Abstract
4-(hydroxyphenyl)retinamide (4-HPR) is a synthetic retinoid with strong apoptotic effect towards different cancer cell lines in vitro, and it is currently tested in clinical trials. Increases of Reactive Oxygen Species (ROS) and modulation of endogenous sphingolipid levels are well-described events observed upon 4-HPR treatment, but there is still a lack of understanding of their relationship and their contribution to cell death. LC/MS analysis of sphingolipids revealed that in human leukemia CCRF-CEM and Jurkat cells, 4-HPR induced dihydroceramide but not ceramide accumulation even at sub-lethal concentrations. Myriocin prevented the 4-HPR-induced dihydroceramide accumulation, but it did not prevent the loss of viability and increase of intracellular ROS production. On the other hand, ascorbic acid, Trolox, and vitamin E reversed 4-HPR effects on cell death but not dihydroceramide accumulation. NDGA, described as a lipoxygenase inhibitor, exerted a significantly higher antioxidant activity than vitamin E and abrogated 4-HPR-mediated ROS. It did not however rescue cellular viability. Taken together, this study demonstrates that early changes observed upon 4-HPR treatment i.e. sphingolipid modulation and ROS production, are mechanistically independent events. Furthermore, the results indicate that 4-HPR-driven cell death may occur even in the absence of dihydroceramide or ROS accumulation. These observations should be taken into account for an improved design of drug combinations.
Keywords: 4-HPR, leukemia, dihydroceramide, ROS, cell death
INTRODUCTION
A large number of studies based on 4-(hydroxyphenyl)retinamide (4-HPR, fenretinide) describe this agent as a promising chemotherapeutic drug, but its mechanism of action remains not fully defined. 4-HPR is a synthetic amide derivative of the all-trans retinoic acid (ATRA) that is being tested against several cancers in vitro (Maurer et al. 1999; Sun et al. 1999; Asumendi et al. 2002; Tiwari et al. 2006) and in vivo as well as in clinical trials (Reynolds and Lemons, 2001; Garaventa et al. 2003; Veronesi et al. 2006; Sabichi et al. 2008). The interest in 4-HPR over other retinoids is based on three major advantages: a) unlike vitamin A and many natural derivatives as ATRA, 4-HPR exerts its cell growth inhibitory effect by mainly inducing cell death rather than differentiation (Ponzoni et al. 1995; Kitareewan et al. 1999), b) it can be applied against retinoic acid resistant cancers (Delia et al. 1993; Kitareewan et al. 1999; Reynolds and Lemons 2001), and c) it shows a favorable systemic toxicity profile compared to other retinoids which allows and supports continuity on in vivo studies as well as further application in clinical trials (Rotmensz et al. 1991).
Despite the positive characteristics of 4-HPR, there is still controversy about its mechanism of action (Hail et al. 2006; Kadara et al. 2007; Tiwari et al. 2008). Previous studies from our group and others described a strong proapoptotic effect of 4-HPR on several T-cell acute lymphoblastic leukemia (T-ALL) cell lines (Asumendi et al. 2002; O’Donnell et al. 2002; Faderl et al. 2003). In agreement with data obtained in several cell models (Suzuki et al. 1999; Kim et al. 2006), oxidative stress (i.e. increase on reactive oxygen species -ROS- production) seemed to be a candidate mediator of 4-HPR driven cell death in our leukemia model (Asumendi et al. 2002). Moreover, we found that upon 4-HPR treatment, RAR/RXR independent mitochondrial apoptosis pathway was activated where enhanced ROS production and modulation of sphingolipids levels were the earliest events detected (Morales et al. 2007). While 4-HPR mediated ROS increase is an established event (Suzuki et al. 1999; Asumendi et al. 2002; Kim et al. 2006; Kadara et al. 2007), the relationship between 4-HPR and sphingolipid metabolism has not been fully elucidated (Maurer et al. 1999; Lovat et al. 2004; Rehman et al. 2004; Morales et al. 2007; Darwiche et al. 2007).
