Abstract
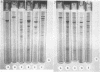
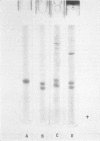
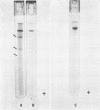
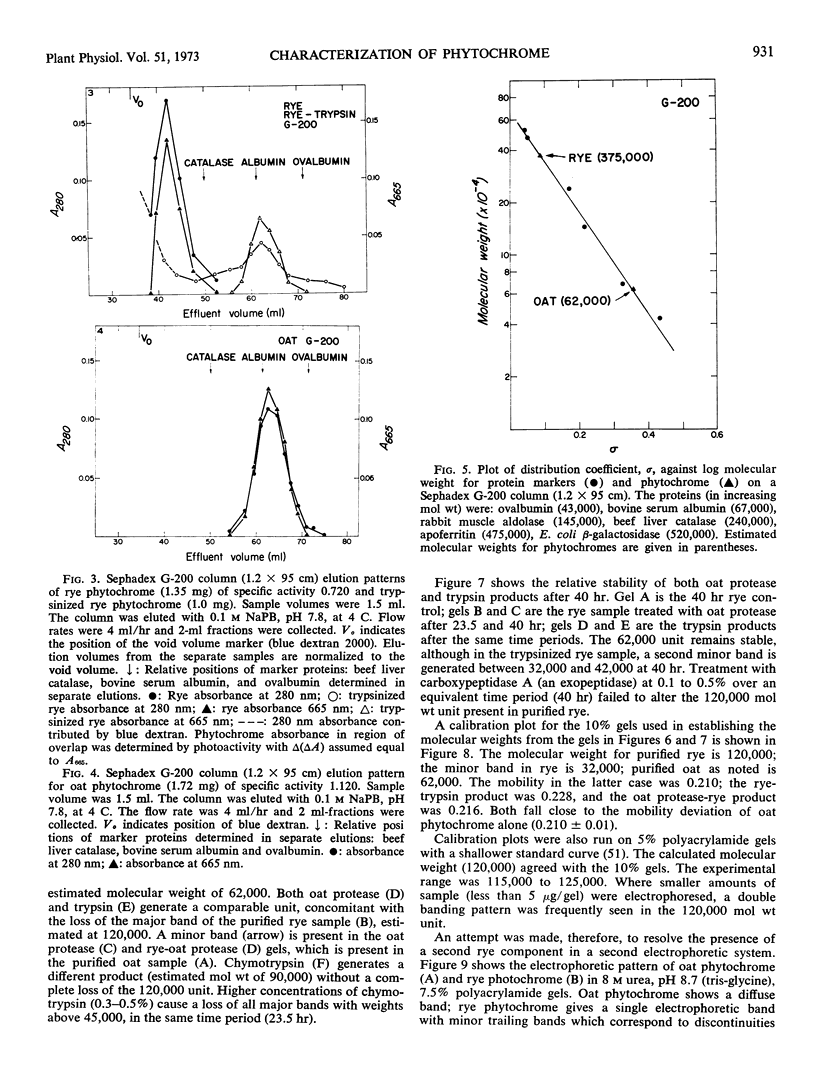
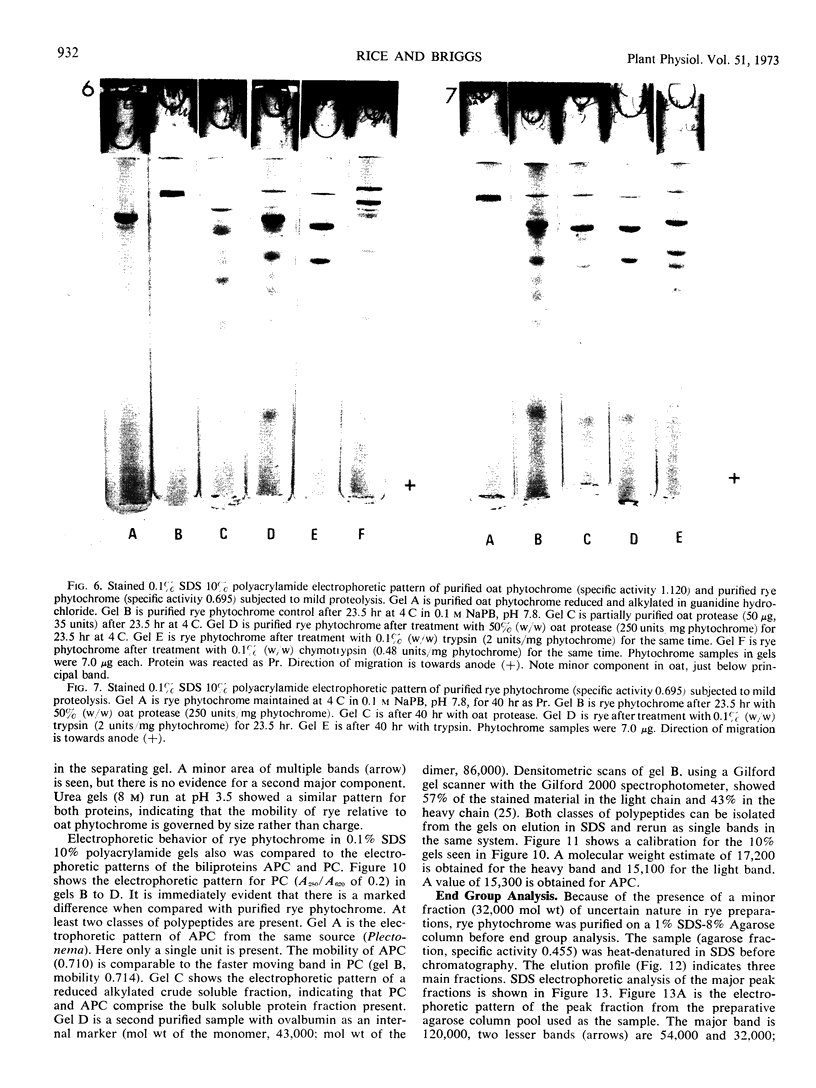
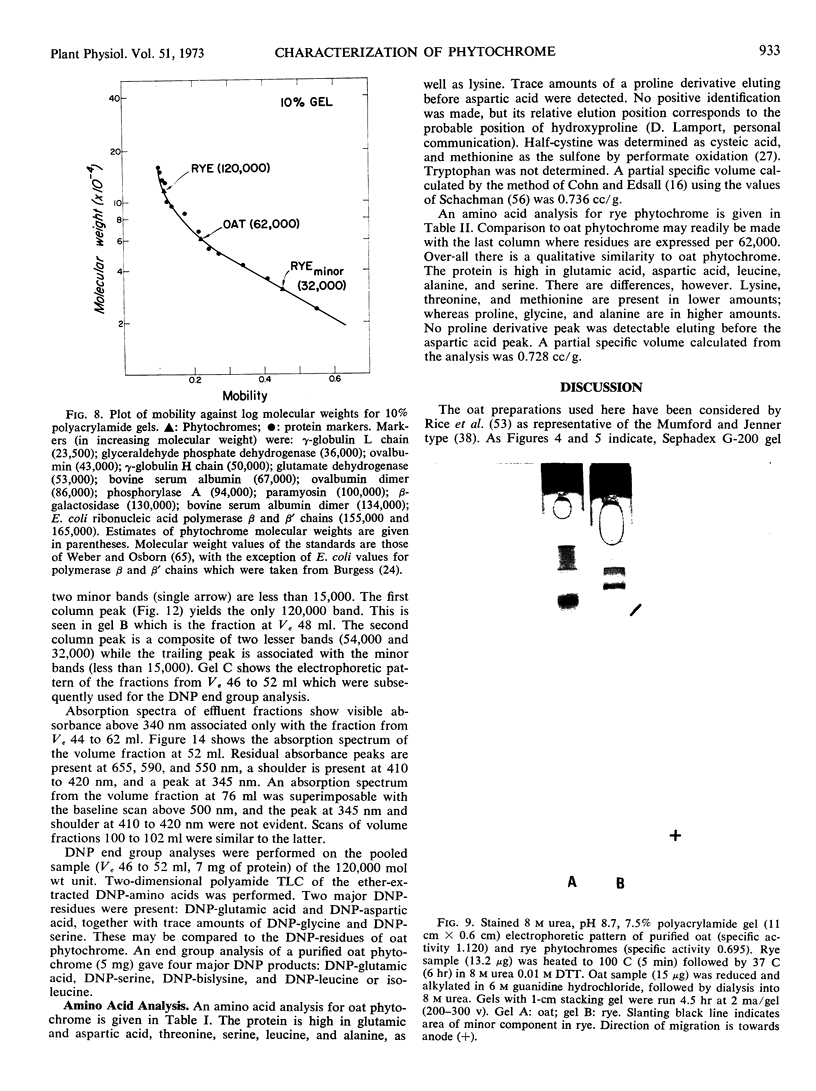
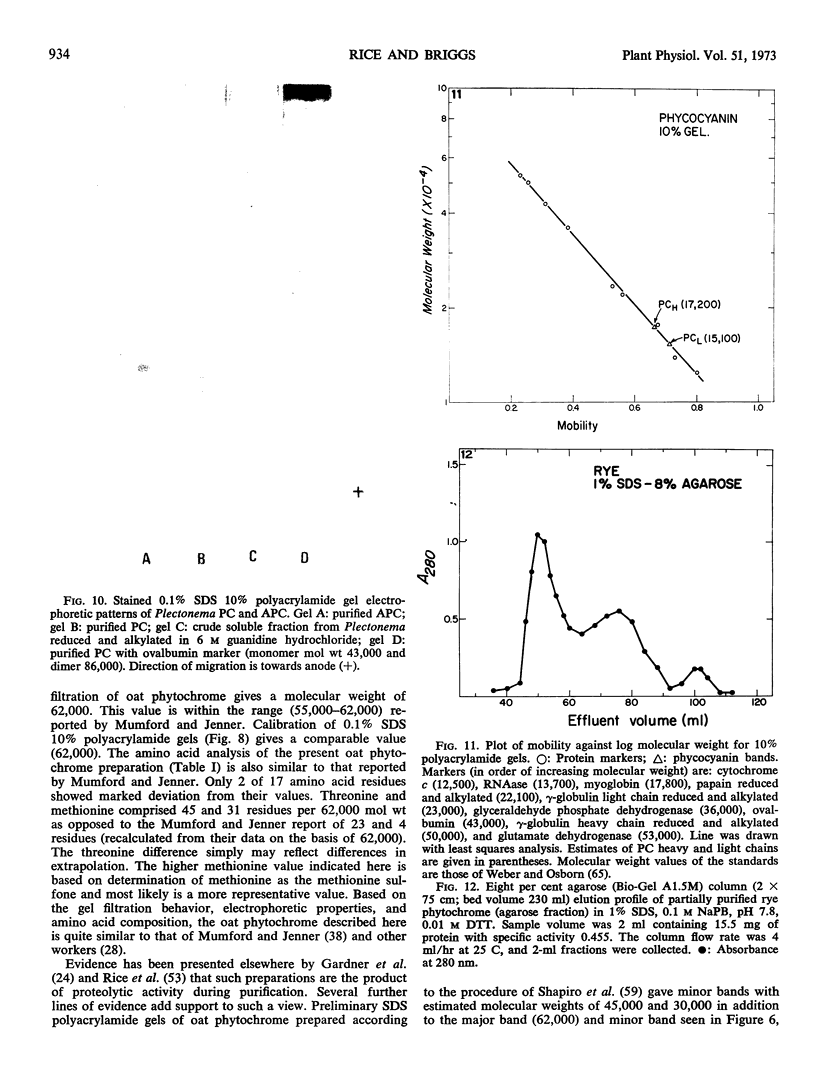
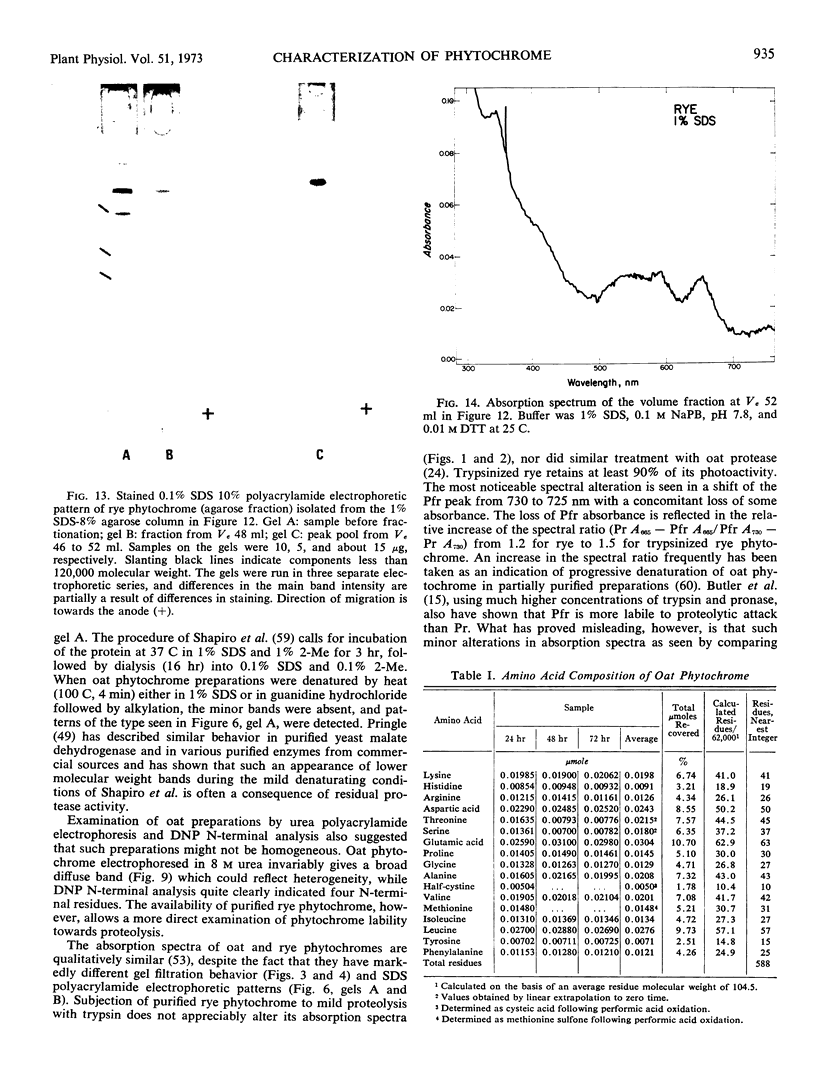
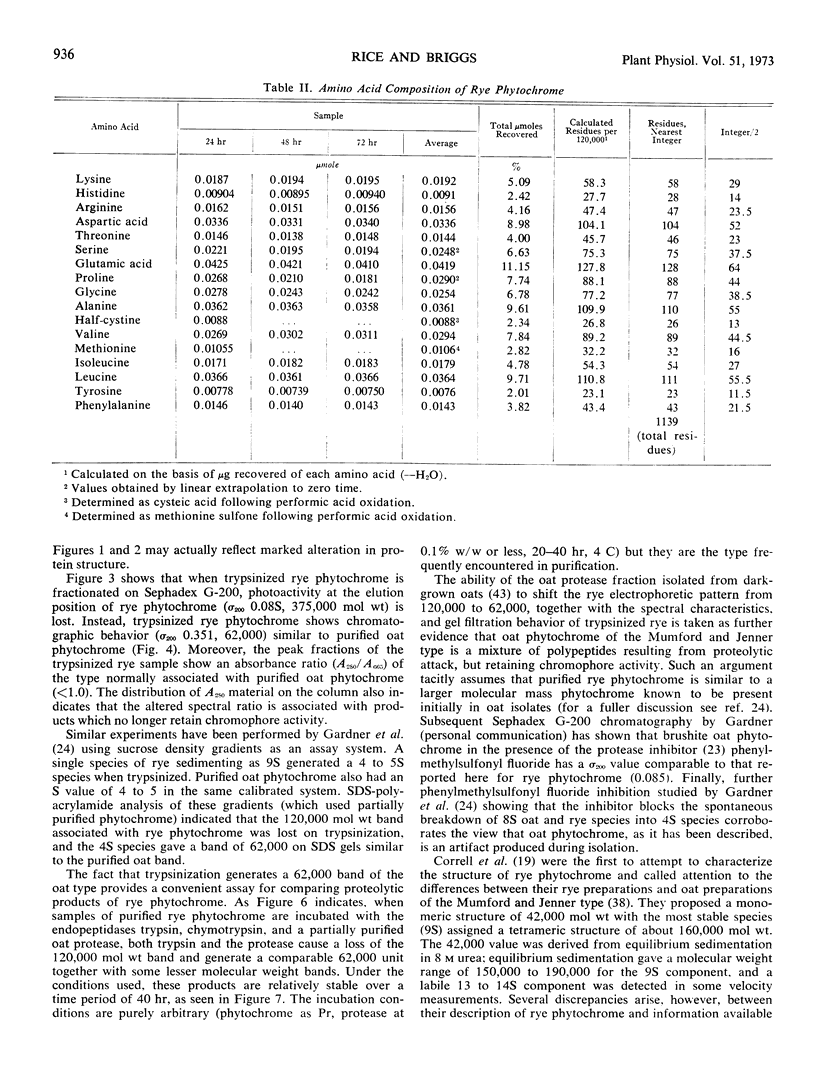
Purified oat and rye phytochrome were examined by analytical gel chromatography, polyacrylamide gel electrophoresis, N-terminal, and amino acid analysis. Purified oat phytochrome had a partition coefficient on Sephadex G-200 (σ200) of 0.350 with an estimated molecular weight of 62,000; sodium dodecyl sulfate polyacrylamide electrophoresis gave an equivalent weight estimate. Purified rye phytochrome had a σ200 value of 0.085 with an estimated molecular weight of 375,000; sodium dodecyl sulfate electrophoresis gave a weight estimate of 120,000, indicating a multimer structure for the nondenatured protein. Comparative sodium dodecyl sulfate electrophoresis with purified phycocyanin and allophycocyanin gave a molecular weight estimate of 15,000 for allophycocyanin, and two constituent classes of subunits for phycocyanin with molecular weights of 17,000 and 15,000. Amino acid analysis of oat phytochrome confirmed a previous report; amino acid analysis of rye phytochrome differs markedly from a previous report. Oat phytochome has four detectable N-terminal residues (glutamic acid, serine, lysine, and leucine, or isoleucine); rye phytochrome has two detectable groups (aspartic and glutamic acids). Model experiments subjecting purified rye phytochrome to