Abstract
Background
An integrated approach to reduce densities of adult Aedes aegypti inside homes is currently being evaluated under experimentally controlled field conditions. The strategy combines a spatial repellent (SR) treatment (applied indoors) with the Biogents Sentinel™ (BGS) mosquito trap positioned in the outdoor environment. In essence, when combined, the goal is to create a push-pull mechanism that will reduce the probability of human-vector contact. The current study measured BGS recapture rates of Ae. aegypti test cohorts that were exposed to either SR or control (chemical-free) treatments within experimental huts. The objective was to define what, if any, negative impact SR may have on BGS trap efficacy (i.e., reduced BGS collection).
Methods
Aedes aegypti females were exposed to SR compounds within experimental huts in the form of either treated fabric (DDT and transfluthrin) or mosquito coil (metofluthrin). Test cohorts were released within individual screen house cubicles, each containing 4 BGS traps, following SR exposure according to treatment. Two separate test cohorts were evaluated: (i) immediate release (IR) exposed from 06:00–12:00 hours and released at 12:00 hours and (ii) delayed release (DR) exposed from12:00–18:00 hours and released at 05:30 hours the following day. BGS recapture was monitored at 09:30, 13:30 and 15:30 hours and the cumulative recapture by time point quantified.
Results
Exposure of Ae. aegypti females to either DDT or metofluthrin did not significantly impact BGS capture as compared to cohorts of non-exposed females. This was true for both IR and DR exposure populations. IR cohorts exposed to transfluthrin resulted in significantly lower BGS recapture compared to matched controls but this effect was primarily due to high mosquito mortality during transfluthrin trials.
Conclusion
Our data indicate no more than minor and short-lived impacts (i.e., reduced attraction) on BGS trap catches following exposure to the pyrethroid compounds transfluthrin and metofluthrin and no change in recapture densities using DDT as compared to matched controls. These findings suggest a combined SR and BGS approach to vector control could function as a push-pull strategy to reduce Ae. aegypti adults in and around homes.
Keywords: Aedes aegypti, Spatial repellents, Screen house, Experimental huts, BG-Sentinel™ trap, Push-pull strategy, Thailand
Background
Dengue and dengue hemorrhagic fever occur in the tropics and subtropics with an estimated 2.5 billion people residing in areas where dengue is endemic [1]. Dengue viruses are transmitted primarily by Aedes aegypti, a day-biting mosquito that feeds and rests indoors and preferentially bites humans [2-5]. Despite years of public health efforts and research progress, an effective vaccine against dengue virus is not yet available. For this reason, disease prevention remains dependent on vector management and control strategies [1,4]. However, controlling Ae. aegypti has proven difficult due to its strong association with domestic and peridomestic human environments that harbor and sustain development sites (artificial containers) for the immatures. Furthermore, control of Ae. aegypti adults is commonly based on indoor and outdoor spraying of insecticides to reduce mosquito abundance and disrupt dengue virus transmission during outbreaks [6-8]. This is complicated by the worldwide rise and increasing impact of resistance of Ae. aegypti to commonly used insecticides, including in Thailand [9-12]. New approaches are urgently needed to improve our capacity to control this mosquito, especially targeting the adult stage in and around the home.
Push-pull strategies, combining a repellent with an attractant, have been effective in the control of some agricultural pests [13,14]. The mechanism underlying a push-pull system includes: (i) behavioral manipulation of the target species to repel or deter (push) them away from a resource (i.e., a crop) using stimuli that renders the resource unsuitable or unattractive and (ii) a device, for example a trap, through which the target species are removed from the environment (pull) [13-15]. Such an approach may also prove effective in the control of pathogen-transmitting mosquitoes, especially in and around the home where many vector-borne pathogens are predominantly transmitted in the developing world. One clear benefit of a push-pull system is that it can be effective in settings where insecticide resistance occurs. This is because the chemical doses that elicit sublethal behavioral responses, such as spatial repellency, are below that required for toxicity, thereby reducing insecticide resistance selection pressure while continuing to prevent human-vector contact [1,16-18].
Spatial repellents are defined as chemicals that function in the vapor phase to affect biting insects at a distance from the treatment source and can inhibit the ability of vectors to locate and track a host [19]. The vapor plume formed by the source of a spatial repellent creates a protective barrier extending to a certain radius from the source of the repellent chemical [20]. This has potential for protection of entire households. Moreover, continuous day and night protection can be provided through formulations that allow slow and continuous evaporation of the repellent substance. Continuous use of spatial repellents is expected to result in prevention of vectors from entering the treated space thereby prolonging times for the mosquito to locate hosts and/or resting places, thus increasing the likelihood of adverse environmental conditions, predation or other causes inducing mortality [21].
In the specific case of Ae. aegypti control, a trap or pull component may pose the greatest challenge within a push-pull system. Several trap designs have been commonly used for adult mosquito surveillance purposes [22,23]. However, many of these have not been satisfactory for Ae. aegypti[24-26]. The development of new, improved traps, such as the BG-Sentinel™ (BGS) and Zumba™ traps, provides an opportunity for improved entomological surveillance and possibly also control of Ae. aegypti[27-31] and Ae. albopictus[32,33]. The BGS trap targets the most important elements of Ae. aegypti host-seeking behavior by combining an olfactory cue (BGS Lure) with a visual cue (black and white contrast) to attract the mosquito. This trap has proven to be an effective tool for surveillance of Ae. aegypti adults, out-performing other collection devices such as the CDC backpack aspirator, the Fay-Prince trap, the Encephalitis Virus Surveillance trap and the Mosquito Magnet Liberty™ trap [29,30].
Based on these findings, the BGS trap was selected for evaluation in a push-pull Ae. aegypti control strategy currently under experimental evaluation. The push component uses spatial repellent chemicals that have been shown to deter Thai Ae. aegypti from entering homes (Ojo et al., unpublished data). The BGS trap provides the pull component to remove repelled Ae. aegypti from the peridomestic environment thereby further reducing human-vector contact. Previous studies have confirmed that the BGS trap effectively removes Ae. aegypti from a controlled environment [34]. However, the effects of exposure to repellents, or sublethal doses of insecticides, on BGS trap collections have not been previously evaluated. For the pull component to be most effective, previous exposure to the spatial repellent being used to push vectors from entering homes should not substantially decrease the likelihood of the mosquito being trapped outdoors.
The objective of the current study was to define the effect of previous exposure of Ae. aegypti to spatial repellents in experimental huts on BGS trap efficacy. This information is important to define potential limitations in strategy success when both tools are used in combination. In addition, as a critical debate in the use of spatial repellents for vector control includes potential diversion or movement of repelled vectors to unprotected human hosts, findings will also provide insight as to how SR exposure may interfere with the host-seeking (i.e. attraction) response.
Methods
Study area and experimental huts
Studies were conducted near Pu Teuy (14°17′N, 99° 11′E), which is a small agricultural village (<1,500 inhabitants) located 150 km northwest of Bangkok in Sai Yok District, Kanchanaburi Province, Thailand. The village is situated in a mountainous area (420 m above sea level) and completely surrounded by dense primary forest, orchards and vegetable plantations. Aedes aegypti is prevalent in Pu Teuy village. The abundance of immatures in artificial water-holding containers is surveyed weekly, by the Thongpaphum District Clinic, and mosquito control interventions include distribution of organophosphate larvicide (temephos). Our experimental site is located >800 m from the closest indigenous home, creating a distance buffer for mark-release-recapture mosquito behavioral studies which exceeds the normal flight range (< 100 m) of Ae. aegypti[5,35]. The experimental huts used in the study have been previously described [36]. The huts mimic indigenous Thai homes in materials and dimensions, and are also used within the larger research program to evaluate Ae. aegypti entering and exiting behaviors as part of the development of the push-pull strategy [37].
Mosquitoes
Immatures of Ae. aegypti were collected weekly from Pu Teuy village and reared to adults at the on-site field insectary. Female, nulliparous, 3–5 d old sugar-starved (i.e. reflecting a host-seeking physiological status) were used for the experimental trials. This age range and starvation treatment increased the probability that mosquitoes would respond to human host and BGS trap cues during evaluations. Mosquito test cohorts (control/treatment) were marked with unique colored fluorescent powder following previous dusting protocols [38].