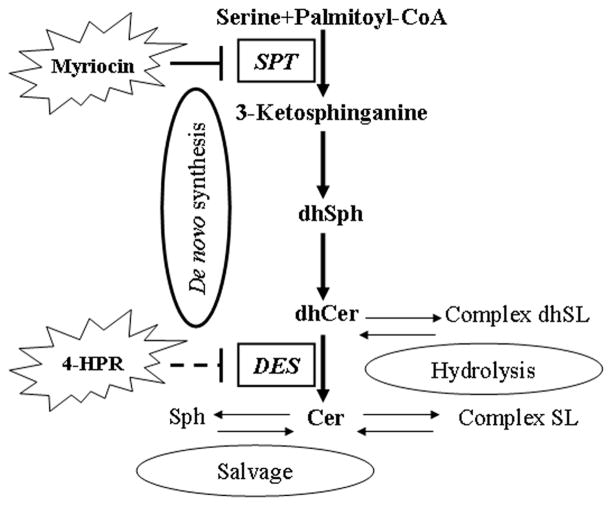
Sphingolipids (SLs) are generally described as sphinganine or sphingosine based lipids that form a large family of molecules with both, structural and signaling functions (Hannun and Obeid 2008). Among them, ceramide (sphingosine-based SL) is currently considered the core of the sphingolipid metabolic network, and much work has been focused on its signaling role in cell death (Obeid et al. 1993; Taha et al. 2006; Carpinteiro et al. 2008). As summarized in Fig. 1, ceramide can be generated by three different pathways i.e. de novo synthesis, sphingomyelin hydrolysis, and the recently described salvage pathway (Hannun and Obeid 2008; Kitatani et al. 2008). Numerous studies have implicated ceramide in 4-HPR mediated cytotoxicity (Maurer et al. 1999; Rehman et al. 2004; Darwiche et al. 2005; Hail et al. 2006; Morales et al. 2007; Jiang et al. 2011) but application of more advance LC/MS (liquid chromatography/mass spectrometry) techniques has recently revealed that 4-HPR induces an increase in dihydroceramide rather than ceramide, at least in some tumor models (Kraveka et al. 2007; Wang et al. 2008; Valsecchi et al. 2010).
Figure 1. General view of sphingolipids synthesis and recycling pathways.
Thicker arrows pointing out the pathway analyzed in the present study. SPT, Serine PalmitoylTransferase; dhSph, dihydrosphingosine (=sphinganine); DES, dihydroceramide desaturase; dhCer, dihydroceramide; Cer, ceramide; Complex dhSL, complex dihydrosphingolipids (including dihydrosphingomyelin, dihydroglucoceramide and derivatives); Complex SL, complex sphingolipids (sphingomyelin, glucoceramide and derivatives); Sph, sphingosine.
The aim of this work was to establish the mechanistic relationship between the 4-HPR-induced oxidative stress, changes in SLs, and cell death. Sphingolipid levels were analyzed by LC/MS technology in the leukemia models previously studied by our group. The analysis revealed accumulation of dihydroceramide (dhCer) (but not ceramide) following 4-HPR treatment. Most importantly, this study defines oxidative stress and dhCer accumulation as two distinct events occurring early after exposure to 4-HPR. On top of that, these data also indicate that -at least in T-ALL cell lines- cell death upon treatment may occur even in the absence of accumulation of dhCer or ROS, indicating that, unlike what was previously proposed, none of these early events are necessarily essential mediators of 4-HPR-mediated cytotoxicity.
MATERIALS AND METHODS
Reagents
RPMI 1640 (#11835-034), red phenol free RPMI 1640 (#11835-063) and heat inactivated fetal bovine serum (#10082-174) were from GIBCO/BRL (Invitrogen). 4- HPR (#H7779), H2O2 (#H1009), myriocin (#M1177) and antioxidants (but baicalein) were purchased from Sigma Chemical Co. (St Louis, MO). 4-HPR was dissolved at 10 mM in DMSO, aliquoted and stored at −80°C. Myriocin (serine-palmitoyl-transference inhibitor) was prepared at 1 mM (in DMSO) and aliquots stored at −20°C. Ascorbic acid, vitamin E, Trolox® and Trolox®-methyl-ether were prepared fresh prior each experiment: vitamin E (#T1539; diluted in ethanol 1:10 prior application), Trolox® ((±)-6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid. #238813; dissolved in culture media to 1mM), Trolox®-methyl-ether (#93510; dissolved in ethanol to 100 mM due to low water solubility) and L-ascorbic acid (#255564; dissolved in water). NDGA (#N5023) stock solution was prepared at 80 mM in DMSO (stored at −20°C). Baicalein (#196322; stock solution in DMSO) and Annexin V-FITC apoptosis Detection Kit (#PF032) were from Calbiochem (San Diego, CA). CM-H2DCFDA (#C6827), BODIPY® 581/591 C11 (D3861)and MitoSOX (#M36008) were obtained from Molecular Probes (Invitrogen, Carlsbad, CA). Cell viability XTT assay was purchased from Roche Molecular Biochemicals, IN, USA (#11465015001). D-erythro- 2-N-[12′-(1″-pyridinium)dodecanoyl]-4,5-dihydrosphingosine bromide (D-erythro-C12- dihydroceramide; C12-dhCCPS) was synthesized in the Lipidomics Core Facility at the Medical University of South Carolina (31). Cells were expose to a final DMSO concentration ≤ 0.1%.