proteinolysis generate a product with the characteristic spectral and weight properties of oat phytochrome, as it has been described in the literature. It is concluded that the structural characteristics of purified rye phytochrome are likely those of the native protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackers G. K. Analytical gel chromatography of proteins. Adv Protein Chem. 1970;24:343–446. doi: 10.1016/s0065-3233(08)60245-4. [DOI] [PubMed] [Google Scholar]
- Anderson G. R., Jenner E. L., Mumford F. E. Optical rotatory dispersion and circular dichroism spectra of phytochrome. Biochim Biophys Acta. 1970 Oct 20;221(1):69–73. doi: 10.1016/0005-2795(70)90198-4. [DOI] [PubMed] [Google Scholar]
- Anderson G. R., Jenner E. L., Mumford F. E. Temperature and pH studies on phytochrome in vitro. Biochemistry. 1969 Mar;8(3):1182–1187. doi: 10.1021/bi00831a052. [DOI] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTLER W. L., SIEGELMAN H. W., MILLER C. O. DENATURATION OF PHYTOCHROME. Biochemistry. 1964 Jun;3:851–857. doi: 10.1021/bi00894a022. [DOI] [PubMed] [Google Scholar]
- Bennett A., Bogorad L. Properties of subunits and aggregates of blue-green algal biliproteins. Biochemistry. 1971 Sep 14;10(19):3625–3634. doi: 10.1021/bi00795a022. [DOI] [PubMed] [Google Scholar]
- Berns D. S. Immunochemistry of biliproteins. Plant Physiol. 1967 Nov;42(11):1569–1586. doi: 10.1104/pp.42.11.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield V., Van Holde K. E., Dalton W. O. Frictional coefficients of multisubunit structures. II. Application to proteins and viruses. Biopolymers. 1967 Feb;5(2):149–159. doi: 10.1002/bip.1967.360050203. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. Major human erythrocyte glycoprotein spans the cell membrane. Nat New Biol. 1971 Jun 23;231(25):229–232. doi: 10.1038/newbio231229a0. [DOI] [PubMed] [Google Scholar]
