Abstract
Objective
To evaluate the prevalence of and factors associated with latent tuberculosis infection (LTBI) based on the tuberculin skin test (TST) and to estimate the boosted reaction rate among newly employed healthcare workers (HCWs).
Design
Newly employed HCWs between January 2010 and July 2012 at Severance Hospital in South Korea were enrolled in this study. A one-step TST was conducted before October 2011, and a two-step TST after October 2011.
Results
Of 2132 participants, 778 (36.5%) had positive TST results. Being older (odds ratio [OR] 1.10, 95% confidence interval [CI] 1.06–1.13, P<0.001), male (OR 1.78, 95% CI 1.21–2.62, P = 0.003), rejoining the hospital workforce (OR 1.58, 95% CI 1.04–2.40, P = 0.032), and having a previous history of tuberculosis (TB) (OR 18.21, 95% CI 2.15–154.10, P = 0.008) during the one-step period, and being older (OR 1.15, 95% CI 1.10–1.21, P<0.001) during the two-step period were significantly associated with a positive TST. A two-step TST was performed in 556 HCWs, and a boosted reaction was observed in 79 (14.2%). The induration size on the first TST (5–9-mm group) was the only factor associated with a boosted reaction on the second TST.
Conclusions
The prevalence of LTBI based on the TST among newly employed HCWs was high. The boosted reaction rate on two-step TST was not low; therefore, the use of two-step TST may be necessary for regular monitoring in countries with an intermediate TB burden and a high rate of Bacillus Calmette-Guérin vaccination.
Introduction
It has long been recognized that healthcare workers (HCWs) are at high risk of a Mycobacterium tuberculosis (M. TB) infection due to occupational exposure [1], [2]. To control tuberculosis (TB) among HCWs, routine screening and appropriate treatment for latent TB infections (LTBIs) are recommended [3]. For regular monitoring, it is important to have baseline data regarding LTBIs in HCWs. However, limited information about LTBIs among newly employed HCWs is available.
Although the interferon-γ release assay (IGRA) was recently developed, the tuberculin skin test (TST) is still widely used to diagnose LTBIs. However, the test has several problems in terms of usage and interpretation. In particular, a boosted reaction may occur on repeated testing, which can cause misinterpretation as a conversion of the TST [4]. A boosted reaction is regarded as correlated with the initial TST reaction and Bacillus Calmette-Guérin (BCG) vaccination [1], [4]–[6]. Therefore, because repeated testing is needed for monitoring, an evaluation of boosted reactions is important among HCWs in areas with a high TB burden and where BCG vaccination is mandatory.
South Korea has an intermediate incidence of TB (85–110/100 000 population per year) [7] and BCG vaccination is given at birth to most infants. Recent Korean guidelines have recommended regular checkups for TB infection among HCWs at high risk for exposure [8]. However, limited data are available regarding LTBIs among newly employed HCWs in South Korea.
The present study was performed to evaluate the prevalence of and associated factors for LTBIs based on the TST, and to estimate the boosted reaction rate among newly employed HCWs at a tertiary referral hospital in South Korea.
Patients and Methods
Ethics Statement
The research protocol was approved by the Institutional Review Board (IRB) of Severance Hospital. The requirement for informed consent was waived by the IRB due to the retrospective nature of the analysis (IRB No. 4-2012-0780).
Study Design and Participants
This study was conducted at Severance Hospital (Seoul, Republic of Korea), a tertiary referral hospital with approximately 2000 beds. About 600 patients with smear- or culture-positive TB are managed at this hospital each year. The participants were HCWs who were newly employed between January 2010 and July 2012. A total of 2160 HCWs were employed during the study period. Because 28 HCWs during the study period were not screened due to pregnancy or administrative omission, 2132 HCWs were enrolled in this study. Each individual underwent a TST with a simple chest radiograph and was examined for comorbidities, previous TB history, occupational category (doctors, nurses, technicians, and others), and previous occupational history as an HCW according to hospital policy. Technicians were defined as technical employees performing services in radiology, laboratory, pathology, and physiotherapy. HCWs who rarely came into contact with patients or who worked in the medical school were defined as others. Previous employment history considered only employment at Severance Hospital; this was expressed as rejoining.
All participants, except retired HCWs, were followed up until September 2012.
Tuberculin Skin Test
A TST was performed on the forearm in accordance with the Mantoux method [4] using a 2-TU dose of the purified protein derivative RT 23 (Statens Serum Institute, Copenhagen, Denmark). Transverse induration was measured in mm between 48 and 72 h after injection.