One day pre-trial, cohorts (n = 50) were placed into individual ‘exposure cages’, mesh screen cages (26 × 26 × 30 cm), to: 1) facilitate transfer between huts and the screen house and 2) to prevent contact with treated surfaces so that chemical exposure would be based entirely on vapor phase particles. For each repellent treatment, two separate exposure cohorts were used: (i) an Immediate Release (IR) cohort exposed during 06:00–12:00 hours and then released into the screen house containing BGS traps at 12:00 hours and (ii) a Delayed Release (DR) cohort exposed during 12:00–18:00 hours and then released at 05:30 hours the following day, thus having a recovery period of nearly 12 h (with access to water soaked cotton pads). Individual screened cages were placed in the center of experimental huts according to exposure time. A matched control (i.e., chemical-free hut) was used simultaneously for each exposure trial.
BG-Sentinel™ (BGS) trap
All BGS traps were baited with the BG-Lure and operated according to the manufacturer’s instructions. The trap consists of a collapsible container made of white plastic sack material. The top of the container is covered with white gauze cloth surrounding a black plastic funnel. This funnel is connected to a mesh catch bag that collects trapped mosquitoes. A 12 volt suction fan below the base of the funnel creates downward suction after connection to an external power source. The air is then pushed upwards passing through the gauze cover creating convection currents [27]. The contrasting black and white colors of the trap provide visual attraction. The accompanying BG-Lure consists of lactic acid, ammonia and caproic acid, compounds that are found in human sweat [39-41]. When the trap fan is operating, the air current carries the lure volatiles out through the gauze cloth cover into the surrounding environment. The BG-Lures were used within 4 months after opening per manufacturer’s recommendation.
Chemical exposure
Two persons were present inside each of the experimental huts during trials to monitor coil burning and conduct collections for push-pull evaluations (Ojo et al. unpublished data). All test chemicals are USEPA registered and as such, have passed mammalian toxicology thresholds for human safety. Informed consent was conducted according to corresponding Uniformed Services University of the Health Sciences and Kasetsart University scientific and ethical review committee approvals.
The following repellent chemicals were evaluated in separate trials: 1) the organochlorine, DDT −1,1 Bis(4-chlorophenyl)-2,2,2- trichloroethane, (CAS 50-29-3,Sigma-Aldrich), 2) the synthetic pyrethroid, transfluthrin-2,3,5,6-tetrafluorobenzy(1R,3S)-3-(2,2-dichlorovinyl) 2,2dimethylcyclopropanecarboxylate (CAS118712-89-3, Bayer, AG) and 3) another synthetic pyrethroid, metofluthrin- 2,3,5,6-Tetrafluoro-4-(methoxymethyl)benzyl2,2-dimethyl-3-(prop-1-en-1-yl) cyclopropanecarboxylate (S.C. Johnson & Son, Inc). These chemicals represent standards in household mosquito control products (i.e., mosquito coils) or use within organized vector control campaigns (i.e. indoor residual spraying) and have been reported to have spatial repellent characteristics [16-18,21,42-55]. Although DDT has been prohibited for use in mosquito control programs in some areas, it was used here based on evidence of effectively controlling pests and mosquitoes transmitting malaria parasites and dengue virus [42,43,55].
Repellent treatments consisted of either chemical-treated fabric panels (DDT and transfluthrin trials) or a standard mosquito coil (metofluthrin trials). Matched control huts contained either chemical-free fabric (solvent only) or blank coil (coil without active ingredient). DDT was applied to fabric to mimic previous experimental hut studies evaluating its spatial repellent characteristics [17] and because this chemical is typically applied as an indoor residual spray to interior walls of houses [44]. Transfluthrin was similarly applied to fabric to match conditions employed for push-pull trials within the larger research project. Metofluthrin exposure was evaluated using a coil as this is a typical delivery format for the compound and volatile insecticides in general [45].
The preparation, treatment and positioning of DDT or transfluthrin-treated fabric inside experimental huts followed procedures previously described [17]. DDT and transfluthrin were applied to fabric panels 48 h pre-testing using solvent solution. The panels were air-dried under a chemical fume hood prior to storage at 4°C until used. Control fabric panels were treated with solvent alone and processed following the same procedure. Fabric panels were fitted onto metal mesh frames positioned along the interior walls of each hut using magnets [17,36]. One set of treated fabric panels was used for each experimental trial. DDT exposure was conducted using field application rate (FAR; 2 g ai/m2) at 75%, 50% and 25% surface area coverage (SAC). Evaluations were also performed using 25% SAC against 0.5 FAR (1 g ai/m2). Transfluthrin evaluations included 1.0 (40 μg ai/cm2), 0.5 (20 μg ai/cm2), 0.125 (5 μg ai/cm2), and 0.062 FAR (2.5 μg ai/cm2) using 25% SAC. The selected coverage during transfluthrin trials was based on data from experiments evaluating the push-pull system that showed 25% coverage to be as effective as coverage at 50% and 100% (Achee et al. unpublished data).
Metofluthrin coils representing high (0.0065% ai) and low (0.003% ai) doses were burned according to manufacturer’s recommendation within a metal dish positioned in the center of the hut. Coils were lit at 05:30 h, or 30 min before exposure cages were introduced, and were replaced at 12:00 h to ensure burning continued until 18:00 h, which represents the typical time range of expected Ae. aegypti biting period. The lag time following initial lighting allowed vaporization of the chemical and thereby distribution within interior air space. Both negative (no coil) and positive (coil without active ingredient) controls were used simultaneously in metofluthrin trials.
On each trial day, one mosquito exposure cage (i.e. one test cohort) was placed in the center of each experimental hut (approximately 2 m from treated fabric panels and 1 m from burning coils) representing either control (chemical-free) or treatment (with spatial repellent) conditions (Figure 1). For trials with mosquito coils, an additional hut containing no coil was used as a negative control. A total of four replicates were performed for each chemical treatment.
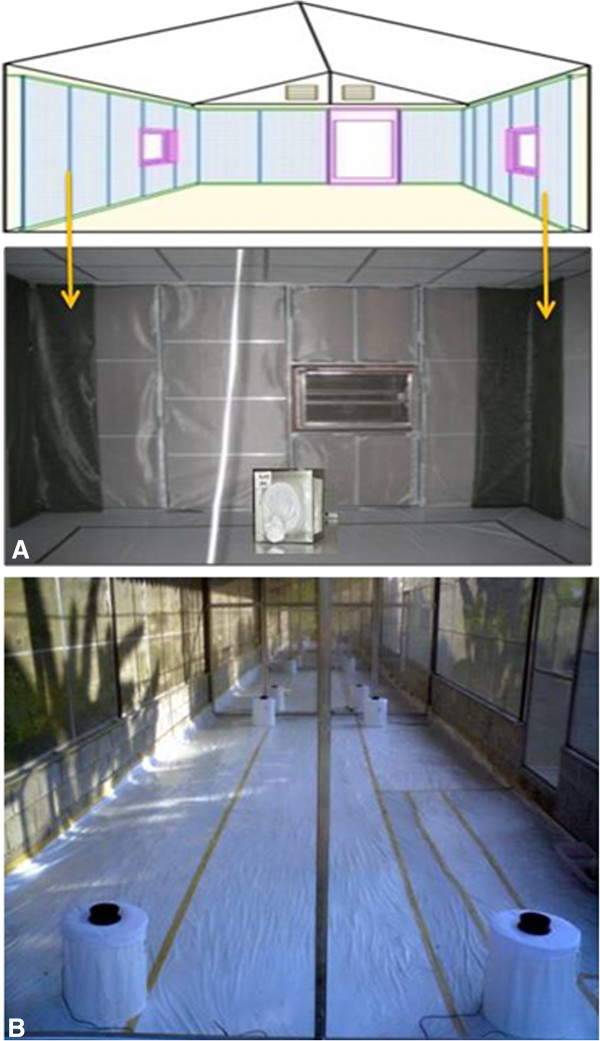
Figure 1.
(A) Positioning of treated material and exposure of Aedes aegypti in huts; (B) Mosquito trapping with BG-Sentinel™ traps in the screen house.
BGS capture evaluation
Post-exposure BGS captures were evaluated under semi-field screen house conditions. The screen house measures 4 m (width) × 3.5 m (height) × 40 m (length) and is located on-site with the experimental huts [34]. The screen house is subdivided into four 10 m long cubicles using metal partitions, each with a space volume of 140 m3 (Figure 1). This is similar to the volume inside and within 2 m outdoors of the experimental huts used for vector behavior studies at the field site [36] and is the expected approximate space volume within which Ae. aegypti primarily would make contact with an outdoor trap at a typical home in a dengue-endemic environment in Thailand [3,5].
