcinoma [11] (Table 1). The introduction of phased-anay coil MRI and the development of T2-weighted fast-spin sequences have enabled accurate determination of prognostic factors and anatomic assessment of the pelvis by delineating rectal tumors through increases in spatial and contrast resolution. Phased-may MRI has overall accuracies for T staging of 65–86%. MRI is very accurate for identifying large T3 and T4 tumors, with sensitivities for prediction of T3 of 80–86% and specificity of 71–76% [7]. However, challenges still exist in the accurate detection of metastatic lymph nodes.
TABLE 1.
TNM Staging in Rectal Cancer
| TNM Stage | Description |
|---|---|
|
| |
| Tumor | |
| T1 | Tumor invades submucosa |
| T2 | Tumor involves muscularis propria but does not cross it |
| T3 | Tumor extends beyond muscularis propria into mesorectal or pericolic fat |
| T4 | Tumor invades adjacent organs or perforates the visceral peritoneum |
| Node | |
| N0 | No nodal metastasis |
| N1 | 1–3 perirectal or pericolic nodes |
| N2 | 4 or more perirectal or pericolic nodes |
| Metastasis | |
| MX | Cannot be assessed |
| M0 | No metastasis |
| M1 | Distant metastasis |
MRI Protocol
Our MRI protocol consists of sagittal T2-weighted single-shot images and T2-weighted turbo spin-echo (TSE) images in the axial and coronal planes. High-resolution images are obtained in the axial and coronal planes with a slice thickness of 3 mm with a small FOV of 18–30. Unenhanced and contrast-enhanced axial and coronal high-resolution T1-weighted fat-saturated images of the rectum are also obtained. A sagittal T2-weighted TSE sequence is obtained first to locate the tumor. On the basis of the sagittal sequence, axial and coronal T2-weighted images, TSE sequences are planned, and they are angled to the plane exactly perpendicular and parallel to the tumor axis. Axial images of the tumor are important because they reduce the overestimation of the tumor depth of invasion noted on oblique imaging [12]. Coronal images help in identifying the relationship of low rectal tumors to the internal and external sphincter [13]. T2-weighted sagittal images help assess the relationship of the tumor to the peritoneal reflection [14].
MRI with an endorectal coil has not been used as the standard imaging modality of choice, and its use poses difficulty in application both for the patients and the MRI team; positioning of the coil is difficult in high and stenosing tumors. Also, it does not allow an accurate evaluation of the circumferential resection margin and the mesorectal nodes at a distance from the coil because of its limited FOV. Administration of gadolinium-based contrast agent has not proven to be beneficial for tumor stage and evaluation of circumferential resection margin status.
The first study to compare 3-T with 1.5-T MRI for T staging of rectal cancer within the same patients was by Maas et al. [15] and found no difference between 3-T and 1.5-T MRI for the distinction between T1–2 and borderline T3 tumors. Also, high-resolution 3-T MRI did not aid in the distinction between desmoplasia in T1–2 tumors and tumor stranding in T3 tumors. However, that study had limited sample size of 13 patients, and further studies with large sample size are required to confirm those findings [15].
Preoperative Staging of Rectal Cancer
Preoperative staging of rectal cancer involves a systematic approach for the interpretation of MRI by the radiologist [16].