A one-step TST was conducted before October 2011; a two-step TST was conducted after October 2011 because the hospital policy changed in October 2011. A total of 1171 HCWs were enrolled during the one-step period, and 961 HCWs were enrolled during the two-step period. The two-step TST was performed as follows: the TST was repeated 1 to 3 weeks later in subjects with negative initial TST results. A positive TST was defined as an induration of ≥10 mm in diameter [4]. A boosted reaction was defined as induration size of <10 mm on the first TST and an induration size of ≥10 mm on the second TST.
Statistical Analysis
All data are shown as numbers (percentages) or medians and interquartile ranges (IQRs). Pearson’s Chi-squared test or Fisher’s exact test was used to compare categorical variables, and the Mann-Whitney U-test was used to compare continuous variables. A logistic regression analysis was performed for multivariate analysis. The data were analyzed using SPSS (v. 18.0; SPSS Inc., Chicago, IL). In all analyses, P<0.05 (two-tailed) was taken to indicate statistical significance. Odds ratios (ORs) and their accompanying 95% confidence intervals (CIs) were estimated from the multivariate logistic regression model. In terms of age, OR was increased per year of age.
Results
Participant Characteristics
The baseline characteristics of the participants according to the period are presented in Table 1. The median age of the HCWs was 27 years (range, 20–65 years), and 1407 (66.0%) of the participants were women. The study population consisted of 650 (30.5%) doctors, 571 (26.8%) nurses, and 376 (17.6%) technicians. Two hundred and seven (9.7%) were rejoining HCWs. Twenty-four (1.1%) had a previous history of TB. Simple chest radiographs showed that 103 (4.8%) of the subjects had previously healed TB, 3 (0.1%) had active TB, and the remaining 2018 (94.7%) had normal findings. The proportion of subjects rejoining the hospital workforce and with a previous TB history was higher and the proportion of male subjects was lower during the one-step period than during the two-step period.
Table 1. Baseline characteristics of participants.
| Characteristics | Period | Total (n = 2132) | P-value | |
| One-step period (n = 1171) | Two-step period (n = 961) | |||
| Age (yrs), median (range) | 27 (21–65) | 27 (20–54) | 27 (20–65) | 0.273 |
| 20–29 | 774 (66.1) | 682 (71.0) | 1456 (68.3) | |
| 30–39 | 357 (30.5) | 258 (26.8) | 615 (28.8) | |
| 40–49 | 36 (3.1) | 20 (2.1) | 56 (2.6) | |
| 50–59 | 2 (0.2) | 1 (0.1) | 3 (0.1) | |
| 60–69 | 2 (0.2) | 0 (0) | 2 (0.1) | |
| Gender; male, n (%) | 369 (31.5) | 356 (37.0) | 725 (34.0) | 0.008 |
| BMI (median, IQR) | 21.0 (19.3–22.9) | 20.5 (19.1–22.5) | 20.8 (19.2–22.7) | 0.011 |
| Rejoined | 146 (12.5) | 61 (6.3) | 207 (9.7) | <0.001 |
| Previous TB history | 20 (1.7) | 4 (0.4) | 24 (1.1) | 0.006 |
| Findings on CXR* | 0.228 | |||
| Normal | 1109 (94.7) | 909 (94.6) | 2018 (94.7) | |
| Previously healed TB | 53 (4.5) | 50 (5.2) | 103 (4.8) | |
| Active pulmonary TB | 3 (0.3) | 0 (0) | 3 (0.1) | |
| Job category | <0.001 | |||
| Doctor | 287 (24.5) | 363 (37.8) | 650 (30.5) | |
| Nurse | 376 (32.1) | 195 (20.3) | 571 (26.8) | |
| Technician$ | 237 (20.2) | 139 (14.5) | 376 (17.6) | |
| Others | 271 (23.1) | 264 (27.5) | 535 (25.1) | |
| TST induration(median, IQR), mm | 5.0 (0.0–12.0) | 6.0 (0.0–12.0) | 5.0 (0.0–12.0) | 0.046 |
| TST ≥5 mm | 589 (50.3) | 421 (43.8) | 1129 (53.0) | 0.007 |
| TST ≥10 mm | 392 (33.5) | 386 (40.2) | 778 (36.5) | 0.002 |
BMI, body mass index; IQR, interquartile range; TB, tuberculosis; CXR, simple chest radiography; TST, tuberculin skin test.