Cell lines and culture conditions
Human CCRF-CEM and Jurkat acute lymphoblastic leukemia cells were grown in RPMI 1640 with 2 mM L-glutamine and supplemented with 10% heat inactivated fetal bovine serum (complete culture medium). Cells were maintained at 37°C in a humidified incubator containing 5% CO2. When required, cells were exposed to 2 h incubation with antioxidants or serine-palmitoyl-transference inhibitor prior adding the drug.
Metabolic activity assay
Standard XTT assay was used to determine cell viability. For 4-HPR-only treatments, cells were plated in 96-well plates at 750,000 cells/ml and 100 μl/well. After 4 h, treatments were added on 50μl/well obtaining a final density of 500,000 cells/ml and final volume of 150 μl/well. Four replicates were used per experimental condition. XTT reagent mixture was added 4 h before the end of selected treatment period and absorbance at 490 nm was determined per each well (according to manufacturer’s instruction). A slightly modified protocol was used for analysis of the effect of myriocin (final concentration of 100 nM) or antioxidant on 4-HPR treatment. Briefly, cells were seeded on 60 mm culture dishes and myriocin or antioxidants added after 4 h. 4-HPR treatment was added 2 h later and cells were plated in quadruplicates in 96 well plates (150 μl/well).
Clonogenic capacity assay
Prior seeding in the semisolid medium, cells were exposed to selected 4-HPR concentrations for 16 h (5×106 cells/sample, approximately 500,000 cells/ml). 16 h time point was selected due to the lack of difference among cells challenged to 16 h and 48 h treatment when compared at 48 h time point (data not shown). 6-well plates were covered by 2 ml/well of pre-warmed 1.2 % methylcellulose medium and a layer of 0.6% methylcellulose medium containing 50,000 cells was placed on top of the 2 ml 1.2 % methylcellulose layer. 6-well plates were maintained at 37°C in a humidified incubator containing 5% CO2 and checked up to a significant colony growth (7–9 days). Clonogenic capacity was determined by counting colonies >50 cells in 3 random areas/well by an inverted Nikon Eclipse TS100 microscope.
Apoptotic cell death
Apoptotic cell death was determined by the annexin V-propidium iodide staining assay according to manufacturer’s RAPID protocol. 10,000 cells/sample were analyzed by Coulter EPICS ELITE ESP flow cytometer. Cells were classified as: a) healthy cells (PI (−) and FITC (−) cells), b) early apoptotic (PI −) and FITC (+) cells), c) late apoptotic/necrotic cells (PI (+) and FITC (+) cell). Results were analyzed by WinMDI 2.8 and Summit v4.3 softwares.
Measurement of general oxidative stress
Intracellular oxidative stress was determined by CM-H2DCFDA. Based on available literature, CM-H2DCFDA and its former versions are able to detect hydrogen peroxide, hydroxyl radicals, peroxyl radicals and peroxynitrite anions. Briefly, cells were seeded in red phenol free RPMI1640-based complete culture media (5×106 cells per treatment). As done for viability assay, myriocin (final concentration of 100 nM) or antioxidants were added after 4 h of incubation. Following 2 h myriocin/antioxidant treatment, 4-HPR was added and cells incubated for selected time points. After treatment, approximately 2×106 cells were resuspended in 0.8 ml PBS containing 10 μM CM-H2DCFDA and incubated for 25 min (37°C, darkness). Samples were transferred to tubes or 96-well plates prior fluorescence measurement. Fluorescence intensity was measured either by plate reader (Fluoroskan Ascent, Labsystems) or by flow cytometer (Coulter EPICS ELITE ESP). Probe-free cells were used as internal negative control and fresh prepared H2O2 (200 μM, 15 min) as positive control.
