Abstract
Plant photosynthesis and photosystem II (PSII) are susceptible to high temperature. However, photosynthetic electron transport process under heat stress remains unclear. To reveal this issue, chlorophyll a fluorescence and modulated 820 nm reflection were simultaneously detected in sweet sorghum. At 43°C, J step in the chlorophyll a fluorescence transient was significantly elevated, suggesting that electron transport beyond primary quinone of PSII (QA) (primary quinone electron acceptor of PSII) was inhibited. PSI (Photosystem I) photochemical capacity was not influenced even under severe heat stress at 48°C. Thus, PSI oxidation was prolonged and PSI re-reduction did not reach normal level. The inhibition of electron transport between PSII and PSI can reduce the possibility of PSI photoinhibition under heat stress. PSII function recovered entirely one day after heat stress at 43°C, implying that sweet sorghum has certain self-remediation capacity. When the temperature reached 48°C, the maximum quantum yield for primary photochemistry and the electron transport from PSII donor side were remarkably decreased, which greatly limited the electron flow to PSI, and PSI re-reduction suspended. The efficiency of an electron transferred from the intersystem electron carrier (plastoquinol, PQH2) to the end electron acceptors at the PSI acceptor side increased significantly at 48°C, and the reason was the greater inhibition of electron transport before PQH2. Thus, the fragment from QA to PQH2 is the most heat sensitive in the electron transport chain between PSII and PSI in sweet sorghum.
Introduction
As a result of greenhouse effect, global warming is predicted to persist in the future, and an increased frequency of periods with exceptionally high temperatures is one of the most important characteristics of global warming [1]. Heat stress is generally defined as a rapid and great elevation in ambient temperature [2]. Unlike moderate high temperature stress, a short period of heat stress is enough to provoke severe cellular injury.
Photosynthesis is susceptible to high temperature, and high temperature is liable to impair photosynthetic apparatus in plants [3]. Photosynthetic electron transport from water to NADP+ is driven by photosystem II (PSII) and photosystem I (PSI). PSII is highly sensitive to high temperature, and heat-induced injury on PSII certainly can inhibit photosynthetic electron transport [2], [3]. However, it is still unclear about the responses of photosynthetic liner electron transport process, particularly the interaction between PSII and PSI in plants under heat stress. Chlorophyll a fluorescence transient (OJIP transient) has been widely used to study PSII performance in plants under environmental stresses, and it is accepted as a convenient tool to diagnose plant health status. Under high temperature stress, PSII performance usually deceased in plants, and OJIP transient could obviously change [4]–[6]. In recent ten years, a series of studies have clearly revealed the biological meanings of kinetic phases in this transient [7]–[9]. In addition, PSI redox change can be detected by the change in modulated 820 nm reflection, as they significantly correlate with each other [8]. At present, simultaneous detecting OJIP and 820 nm reflection transients served as a feasible way to explore photosynthetic electron transport process and the interaction between PSII and PSI. By using this technique, the effects of chilling and dehydration on photosynthetic electron transport chain were recently reported in apple and cucumber leaves [10], [11], but responses of photosynthetic electron transport chain to heat stress remains to be elucidated.
Sweet sorghum is an annual C4 crop with fast growth rate and high biomass yield. Sweet sorghum is consumed as human food and livestock feed. In addition, it is an important bio-energy crop, as the stalks are rich in fermentable sugars. To date, many studies focus on the procedure of producing bio-energy with sweet sorghum as materials (e.g., [12]–[14]) under the tendency of gradual shrink of ordinary energy source such as coal, oil and natural gas. However, a few of studies pay attention to the relationship between environmental stresses and physiological responses in sweet sorghum, and moreover, these studies mainly associate with salt stress [15]–[17]. To our knowledge, effects of high temperature on sweet sorghum have not been reported. Sweet sorghum has been recognized as a promising crop species for exploiting saline land in coastal zone in China, and air temperature can rapidly rise to extremely high level in summer midday in this region due to low vegetation coverage. Therefore, heat stress studies on sweet sorghum may provide a scientific reference for the practice of saline land exploitation in coastal zone.
Djanaguiraman et al. [18] demonstrated that long-term high temperature (45 days, 40/30°C day/night) reduced photosynthetic rate and PSII photochemical efficiency in the leaves of grain sorghum. However, heat stress on plants may be different compared with long-term high temperature treatment. In this study, we aimed to explore photosynthetic electron transport process in the leaves of sweet sorghum under heat stress by simultaneously examining 820 nm reflection and chlorophyll a fluorescence.