T Stage and Extramural Depth of Invasion
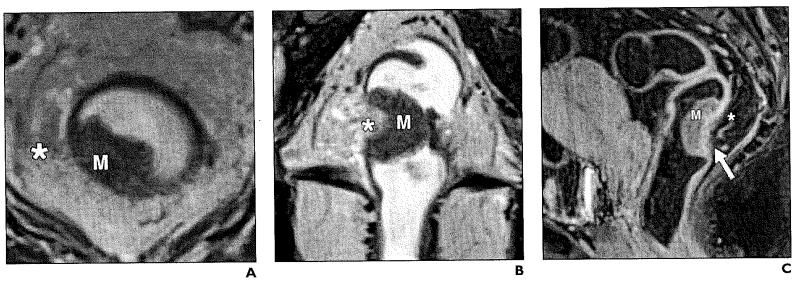
Phased-array MRI has shown accuracy of 65–86% for T staging. MRI is also accurate for identifying T3 and T4 tumors, with sensitivity for T3 tumors of 80–86% and specificity of 71–76%. The depth of extramural spread is a key factor in determining prognosis and aiding selection for treatment. Table 1 describes the TNM staging for rectal cancer. Patients with stage I (T1–2, N0) cancer (Figs. 3 and 4) benefit from surgical treatment, whereas patients with stage T3–4 tumors require preoperative chemoradiation because it reduces the rate of local recurrence (Figs. 5-8). Treatment options also do not depend merely on differentiating stage T2 from T3 cancer, but studies have found that patients with extramural invasion greater than 5 mm have a cancer-specific 5-year survival rate of 54%, compared with 85% in patients who have depth of less than 5 mm [17]. MRI can accurately determine the depth of extramural invasion and thus aid in appropriate treatment selection. Phased-array MRI poses difficulty in differentiating between T1 and T2 tumors and to differentiate T1–2 from borderline T3 stage tumors.
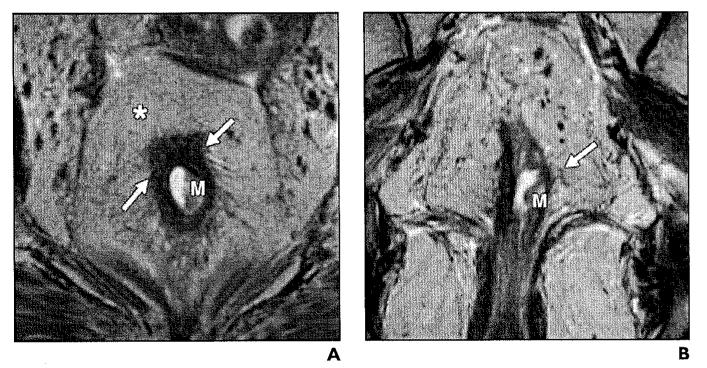
Fig. 3.