Data could not be obtained for eight participants.
Technicians were defined as technical employees performing radiologic, laboratory, pathologic, and physiotherapeutic services.
TST Results and Associated Factors
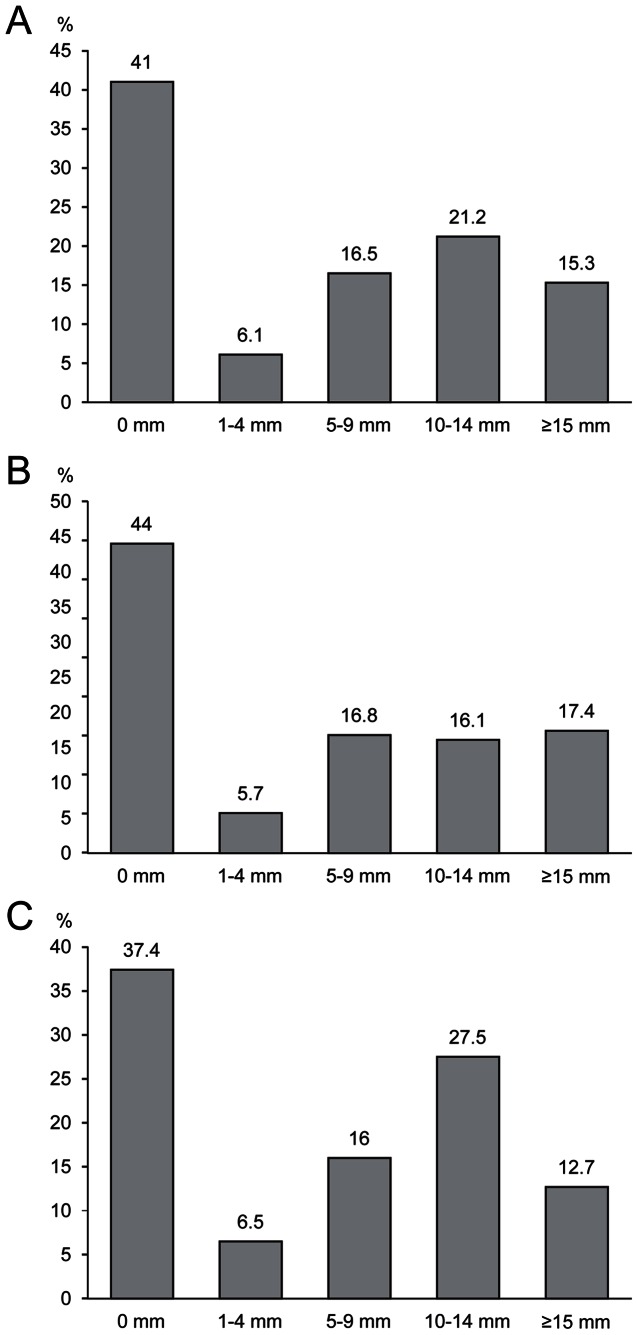
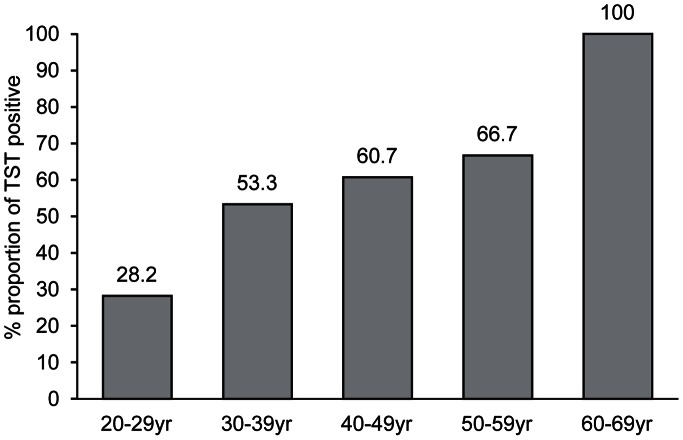
The proportion of positive TST was 36.5% overall. The prevalence of TST positivity was 33.5%, and the median TST induration size was 5.0 mm (IQR, 0.0–12.0 mm) during the one-step period. During the two-step period, the prevalence of TST positivity was 40.2%, and the median TST induration size was 6.0 mm (IQR, 0.0–12.0 mm). Figure 1 shows the distribution of TST reactions in each period. Eight hundred and four (41%) subjects had no measurable indurations, and the other distribution was concentrated around 10 mm. Tables 2 and 3 present the factors associated with TST positivity as determined by univariate and multivariate analyses according to the period. Finally, being older (OR 1.10, 95% CI 1.06–1.13, P<0.001), male (OR 1.78, 95% CI 1.21–2.62, P = 0.003), rejoining the hospital workforce (OR 1.58, 95% CI 1.04–2.40, P = 0.032), and having a previous history of TB (OR 18.21, 95% CI 2.15–154.10, P = 0.008) were significantly associated with positive TST results by multivariate analysis during the one-step period. Being older (OR 1.15, 95% CI 1.10–1.21, P<0.001) was significantly associated with positive TST results by multivariate analysis during the two-step period. The trend of TST positivity according to age is presented in Figure 2. Job category and the presence of previously healed TB on simple chest radiography showed no association on multivariate analysis.