Materials and Methods
Plant material and heat treatment
Seeds of sweet sorghum (Sorghum bicolor (L.) Moench. cv. YaJin) were immersed in 30°C water for 2 h. Then, fifty seeds were placed in each Petri dish in the dark between two sheets of filter paper at 25°C to germinate, and the filter paper was kept wet by spraying Hoagland nutrient solution (pH 5.7). After 2 days, seeds with similar buds (about 0.6 cm) were transferred to plastic pots filled with vermiculite (one bud in each pot) and grown in artificial climatic chambers (Huier, China). The photon flux density was approximately 200 μmol m−2 s−1 (12 h per day from 07:00 to 19:00), and day/night temperature and humidity were controlled at 25/18°C and 65%. The seedlings were daily watered with Hoagland nutrient solution (pH 5.7). After 30 days, plants with uniform growth pattern (about 30 cm height and 0.8 cm diameter of the stem) were selected as experimental materials.
Heat stress treatments were conducted in artificial climatic chamber. Seedlings were subjected to 38°C, 43°C and 48°C for 2 h with the light (200 μmol m−2 s−1, which was equivalent of growth light intensity), and seedlings growing at 25°C were taken as control. Five replicate seedlings were used for each treatment, and the newest fully expanded leaves were used for the following measurements.
Analysis of photosynthetic rate
Measurement of photosynthetic rate (Pn) was carried out by using an open photosynthetic system (LI-6400XT, Li-Cor, Lincoln, NE, USA) equipped with a LED leaf chamber (6400-02B). Photon flux density was set at 800 μmol m−2 s−1 in leaf cuvette and the temperature, CO2 concentration and relative humidity were not controlled and depended on ambient conditions.
Measurements of chlorophyll a fluorescence transient and modulated 820 nm reflection
The measurements were conducted by using a multifunctional plant efficiency analyzer (M-PEA, Hansatech, UK). Monitoring modulated reflection change near 820 nm is a very convenient way to follow the redox state of PSI (reaction center + plastocyanin). This instrument was elucidated by Strasser et al. [19] in detail. In this study, leaves were dark adapted for 30 min before they were measured. Dark-adapted leaves were illuminated with 1 s pulse of continuous red light (627 nm, 5000 μmol photons m−2 s−1) and subsequently, with 10 s far-red light (735 nm, 200 μmol photons m−2 s−1). Chlorophyll a fluorescence and modulated 820 nm reflection were recorded during the illumination. At the onset of the red light illumination (0.7 ms), PSI (reaction center + plastocyanin) was entirely in reduced state. After the far-red illumination, PSI was completely oxidized. The declined amplitude of modulated 820 nm reflection intensity due to PSI redox change can reflect PSI photochemical capacity [10], [20].
Chlorophyll a fluorescence transients were quantified according to the JIP test by using the following original data: (1) fluorescence intensity at 20 μs (Fo, when all reaction centers of PSII are open); (2) the maximum fluorescence intensity (Fm, when all reaction centers of PSII are closed) and (3) fluorescence intensities at 300 μs (K step), 2 ms (J step) and 30 ms (I step). Using these original data, some parameters can be calculated for quantifying PSII behavior [19]. These parameters are listed in Table 1.
Table 1. Formulae and terms used in the analysis of the OJIP fluorescence transient.
| Data extracted from the recorded fluorensence transient OJIP | |
| Ft | Fluorescence intensity at time t after onset of actinic illumination |
| Fo = F20 μs | Minimal recorded fluorescence intensity |
| Fk = F300 μs | Fluorescence intensity at 300 μs |
| FJ = F 2ms | Fluorescence intensity at the J step |
| FI = F30 ms | Fluorescence intensity at the I step |
| Fm = FP | Maximal recorded fluorescence intensity |
QA: primary quinone; PSI: photosystem I; PSII: photosystem II.
Statistical analysis
One-way ANOVA was carried out using SPSS 16.0 (SPSS Inc., Chicago, IL, USA) for all sets of data, and significant differences between means were determined through LSD test. Differences were considered statistically significant when P<0.05.