- Briggs W. R. Long-lived Intermediates in Phytochrome Transformation I: In Vitro Studies. Plant Physiol. 1969 Aug;44(8):1081–1088. doi: 10.1104/pp.44.8.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs W. R. Long-lived Intermediates in Phytochrome Transformation II: In Vitro and In Vivo Studies. Plant Physiol. 1969 Aug;44(8):1089–1094. doi: 10.1104/pp.44.8.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. L., Lane H. C., Siegelman H. W. Nonphotochemical Transformations of Phytochrome in Vivo. Plant Physiol. 1963 Sep;38(5):514–519. doi: 10.1104/pp.38.5.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope B. T., Smith U., Crespi H. L., Katz J. J. Studies on the identity of ordinary and deuterio-phycocyanins: end-groups, amino acid compositions, minimum molecular weights, peptide maps. Biochim Biophys Acta. 1967 Apr 11;133(3):446–453. doi: 10.1016/0005-2795(67)90548-x. [DOI] [PubMed] [Google Scholar]
- Correll D. L., Edwards J. L., Shropshire W., Jr Multiple chromophore species in phytochrome. Photochem Photobiol. 1968 Nov;8(5):465–475. doi: 10.1111/j.1751-1097.1968.tb05890.x. [DOI] [PubMed] [Google Scholar]
- Correll D. L., Steers E., Jr, Towe K. M., Shropshire W., Jr Phytochrome in etiolated annual rye. IV. Physical and chemical characterization of phytochrome. Biochim Biophys Acta. 1968 Sep 10;168(1):46–57. doi: 10.1016/0005-2795(68)90232-8. [DOI] [PubMed] [Google Scholar]