Figure 1. Distribution of tuberculin skin test reactions.
A. Overall. B. One-step period. C. Two-step period.
Table 2. Clinical factors associated with a positive TST by univariate and multivariate analyses during the one-step period.
| TST | Univariate analysis | Multivariate analysis | ||||||||
| Clinical factor | Negative (n = 779) | Positive (n = 392) | OR | 95% CI | P | OR | 95% CI | P | ||
| Age (yrs), median (range) | 25 (21–53) | 29 (21–65) | 1.12 | 1.10–1.15 | <0.001 | 1.10 | 1.06–1.13 | <0.001 | ||
| Gender; male, n (%) | 200 (25.7) | 169 (43.1) | 2.19 | 1.70–2.84 | <0.001 | 1.78 | 1.21–2.62 | 0.003 | ||
| BMI (median, IQR) | 20.8 (19.1–22.6) | 21.5 (19.7–23.3) | 1.07 | 1.02–1.13 | 0.004 | 0.99 | 0.93–1.05 | 0.726 | ||
| Rejoined | 77 (9.9) | 69 (17.6) | 1.95 | 1.37–2.77 | <0.001 | 1.58 | 1.04–2.40 | 0.032 | ||
| Previous TB history | 2 (0.3) | 18 (4.6) | 18.70 | 4.32–81.00 | <0.001 | 18.21 | 2.15–154.10 | 0.008 | ||
| Finding of CXRs* | ||||||||||
| Normal | 751 (96.9) | 358 (91.8) | 1.00 | 1.00 | ||||||
| Previously healed TB | 24 (3.1) | 29 (7.4) | 2.54 | 1.46–4.42 | 0.001 | 1.15 | 0.55–2.43 | 0.711 | ||
| Active pulmonary TB | 0 (0.0) | 3 (0.8) | ||||||||
| Job category | ||||||||||
| Doctor | 154 (19.8) | 133 (33.9) | 1.55 | 1.10–2.18 | 0.012 | 1.05 | 0.69–1.60 | 0.821 | ||
| Nurse | 273 (35.0) | 103 (26.3) | 0.68 | 0.49–0.95 | 0.023 | 1.26 | 0.81–1.97 | 0.313 | ||
| Technician$ | 178 (22.8) | 59 (15.1) | 0.60 | 0.41–0.87 | 0.008 | 0.82 | 0.52–1.31 | 0.408 | ||
| Others | 174 (22.3) | 97 (24.7) | 1.00 | 1.00 | ||||||
TST, tuberculin skin test; BMI, body mass index; IQR, interquartile range; TB, tuberculosis; CXR, simple chest radiography; OR, odds ratio; CI, confidence interval.
Data could not be obtained for six participants.
Technicians are defined as technical employees performing radiologic, laboratory, pathologic, and physiotherapeutic services.
Table 3. Clinical factors associated with a positive TST through univariate and multivariate analyses during the two-step period.