Results
Effects of heat stress on relative variable chlorophyll a fluorescence and 820 nm transmission transients
As shown in Fig. 1, chlorophyll a fluorescence and 820 nm reflection transients were not affected by heat stress at 38°C. J step was significantly elevated at 43°C, and chlorophyll a fluorescence transient changed greatly at 48°C (Fig. 1A).
Figure 1. Chlorophyll a fluorescence and modulated 820 nm reflection transients at high temperatures.
O, J, I and P indicate the specific steps in chlorophyll a fluorescence transient. The modulated 820 nm reflection signals are presented by MRt/MR0 ratio. MRt indicates modulated 820 nm reflection intensity at time t. MR0 is the value at the onset of actinic illumination (at 0.7 ms).
The modulated 820 nm reflection signals are presented by MRt/MR0 ratio (Fig. 1B). MRt indicates modulated 820 nm reflection intensity at time t, and MR0 is the value at the onset of actinic illumination (at 0.7 ms). Decrease in MRt/MR0 from MR0 (at 0.7 ms) to the minimal value (at about 12 ms) reflects PSI oxidation process. The minimal value point is a transitory steady state with equal oxidation and re-reduction rate of PSI. Subsequently, increase in MRt/MR0 indicates PSI re-reduction. PSI oxidation amplitude increased at 43°C, and the following re-reduction did not reach the normal level at 25°C (Fig. 1B). Under the severe stress at 48°C, 820 nm reflection transient changed greatly, and PSI re-reduction nearly suspended (Fig. 1B).
Effects of heat stress on photosynthetic rate, PSII performance and PSI photochemical capacity
Pn and PI(abs)(PSII performance index on absorption basis) were significantly decreased by heat stress at 43°C (P<0.05), and the decrease become greater at 48°C (P<0.05) (Fig. 2A and B). In contrast, PSI photochemical capacity was not inhibited by heat stress even at 48°C (Fig. 2C).
Figure 2. (A) Photosynthetic rate (Pn), (B) PSII performance index (PI(abs)) and (C) PSI photochemical capacity at high temperatures.

Data in the figure indicate mean of five replicates (± SD). Different letters on error bars indicate significant difference at P<0.05.
Effects of heat stress on PSII behaviors
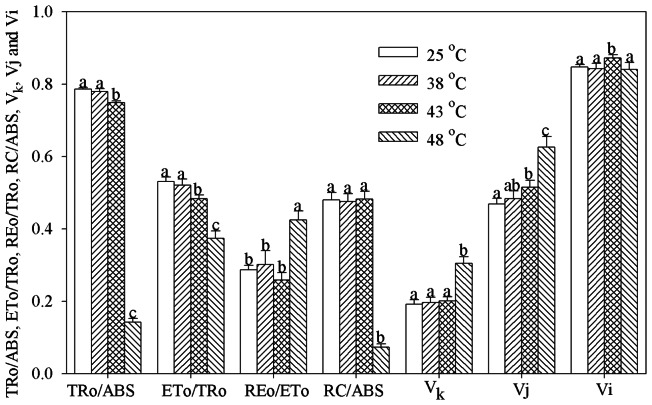
At 48°C, RC/ABS significantly decreased (P<0.05), whereas Vk (relative variable fluorescence intensity at 300 μs)increased significantly (P<0.05) (Fig. 3). Vj and Vi remarkably increased at 43°C (P<0.05), whereas TRo/ABS(maximum quantum yield for primary photochemistry) and ETo/TRo (probability that an electron moves further than QA)significantly decreased (P<0.05), and the decrease became greater at 48°C (Fig. 3).
Figure 3. TRo/ABS, ETo/TRo, REo/ETo, RC/ABS, Vk, Vj and Vi at high temperatures.
The definition for these parameters is in Table. 2. Data in the figure indicate mean of five replicates (± SD). Different letters on error bars indicate significant difference at P<0.05.
Recovery of photosynthetic apparatus one day after heat stress
PI(abs), Vj(relative variable fluorescence intensity at 2 ms), Vi(relative variable fluorescence intensity at 30 ms), TRo/ABS and ETo/TRo recovered to the normal level (25°C) one day after heat stress at 43°C for 2 h (Table. 2). However, no recovery was observed in PI(abs), TRo/ABS, ETo/TRo, Vk, Vj, Vi and RC/ABS (QA reducing reaction centers per PSII antenna chlorophyll)one day after the severe heat stress at 48°C (Table. 2), and the leaves became curly and parching and tended to die.