54-year-old woman with stage T1 rectal adenocarcinoma.
A and B, Axial (A) and coronal (B) T2-weighted high-resolution images show exophytic hypointense mass (M) on left lateral rectal wall. Muscular layer (arrows) appears to be spared of tumor. Mesorectal fat and mesorectal fascia (asterisk, A) are intact.
Fig. 4.
Stage T2 rectal cancer.
A and B, Axial (A) and coronal (B) T2-weighted high-resolution images show asymmetric thickening of rectal wall (arrows) involving mucosa, submucosa, and muscularis propria (M, A). Boundary between muscularis and submucosa are ill defined at right lateral wall (line, B). Mesorectal fat (asterisk, A) is not involved.
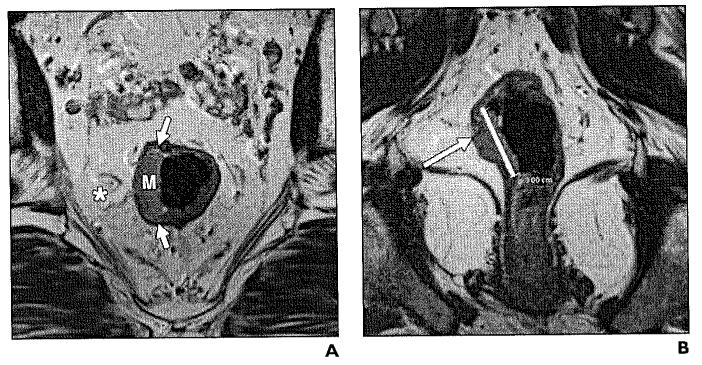
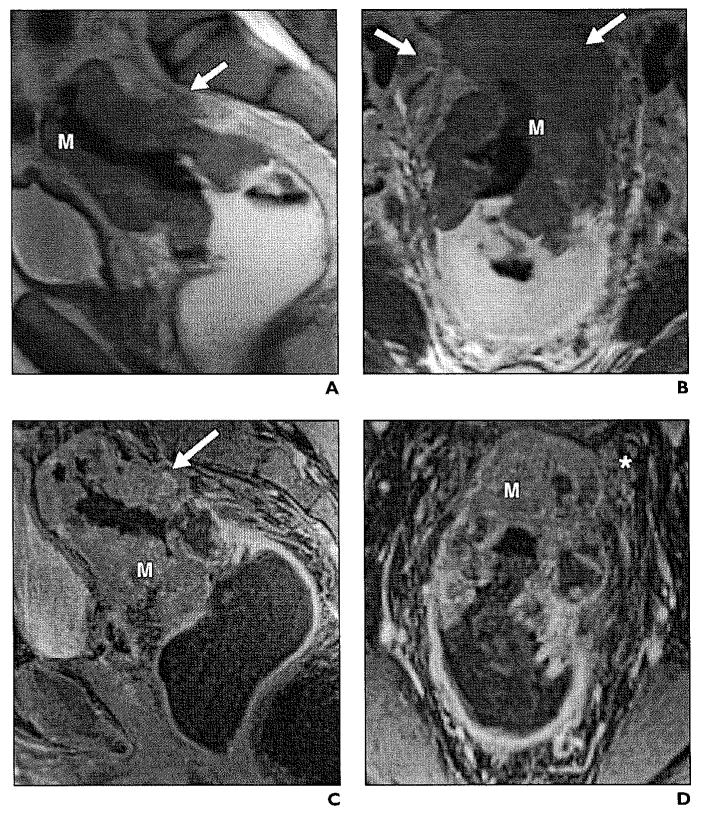
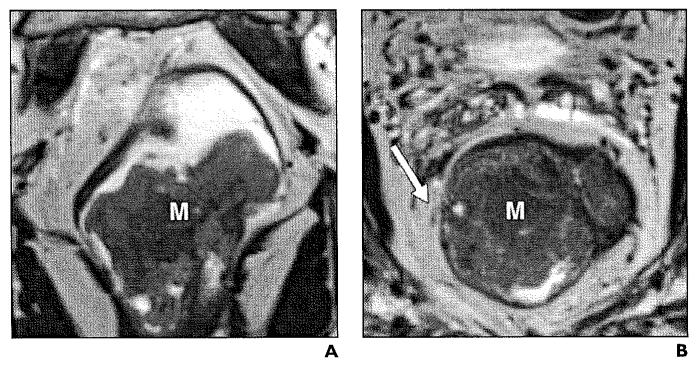
Fig. 5.
44-year-old woman with stage T3 moderately differentiated rectal adenocarcinoma.
A and B, Axial (A) and coronal (B) T2-weighted high-resolution images show exophytic mass (M) with transmural involvement of rectal layers infiltrating mesorectal fat (asterisk).
C, Sagittal T1-weighted fat-saturated image shows enhancing rectal tumor (M) along posterior wall with edema (asterisk) and enhancement of mesorectal fat (arrow).
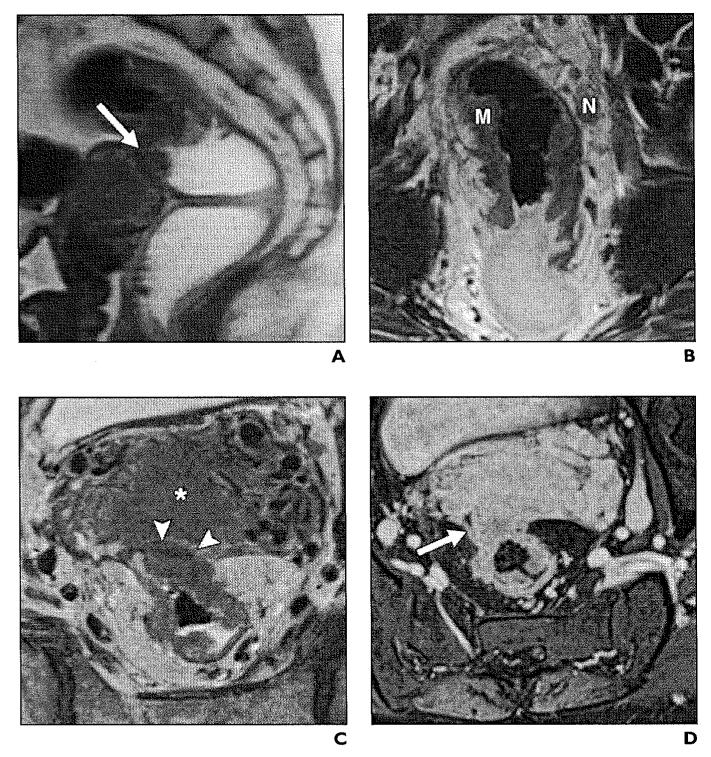
Fig. 8.