- Cross D. R., Linschitz H., Kasche V., Tenenbaum J. Low-temperature studies on phytochrome: light and dark reactions in the red to far-red transformation and new intermediate forms of phytochrome. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1095–1101. doi: 10.1073/pnas.61.3.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Everett M. S., Briggs W. R. Some spectral properties of pea phytochrome in vivo and in vitro. Plant Physiol. 1970 Jun;45(6):679–683. doi: 10.1104/pp.45.6.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner G., Pike C. S., Rice H. V., Briggs W. R. "Disaggregation" of phytochrome in vitro-a consequence of proteolysis. Plant Physiol. 1971 Dec;48(6):686–693. doi: 10.1104/pp.48.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorovsky M. A., Carlson K., Rosenbaum J. L. Simple method for quantitive densitometry of polyacrylamide gels using fast green. Anal Biochem. 1970 Jun;35(2):359–370. doi: 10.1016/0003-2697(70)90196-x. [DOI] [PubMed] [Google Scholar]
- Hopkins D. W., Butler W. L. Immunochemical and spectroscopic evidence for protein conformational changes in phytochrome transformations. Plant Physiol. 1970 May;45(5):567–570. doi: 10.1104/pp.45.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao O., Berns D. S. The monomer molecular weight of C-phycocyanin. Biochem Biophys Res Commun. 1968 Nov 8;33(3):457–462. doi: 10.1016/0006-291x(68)90595-0. [DOI] [PubMed] [Google Scholar]
- Kroes H. H. Reversible changes in the circular dichroism of phytochrome during photoisomerisation of the pigment. Biochem Biophys Res Commun. 1968 Jun 28;31(6):877–883. doi: 10.1016/0006-291x(68)90533-0. [DOI] [PubMed] [Google Scholar]