Screen house cubicles were designated as control or treatment for evaluation of unexposed cohorts (chemical-free huts) and repellent-exposed cohorts, respectively. Within each cubicle, 4 BGS traps were operated simultaneously. The traps were monitored for mosquitoes based on sampling periods established from previous studies [35]. Those used for IR cohorts included: 13:30 and 17:30 hours Day of exposure (Day 1) and 05:30, 09:30, 13:30 and 17:30 hours Day following exposure (Day 2). Those used for DR cohorts included: 09:30, 13:30 and 17:30 hours Day 2 and 05:30, 09:30, 13:30 and 17:30 hours two days following exposure (Day 3).
Environmental parameters (temperature, relative humidity and light intensity) were recorded inside each cubicle using HOBO data loggers (HOBO U12-012 Model, Onset Computer Corporation, Bourne, MA). Baseline experiments were conducted to measure environmental variables among cubicles to ensure comparability before exposure studies were performed [34].
Data analyses
Cumulative percentage BGS recapture of each test cohort were computed after correcting for the number of knocked down mosquitoes following exposure and prior to release. A Kruskal-Wallis statistical test was used to compare percentage recapture between treatment and control cohorts. The Mann–Whitney statistical test was used to compare cumulative BGS densities between IR and DR exposure cohorts. For all analyses, a p-value of 0.05 or less was considered statistically significant. Statistical analyses were performed in STATA 11.2 using the ranksum and kwallis syntax for Mann–Whitney test and Kruskal Wallis tests, respectively.
Results
DDT exposure
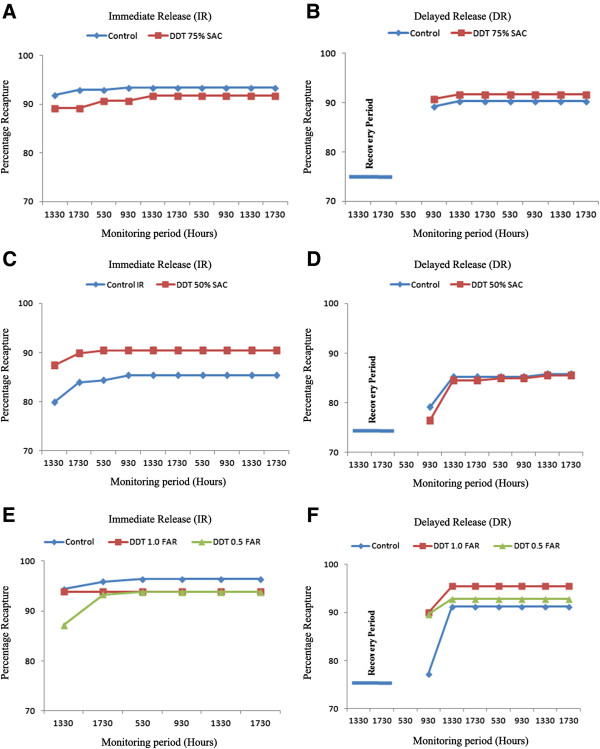
Cumulative BGS recapture, across application dose and SAC, ranged from 86-94% for DDT-exposed mosquitoes and 85-95% for control cohorts (Figure 2, Table 1). There was no significant difference in BGS recapture between DDT-exposed and control cohorts for either IR or DR populations (Figure 2, Table 1). This was true for evaluations using 75%, 50% and 25% SAC 1.0 FAR (2 g/m2) exposure conditions. Similar results were seen for trials using 25% SAC at 0.5 FAR (1 g/m2).
Figure 2.
Cumulative BG-Sentinel™trap recaptures for Ae. aegypti females in trials using immediate release or delayed release of mosquitoes previously exposed to DDT (2 g ai/m2) A and B - 75% SAC; C and D - 50% SAC) or DDT (2 g and 1 ai/m2) E and F - 25% SAC exposed populations.
Table 1.
Cumulative BG-Sentinel™ trap catches for immediate release (IR)1 and delayed release (DR)2 trials with Ae. aegypti3 exposed to DDT-treated fabrics
| Cumulative mean percentage (±SD) of released Ae. aegypti recaptured by time point4 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Surface area coverage |
Release5/Treatments |
←‒‒‒Day 1‒‒‒‒‒→ |
←‒‒‒‒‒‒‒‒‒‒‒‒‒‒‒Day 2‒‒‒‒‒‒‒‒‒‒‒‒‒‒‒‒→ |
←‒‒‒‒‒‒‒‒‒‒‒‒‒‒‒‒‒Day 3‒‒‒‒‒‒‒‒‒‒‒‒‒‒‒‒‒‒‒→ |
|
Mean day-time (12 hr) conditions |
|||||||||
| |
|
13:30 h |
17:30 h |
05:30 h |
09:30 h |
13:30 h |
17:30 h |
05:30 h |
09:30 h |
13:30 h |
17:30 h |
N6 |
Temp (°C) |
RH (%) |
Light intensity (lx/ft2) |
|
75% |
IRa |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Control |
91.9a (±4.5) |
92.9a (±3.5) |
92.9a (±3.5) |
93.4a (±3.5) |
93.4a (±3.5) |
93.4a (±3.5) |
- |
- |
- |
- |
185/198 |
33.4 |
25.9 |
382.1 |
|
| DDT (2 g ai/m2) |
89.2a (±5.3) |
89.2a (±5.3) |
90.7a (±4.1) |
90.7a (±4.1) |
91.8a (±3.8) |
91.8a (±3.8) |
- |
- |
- |
- |
178/194 |
31.9 |
28.6 |
331.6 |
|
|
DRa |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Control |
- |
- |
- |
89.3a (±4.8) |
90.3a (±3.7) |
90.3a (±3.7) |
90.3a (±3.7) |
90.3a (±3.7) |
90.3a (±3.7) |
90.3a (±3.7) |
177/196 |
33.5 |
25.9 |
371.7 |
|
| DDT (2 g ai/m2) |
- |
- |
- |
90.7a (±5.2) |
91.8a (±3.8) |
91.8a (±3.8) |
91.8a (±3.8) |
91.8a (±3.8) |
91.8a (±3.8) |
91.8a (±3.8) |
178/194 |
31.9 |
28.4 |
318.8 |
|
| 50% |
IRa |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Control |
79.9a (±10.6) |
83.9a (±4.9) |
84.4a (±5.9) |
85.4a (±5.5) |
85.4a (±5.5) |
85.4a (±5.5) |
|
|
|
|
170/199 |
28.7 |
31.9 |
479.0 |
|
| DDT (2 g ai/m2) |
87.4a (±9.6) |
89.9a (±7.7) |
90.4a (±7.7) |
90.4a (±7.7) |
90.4a (±7.7) |
90.4a (±7.7) |
- |
- |
- |
- |
178/198 |
29.7 |
69.1 |
454.5 |
|
|
DRa |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Control |
|
|
|
79.2a (±10.8) |
85.3a (±5.9) |
85.3a (±5.9) |
85.3a (±5.9) |
85.3a (±5.9) |
85.8a (±6.3) |
85.8a (±6.3) |
169/197 |
30.8 |
66.7 |
494.2 |
|
| DDT (2 g ai/m2) |
|
|
|
76.5a (±11.1) |
84.5a (±3.4) |
84.5a (±3.4) |
85.0a (±3.5) |
85.0a (±3.5) |
85.5a (±3.5) |
85.5a (±3.5) |
171/200 |
29.2 |
71.8 |
501.2 |
|
| 25% |
IRa |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Control |
94.4a (±4.7) |
95.9a (±4.5) |
96.4a (±4.6) |
96.4a (±4.6) |
96.4a (±4.6) |
96.4a (±4.6) |
- |
- |
- |
- |
189/196 |
28.1 |
24.4 |
192.16 |
|
| DDT (2 g ai/m2) |
93.9a (±3.0) |
93.9a (±3.0) |
93.9a (±3.0) |
93.91a (±2.95) |
93.9a (±3.0) |
93.9a (±3.0) |
- |
- |
- |
- |
185/197 |
27.4 |
26.0 |
126.2 |
|
| DDT (1 g ai/m2) |
87.2a (±14.6) |
93.3a (±3.9) |
93.9a (±3.6) |
93.9a (±3.6) |
93.9a (±3.6) |
93.9a (±3.6) |
|
|
|
|
183/195 |
27.1 |
31.7 |
76.4 |
|
|
DRa |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Control |
- |
- |
- |
77.2a (±8.9) |
91.2a (±3.7) |
91.2a (±3.7) |
91.2a (±3.7) |
91.2a (±3.7) |
91.2a (±3.7) |
91.2a (±3.7) |
176/193 |
28.4 |
25.3 |
205.5 |
|
| DDT (2 g ai/m2) |
- |
- |
- |
90.0a (±5.4) |
95.5a (±2.6) |
95.5a (±2.6) |
95.5a (±2.6) |
95.5a (±2.6) |
95.5a (±2.6) |
95.5a (±2.6) |
190/199 |
27.8 |
26.6 |
135.2 |
|
| DDT (1 g ai/m2) | - | - | - | 89.6a (±4.7) | 92.8a (±4.11) | 92.8a (±4.1) | 92.8a (±4.1) | 92.8a (±4.1) | 92.8a (±4.1) | 92.8a (±4.1) | 181/195 | 27.5 | 32.8 | 85.6 | |
1 Cohort exposed from 0600–1200 hours and released immediately afterwards.
2 Cohort exposed from 1200–1800 hours but released only after a holding period of 12 h.
3 3–5 day old starved females.