Table 2. Recovery of PI(abs), TRo/ABS, Vk, Vj, Vi and RC/ABS in the leaves one day after heat stress at 43°C and 48°C for 2 h.
| Parameters | Control (25°C) | 43°C for 2 h | 48°C for 2 h |
| PI(abs) | 1.99±0.18a | 1.92±0.20a | 0.0066±0.0020b |
| TRo/ABS | 0.79±0.0034a | 0.78±0.0013a | 0.14±0.020b |
| Vk | 0.19±0.012a | 0.20±0.013a | 0.35±0.038b |
| Vj | 0.47±0.013a | 0.47±0.019a | 0.72±0.050b |
| Vi | 0.85±0.015a | 0.84±0.013a | 0.93±0.047b |
| RC/ABS | 0.48±0.021a | 0.47±0.012a | 0.072±0.0070b |
Discussion
Photosynthesis and PSII were negatively affected by heat stress in sweet sorghum, as a significant decrease in Pn and PI (abs) was noted (Fig. 2A and B). In contrast, PSI photochemical capacity was not influenced by heat stress, suggesting higher heat tolerance in PSI than PSII (Fig. 2C). Heat sensitivity of photosynthesis and PSII has been extensively reported in other crops in previous studies [21]–[23]. However, a few studies demonstrated that PSI was more heat tolerant than PSII in Triticum aestivum, Spinacia oleracea, Haberlea rhodopensis and Arabidopsis [24]–[27]. This study on sweet sorghum further confirmed the heat resistance of PSI.
When temperature rose to 43°C, PSI oxidation amplitude increased and PSI re-reduction could not reach the normal level in the first 1 s red illumination (Fig. 1B). The reason was mainly attributed to the heat-induced decrease in electron donation from PSII, and in other words, the imbalance between PSII and PSI appeared. J step suggests the kinetic bottlenecks of the electron transport chain resulting in the momentary maximum accumulation of QA − [8], and I step also represents the subsequent kinetic bottlenecks of the electron transport chain but due to the limitation of plastoquinol (PQH2) re-oxidation [9]. Both J and I steps were elevated significantly at 43°C, suggesting that electron transport beyond QA and beyond PQH2 were both inhibited, and consequently, ETo/TRo decreased significantly, however, REo/ETo(probability with which an electron from the intersystem electron carriers is transferred to reduce end electron acceptors at the PSI acceptor side.) did not change at this temperature and even increased greatly at 48°C (Fig. 3). REo/ETo depends on the electrons transferred to PSI from PQH2 and the electron influx from upper electron carrier. Heat-induced increase in REo/ETo resulted from the less electrons donated to reduce PQH2. Thus, the electron transport chain beyond PQH2 is less sensitive to heat stress than that from QA to PQH2 in sweet sorghum.
Oxygen-evolving complex (OEC) is considered as the most heat sensitive component of PSII [3]. Increase in Vk is a specific indicator for heat-induced damage to OEC [28], [29]. No significant change in Vk at 43°C (Fig. 3) indicated that OEC was not affected and the electron transport from PSII donor side to PSII reaction center was not inhibited. In disagreement with previous studies [4], [30], the above results illustrate that electron transport chain of PSII acceptor side is more susceptible to heat stress compared with PSII donor side in sweet sorghum. In our opinion, the conflict derives from different treatment protocol. Heat treatment was conducted in dark in previous studies, whereas heat stress in this study was performed under growth light intensity in order to make the heat treatment more physiologically relevant. Yang et al. [29] pointed out that the mechanisms of PSII inactivation were different under heat stress with and without light. High light damaged the PSII acceptor side more severely than the PSII donor side [31]. Chen et al. [32] proved that high temperature combined with light damaged the acceptor side of electron transport chain to a greater degree than high temperature alone in apple peel. Therefore, it is conceivable that electron transport in PSII acceptor side was earlier inhibited in this study. PSI photoinhibition is more dangerous than PSII photoinhibition because