Mitochondrial superoxide production
Mitochondrial superoxide production was estimated by MitoSOX oxidation. Cells were seeded and treated as specified for the analysis of the intracellular oxidative stress. After treatment, approximately 2×106 cells were resuspended in 0.8 ml Hank’s Buffered Salt Solution containing 5 μM MitoSOX and incubated for 15 min (37°C, darkness). Samples were washed with PBS and transferred to tubes or 96-well plates prior fluorescence measurement. Fluorescence intensity was measured either by plate reader (Fluoroskan Ascent, Labsystems) or by flow cytometer (Coulter EPICS ELITE ESP).
Intracellular lipid peroxidation
This assay was based on the oxidation-mediated fluorescence shift of an externally added undecanoic acid (BODIPY 581/591 C11). Oxidation of the polyunsaturated butadienyl portion of the BODIPY 581/591 dye truncates the conjugated π-electron system, resulting in a shift of the fluorescence emission peak from ~590 nm to ~510 nm.
Briefly, cells were seeded and treated as described for intracellular oxidative stress and mitochondrial superoxide estimation. 3 h incubation (37°C and darkness) was required for the proper internalization of the probe (10 μM BODIPY 581/591 C11) and treatments were added before or after the probe depending on the selected treatment exposure time. BODIPY and treatment-containing culture medium was replaced by 1 ml PBS/sample prior measuring fluorescence. Flow cytometric analysis of lipid peroxidation was performed in the General Research Services SGIker of the UPV/EHU (http://www.ikerkuntza.ehu.es/p273-sgikerhm/en/). 10,000 cells were analyzed per sample.
In situ dihydroceramide desaturase assay
C12-dhCCPS was used as a substrate for the dihydroceramide desaturase enzyme. The synthetic analogue was dissolved in 100% ethanol at a 100 mM stock concentration and stored at −20°C, protected from light. Stock concentration was diluted and added to cells in complete culture medium. Final C12-dhCCPS concentration in medium was 500 nM. When required, C12-dhCCPS was added together with 4-HPR and incubated for the selected time points. Levels of C12-dhCCPS and its product (C12- CCPS) were analyzed by LC/MS as described by Szulc et al. 2006 and Bielawski et al. 2006.
LC/MS analysis of endogenous sphingolipid species
Cells were seeded and treated in 60 mm culture dishes as described above. After treatment, cells were washed twice with PBS to avoid external sphingolipid contamination from media and further analysis were performed in the Lipidomics Core Facility at the Medical University of South Carolina (hcc.musc.edu/research/shared_resources/lipidomics.cfm) as described (Bielawski et al. 2006; Szulc et al. 2006). Total values were normalized to inorganic phosphates by an adapted Bligh and Dyer lipid extraction (Van Veldhoven et al. 1988).
Statistics
Results were represented as the mean ± standard deviation (SD). The number of replicates was specified for each experiment. Statistical analysis was performed by SPSS 15.0 software. Depending on the compared set of data, Student t-test (for pairwise mean comparison) or ANOVA plus Bonferroni/Tamhane post hoc (based on variance homogeneity test) analysis were applied. Statistical significance was settled at 95% (p<0.05) or 99% (p<0.01) as indicated for each figure.