51-year-old woman with stage T4 infiltrating adenocarcinoma.
A, Sagittal T2-weighted image illustrates large mass (arrow) causing thickening of rectal wall, disruption of mesorectal fascia, and infiltration of uterus.
B, High-resolution coronal T2-weighted image shows circumferential mass (M) in distal sigmoid rectum, with associated suspicious mesorectal node (N).
C, In axial plane, there is tumor extension to fundus of uterus (asterisk) and no visible fat plane (arrowheads).
D, Axial gadolinium-enhanced image clearly demonstrates invasion of tumor (arrow) to retroverted uterus.
N Stage: Nodal Drainage
The lymphatic drainage of the upper rectum is different from that of the lower rectum. In the upper rectum, the lymphatics are thought to ascend cranially through the pararectal (mesorectal) lymph nodes in the mesorectum to lower sigmoid mesocolon nodes and along the superior rectal artery. Lymphatics from the lower half of the rectum and the anal canal above its mucocutaneous junction accompany the middle rectal vessels to the internal iliac nodes. Some lymphatics have been described traversing the levator ani muscle into the ischiorectal fossa, to accompany the inferior rectal and internal pudendal vessels to the internal iliac nodes [18].
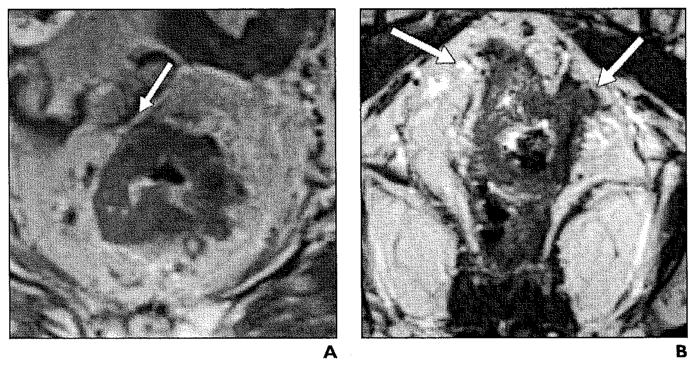
Node-positive disease is generally an indication for preoperative chemoradiation. Detection of lymph node metastasis still remains a challenge to the currently available imaging modalities, including MRI, which is only moderately accurate for preoperative infiltrated lymph node imaging with reported accuracy rates of 71–91% [19]. Size criteria alone are not sufficient for the diagnosis of lymph node metastasis, because 94% of the involved nodes will be as small as 5 mm [20]. The characterization of lymph nodes as malignant is more accurate for larger nodes (≥ 5 mm) that can be evaluated for size, shape (ovoid nodes > 5–6 mm), border (spiculated or indistinct borders), and signal intensity (heterogeneous signal) (Figs. 9 and 10). Chun et al. [21] reported sensitivities of 63.6% for 3-T MRI and 57.6% for endoscopic ultrasound and specificities of 92.3% for 3-T MRI and 82.1% for endoscopic ultrasound in lymph node staging, with tumor histopathology as the reference standard.