4 Different lowercase letters in the same column indicate significant differences between mean recapture percentages for released females within release trial types (IR or DR; Kruskal-Wallis 95% confidence limit ) or between IR and DR trials (Mann-U Whitney Test, 95% confidence limit).
5 Different lowercase letters in the same column indicate significant differences between mean recapture percentages for released females between IR and DR trials (Mann-U Whitney Test, 95% confidence limit).
6Total recaptured /total released minus total knock down.
Metofluthrin exposure
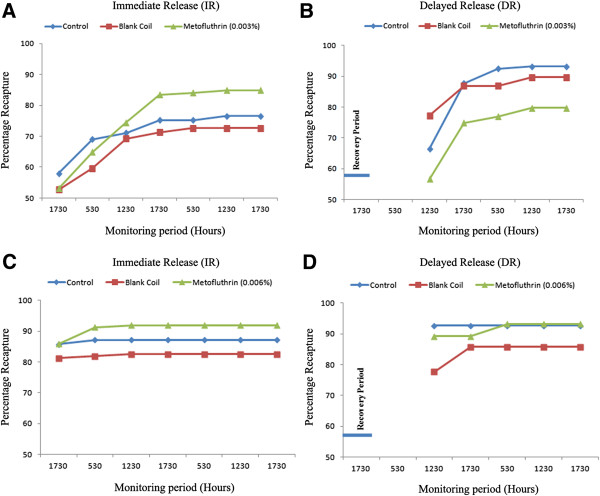
There were no significant differences in BGS recapture rates among metofluthrin-exposed and both positive (blank coil) and negative control (no coil) cohorts for either IR or DR populations. This was true using both high (0.0065%) and low dose (0.003%) coils (Figure 3A-D, Tables 2). Cumulative recaptures ranged from 77-93% for negative controls, 73-90% for positive controls and a combined range of 80-93% for cohorts exposed to both low or high dose coils (Figure 3A-B, Table 2).
Figure 3.
Cumulative BG-Sentinel™trap recaptures for Ae. aegypti females in trials using immediate release or delayed release of mosquitoes previously exposed to metofluthrin: A and B - low dose (0.003%) and C and D - high dose (0.006%).
Table 2.
Cumulative BG-Sentinel™ trap catches for immediate release (IR)1 and delayed release (DR)2 trials with Ae. aegypti3 exposed to metofluthrin coils
| Cumulative mean percentage (±SD) of released Ae. aegypti recaptured by time point4 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Release5/Treatments |
Day 1 |
←‒‒‒‒‒‒‒‒‒‒‒‒‒‒‒Day 2‒‒‒‒‒‒‒‒‒‒‒‒‒‒‒‒‒→ |
←‒‒‒‒‒‒‒‒‒‒‒‒‒‒Day 3‒‒‒‒‒‒‒‒‒‒‒‒‒‒‒‒‒‒‒→ |
|
Mean day-time (12 hr) conditions |
||||||
|
Dose |
|
17:30 h |
05:30 h |
12:30 h |
17:30 h |
05:30 h |
12:30 h |
17:30 h |
N6 |
Temp (°C) |
RH (%) |
Light intensity (lx/ft2) |
| Low dose (0.00312%) |
IRa |
|
|
|
|
|
|
|
|
|
|
|
| Control |
57.9a (±5.6) |
69.0a (±6.5) |
71.0a (±6.3) |
75.2a (±7.9) |
75.2a (±7.9) |
76.6a (±7.9) |
76.6a (±7.9) |
111/145 |
24.9 |
60.2 |
167.6 |
|
| Blank Coil |
52.7a (±7.9) |
59.6a (±13.6) |
69.2a (±18.7) |
71.2a (±6.3) |
72.6a (±8.7) |
72.6a (±8.7) |
72.6a (±8.7) |
106/146 |
25.2 |
59.9 |
177.9 |
|
| Metofluthrin |
53.1a (±11.0) |
64.8a (±8.2) |
74.2a (±8.9) |
83.5a (±1.3) |
84.2a (±0.3) |
84.8a (±1.1) |
84.8a (±1.1) |
123/145 |
24.1 |
61.8 |
66.6 |
|
|
DRa |
|
|
|
|
|
|
|
|
|
|
|
|
| Control |
- |
- |
66.4a (±18.8) |
87.7a (±9.5) |
92.5a (±2.6) |
93.2a (±3.4) |
93.2a (±3.4) |
136/146 |
26.2 |
63.0 |
178.5 |
|
| Blank Coil |
- |
- |
77.2a (±14.6) |
86.9a (±3.7) |
86.9a (±3.7) |
89.9a (±1.7) |
89.9a (±1.7) |
130/145 |
26.3 |
62.7 |
173.9 |
|
| Metofluthrin |
- |
- |
56.6a (±7.4) |
74.8a (±10.3) |
76.9a (±11.5) |
79.7a (±13.8) |
79.7a (±13.9) |
113/143 |
25.2 |
65.7 |
61.5 |
|
| High Dose (0.00625%) |
IRa |
|
|
|
|
|
|
|
|
|
|
|
| Control |
85.8a(±5.3) |
87.2a (±5.8) |
87.2a (±5.8) |
87.2a (±5.8) |
87.2a (±5.8) |
87.2a (±5.8) |
87.2a (±5.8) |
123/148 |
28.1 |
65.6 |
167.6 |
|
| Blank Coil |
81.2a (±8.9) |
81.9a (±7.9) |
82.6a (±6.9) |
82.55a (±7.0) |
82.6a (±6.9) |
82.6a (±6.9) |
82.6a (±6.9) |
123/149 |
28.0 |
65.8 |
185.1 |
|
| Metofluthrin |
85.9a (±6.0) |
91.3a (±1.1) |
92.0a (±1.9) |
92.0a (±1.9) |
92.0a (±1.9) |
92.0a (±1.9) |
92.0a (±1.9) |
137/149 |
26.7 |
69.2 |
63.7 |
|
|
DRa |
|
|
|
|
|
|
|
|
|
|
|
|
| Control |
- |
- |
92.7a (±4.2) |
92.7a (±4.2) |
92.7a (±4.2) |
92.7a (±4.2) |
92.7a (±4.2) |
144/150 |
28.0 |
65.8 |
194.8 |
|
| Blank Coil |
- |
- |
77.7a (±1.6) |
85.8a (±5.4) |
85.8a (±5.4) |
85.8a (±5.4) |
85.8a (±5.4) |
126/148 |
29.7 |
65.9 |
186.8 |
|
| Metofluthrin | - | - | 89.2a (±5.4) | 89.2a (±5.4) | 93.2a (±3.0) | 93.2a (±3.0) | 93.2a (±3.0) | 138/148 | 26.7 | 69.4 | 61.7 | |
1 Cohort exposed from 0600–1200 hours and released immediately afterwards.
2 Cohort exposed from 1200–1800 hours but released only after a holding period of 12 h.
3 3–5 day old starved females.
4 Different lowercase letters in the same column indicate significant differences between mean recapture percentages for released females within release trial types (IR or DR; Kruskal-Wallis 95% confidence limit ) or between IR and DR trials (Mann-U Whitney Test, 95% confidence limit).
5 Different lowercase letters in the same column indicate significant differences between mean recapture percentages for released females between IR and DR trials (Mann-U Whitney Test, 95% confidence limit).
6Total recaptured /total released minus total knock down.