of the very slow recovery rate of PSI [33]. PSI photoinhibition is mainly induced by reactive oxygen species produced at the acceptor side of PSI through Mehler reaction in vivo [34]. Thus, electron flow from PSII is responsible for PSI photoinhibition, and the addition of 3-(3,4-dichlorophenyl)-1,1-dimethylurea, an inhibitor of QA oxidation, can completely suppress PSI photoinhibition and help PSI recovery after chilling stress [11], [35]. Carbon fixation process has been proved highly susceptible to heat stress [36], which can reduce the NADPH production through linear electron transport, and in consequence, the possibility of PSI photoinhibition may rise due to more electrons transferred to Mehler reaction. Therefore, decrease in ETo/TRo at 43°C could help to reduce the possibility of PSI photoinhibition by limiting the production of reactive oxygen species from Mehler reaction in this study. Thus, we suppose that decrease in ETo/TRo is a self-protection strategy in sweet sorghum at 43°C. RC/ABS was not affected at 43°C (Fig. 3), suggesting that the effective antenna size and active reaction centers were not affected. The significant decrease in TRo/ABS at 43°C indicated that PSII photoinhibition occurred in sorghum (Fig. 3). The declined TRo/ABS also could be considered as positive adaption for down-regulating the photosynthetic excited pressure, and it might be induced by the photo-protective mechanisms including non-photochemical dissipation or state transition [37]–[39]. The photosynthetic proteins and lipids might not be greatly affected at this temperature. PSII function recovered entirely one day after heat stress at 43°C (Table. 2), implying that sweet sorghum has certain capacity to protect itself against heat stress through physiological regulation.
When temperature reached 48°C, OEC and PSII reaction center were damaged, as increase in Vk and decrease in RC/ABS were greatly significant (Fig. 3). As a result, photosynthetic electron donation to PSI was sharply lowered, and PSI re-reduction became impossible (Fig. 1B). One day after the stress, the leaves could not recover and tended to die. The inreversible damage on PSII at this temperature should result from heat-induced protein denaturation and lipid oxidation.
In conclusion, PSI photochemical capacity was not affected by heat stress in sweet sorghum. Electron transport of PSII acceptor side was initially inhibited by heat stress, and the fragment from QA to PQH2 is the most heat sensitive in the electron transport chain between PSII and PSI. The decrease in electron transport between PSII and PSI may play a self-protection role in reducing the possibility of PSI photoinhibition.
Acknowledgments
We express our sincere thanks to Prof. Huiyuan Gao for critically reading this manuscript.
Funding Statement
This work was jointly supported by the National Natural Science Foundation of China (41201292; 41171216), One Hundred-Talent Plan of Chinese Academy of Sciences (CAS), the Opening Foundation of the State Key Lab of Crop Biology, Shandong Agriculture University (2011KF02), the Chinese Academy of Sciences (CAS) Visiting Professorship for Senior International Scientists (2012T1Z0010), the Science & Technology Development Plan of Shandong Province (2010GSF10208), the Science & Technology Development Plan of Yantai City (2011016), the CAS/SAFEA International Partnership Program for Creative Research Teams, The Strategic Priority Research Program of the Chinese Academy of Sciences (XDA01020304), Yantai Double–Hundred High–end Talent Plan (XY-003-02) and 135 Development Plan of YIC-CAS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Meehl GA, Stocker TF, Collins W, Friedlingstein P, Gaye A, et al.. (2007) Global climate projections Climate Change 2007: The Physical Science Basis. In Qin D, Manning M, Chen Z, Marquis M, Averyt K, Tignor M, HL Miller, editors. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press. 747–845.