RESULTS
4-HPR cytotoxicity in T-ALL cell lines
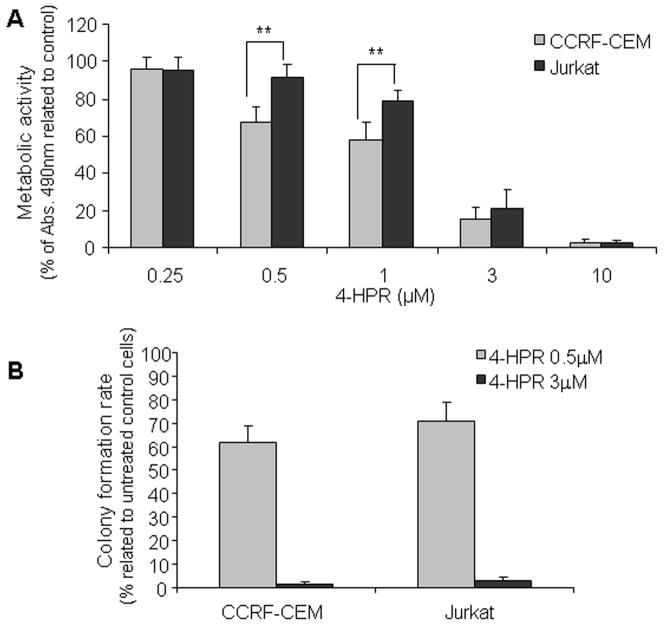
Previous studies demonstrated the strong apoptotic effect of 4-HPR in leukemia cells as well as the dose and time-dependence of the cytotoxicity (Asumendi et al. 2002; Morales et al. 2005). In this study we selected 48 h treatment exposure in order to determine acute toxicity profiles upon a wide range of 4-HPR concentrations while long term toxicity was determined by clonogenic assay. As shown in Fig. 2A, 4-HPR concentrations ≥1 μM induced acute loss of viability in both leukemia cell lines; after 48 h at 3 μM, the number of CCRF-CEM and Jurkat viable cells decreased to 15.3% ± 6.3 and 21.1% ± 10.2, respectively, and cell death was nearly total with 10 μM as corroborated by annexin V-propidium iodide staining (data not shown). Despite observed differences in drug-driven acute toxicity (Fig. 2A), 4- HPR decreased clonogenic capacity in a similar fashion in both cell lines (p>0.05; Fig. 2B), even at concentrations as low as 0.5 μM. Next, we determined the effect of 4-HPR on the cell cycle in order to evaluate cell cycle arrest as a possible mechanism underlying observed decrease of the clonogenic capacity upon treatment. Propidium iodide labeling mediated analysis of the cell cycle following 24–48 h exposure to 4-HPR (0.5 μM) revealed no significant differences among treated and untreated CCRF-CEM and Jurkat cells (data not shown). Thus, 4-HPR exerted not just acute but also long term antitumor activity in selected T-ALL cell lines.
Figure 2. Effect of 4-HPR on cell viability (A) and clonogenic capacity (B).
(A) Cell viability was estimated by means of metabolic activity. Cells were treated with indicated concentration of 4-HPR for 48 h and viability was determined by XTT assay. Values are shown as percentage of untreated control cells ± SD of at least three independent experiments performed in quadruplicates (n ≥ 12). For clonogenic capacity (B) cells were treated in normal culture conditions for 16 h with indicated 4-HPR concentrations. After 16 h, treatment was removed and cells seeded on semisolid methylcellulose medium as mentioned in Materials and Methods. Colonies were counted upon 7–9 days. As internal assay controls, cells for clonogenic assay were also subjected to 16 h viability estimation (XTT) and apoptosis (Annexin V-PI) determination (data not shown). Data represent average of colony-number percentage related to untreated cells ± SD of three independent experiments. **p<0.01
Dihydroceramide desaturase (DES) activity and endogenous dihydroceramide levels upon 4-HPR treatment
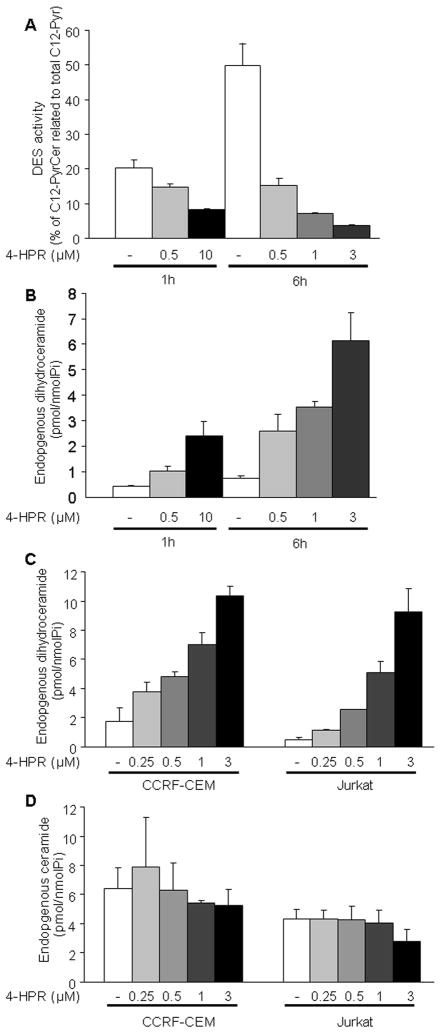