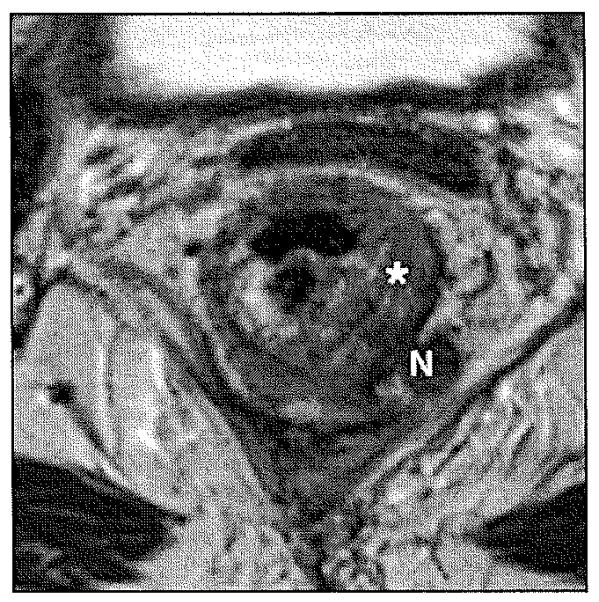
Fig. 9.
Stage IIIB (T3, Nl) rectal carcinoma with tumor invasion into mesorectal fat. Axial T2-weighted image illustrates asymmetrical thickening (asterisk) of left lateral rectal wall with metastatic mesorectal lymphadenopathy (N) measuring 1.1 cm in size.
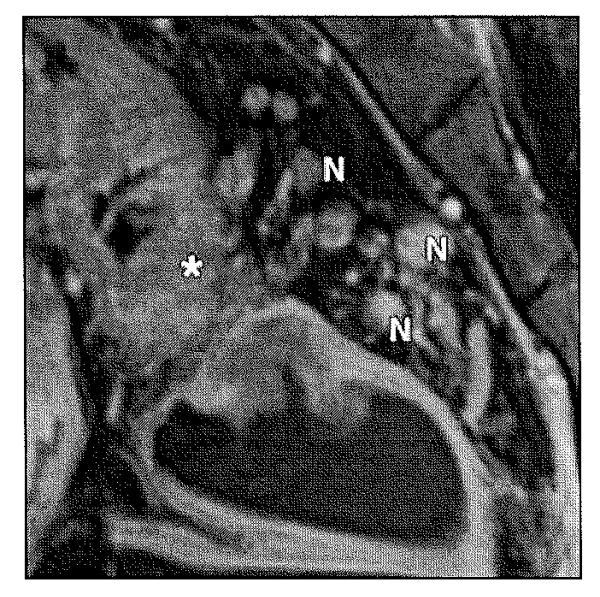
Fig. 10.
Stage IIIC (T3, N2) rectal carcinoma.
Sagittal T2-weighted image shows enhancement of thickened rectal wall (asterisk) with multiple metastatic mesorectallymphadenopathy (N).
Ultrasmall Superparamagnetic Iron Oxide MRI and Diffusion-Weighted MRI
Recent studies have evaluated the ability of ultrasmall superparamagnetic iron oxide MRI for lymph node characterization [22]. In a recent multicenter study, ultrasmall superparamagnetic iron oxide MRI showed a high sensitivity (91%) and specificity (93%) in assessing lymph node status in rectal cancer. However, ultrasmall superparamagnetic iron oxide MRI is not approved by the U.S. Food and Drug Administration and thus will not be available for commercial use in the near future. Diffusion-weighted imaging has been used to assess treatment response to preoperative chemoradiation. Preliminary studies have found lower apparent diffusion coefficient values in malignant lymph nodes and seem to be promising tools in characterization of lymph node metastasis [23].
Circumferential Resection Margin and Recurrence
The circumferential resection margin is defined as the distance from the edge of the tumor to the margin of the resected specimen. Circumferential resection margin involvement is one of the most important independent prognostic factors in the treatment of patients with rectal cancer [24]. High-resolution MRI has the ability to detect involvement of the surgical circumferential resection margin. Circumferential resection margin involvement has been defined as tumor within 1 mm of the mesorectal fascia (Figs. 11 and 12). Beets-Tan et al. [25] found that a tumor-free margin of at least 1 mm can be predicted with a high degree of certainty when the measured distance on MRI is at least 5 mm and a margin of at least 2 mm when the MRI distance is at least 6 mm. The authors also found high agreement within and between observers. MRI has a sensitivity of 60–88% and a specificity of 73–100% for determining circumferential resection margin status [26]. Total mesorectal excision is currently the reference standard for surgical treatment of rectal cancer, and it involves resection of the rectum and mesorectum with an intact mesorectal fascia. The frequency of recurrence is higher in patients with positive margins (19–22%) than in patients with negative margins (3–5%).