- 2. Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: An overview. Environ Exp Bot 61: 199–223. [Google Scholar]
- 3. Allakhverdiev SI, Kreslavski VD, Klimov VV, Los DA, Carpentier R, et al. (2008) Heat stress: an overview of molecular responses in photosynthesis. Photosynth Res 98: 541–550. [DOI] [PubMed] [Google Scholar]
- 4. Li PM, Cheng LL, Gao HY, Jiang CD, Peng T (2009) Heterogeneous behavior of PSII in soybean (Glycine max) leaves with identical PSII photochemistry efficiency under different high temperature treatments. J Plant Physiol 166: 1607–1615. [DOI] [PubMed] [Google Scholar]
- 5. Mathur S, Allakhverdiev SI, Jajoo A (2011) Analysis of high temperature stress on the dynamics of antenna size and reducing side heterogeneity of Photosystem II in Wheat leaves (Triticum aestivum). Bba-Bioenergetics 1807: 529–529. [DOI] [PubMed] [Google Scholar]
- 6. Yan K, Chen P, Shao H, Zhang L, Xu G (2011) Effects of short-term high temperature on photosynthesis and photosystem II performance in sorghum. J Agron Crop Sci 197: 400–408. [Google Scholar]
- 7. Ceppi MG, Oukarroum A, Cicek N, Strasser RJ, Schansker G (2012) The IP amplitude of the fluorescence rise OJIP is sensitive to changes in the photosystem I content of leaves: a study on plants exposed to magnesium and sulfate deficiencies, drought stress and salt stress. Physiol Plant 144: 277–288. [DOI] [PubMed] [Google Scholar]
- 8. Schansker G, Srivastava A, Govindjee, Strasser RJ (2003) Characterization of the 820-nm transmission signal paralleling the chlorophyll a fluorescence rise (OJIP) in pea leaves. Funct Plant Biol 30: 785–796. [DOI] [PubMed] [Google Scholar]
- 9. Schansker G, Toth SZ, Strasser RJ (2005) Methylviologen and dibromothymoquinone treatments of pea leaves reveal the role of photosystem I in the Chl a fluorescence rise OJIP. Biochim Biophys Acta 1706: 250–261. [DOI] [PubMed] [Google Scholar]
- 10. Li PM, Ma FW (2012) Different effects of light irradiation on the photosynthetic electron transport chain during apple tree leaf dehydration. Plant Physiol Bioch 55: 16–22. [DOI] [PubMed] [Google Scholar]
- 11. Zhang ZS, Jia YJ, Gao HY, Zhang LT, Li HD, et al. (2011) Characterization of PSI recovery after chilling-induced photoinhibition in cucumber (Cucumis sativus L.) leaves. Planta 234: 883–889. [DOI] [PubMed] [Google Scholar]
- 12. Ntaikou I, Gavala HN, Kornaros M, Lyberatos G (2008) Hydrogen production from sugars and sweet sorghum biomass using Ruminococcus albus. Int J Hydrogen Energ 33: 1153–1163. [Google Scholar]
- 13. She DA, Xu F, Geng ZC, Sun RC, Jones GL, et al. (2010) Physicochemical characterization of extracted lignin from sweet sorghum stem. Ind Crop Prod 32: 21–28. [Google Scholar]
- 14. Shi XX, Song HC, Wang CR, Tang RS, Huang ZX, et al. (2010) Enhanced bio-hydrogen production from sweet sorghum stalk with alkalization pretreatment by mixed anaerobic cultures. Int J Energ Res 34: 662–672. [Google Scholar]
- 15. Almodares A, Hadi MR, Ahmadpour H (2008) Sorghum stem yield and soluble carbohydrates under different salinity levels. Afr J Biotechnol 7: 4051–4055. [Google Scholar]
- 16. Chai YY, Jiang CD, Shi L, Shi TS, Gu WB (2010) Effects of exogenous spermine on sweet sorghum during germination under salinity. Biol Plantarum 54: 145–148. [Google Scholar]
- 17. Koyro HW (1997) Ultrastructural and physiological changes in root cells of sorghum plants (Sorghum bicolor x S-sudanensis cv Sweet Sioux) induced by NaCl. J Exp Bot 48: 693–706. [Google Scholar]
- 18. Djanaguiraman M, Prasad PVV, Seppanen M (2010) Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiol Bioch 48: 999–1007. [DOI] [PubMed] [Google Scholar]
- 19. Strasser RJ, Tsimilli-Michael M, Qiang S, Goltsev V (2010) Simultaneous in vivo recording of prompt and delayed fluorescence and 820 nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. Biochim Biophys Acta 1797: 122–122. [DOI] [PubMed] [Google Scholar]
- 20. Yan K, Chen P, Shao HB, Zhao SJ, Zhang LH, et al. (2012) Photosynthetic characterization of Jerusalem artichoke during leaf expansion. Acta Physiol Plant 34: 353–360. [Google Scholar]