| Clinical factor | TST | Univariate analysis | Multivariate analysis | |||||
| Negative (n = 575) | Positive (n = 386) | OR | 95% CI | P | OR | 95% CI | P | |
| Age (yrs), median (range) | 25 (21–47) | 29 (20–54) | 1.16 | 1.13–1.20 | <0.001 | 1.15 | 1.10–1.21 | <0.001 |
| Gender; male, n (%) | 191 (33.2) | 165 (42.7) | 1.50 | 1.15–1.96 | 0.003 | 1.21 | 0.78–1.87 | 0.405 |
| BMI (median, IQR) | 20.2 (18.9–22.3) | 20.9 (19.5–22.8) | 1.09 | 1.03–1.15 | 0.005 | 1.02 | 0.95–1.10 | 0.541 |
| Rejoined | 26 (4.5) | 35 (9.1) | 2.11 | 1.25–3.56 | 0.005 | 0.90 | 0.46–1.77 | 0.769 |
| Previous TB history | 0 (0.0) | 4 (1.0) | – | – | ||||
| Findings of CXRs* | ||||||||
| Normal | 551 (96.2) | 358 (92.7) | 1.00 | 1.00 | ||||
| Previously healed TB | 22 (3.8) | 28 (7.3) | 1.96 | 1.10–3.48 | 0.022 | 1.66 | 0.78–3.52 | 0.187 |
| Active pulmonary TB | 0 (0.0) | 0 (0.0) | ||||||
| Job category | ||||||||
| Doctor | 205 (35.7) | 158 (40.9) | 1.08 | 0.78–1.49 | 0.642 | |||
| Nurse | 125 (21.7) | 70 (18.1) | 0.78 | 0.54–1.15 | 0.211 | |||
| Technician$ | 91 (15.8) | 48 (12.4) | 0.74 | 0.48–1.13 | 0.164 | |||
| Others | 154 (26.8) | 110 (28.5) | 1.00 | |||||
TST, tuberculin skin test; BMI, body mass index; IQR, interquartile range; TB, tuberculosis; CXR, simple chest radiography; OR, odds ratio; CI, confidence interval.
Data could not be obtained for two participants.
Technicians were defined as technical employees performing radiologic, laboratory, pathologic, and physiotherapeutic services.
Figure 2. Trend of TST positivity according to age.
Boosted Reaction and Associated Factors
A two-step TST was performed in 556 HCWs, and a boosted reaction was observed in 79 (14.2%). Older or male subjects, or subjects with previously healed TB on chest radiography or a higher induration size on the first TST (especially in the 5–9-mm group) tended to show a boosted reaction on the TST. By multivariate analysis, induration size on the first TST (5–9-mm group) was the only factor associated with a boosted reaction on the second TST (Table 4).
Table 4. Clinical factors associated with a boosted TST reaction.
| Characteristics | Boosted reaction | OR (95% CI) | P | |
| Absence | Presence | |||
| Subjects | 477 (85.8) | 79 (14.2) | ||
| Age (yrs) | 25 (21–47) | 26 (20–54) | ||
| 20–24 | 170 (35.6) | 17 (21.5) | 1.00 | |
| 25–29 | 218 (45.7) | 38 (48.1) | 1.63 (0.72–3.70) | 0.244 |
| 30–34 | 66 (13.8) | 16 (20.3) | 2.30 (0.85–6.26) | 0.103 |
| 35–39 | 14 (2.9) | 6 (7.6) | 1.63 (0.22–11.98) | 0.630 |
| >40 | 9 (1.9) | 2 (2.5) | – | 0.99 |
| Gender; male, n (%) | 135 (28.3) | 33 (41.8) | 1.47 (0.63–3.45) | 0.372 |
| BMI (median, IQR) | 20.0 (18.9–22.1) | 21.7 (19.8–22.7) | 1.16 (0.99–1.36) | 0.060 |
| Rejoined | 19 (4.0) | 3 (3.8) | 0.23 (0.02–2.26) | 0.207 |
| Previous TB history | 0 (0) | 0 (0) | – | – |
| Previously healed TB on CXR* | 18 (3.8) | 6 (7.6) | 1.65 (0.40–6.74) | 0.485 |
| TST induration (median, IQR), mm | 0 (0–3) | 6 (0–8) | ||
| 0 mm | 331 (69.4) | 25 (31.6) | 1.00 | |
| 1–4 mm | 45 (9.4) | 6 (7.6) | 2.53 (0.83–7.75) | 0.103 |
| 5–9 mm | 101 (21.2) | 48 (60.8) | 6.51 (3.13–13.53) | <0.001 |
| Job category | ||||
| Doctor | 129 (27.0) | 17 (21.5) | ||
| Nurse | 121 (25.4) | 17 (21.5) | ||
| Technician$ | 90 (18.9) | 14 (17.7) | ||
| Others | 137 (28.7) | 31 (39.2) | ||
| Interval between 1st and 2nd TST days(median, range) | 9 (4–65) | 10 (7–35) | ||
TST, tuberculin skin test; OR, odds ratio; CI, confidence interval; BMI, body mass index; IQR, interquartile range; TB, tuberculosis; CXR, simple chest radiography.
Data could not be obtained for one participant.