Transfluthrin exposure
Exposure to transfluthrin at 1.0 FAR (40 μg ai/cm2) using 100, 50 or 25% SAC resulted in high mortality rates of Ae. aegypti cohorts. Mortality ranged from 95-100% and prevented BGS trap evaluations (Figure 4, Table 3). Trials using 0.125 FAR (5 μg ai/cm2) and 0.062 FAR (2.5 μg ai/cm2) at 25% SAC, however, did not have the same killing effect (23-69% mortality). Exposure to these treatment conditions resulted in significantly reduced BGS recapture for IR cohorts as compared to control; in contrast, there was no significant difference between BGS recapture of DR and control cohorts (Figure 4A-B, Table 3). Overall, BGS recapture was higher for DR as compared to IR cohorts (Figure 4A-B, Table 3). BGS trap catches for IR were reduced following exposure to the higher transfluthrin concentration (0.125 FAR), where only 45% of released females were recaptured as compared to 76% using 0.0625 FAR (p = 0.01).
Figure 4.
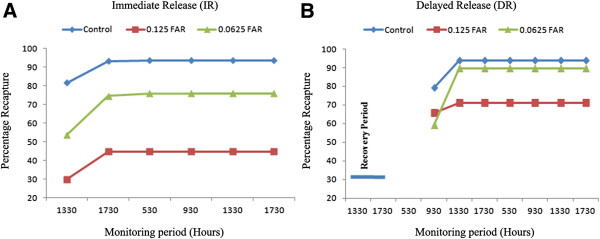
Cumulative BG-Sentinel™trap recaptures for Ae. aegypti females in trials using immediate release (A) or delayed release (B) of mosquitoes previously exposed to transfluthrin.
Table 3.
Cumulative BG-Sentinel™ trap catches for immediate release (IR)1 and delayed release (DR)2 trials with Ae. aegypti3 exposed to transfluthrin-treated fabrics
| Cumulative mean percentage (±SD) of released Ae. aegypti recaptured by time point4 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Release5/Treatments |
←‒‒‒‒‒Day 1‒‒‒‒‒→ |
←‒‒‒‒‒‒‒‒‒‒‒‒‒‒‒Day 2‒‒‒‒‒‒‒‒‒‒‒‒‒‒‒‒‒→ |
←‒‒‒‒‒‒‒‒‒‒‒‒‒‒‒‒‒‒‒Day 3‒‒‒‒‒‒‒‒‒‒‒‒‒‒‒‒‒‒→ |
|
Mean day-time (12 hr) conditions |
|||||||||
| |
13:30 h |
17:30 h |
05:30 h |
09:30 h |
13:30 h |
17:30 h |
05:30 h |
09:30 h |
13:30 h |
17:30 h |
N6 |
Temp (°C) |
RH (%) |
Light intensity (lx/ft2) |
|
IRa Control |
81.8a (±11.1) |
93.3a (±8.6) |
93.8a (±7.6) |
93.8a (±7.6) |
93.8a (±7.6) |
93.8a (±7.6) |
- |
- |
- |
- |
180 /192 |
28.7 |
77.1 |
314.7 |
| Transfluthrin (5 μg ai/cm2) |
29.9c (±11.8) |
44.8c (±18.7) |
44.8c (±18.7) |
44.8c (±18.7) |
44.8c (±18.7) |
44.8c (±18.7) |
- |
- |
- |
- |
39/87 |
27.2 |
83.4 |
97.1 |
| Transfluthrin (2.5 μg ai/cm2) |
53.9b (±16.0) |
74.7b (±9.3) |
76.0b (±9.3) |
76.0b (±9.3) |
76.0b (±9.3) |
76.0b (±9.3) |
|
|
|
|
117/154 |
26.1 |
37.1 |
120.3 |
|
DRb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Control |
- |
- |
- |
79.2a (±9.4) |
93.9a (±4.3) |
93.9a (±4.3) |
93.9a (±4.3) |
93.9a (±4.3) |
93.9a (±4.3) |
93.9a (±4.3) |
185/197 |
28.0 |
78.6 |
308.3 |
| Transfluthrin (5 μg ai/cm2) |
- |
- |
- |
65.6a (±18.3) |
70.9a (±16.4) |
70.9a (±16.4) |
70.9a (±16.4) |
70.9a (±16.4) |
70.9a (±16.4) |
70.9a (±16.4) |
43/61 |
26.8 |
85.8 |
98.0 |
| Transfluthrin (2.5 μg ai/cm2) | - | - | - | 59.2a (±28.1) | 89.6a (±4.6) | 89.6a (±4.6) | 89.6a (±4.6) | 89.6a (±4.6) | 89.6a (±4.6) | 89.6a (±4.6) | 92/138 | 25.7 | 37.7 | 126.8 |
1 Cohort exposed from 0600–1200 hours and released immediately afterwards.
2 Cohort exposed from 1200–1800 hours but released only after a holding period of 12 h.
3 3–5 day old starved females.
4 Different lowercase letters in the same column indicate significant differences between mean recapture percentages for released females within release trial types (IR or DR; Kruskal-Wallis 95% confidence limit ) or between IR and DR trials (Mann-U Whitney Test, 95% confidence limit).
5 Different lowercase letters in the same column indicate significant differences between mean recapture percentages for released females between IR and DR trials (Mann-U Whitney Test, 95% confidence limit).
6Total recaptured /total released minus total knock down.
Environmental parameters
No significant differences (p > 0.05) were found among mean daily temperature, relative humidity or light intensity variables measured within screen house cubicles designated as either control (for release of unexposed cohorts) or treatment (for release of repellent-exposed cohorts) for all trials (Tables 1, 2, 3).
Discussion
The primary objective of the current study was to quantify the effects of exposure of Ae. aegypti to spatial repellent compounds on catch rates from a validated adult mosquito trap – the Biogents Sentinel™ (BGS) . The purpose was to generate critical information regarding how a spatial repellent may interfere with the efficacy of the BGS when the two tools are used in combination as a push-pull strategy. We specifically sought to determine: (i) if Ae. aegypti females exposed to spatial repellent chemicals (DDT, metofluthrin and transfluthrin) have a reduced likelihood of being captured with BGS traps (i.e., effect host-attraction) and (ii) if such an effect, should it occur, is immediate but short-lived or potentially latent.
Metofluthrin has been previously evaluated for repellency against Ae. aegypti[46,47], Culex quinquefasciatus[48,49] and An. balabacensis[48,49] and metofluthrin coils have been reported to significantly reduce landing counts of Ae. aegypti[46]. Transfluthrin is a fast acting insecticide that exhibits high volatility and knock down activity at high concentrations [50]. It is used in household products against various pest insects, such as mosquitoes, flies and moths. Evaluations have been conducted using transfluthrin to repel Cx. quinquefasciatus[51,52], An. arabiensis[53] and Ae. albopictus[54,56]. DDT has spatial repellent qualities as indicated in previous experimental hut studies [17,42,43].
The BGS trap has previously been validated as an effective tool for the monitoring and surveillance of the dengue virus vector Ae. aegypti[26-31]. However, the efficacy of the BGS trap to attract and catch (or pull) chemically-repelled or insecticide-exposed mosquitoes is not known. Many chemicals can elicit repellent behavioral responses in Ae. aegypti at doses well below those required for toxic outcomes [1,16,17], but the effects of such exposures on the mosquito’s host-seeking behavior are poorly understood. For another important dengue virus vector, Ae. albopictus, changes in both host-seeking and blood-feeding behaviors upon exposure to plant volatiles under laboratory conditions have been described [57]. Aedes albopictus females surviving exposure to geraniol, citral, eugenol, or anisaldehyde for 24 and 48 h all showed different degrees of reduction in host-seeking ability (e.g., increased times to reach a target location and to search for a suitable feeding site and insert the stylet). After 48 h of exposure to 0.250 μg/cm3 of anisaldehyde, 100% of the mosquitoes showed loss of host-seeking ability, through impacts on the time to host-seeking activation, orientation, probing and engorgement compared to unexposed controls. In another study, Ae. aegypti females were exposed to sublethal levels (LD25) of pyrethroid insecticides to evaluate the effects of the neurotoxicants 24 h post-exposure. A significant reduction in time of activation to flight was observed in mosquitoes exposed to deltamethrin and permethrin [58]. Similarly, excito-repellency studies using lower concentrations of deltamethrin showed that mated Ae. aegypti exhibited significant differences in escape responses with and without hosts present [59]. This type of knowledge is critical to define the expected efficacy of a BGS trap in a repellent focused push-pull strategy.
Holding female Ae. aegypti in experimental huts with DDT-treated fabric did not significantly impact subsequent BGS capture compared to non-exposed females, regardless of whether the exposed females were evaluated immediately following DDT exposure or following a 12 h recovery period in a repellent–free setting. Thus, there was no immediate or latent negative impact on host-seeking ability, as estimated by BGS trap catches, using this DDT exposure route. Similarly, mosquitoes exposed to metofluthrin coils with high (0.0065%) or low (0.003%) concentrations were as likely to be captured with the BGS traps as non-exposed control mosquitoes. Previous exposure to transfluthrin at 0.125 and 0.062 FAR resulted in significantly lower trap catches, compared to control mosquitoes, for mosquitoes released immediately following exposure but not for those allowed to recover for 12 h before BGS trap evaluation.