The effect of 4-HPR on DES was described by Kraveka and colleagues (2007) in neuroblastoma cells. As shown in Fig. 3A, 4-HPR inhibited DES activity in CCRF-CEM leukemia cells in a dose (p<0.01) and time (p<0.05) dependent manner, leading to a concomitant increase of the endogenous cellular dhCer content (Fig. 3B). Interestingly enough, DES inhibition occurred within the first hour of treatment at 4-HPR concentrations as low as 0.5 μM and was sustained as shown by the persistent effect on the in situ conversion of C12-PyrdhCer to C12-PyrCer during 4-HPR exposure (i.e. 14.8±0.6 after 1 h vs. 15.3±1.9 after 6 h exposure to 4-HPR; Fig. 3A). LC-MS analysis of endogenous SLs revealed a similar pattern of dhCer accumulation in both CCRF-CEM and Jurkat cells (Fig. 3C) at both, sub-lethal and cytotoxic 4-HPR concentrations. Of note, we also observed an increase in the level of endogenous dihydrosphingosine (dhSph; also known as sphinganine. Supplementary Figure 2A) in CCRF-CEM cells at 4-HPR concentrations ≥ 1 μM. Similar dhSph accumulation required higher 4-HPR concentrations (6 h, 10 μM; data not shown) in Jurkat cells. Under the same conditions, a slight accumulation of sphingosine (Sph) was also observed in CCRF-CEM cells (Supplementary Figure 3A) but not in Jurkat cells (data not shown). Moreover, no increase of endogenous ceramide (Cer) levels was observed in any of the tested cell lines and conditions (Fig. 3D), consistent with recent studies (Kraveka et al. 2007). These data demonstrated that in our leukemia model, 4-HPR driven rapid inhibition of cellular DES is the likeliest source for observed accumulation of endogenous dhCer. Of note, the increase in dhCer levels occurred even at 4-HPR concentrations below those required for the acute toxicity.
Figure 3. Effect of 4-HPR on dihydroceramide desaturase (DES) activity and endogenous dihydroceramide/ceramide levels.
CCRF-CEM cells were incubated 1 h with the synthetic dihydroceramide analogue (C12-PyrdhCer or C12-dhCPPS) prior to adding indicated 4-HPR concentrations. Cells were collected after 1 h or 6 h 4-HPR exposure. Synthetic (A) and endogenous (B) sphingolipids were measured by LC/MS. DES activity was estimated as % of desaturated product (C12-PyrCer) related to total C12-Pyr (C12-PyrdhCer plus C12-PyrCer) in cells. Dose dependent effect of 4-HPR on endogenous dihydroceramide (dhCer) and ceramide (Cer) levels are represented in figures C and D. Cells were treated with low (0.25–0.5 μM) and moderate (1–3 μM) 4- HPR concentrations and endogenous total dihydroceramide (C) and ceramide (D) levels measured by LC/MS upon 6 h exposure. Data represent mean ± SD of two independent experiments performed in duplicates.