Technicians were defined as technical employees performing radiologic, laboratory, pathologic, and physiotherapeutic services.
Follow-up
The median length of follow-up was 9.9 months (range, 3.5–30.9 months). Active TB developed in two HCWs during the follow-up period. Both subjects were women, 27 and 30 years old, respectively. One was a nurse and the other was a doctor, and the doctor had rejoined the hospital. Simple chest radiography at employment showed that one had previously healed TB and the other had normal finding. The results of a TST at employment were positive in two subjects (induration sizes, 15 and 24 mm).
Discussion
In the present study, the prevalence of an LTBI based on a TST among newly employed HCWs was 36.5%, and factors associated with TST positivity were older age, male, rejoining the hospital workforce, and a previous history of TB. In addition, we showed that the boosted reaction rate on a two-step TST was not low (14.2%), and that a boosted reaction was related to the induration size on the first TST (5–9-mm group).
The reported TST positivity among HCWs can vary according to the characteristics of the participants. TST positivity in this study was similar to that in a previous study performed at our institution (34% among subjects at low risk of exposure to TB) [9]. The reported rates of TST positivity among newly employed HCWs at other institutions in South Korea have been higher (51.5%) and lower (26%) than ours [10], [11]. This discrepancy may be due to the different characteristics of HCWs or setting. One previous study that reported higher TST positivity included a relatively high proportion of HCWs with exposure to TB and prior anti-TB treatment [11]. In contrast, lower positivity was reported in the setting of a one-step TST [10]. Considering these differences in study design and setting, the rate of LTBIs based on the TST in our study was relatively considerable.
There is presently no gold standard diagnostic test for LTBI. Nevertheless, our study showed that being older, male, rejoining the hospital workforce, and having a previous history of TB were associated with TST positivity, although there were slight differences according to the period due to the small discrepancy in baseline characteristics during the one-step and two-step periods. Cristopoulos et al. and Altunoren et al. reported that older age is a risk factor for TB [12], [13]. Jimenez-Corona et al. reported that there may be sex-related differences in incidence rates of TB in the general population due to the dynamics of local spread or the degree of environmental exposure [14]. Rejoining the hospital workforce was a reasonable associated factor for TST positivity because occupational exposure is a known risk factor for M. TB infection [1], [2]. Previous TB history was also considered a risk factor for an LTBI in other studies [15], [16]. Taken together, our data suggest that the TST may reflect the risk of LTBI among HCWs, and the observation that the HCWs who developed active TB after employment had a positive TST at employment supports this hypothesis.
Recently, the IGRAs, QuantiFERON-TB Gold In-Tube test (Cellestis Ltd., Carnegie, Victoria, Australia), and T.SPOT TB test (Oxford Immunotec, Abingdon, UK) have been developed and used in clinical practice, and many studies have suggested that IGRAs could overcome the limitations of the TST. However, serial IGRAs can reveal variations and serial monitoring in HCWs by IGRAs is challenging [17], [18]; thus, the TST is still useful despite the possibility of a boosted reaction, particularly in a BCG-vaccinated population [4]–[6], [19].
The boosted reaction rate for the two-step TST varies according to the characteristics of the study population (e.g., age distribution, previous exposure to TB or nontuberculous mycobacteria, and BCG vaccination status) [20]–[24]. The boosted reaction rate (14.2%) in our study was intermediate between those of populations in which BCG vaccination was given at infancy (8%) and at 5 years or older (18%) [19]. In South Korea until 1997, the BCG vaccine was given at birth and again at the age of 12 or 13 years if the child proved to be a TST non-responder. BCG revaccination was stopped in 1997 according to the recommendation of the WHO in 1995 due to the lack of evidence [25]. Considering the ages of the participants, the HCWs in our study would have consisted of a mixture of those vaccinated with BCG once and twice. Therefore, the observed boosted reaction rate among newly employed HCWs in this study is reasonable and not low compared to the general population.
The observation that induration size on the first TST, especially 5–9 mm, was the only factor associated with a boosted reaction is consistent with previous reports [26], [27]. Based on the observed rate of a boosted reaction and associated factors, the two-step TST may be useful in newly employed HCWs for regular TST monitoring, and interpretation of the TST should be performed with caution in some groups. However, a substantial number of boosted reactions developed in HCWs with a 0-mm induration size at the first TST. This result indicates that the two-step TST cannot be performed on only a selected group and should be performed on all individuals without a prior skin test in the 12-month period before beginning employment, as recommended by the US Centers for Disease Control and Prevention (CDC) TB infection control guidelines [3].