- Linschitz H., Kasche V. KINETICS OF PHYTOCHROME CONVERSION: MULTIPLE PATHWAYS IN THE P(r) TO P(fr) REACTION, AS STUDIED BY DOUBLE-FLASH TECHNIQUE. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1059–1064. doi: 10.1073/pnas.58.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linschitz H., Kasche V. The kinetics of phytochrome conversion. J Biol Chem. 1966 Jul 25;241(14):3395–3403. [PubMed] [Google Scholar]
- McKee P. A., Mattock P., Hill R. L. Subunit structure of human fibrinogen, soluble fibrin, and cross-linked insoluble fibrin. Proc Natl Acad Sci U S A. 1970 Jul;66(3):738–744. doi: 10.1073/pnas.66.3.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore G. L. Use of azo-dye-bound collagen to measure reaction velocities of proteolytic enzymes. Anal Biochem. 1969 Oct 15;32(1):122–127. doi: 10.1016/0003-2697(69)90111-0. [DOI] [PubMed] [Google Scholar]
- Mumford F. E., Jenner E. L. Catalysis of the phytochrome dark reaction by reducing agents. Biochemistry. 1971 Jan 5;10(1):98–101. doi: 10.1021/bi00777a015. [DOI] [PubMed] [Google Scholar]
- Mumford F. E. Studies on the phytochrome dark reaction in vitro. Biochemistry. 1966 Feb;5(2):522–524. doi: 10.1021/bi00866a018. [DOI] [PubMed] [Google Scholar]
- Neufeld G. J., Riggs A. F. Aggregation properties of C-Phycocyanin from Anacystis nidulans. Biochim Biophys Acta. 1969 May;181(1):234–243. doi: 10.1016/0005-2795(69)90246-3. [DOI] [PubMed] [Google Scholar]