Role of endogenous dhCer in 4-HPR-induced oxidative stress and cytotoxicity
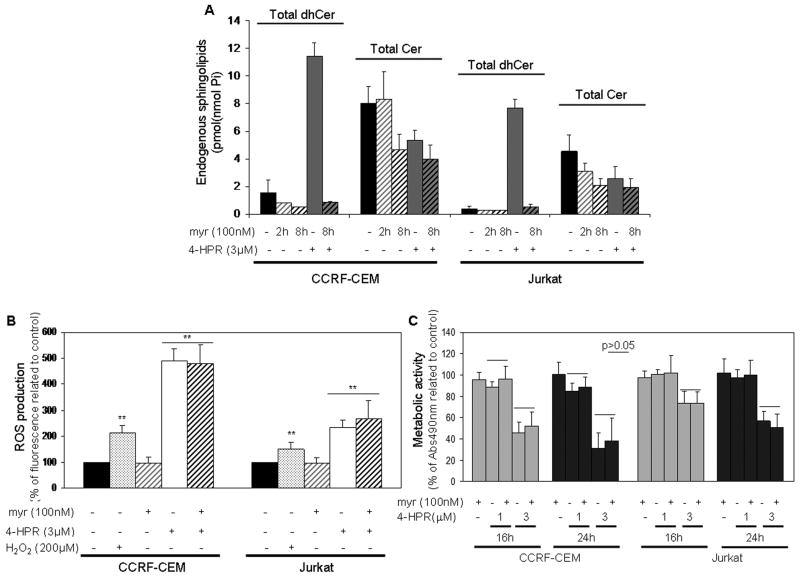
Preincubation with the SPT inhibitor myriocin (myr) was performed in order to evaluate the role of dhCer accumulation in 4-HPR mediated toxicity and ROS production. For this set of experiments, 4-HPR concentration and treatment exposure were established based on the high lethality observed with 3 μM 4-HPR following 48 h incubation (Fig. 2A), and the robust dhCer accumulation obtained after 6 h exposure to 4-HPR (Fig. 3B– C). As shown in Fig. 4A, myriocin (100 nM) effectively blocked de novo SL synthesis pathway and completely abolished the 4-HPR (3 μM)-induced dhCer accumulation in both CCRF-CEM and Jurkat cells (4-HPR vs. myr+4-HPR) as well as previously described dhSph and Sph accumulations (Supplementary Figure 2B and Supplementary Figure 3B.1-B.2, respectively). As expected, inhibition of de novo synthesis pathway decreased endogenous Cer levels in both cell lines; no difference in Cer content was observed in the 4-HPR-treated and untreated cells (Fig. 4A).
Figure 4. Role of the de novo sphingolipid synthesis in 4-HPR mediated ROS increase and cell death.
Cells were treated with 100 nM myriocin (myr) for 2 h, followed by 4-HPR treatment. Cellular levels of dihydroceramide and ceramide were measured by LC/MS upon 6 h 4-HPR (3 μM) treatment (A); general ROS production was determined by CM-H2DCFDA after 30 min 4-HPR (3 μM) exposure (B) and cellular viability was estimated by XTT assay (upon 16 h and 24 h exposure; C). Values are presented as mean ± SD of two independent experiments (A) or percentage related to corresponding control cells ± SD of at least three independent experiments performed in quadruplicates (n ≥ 12; B–C). 15 min H2O2 (200 μM) exposure was used as internal positive control for ROS production (B). **p<0.01; p>0.05 represents lack of significant differences.
Next, the role of 4-HPR-mediated dhCer increase in the observed oxidative stress induction (Supplementary Figure 1A–B) was evaluated by employing myriocin followed by 30 min 4-HPR treatment. As shown in Fig. 4B, 30 min treatment with 3 μM 4-HPR was enough to induce 4.91 fold ± 0.44 (CCRF-CEM) and 2.35 fold ± 0.27 (Jurkat) increase in ROS production relative to untreated control cells, while 10 μM 4- HPR induced an even higher ROS accumulation (data not shown). No difference was observed between untreated control and myriocin treated cells (p>0.05), and myriocin pretreatment did not prevent 4-HPR induced oxidative stress in any of the 4-HPR concentrations or cell lines (4-HPR vs. myr+4-HPR; p>0.05). 15 min H2O2 exposure was chosen as internal positive control for ROS detection. Thus, inhibition of accumulation of dhCer and free sphingoid bases did not affect the production of ROS, negating a role for dhCer and free sphingoid bases in the ROS response to 4-HPR.
We next analyzed the effect of myriocin on 4-HPR-induced acute loss of viability upon 16–24 h exposure in order to assess any early or transient effect of myriocin on 4-HPR- treatment. Myriocin treatment did not prevent 4-HPR-mediated cytotoxicity in either cell line (CCRF-CEM, Jurkat), and any time point (16 h, 24 h) or 4-HPR concentration (1 μM, 3 μM) (p>0.05; Fig. 4C). Therefore, the results suggest that observed 4-HPR-induced dhCer, dhSph, and Sph do not mediate acute cell toxicity.