Fig. 11.
Rectal cancer.
Axial T2-weighted image shows polypoid tumor (M) with less than 1 mm invasion of mesorectal fat (double arrow). Circumferential resection margin is preserved with intact meso rectal fascia.
Fig. 12.
Rectal cancer.
A and B, Axial (A) and coronal (B) T2-weighted high-resolution images show significant infiltration to meso rectal fat (arrows) threatening circumferential resection margin.
Extramural Vascular Invasion
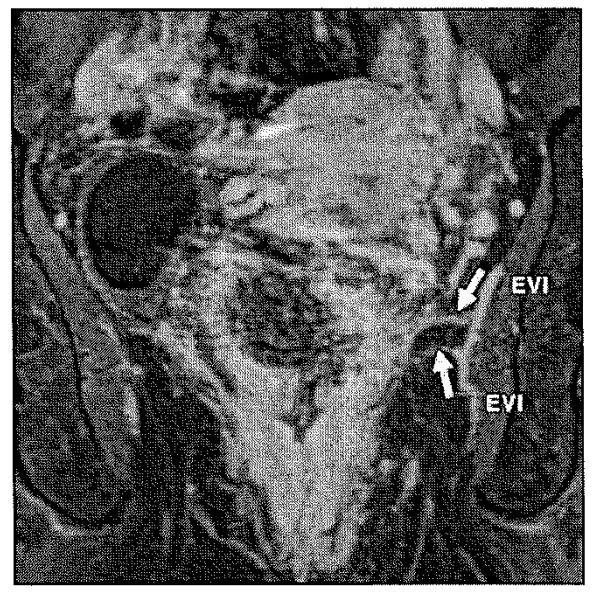
Vascular invasion is defined as the presence of tumor cells in a blood vessel beyond the muscularis propria in the region of primary tumor. Studies have found that vascular invasion independently predicts poor survival and that tumor extramural vascular invasion is an important predictor of both local and distant failure [27]. Brown et al. [28] described the imaging characteristics of extramural vascular invasion (κ = 0.64) compared with histopathology. Extramural invasion presents as serpiginous tumor signal extending through the bowel wall adjacent to the signal void of avessel on MRI. A five-point grading system using four prognostic factors, including tumor margin, tumor location relative to vessels, vessel size, and vessel border, has been proposed for the detection of extramural vascular invasion when MRI features were compared with histopathologic reference standard [29] (Fig. 13).
Fig. 13.
Rectal cancer. Enhanced coronal T1-weighted MRI shows vascular tumor invasion of middle rectal artery branches (arrows). EVI = extramural vascular invasion.
Conclusion
High-resolution MRI plays a vital role in the multimodality imaging approach in the treatment of patients with rectal cancer. Accurate preoperative staging is crucial for making effective therapeutic decisions. High-resolution MRI can accurately delineate the extent of primary tumor, providing physicians with information regarding depth of tumor invasion, relationship of the tumor to mesorectal fascia, status of circumferential resection margin (threatened or not), extramural vascular invasion, and lymph node status, thus en-abling physicians to make effective decisions in terms of patient treatment and contributing to improving overall survival and quality of life in patients with rectal cancer.
Fig. 6.
45-year-old man with stage T3 rectal adenocarcinoma.
A and B, Sagittal (A) and coronal (B) T2-weighted images show asymmetrical circumferential wall thickening (M) obliterating rectal lumen. There is mesorectal fat involvement (arrows).
C and D, Contrast-enhanced sagittal (C) and coronal (D) T1-weighted images show heterogeneous enhancement (arrow, C) of tumor (M) and mesorectal fat (asterisk, D).
Fig. 7.