- Pike C. S., Briggs W. R. Partial Purification and Characterization of a Phytochrome-degrading Neutral Protease from Etiolated Oat Shoots. Plant Physiol. 1972 Apr;49(4):521–530. doi: 10.1104/pp.49.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike C. S., Briggs W. R. The dark reactions of rye phytochrome in vivo and in vitro. Plant Physiol. 1972 Apr;49(4):514–520. doi: 10.1104/pp.49.4.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt-Rivers R., Impiombato F. S. The binding of sodium dodecyl sulphate to various proteins. Biochem J. 1968 Oct;109(5):825–830. doi: 10.1042/bj1090825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt L. H., Butler W. L. Phytochrome conversion by ultraviolet light. Photochem Photobiol. 1970 Jun;11(6):503–509. doi: 10.1111/j.1751-1097.1970.tb06021.x. [DOI] [PubMed] [Google Scholar]
- Pratt L. H., Butler W. L. Stabilization of phytochrome intermediates by low temperature. Photochem Photobiol. 1968 Nov;8(5):477–485. doi: 10.1111/j.1751-1097.1968.tb05891.x. [DOI] [PubMed] [Google Scholar]
- Pratt L. H., Butler W. L. The temperature dependence of phytochrome transformations. Photochem Photobiol. 1970 May;11(5):361–369. doi: 10.1111/j.1751-1097.1970.tb06008.x. [DOI] [PubMed] [Google Scholar]
- Pringle J. R. The molecular weight of the undegraded polypeptide chain of yeast hexokinase. Biochem Biophys Res Commun. 1970 Apr 8;39(1):46–52. doi: 10.1016/0006-291x(70)90755-2. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Tanford C. Binding of dodecyl sulfate to proteins at high binding ratios. Possible implications for the state of proteins in biological membranes. Proc Natl Acad Sci U S A. 1970 Jul;66(3):1002–1007. doi: 10.1073/pnas.66.3.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice H. V., Briggs W. R. Immunochemistry of phytochrome. Plant Physiol. 