The comparison of results for mosquitoes released immediately following exposure versus those allowed to recover for 12 h before being released into the environment with the BGS traps indicate that this is a temporary phenomenon. This is suggestive of effects on sensory pathways used to detect host cues that resolve following the 12 h holding period. Similarly, Hao et al.[57] noted that a reduction in host-seeking ability in Ae. albopictus in the laboratory was reversible following recovery times that were dependent on chemical and concentration specific exposure conditions. However, we cannot rule out the possibility that the observed increase in host-seeking activity/trap catch rates for the delayed release females resulted, in part, from that they were only supplied with water during the recovery phase and thus were more motivated to locate a food source at the end of the recovery period.
Repellents have been shown to induce changes in responses of olfactory receptor neurons of female mosquitoes [60], specifically involving the grooved peg sensilla and sensilla trichodea, which are located on the mosquito antennae [61-64]. This neuronal activation disrupts the mosquito’s ability to detect host-seeking kairomones, components of human sweat and presumably also the BGS trap lure (BG Lure). The decline in sensitivity of Ae. aegypti to human odor as a result of repellent exposure might be a mechanism for the temporary suppression of host-seeking behavior [65], as seen for the IR mosquitoes exposed to transfluthrin. Most evidence published to date on the basis of action of repellent compounds (e.g., DEET) are clearly conflicting and support either hypotheses indicating that repellents mask odors by blocking their receptors or act as true odorants that seem to be avoided by pests. However, none of these studies refer to insecticides similar to those tested in the present study. Several insecticides have been described that induce hyperactivity at sublethal doses and even promote the avoidance of impregnated areas. However, no clear evidence exists to date to link these effects to those of known repellents acting on insect chemoreceptors. The specific mechanism of action behind the observed change in BGS recapture rates over time following exposure to transfluthrin, could not be addressed in the current study, but highlights the need to integrate laboratory and field evaluations as a model for translational research.
There are several study design biases that could have influenced our results. This includes innate differences in spatial repellent actives, such as volatility, and the fact that trials were performed independently at various times of year under varying temperatures that could also affect chemical volatility. Although the two treatment formats varied (treated fabric vs. coils), each format represented the typical exposure method that target vector mosquitoes would experience under operational implementation for these interventions. This allowed for a more accurate assessment of exposure effects of the spatial repellents as would be expected under natural conditions. In addition, the exposure methods used – where mosquitoes were held in screened cages placed inside experimental huts containing spatial repellent treatments (treated fabrics or coils) mimicked expected exposure routes for airborne repellent molecules where direct contact with treated surfaces does not occur. Despite these potential study design biases, it is clear that mosquito behavior is an area of research that will continue to be of high importance, especially as development of novel vector control tools are necessary in order to combat diseases such as malaria and dengue [66].
Conclusion
Overall, data indicate that exposure of Ae. aegypti to the test repellents had no more than minor and short-lived impacts on BGS capture rates. This finding suggests the use of these repellents may not negatively impact the ability of the BGS to remove deterred Ae. aegypti adults from outdoor areas around treated homes. Deriving maximum benefits from an outdoor trap (pull) component within a repellent-focused combination push-pull system requires that previous exposure to a repellent chemical, used as the push component, will not substantially reduce trap efficacy to capture vectors from the peridomestic environment. We show here that exposure to DDT, metofluthrin or transfluthrin results in no more than minor and short-lived reductions in the efficacy of the Biogents Sentinel™ trap to recapture Ae. aeygpti females. However, using BGS recapture rates as a proxy for host-seeking, exposure to the highly volatile pyrethroid compound, transfluthrin, appeared to have some impact on test populations’ attraction or movement towards the trap immediately following exposure, (i.e., IR populations) based on reduced recapture rates but this effect was absent following a 12 h recovery period (i.e., DR populations). This delay may have significant impact on disease reduction, as several models have shown that the time period required for the mosquito to regain its ability to seek a host after repellent exposure likely increases the probability of mortality due to adverse environmental factors or predation [55]. This study has begun to elucidate an understanding of: (i) the selection of spatial repellent chemicals best suited for inclusion in a combined intervention system in which traps are required to function in removing repelled vectors from the peridomestic environment and (ii) the estimation of recovery time a vector may require to respond to an attractant source following repellent exposure.
Competing interests
The authors declare no competing interests.
Authors’ contributions
All the authors have contributed significantly to this study. FVS, NLA, JPG and TC contributed to conceptualization the study and wrote the manuscript. AP performed analysis the data. TAO, LE, CD and SP participated in its study and performed the coordination and helped the draft of the manuscript. All authors read and approved the final manuscript. Some of the authors are U.S. government employees. The opinions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the Department of Defense or the Uniformed Services University of the Health Sciences. Mention of specific commercial products does not constitute an endorsement or recommendation.
Contributor Information
Ferdinand V Salazar, Email: rdsalazarvil@gmail.com.
Nicole L Achee, Email: nicole.achee@usuhs.edu.
John P Grieco, Email: john.grieco@usuhs.edu.
Atchariya Prabaripai, Email: faasatp@ku.ac.th.
Tolulope A Ojo, Email: tolu.ojo@gmail.com.
Lars Eisen, Email: lars.eisen@colostate.edu.
Christine Dureza, Email: chris91@hotmail.com.
Suppaluck Polsomboon, Email: autumnalheart@hotmail.com.
Theeraphap Chareonviriyaphap, Email: faasthc@ku.ac.th.
Acknowledgements
We thank the Armed Forces Development Command, Sai Yok District, Kanchanaburi Province, Thailand for support of the research program by providing land to serve as the study site. We also thank Dr. Maude Christian Meier, Senior Research Scientist, Insect Control RD&E, of SC Johnson A Family Company, Racine WI for her contribution of metofluthrin coils. Funding for this research was provided by the Bill & Melinda Gates Foundation (Grant #48513) and the Thailand Research Fund (RTA5280007).