In our hospital, the Department of Infectious Disease Control manages the infection control policy. Rooms for infectious patients with TB and the area for high-risk procedures such as bronchoscopy are isolated and equipped with negative-pressure ventilation. Patients who are confirmed to have infectious TB or are suspicious for infectious TB are immediately isolated. HCWs or visitors entering such rooms should wear N95 respirators.
In addition, we have a policy for monitoring TB infection in HCWs. We perform TSTs and chest radiographs in newly employed HCWs and repeatedly perform TSTs and chest radiographs in HCWs who are exposed to patients with TB. We also annually perform TSTs and chest radiographs in HCWs who work in departments related to TB. However, because there is no consensus regarding the optimal screening strategy for LTBI in HCWs in Korea, this study will provide baseline data.
Our study has several limitations. First, this study was conducted at a single institution. Second, we could not obtain data regarding the history of BCG vaccination. However, a high rate of BCG vaccination can be expected in our participants because BCG vaccination is mandatory in South Korea and previous data indicate that the prevalence of BCG scars in Korean infants is about 88% [28]. Third, there was no information about TB exposure before employment. Fourth, we had no information about previous occupational history as an HCW except at our institution. Occupational exposure is an important risk factor for TB infection in HCWs, and information about employment history is necessary to accurately evaluate the risk factors for an LTBI. Fifth, the mixed one- and two-step TST group was a limitation in evaluating the prevalence and risk factors for having a positive test. The prevalence of LTBI could have been underestimated during the one-step period, and the boosted reaction could have been due to a combination of LTBI and prior BCG vaccination. This mixture was due to the change in the hospital policy during the study period.
Conclusions
The prevalence of LTBI based on the TST among newly employed HCWs was high. The boosted reaction rate on the two-step TST was not low; therefore, the use of the two-step TST may be necessary for regular monitoring in countries with an intermediate TB burden and a high rate of BCG vaccination.
Funding Statement
The authors have no support or funding to report.
References
- 1. Menzies D, Fanning A, Yuan L, Fitzgerald M (1995) Tuberculosis among health care workers. N Engl J Med 332: 92–8. [DOI] [PubMed] [Google Scholar]
- 2. Menzies D, Joshi R, Pai M (2007) Risk of tuberculosis infection and disease associated with work in health care settings. Int J Tuberc Lung Dis 11: 593–605. [PubMed] [Google Scholar]
- 3. Jensen PA, Lambert LA, Iademarco MF, Ridzon R (2005) Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep 54: 1–141. [PubMed] [Google Scholar]
- 4. Anonymous (2000) Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med 161: S221–47. [DOI] [PubMed] [Google Scholar]
- 5. Farhat M, Greenaway C, Pai M, Menzies D (2006) False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis 10: 1192–204. [PubMed] [Google Scholar]
- 6. Joos TJ, Miller WC, Murdoch DM (2006) Tuberculin reactivity in bacille Calmette-Guerin vaccinated populations: a compilation of international data. Int J Tuberc Lung Dis 10: 883–91. [PubMed] [Google Scholar]
- 7.WHO (2011). Global tuberculosis control: WHO report 2011. WHO/HTM/TB/2011. Geneva, Switzerland: WHO.
- 8.Joint committee for the development of Korean guidelines for tuberculosis (2011) Korea Centers for Disease Control and Prevention. Korean guidelines for tuberculosis, 1st edition.