50-year-old man with stage T3 rectal adenocarcinoma arising in association with tubulovillous adenoma with high-grade dysplasia.
A and B, Coronal (A) and axial (B) T2-weighted high-resolution images show polypoid mass (M) along anterior wall of rectum 3.5 cm superior to anal verge. Tumor (arrow, B) invades mesorectal fat and fascia anteriorly.
References
- 1.American Cancer Society . Cancer facts & figures 2011. American Cancer Society; Atlanta, GA: 2011. [Google Scholar]
- 2.Shihab OC, Moran BJ, Heald RJ, Quirke P, Brown G. MRI staging of low rectal cancer. Eur Radial. 2009;19:643–650. doi: 10.1007/s00330-008-1184-6. [DOI] [PubMed] [Google Scholar]
- 3.MERCURY Study Group Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ. 2006;333:779. doi: 10.1136/bmj.38937.646400.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vliegen RF, Beets GL, Lammering G, et al. Mesorectal fascia invasion after neoadjuvant chemotherapy and radiation therapy for locally advanced rectal cancer: accuracy of MR imaging for prediction. Radiology. 2008;246:454–462. doi: 10.1148/radiol.2462070042. [DOI] [PubMed] [Google Scholar]
- 5.Williamson PR, Hellinger MD, Larach SW, Ferrara A. Endorectal ultrasound of T3 and T4 rectal cancers after preoperative chemoradiation. Dis Colon Rectum. 1996;39:45–49. doi: 10.1007/BF02048268. [DOI] [PubMed] [Google Scholar]
- 6.Akasu T, Kondo H, Moriya Y, et al. Endorectal ultrasonography and treatment of early stage rectal cancer. World J Surg. 2000;24:1061–1068. doi: 10.1007/s002680010151. [DOI] [PubMed] [Google Scholar]
- 7.Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging-a meta-analysis. Radiology. 2004;232:773–783. doi: 10.1148/radiol.2323031368. [DOI] [PubMed] [Google Scholar]
- 8.Thaler W, Watzka S, Martin F, et al. Preoperative staging of rectal cancer by endoluminal ultrasound vs. magnetic resonance imaging: preliminary results of a prospective, comparative study. Dis Colon Rectum. 1994;37:1189–1193. doi: 10.1007/BF02257780. [DOI] [PubMed] [Google Scholar]
- 9.Herzog U, von Flue M, Tondelli P, Schuppisser JP. How accurate is endorectal ultrasound in the preoperative staging of rectal cancer? Dis Colon Rectum. 1993;36:127–134. doi: 10.1007/BF02051167. [DOI] [PubMed] [Google Scholar]
- 10.Solomon MJ, McLeod RS. Endoluminal transrectal ultrasonography: accuracy, reliability, and validity. Dis Colon Rectum. 1993;36:200–205. doi: 10.1007/BF02051183. [DOI] [PubMed] [Google Scholar]
- 11.Jessup J, Gunderson L, Compton C. Colon and rectum. In: Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7th ed. Springer-Verlag; New York: 2010. pp. 143–164. [Google Scholar]
- 12.Brown G, Richards CJ, Newcombe RG, et al. Rectal carcinoma: thin-section MR imaging for staging in 28 patients. Radiology. 1999;211:215–222. doi: 10.1148/radiology.211.1.r99ap35215. [DOI] [PubMed] [Google Scholar]
- 13.Shihab OC, Heald RJ, Rullier E, et al. Defining the surgical planes on MRI improves surgery for cancer of the low rectum. Lancet Oneal. 2009;10:1207–1211. doi: 10.1016/S1470-2045(09)70084-1. [DOI] [PubMed] [Google Scholar]
- 14.Brown G, Daniels IR, Richardson C, Revell P, Peppercorn D, Bourne M. Techniques and trouble-shooting in high spatial resolution thin slice MRI for rectal cancer. Br J Radiol. 2005;78:245–251. doi: 10.1259/bjr/33540239. [DOI] [PubMed] [Google Scholar]