References
- WHO [World Health Organization] WHO recommended insecticides for indoor residual spraying against malaria vectors. Geneva, Switzerland: World Health Organization; 2009. http://apps.who.int/malaria/cmc_pload/0/000/012/604/IRS Insecticides.htm. [Google Scholar]
- Scott TW, Chow E, Strickman D, Kittayapong P, Wirtz RA, Lorenz LH, Edman JD. Blood-feeding patterns of Aedes aegypti (Diptera: Culicidae) collected in a rural Thai village. J Med Entomol. 1993;30:922–927. doi: 10.1093/jmedent/30.5.922. [DOI] [PubMed] [Google Scholar]
- Scott TW, Amerasinghe PH, Morrison AC, Lorenz LH, Clark GG, Strickman D, Kittayapong P, Edman JD. Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: Blood feeding frequency. J Med Entomol. 2000;37:89–101. doi: 10.1603/0022-2585-37.1.89. full/10.1603/0022-2585-37.1.89. [DOI] [PubMed] [Google Scholar]
- Gubler D. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LC, Scott TW, Lerdthusnee K, Coleman RC, Costero A, Clark GG, Jones JJ, Kitthawee K, Kittayapong P, Sithiprasasna R, Edman JD. Dispersal of the dengue vector Aedes aegypti within and between rural communities. AmJTrop Med Hyg. 2005;72:209–220. [PubMed] [Google Scholar]
- WHO [World Health Organization] Dengue haemorrhagic fever: diagnosis, treatment, prevention and control. 2. Geneva: World Health Organization; 2007. [Google Scholar]
- Gratz NG. Lessons of Aedes aegypti control in Thailand. Med Vet Entomol. 1993;7:1–10. doi: 10.1111/j.1365-2915.1993.tb00644.x. [DOI] [PubMed] [Google Scholar]
- Gratz NG. Space sprays for the control of Aedes aegypti in South-East Asia and the Western Pacific. Dengue Bull. 1999;23:80–84. [PMC free article] [PubMed] [Google Scholar]
- Somboon P, Prappanthadara L, Suwanakerd W. Insecticide susceptibility tests of Anopheles minimus, Aedes aegypti, Aedes albopictus and Culex quinquefasciatus in northern Thailand. Southeast Asian J Trop Med Public Health. 2003;34:87–93. [PubMed] [Google Scholar]
- Sathantriphop S, Paeporn P, Supaphathom K. Detection of insecticides resistance status in Culex quinquefasciatus and Aedes aegypti to four major groups of insecticides. Trop Biomed. 2006;23:97–101. [PubMed] [Google Scholar]
- Ponlawat A, Scott JG, Harrington LA. Insecticide susceptibility of Aedes aegypti and Aedes albopictus across Thailand. J Med Entomol. 2005;42:821–825. doi: 10.1603/0022-2585(2005)042[0821:ISOAAA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Jirakanjanakit N, Rongnoparut P, Saengtharatip S, Chraeonviriyaphap T, Duchon S, Bellec C, Yoksan S. Insecticide susceptible/resistance status in Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus (Diptera: Culicidae) In Thailand 2003–2005. J Econ Entomol. 2007;100:545–550. doi: 10.1603/0022-0493(2007)100[545:IRSIAS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Miller JR, Cowles RS. Stimulo-deterrent diversion: a concept and its possible application to onion maggot control. J Chemical Ecol. 1990;16:3197–3212. doi: 10.1007/BF00979619. [DOI] [PubMed] [Google Scholar]
- Midega CAO, Khan ZR, Van Den Berg J, Ogol CKPO, Pickett JA, Wadhams J. Maize stemborer predator activity under ‘push – pull’ system and Bt-maize: A potential component in managing Bt resistance. Int J Pest Manag. 2006;52(1):1–10. doi: 10.1080/09670870600558650. [DOI] [Google Scholar]
- Nielsen F. The Push-Pull system – a viable alternative to Bt maize. LEISA Magazine. 2001;4:17–18. [Google Scholar]
- Achee NL, Sardelis MR, Dusfour I, Chauhan KR, Grieco JP. Characterization of spatial repellent, contact irritant, and toxicant chemical actions of standard vector control compounds. J Am Mosq Contr Assoc. 2009;25:156–167. doi: 10.2987/08-5831.1. [DOI] [PubMed] [Google Scholar]
- Grieco JP, Achee NL, Chareonviriyaphap T, Suwonkerd W, Chauhan K, Sardelis MR, Roberts DR. A new classification system for the actions of IRS chemicals traditionally used for malaria control. PLoS One. 2007;8:e716. doi: 10.1371/journal.pone.0000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan EB, Hossignol PA. An olfactometer for discriminating between attraction, inhibition, and repellency in mosquitoes (Diptera: Culicidae) J Med Entomol. 1999;36:788–793. doi: 10.1093/jmedent/36.6.788. [DOI] [PubMed] [Google Scholar]
- Schreck CE, Gilbert IH, Weidhaas DE, DE Posey DE. Spatial action of mosquito repellents. J Econ Entomol. 1970;63:1576–1578. doi: 10.1093/jee/63.5.1576. [DOI] [PubMed] [Google Scholar]
- Rupp HR, Jobbins DM. Equipment for mosquito surveys: two recent developments. Atlantic City, NJ. USA: ; 1969. pp. 183–188. (Proceedings of the 56th Annual Meeting of the New Jersey Mosquito Extermination Association). [Google Scholar]
- Achee NL, Bangs MJ, Farlow R, Killeen GF, Lindsay S, Logan JG, Moore SJ, Rowland M, Sweeney K, Torr SJ, Zwiebel LJ, Grieco JP. Spatial repellents: from discovery and development to evidence-based validation. Malaria J. 2012;11(1):164. doi: 10.1186/1475-2875-11-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline DL. Traps and trapping techniques for adult mosquito control. J. Am Mosq Contr Assoc. 2006;22:490–496. doi: 10.2987/8756-971X(2006)22[490:TATTFA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Service MW. Mosquito ecology: Field sampling methods. 2. London, U.K: Elsevier Applied Science; 1993. [Google Scholar]
- Scott TW, Morrison AC. In: Ecological Aspects for Application of Genetically Modified Mosquitoes. Takken W, Scott TW, editor. Dordrecht: Kluwer Academic Publishers; 2003. Aedes aegypti density and the risk of dengue-virus transmission; pp. 187–206. [Google Scholar]
- Facchinelli L, Koenraadt CJM, Fanello C, Kijchalao U, Valerio L, Jones JW, Scott TW. della Torre A. Evaluation of a sticky trap for collecting Aedes (Stegomyia) adults in a dengue-endemic area in Thailand. Am J Trop Med Hyg. 2008;78:904–909. [PubMed] [Google Scholar]
- Krockel U, Rose A, Eiras AE, Geier M. New tools for surveillance of adult yellow fever mosquitoes: comparison of trap catches with human landing rates in urban environment. J Am Mosq Contr Assoc. 2006;22:229–238. doi: 10.2987/8756-971X(2006)22[229:NTFSOA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Maciel-de-Freitas R, Eiras EA, Lourenço-de-Oliveira R. Field evaluation of effectiveness of the BG-Sentinel, a new trap for capturing adult Aedes aegypti (Diptera: Culicidae) Mem Inst Oswaldo Cruz. 2006;101:321–325. doi: 10.1590/s0074-02762006000300017. [DOI] [PubMed] [Google Scholar]
- Williams CR, Long SA, Webb CE, Bitzhenner M, Geier M, Russel RC, Ritchie SA. Aedes aegypti population sampling using BG-Sentinel traps in north Queensland, Australia: statistical considerations for trap deployment and sampling strategy. J Med Entomol. 2007;44:345–350. doi: 10.1603/0022-2585(2007)44[345:AAPSUB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Williams CR, Long SA, Russell RC, Ritchie SA. Field efficacy of the BG-Sentinel compared with CDC backpack aspirators and CO2 baited EVS traps for collection of adult Aedes aegypti in Cairns, Queensland, Australia. J Am Mosq Contr Assoc. 2006;22:296–300. doi: 10.2987/8756-971X(2006)22[296:FEOTBC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Ball TS, Ritchie SA. Evaluation of BG-Sentinel trap trapping efficacy for Aedes aegypti (Diptera: Culicidae) in a visually competitive environment. J Med Entomol. 2010;47:657–663. doi: 10.1603/ME09242. [DOI] [PubMed] [Google Scholar]
- Bhalala H, Arias JR. The Zumba™ mosquito trap and BG-Sentinel™ trap: novel surveillance tools for host-seeking mosquitoes. J Am Mosq Contr Assoc. 2009;25:134–139. doi: 10.2987/08-5821.1. [DOI] [PubMed] [Google Scholar]
- Ritchie SA, Moore P, Carruthers M, Williams C, Montgomery B, Foley P, Ahboo S, van den Hurk AF, Lindsay MD, Cooper B, Beebe N, Russell RC. Discovery of a widespread infestation of Aedes albopictus in the Torres Strait, Australia. J Am Mosq Contr Assoc. 2006;22:358–365. doi: 10.2987/8756-971X(2006)22[358:DOAWIO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Farajollahi A, Kesavaraju B, Price DC, Williams GM, Healy SP, Gaugler R, Nelder MP. Field efficacy of BG-Sentinel and industry-standard traps for Aedes albopictus (Diptera: Culicidae) and West Nile virus surveillance. J Med Entomol. 2009;46:919–925. doi: 10.1603/033.046.0426. [DOI] [PubMed] [Google Scholar]