1973 May;51(5):939–945. doi: 10.1104/pp.51.5.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice H. V., Briggs W. R., Jackson-White C. J. Purification of oat and rye phytochrome. Plant Physiol. 1973 May;51(5):917–926. doi: 10.1104/pp.51.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux S. J. Chemical evidence for conformational differences between the red- and far-red-absorbing forms of oat phytochrome. Biochemistry. 1972 May 9;11(10):1930–1936. doi: 10.1021/bi00760a030. [DOI] [PubMed] [Google Scholar]
- SELA M., WHITE F. H., Jr, ANFINSEN C. B. The reductive cleavage of disulfide bonds and its application to problems of protein structure. Biochim Biophys Acta. 1959 Feb;31(2):417–426. doi: 10.1016/0006-3002(59)90016-2. [DOI] [PubMed] [Google Scholar]
- SIEGELMAN H. W., FIRER E. M. PURIFICATION OF PHYTOCHROME FROM OAT SEEDLINGS. Biochemistry. 1964 Mar;3:418–423. doi: 10.1021/bi00891a019. [DOI] [PubMed] [Google Scholar]
- STARK G. R., SMYTH D. G. The use of cyanate for the determination of NH2-terminal residues in proteins. J Biol Chem. 1963 Jan;238:214–226. [PubMed] [Google Scholar]
- Sanger F. The free amino groups of insulin. Biochem J. 1945;39(5):507–515. doi: 10.1042/bj0390507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott E., Berns D. S. Protein-protein interaction. The phycocyanin system. Biochemistry. 1965 Dec;4(12):2597–2606. doi: 10.1021/bi00888a008. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- THOMPSON E. O. The N-terminal sequence of serum albumins; observations on the thiohydantoin method. J Biol Chem. 1954 Jun;208(2):565–572. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]