- 15.Maas M, Lambregts DM, Lahaye MJ, et al. T-staging of rectal cancer: accuracy of 3.0 Tesla MRI compared with 1.5 Tesla. Abdom Imaging. 2011 doi: 10.1007/s00261-011-9770-5. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor FG, Swift RI, Blomqvist L, Brown G. A systematic approach to the interpretation of preoperative staging MRI for rectal cancer. AJR. 2008;191:1827–1835. doi: 10.2214/AJR.08.1004. [DOI] [PubMed] [Google Scholar]
- 17.Merkel S, Mansmann U, Siassi M, Papadopoulos T, Hohenberger W, Hermanek P. The prognostic inhomogeneity in pT3 rectal carcinomas. Int J Colo rectal Dis. 2001;16:298–304. doi: 10.1007/s003840100309. [DOI] [PubMed] [Google Scholar]
- 18.Williams PL. Gray’s anatomy: the anatomical basis of medicine and surgery. 38th ed. Churchill Livingston; London: 1995. [Google Scholar]
- 19.Bellows CF, Jaffe B, Bacigalupo L, Pucciarelli S, Gagliardi G. Clinical significance of magnetic resonance imaging findings in rectal cancer. World J Radiol. 2011;3:92–104. doi: 10.4329/wjr.v3.i4.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang C, Zhou Z, Wang Z, et al. Patterns of neoplastic foci and lymph node micrometastasis within the mesorectum. Langenbecks Arch Surg. 2005;390:312–318. doi: 10.1007/s00423-005-0562-7. [DOI] [PubMed] [Google Scholar]
- 21.Chun HK, Choi D, Kim MJ, et al. Preoperative staging of rectal cancer: comparison of 3-T high-field MRI and endorectal sonography. AJR. 2006;187:1557–1562. doi: 10.2214/AJR.05.1234. [DOI] [PubMed] [Google Scholar]
- 22.Will O, Purkayastha S, Chan C, et al. Diagnostic precision of nanoparticle-enhanced MRI for lymph-node metastases: a meta-analysis. Lancet Oncol. 2006;7:52–60. doi: 10.1016/S1470-2045(05)70537-4. [DOI] [PubMed] [Google Scholar]
- 23.Mizukami Y, Ueda S, Mizumoto A, et al. Diffusion-weighted magnetic resonance imaging for detecting lymph node metastasis of rectal cancer. World J Surg. 2011;35:895–899. doi: 10.1007/s00268-011-0986-x. [DOI] [PubMed] [Google Scholar]
- 24.Wibe A, Rendedal PR, Svensson E, et al. Prognostic significance of the circumferential resection margin following total mesorectal excision for rectal cancer. Br J Surg. 2002;89:327–334. doi: 10.1046/j.0007-1323.2001.02024.x. [DOI] [PubMed] [Google Scholar]
- 25.Beets-Tan RG, Beets GL, Vliegen RF, et al. Accuracy of magnetic resonance imaging in prediction of tumour-free resection margin in rectal cancer surgery. Lancet. 2001;357:497–504. doi: 10.1016/s0140-6736(00)04040-x. [DOI] [PubMed] [Google Scholar]
- 26.Lahaye MJ, Engelen SM, Nelemans PJ, et al. Imaging for predicting the risk factors—the circumferential resection margin and nodal disease—of local recurrence in rectal cancer: a meta-analysis. Semin Ultrasound CT MR. 2005;26:259–268. doi: 10.1053/j.sult.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Horn A, Dahl O, Morild I. The role of venous and neural invasion on survival in rectal adenocarcinoma. Dis Colon Rectum. 1990;33:598–601. doi: 10.1007/BF02052215. [DOI] [PubMed] [Google Scholar]
- 28.Brown G, Radcliffe AG, Newcombe RG, Dallimore NS, Bourne MW, Williams GT. Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging. Br J Surg. 2003;90:355–364. doi: 10.1002/bjs.4034. [DOI] [PubMed] [Google Scholar]
- 29.Smith NJ, Barbachano Y, Norman AR, Swift RI, Abulafi AM, Brown G. Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br J Surg. 2008;95:229–236. doi: 10.1002/bjs.5917. [DOI] [PubMed] [Google Scholar]