- 9. Lee KJ, Kang YA, Kim YM, Cho SN, Moon JW, et al. (2010) Screening for latent tuberculosis infection in South Korean healthcare workers using a tuberculin skin test and whole blood interferon-gamma assay. Scand J Infect Dis 42: 672–8. [DOI] [PubMed] [Google Scholar]
- 10. Park HY, Jeon K, Suh GY, Kwon OJ, Chung DR, et al. (2010) Interferon-gamma release assay for tuberculosis screening of healthcare workers at a Korean tertiary hospital. Scand J Infect Dis 42: 943–5. [DOI] [PubMed] [Google Scholar]
- 11. Lee K, Han MK, Choi HR, Choi CM, Oh YM, et al. (2009) Annual incidence of latent tuberculosis infection among newly employed nurses at a tertiary care university hospital. Infect Control Hosp Epidemiol 30: 1218–22. [DOI] [PubMed] [Google Scholar]
- 12. Christopoulos AI, Diamantopoulos AA, Dimopoulos PA, Goumenos DS, Barbalias GA (2009) Risk factors for tuberculosis in dialysis patients: a prospective multi-center clinical trial. BMC Nephrol 10: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Altunoren O, Kahraman H, Sayarlioglu H, Yavuz YC, Dogan E, et al. (2012) The affecting factors and comparison of tuberculin skin test in peritoneal dialysis and hemodialysis patients. Ren Fail 34: 304–7. [DOI] [PubMed] [Google Scholar]
- 14. Jimenez-Corona ME, Garcia-Garcia L, DeRiemer K, Ferreyra-Reyes L, Bobadilla-del-Valle M, et al. (2006) Gender differentials of pulmonary tuberculosis transmission and reactivation in an endemic area. Thorax 61: 348–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Manuel O, Humar A, Preiksaitis J, Doucette K, Shokoples S, et al. (2007) Comparison of quantiferon-TB gold with tuberculin skin test for detecting latent tuberculosis infection prior to liver transplantation. Am J Transplant 7: 2797–801. [DOI] [PubMed] [Google Scholar]
- 16. Kim SY, Jung GS, Kim SK, Chang J, Kim MS, et al. (2012) Comparison of the tuberculin skin test and interferon-gamma release assay for the diagnosis of latent tuberculosis infection before kidney transplantation. Infection 41: 103–10. [DOI] [PubMed] [Google Scholar]
- 17. van Zyl-Smit RN, Zwerling A, Dheda K, Pai M (2009) Within-subject variability of interferon-g assay results for tuberculosis and boosting effect of tuberculin skin testing: a systematic review. Plos One 4: e8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Park JS, Lee JS, Kim MY, Lee CH, Yoon HI, et al. (2012) Monthly follow-ups of interferon-gamma release assays among healthcare workers in contact with TB patients. Chest 142: 1461–8. [DOI] [PubMed] [Google Scholar]
- 19. Menzies D (1999) Interpretation of repeated tuberculin tests. Boosting, conversion, and reversion. Am J Respir Crit Care Med 159: 15–21. [DOI] [PubMed] [Google Scholar]
- 20. Al Mazrou AM (2004) Booster effect of two-step tuberculin skin testing among hospital employees from areas with a high prevalence of tuberculosis. Infect Control Hosp Epidemiol 25: 1117–20. [DOI] [PubMed] [Google Scholar]
- 21. Hizel K, Maral I, Karakus R, Aktas F (2004) The influence of BCG immunisation on tuberculin reactivity and booster effect in adults in a country with a high prevalence of tuberculosis. Clin Microbiol Infect 10: 980–3. [DOI] [PubMed] [Google Scholar]
- 22. Salles CG, Ruffino-Netto A, Lapa-e-Silva JR, Kritski AL, Cailleaux-Cesar M, et al. (2007) The presence of a booster phenomenon among contacts of active pulmonary tuberculosis cases: a retrospective cohort. BMC Public Health 7: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cauthen CM, Snider DE, Onorato IM (1994) Boosting of Tuberculin Sensitivity among Southeast-Asian Refugees. Am J Respir Crit Care Med 149: 1597–600. [DOI] [PubMed] [Google Scholar]
- 24. Frenzel EC, Thomas GA, Hanna HA (2006) The importance of two-step tuberculin skin testing for newly employed healthcare workers. Infect Control Hosp Epidemiol 27: 512–4. [DOI] [PubMed] [Google Scholar]
- 25. WHO (1995) Global Tuberculosis Programme and Global Programme on Vaccines: Statement on BCG revaccination for the prevention of tuberculosis. WHO Wkly Epidem Rec 70: 229–36. [PubMed] [Google Scholar]
- 26. Menzies R, Vissandjee B, Rocher I, St Germain Y (1994) The booster effect in two-step tuberculin testing among young adults in Montreal. Ann Intern Med 120: 190–8. [DOI] [PubMed] [Google Scholar]
- 27. Jeon K, Ji SH, Oh SY, Lee JB, Kim HJ, et al. (2008) Boosted reaction on two-step tuberculin skin test among military personnel in South Korea, a setting with an intermediate burden of tuberculosis and routine bacille Calmette-Guerin vaccination. J Korean Med Sci 23: 402–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hong YP, Kim SJ, Lew WJ, Lee EK, Han YC (1998) The seventh nationwide tuberculosis prevalence survey in Korea, 1995. Int J Tuberc Lung Dis 2: 27–36. [PubMed] [Google Scholar]