- Salazar FV, Achee NL, Grieco JP, Prabaripai A, Eisen L, Shah P, Chareonviriyaphap T. Evaluation of a peridomestic trap for integration into an Aedes aegypti (Diptera:Culicidae) push-pull control strategy. J Vector Ecol. 2012;37:8–19. doi: 10.1111/j.1948-7134.2012.00195.x. [DOI] [PubMed] [Google Scholar]
- Muir LE, Kay BH. Aedes aegypti survival and dispersal estimated by mark-release-recapture in northern Australia. Am J Trop Med Hyg. 1998;58:277–282. doi: 10.4269/ajtmh.1998.58.277. [DOI] [PubMed] [Google Scholar]
- Chareonviriyaphap T, Grieco JP, Suwonkerd W, Prabaripai A, Polsomboon S. An improved experimental hut design for the study of Aedes aegypti (Diptera: Culicidae) movement patterns in Thailand. J Vector Ecol. 2010;35:428–431. doi: 10.1111/j.1948-7134.2010.00102.x. [DOI] [PubMed] [Google Scholar]
- Manda H, Arce LM, Foggie T, Shah P, Grieco JP, Achee NL. Effects of irritant chemicals on Aedes aegypti resting behavior: is there a simple shift to untreated “safe sites”? PLoS Negl Trop Dis. 2011. p. e1243. Epub 2011. [DOI] [PMC free article] [PubMed]
- Achee NL, Grieco JP, Andre RG, Rejmankova E, Roberts DR. A mark-release-recapture study using a novel portable hut design to define the flight behaviour of Anopheles darlingi in Belize, Central America. J Am Mosq Contr Assoc. 2005;21:366–379. doi: 10.2987/8756-971X(2006)21[366:AMSUAN]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Geier M, Bosch OJ, Boeckh J. Ammonia as an attractive component of host odour for the yellow fever mosquito, Aedes aegypti. Chem Senses. 1999;24:647–653. doi: 10.1093/chemse/24.6.647. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Geier M, Boeckh J. Contribution of fatty acids to olfactory host finding of female Aedes aegypti. Chem Senses. 2000;25:323–330. doi: 10.1093/oxfordjournals.chemse.a014042. [DOI] [PubMed] [Google Scholar]
- Steib BM, Boeckh J. M Geier M. The effect of lactic acid on odour-related host preference of yellow fever mosquitoes. Chem Senses. 2001;26:523–528. doi: 10.1093/chemse/26.5.523. [DOI] [PubMed] [Google Scholar]
- Roberts DR, Alecrim WD. Behavioral response of Anopheles darlingi; to DDT-sprayed house walls in Amazonia. PAHO Bull. 1991;25:210–217. [PubMed] [Google Scholar]
- Grieco JP, Achee NL, Andre RG, Roberts DR. A comparison study of house entering and exiting behavior of Anopheles vestitipennis (Diptera: Culicidae) using experimental huts sprayed with DDT or deltamethrin in the southern district of Toledo, Belize. J Vector Ecol. 2000;25:62–73. [PubMed] [Google Scholar]
- WHO [World Health Organization] Indoor residual spraying: use of indoor residual spraying for scaling up global malaria control and elimination. Geneva, Switzerland: WHO/HTM/MAL/2006.1112; 2006. [Google Scholar]
- WHO [World Health Organization] WHO: Guidelines for efficacy testing of household insecticides products. Mosquito coils, vaporizer mats, liquid vaporizers, ambient emanators and aerosols. Geneva, Switzerland: World Health Organization; (Guidelines for efficacy testing of household insecticides products. Mosquito coils, vaporizer mats, liquid vaporizers, ambient emanators and aerosols). WHO/HTM/NTD/WHOPES/2009.3. [Google Scholar]
- Shono Y, Kubota S, Sugano M, Yap HH, Tsushima K. Field evaluation of papers strip and mosquito coil formulation impregnated metofluthrin for mosquito control in Malaysia. The Abstract Book, 70th Annual Meeting of American Mosquito Control Association. Savannah, GA. Eatontown, NJ: American Mosquito Control Association; 2004. p. 40. [Google Scholar]
- Rapley LP, Russell RC, Montgomery BL, Ritchie SA. The effects of sustained release metofluthrin on the biting, movement, and mortality of Aedes aegypti in a domestic setting. Am Trop Med Hyg. 2009;81:94–99. [PubMed] [Google Scholar]
- Kawada H, Maekawa Y, Tsuda Y, Takagi M. Trial of spatial repellency of metofluthrin-impregnated paper strip against Anopheles and Culex in shelters without walls in Lombok, Indonesia. J Am Mosq Contr Assoc. 2004;20:434–437. [PubMed] [Google Scholar]
- Kawada H, Thi Yen N, Thuy Hoa N, Minh Sang T, Van Dan N, Takagi M. Field evaluation of spatial repellency of metofluthrin impregnated plastic strips against mosquitoes in Hai Phong City, Vietnam. Am J Trop Med Hyg. 2005;73:350–353. [PubMed] [Google Scholar]
- WHO [World Health Organization] WHO Specifications and Evaluations. 2006. http://www.who.int/entity/whopes/…/Transfluthrin_eval_only.
- Yap HH, Chong CY, Yahaya NL, Baba AM, Awang AH. Performance of mosquito coils containing transfluthrin against Culex quinquefasciatus (Say) in an urban squatter environment. Research Note Trop Biomed. 1996;13:101–103. [Google Scholar]
- Pates HV, Line JD, Keto AJ, Miller JE. Personal protection against mosquitoes in Dar Es Salaam, Tanzania by using a kerosene oil to vaporize transfluthrin. Med Vet Entomol. 2002;16:277–284. doi: 10.1046/j.1365-2915.2002.00375.x. [DOI] [PubMed] [Google Scholar]
- Ogoma SB, Ngonyani H, Simfukwe ET, Mseka A, Moore J, GF Killeen GF. Spatial repellency of transfluthrin-treated hessian strips against laboratory-reared Anopheles arabiensis mosquitoes in a semi-field tunnel cage. Parasit Vectors. 2012;5:54–61. doi: 10.1186/1756-3305-5-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argueta TBO, Kawada H, Takagi M. Spatial repellency of metofluthrin-impregnated multilayer paper strip against Aedes albopictus under outdoor conditions, Nagasaki, Japan. Med Entomol Zool. 2004;55:211–216. [Google Scholar]
- Roberts DR, Tren R, Bates R, Zambone J. The excellent powder: DDT’s political and scientific history. Indianapolis: Dog ear publishing. LLC.; 2010. [Google Scholar]
- Lee DK. Lethal and repellent effects of transfluthrin and metofluthrin used in portable blowers for personal protection against Ochlerotatus togoi and Aedes albopictus (Diptera: Culicidae) Entomol Res. 2007;37:173–179. doi: 10.1111/j.1748-5967.2007.00109.x. [DOI] [Google Scholar]
- Hao H, Wee J, Dai J, Du J. Host-seeking and blood-feeding behavior of Aedes albopictus (Diptera: Culicidae) exposed to vapors of geraniol, citral, citronellal, eugenol, or anisaldehyde. J Med Entomol. 2008;45:533–539. doi: 10.1603/0022-2585(2008)45[533:HABBOA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Cohnstaedt LW, Allan SA. Effects of sublethal pyrethroid exposure on the host-seeking behavior of female mosquitoes. J Vector Ecol. 2011;36:395–403. doi: 10.1111/j.1948-7134.2011.00180.x. [DOI] [PubMed] [Google Scholar]
- Boonyuan W, Kongmee M, Bangs MJ, Prabaripai A, Chareonviriyaphap T. Host feeding responses of Aedes aegypti (L.) exposed to deltamethrin. J Vector Ecol. 2011;36:361–362. doi: 10.1111/j.1948-7134.2011.00177.x. [DOI] [PubMed] [Google Scholar]
- Zwiebel LJ, Takken W. Olfactory regulation of mosquito–host interactions. Insect Biochem Mol Biol. 2004;34:645–652. doi: 10.1016/j.ibmb.2004.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijerink I, Braks MAH, Van Loon JJA. Olfactory receptors on the antennae of the malaria mosquito Anopheles gambiae are sensitive to ammonia and other sweat-borne components. J Insect Physiol. 2001;47:455–464. doi: 10.1016/S0022-1910(00)00136-0. [DOI] [PubMed] [Google Scholar]
- Qiu YT. Sensory and behavioral responses of the malaria mosquito Anopheles gambiae to human odours. The Netherlands: Wageningen University, Ponsen & Looijen E.V; 2005. (M.S. Thesis). [Google Scholar]
- van den Broek IVF, den Otter CJ. Odour sensitivity of antennal olfactory cells underlying grooved pegs of Anopheles gambiae s.s. and An. quadrimaculatus. Entomol Exp Appl. 2000;96:167–175. doi: 10.1046/j.1570-7458.2000.00692.x. [DOI] [Google Scholar]
- Diehl PA, Vlimant M, Guerenstein P, Guerin PM. Ultrastructure and receptor cell responses of the antennal grooved peg sensilla of Triatoma infestans (Hemiptera: Reduviidae) Arthropod Struct Dev. 2003;31:271–285. doi: 10.1016/S1467-8039(03)00004-5. [DOI] [PubMed] [Google Scholar]
- Fox AN, Pitts RJ, Robertson HM, Carlson JR, Zwiebel LJ. Candidate odorant receptors from the malaria vector mosquito Anopheles gambiae and evidence of down regulation in response to blood feeding. Proc Natl Acad Sci USA. 2001;98:14693–14697. doi: 10.1073/pnas.261432998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The malERA Consultative Group on Vector Control. A research agenda for malaria eradication: Vector control. PLoS Med. 2011;8:e1000401. doi: 10.1371/journal.pmed.1000401. [DOI] [PMC free article] [PubMed] [Google Scholar]