- 21. Sinsawat V, Leipner J, Stamp P, Fracheboud Y (2004) Effect of heat stress on the photosynthetic apparatus in maize (Zea mays L.) grown at control or high temperature. Environ Exp Bot 52: 123–129. [Google Scholar]
- 22. Yang XH, Chen XY, Ge QY, Li B, Tong YP, et al. (2006) Tolerance of photosynthesis to photoinhibition, high temperature and drought stress in flag leaves of wheat: A comparison between a hybridization line and its parents grown under field conditions. Plant Sci 171: 389–397. [DOI] [PubMed] [Google Scholar]
- 23. Yin Y, Li S, Liao W, Lu Q, Wen X, et al. (2010) Photosystem II photochemistry, photoinhibition, and the xanthophyll cycle in heat-stressed rice leaves. J Plant Physiol 167: 959–966. [DOI] [PubMed] [Google Scholar]
- 24. Sayed OH, Earnshaw MJ, Emes MJ (1989) Photosynthetic responses of different varieties of wheat to high temperature. I. effect of heat stress on photosynthetic electron transport. J Exp Bot 40: 633–638. [Google Scholar]
- 25. Boucher N, Carpentier R (1993) Heat-stress stimulation of oxygen uptake by Photosystem I involves the reduction of superoxide radicals by specific electron donors. Photosynth Res 35: 213–218. [DOI] [PubMed] [Google Scholar]
- 26. Mihailova G, Petkova S, Buchel C, Georgieva K (2011) Desiccation of the resurrection plant Haberlea rhodopensis at high temperature. Photosynth Res 108: 5–13. [DOI] [PubMed] [Google Scholar]
- 27. Essemine J, Govindachary S, Ammar S, Bouzid S, Carpentier R (2012) Enhanced sensitivity of the photosynthetic apparatus to heat stress in digalactosyl-diacylglycerol deficient Arabidopsis. Environ Exp Bot 80: 16–26. [Google Scholar]
- 28. Wen XG, Qiu NW, Lu QT, Lu CM (2005) Enhanced thermotolerance of photosystem II in salt-adapted plants of the halophyte Artemisia anethifolia. Planta 220: 486–497. [DOI] [PubMed] [Google Scholar]
- 29. Yang XH, Wen XG, Gong HM, Lu QT, Yang ZP, et al. (2007) Genetic engineering of the biosynthesis of glycinebetaine enhances thermotolerance of photosystem II in tobacco plants. Planta 225: 719–733. [DOI] [PubMed] [Google Scholar]
- 30. Lu CM, Zhang JH (2000) Heat-induced multiple effects on PSII in wheat plants. J Plant Physiol 156: 259–265. [Google Scholar]
- 31. Song YG, Liu B, Wang LF, Li MH, Liu Y (2006) Damage to the oxygen-evolving complex by superoxide anion, hydrogen peroxide, and hydroxyl radical in photoinhibition of photosystem II. Photosynth Res 90: 67–78. [DOI] [PubMed] [Google Scholar]
- 32. Chen LS, Li P, Cheng L (2008) Effects of high temperature coupled with high light on the balance between photooxidation and photoprotection in the sun-exposed peel of apple. Planta 228: 745–756. [DOI] [PubMed] [Google Scholar]
- 33. Kudoh H, Sonoike K (2002) Irreversible damage to photosystem I by chilling in the light: cause of the degradation of chlorophyll after returning to normal growth temperature. Planta 215: 541–548. [DOI] [PubMed] [Google Scholar]
- 34. Sonoike K (2011) Photoinhibition of photosystem I. Physiol Plant. 142: 56–64. [DOI] [PubMed] [Google Scholar]
- 35. Sonoike K (1996) Degradation of psaB gene product, the reaction center subunit of photosystem I, is caused during photoinhibition of photosystem I: Possible involvement of active oxygen species. Plant Sci 115: 157–164. [Google Scholar]
- 36. Salvucci ME, Crafts-Brandner SJ (2004) Relationship between the heat tolerance of photosynthesis and the thermal stability of rubisco activase in plants from contrasting thermal environments. Plant Physiol 134: 1460–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baker NR, Harbinson J, Kramer DM (2007) Determining the limitations and regulation of photosynthetic energy transduction in leaves. Plant Cell Environ 30: 1107–1125. [DOI] [PubMed] [Google Scholar]
- 38. Raven JA (2011) The cost of photoinhibition. Physiol Plant 142: 87–104. [DOI] [PubMed] [Google Scholar]
- 39. Rochaix JD (2011) Regulation of photosynthetic electron transport. Biochim Biophys Acta 1807: 375–383. [DOI] [PubMed] [Google Scholar]