Effect of radical scavengers on 4-HPR-driven dhCer accumulation and antitumoral activity
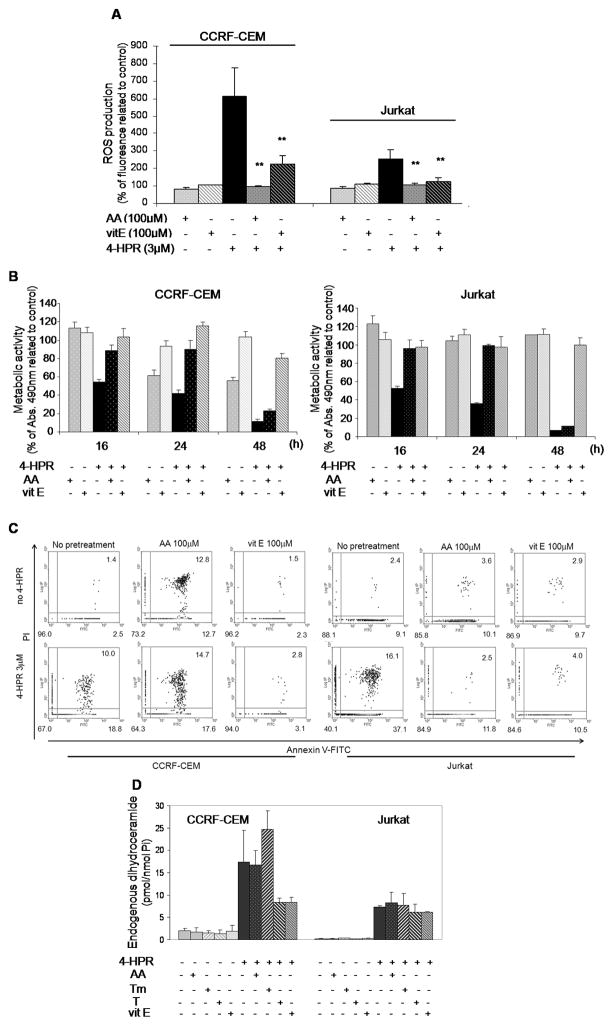
As shown in Fig. 5A, ascorbic acid (AA) and (+)-α-tocopherol (vitamin E, vitE) significantly decreased 4-HPR (3 μM) triggered increase in ROS production in both cell lines (p<0.01) although complete inhibition was achieved only with ascorbic acid. Despite partial ROS buffering capacity, vitamin E provided a sustained capacity to abolish 4-HPR-mediated cell death as evaluated by both metabolic activity (Fig. 5B) and annexin V-propidium iodide staining (Fig. 5C) measurements. Protection by ascorbic acid, on the other hand, lasted no longer than 24 h in either cell line (Fig. 5B).
Figure 5. Effect of antioxidants on 4-HPR-mediated cytotoxicity and dihydroceramide accumulation.
CCRF-CEM and Jurkat cells were incubated 2 h with 100μM ascorbic acid (AA) or vitamin E (vitE) prior to 3 μM 4-HPR treatment. Effect of radical scavengers on ROS upon 4-HPR (30 min) exposure was measured by CM- H2DCFDA oxidation (A). Values are presented as percentage of corresponding control cells ± SD of three independent experiments performed in quadruplicates (n = 12). **p<0.01, related to cells exposed to 4-HPR (3 μM). Cell viability following 4- HPR±antioxidants was determined by XTT assay (B) and apoptotic cell death (C) estimated by annexin V-FITC/propidium iodide (PI) labeling. Data representative of two independent experiments. Endogenous total dihydroceramide levels (D) were measured by LC/MS as described in Materials and Methods. Data represent mean ± SD of two independent experiments.
Next, the effects of antioxidants on endogenous dhCer and Cer levels were determined by LC/MS. Trolox (T; synthetic derivative of vitamin E) and Trolox-methylether (Tm; modified Trolox molecule without antioxidant activity) were tested together with the previously mentioned ascorbic acid and vitamin E. Cells were subjected to 2 h preincubation with antioxidants prior to adding 4-HPR (3 μM) as performed for the analysis of oxidative stress and cytotoxicity. Regardless of their capacity to buffer 4-HPR-induced ROS increase or cell death, antioxidants were able neither to block endogenous dhCer accumulation (Fig. 5D), nor to substantially modify Cer levels (data not shown) upon 4-HPR treatment. Of note, Trolox but not Trolox-methylether rescued cells from 4-HPR-induced death (data not shown). Thus, we concluded that cellular ROS increase was not a direct modulator of endogenous dhCer levels. On the other hand, clear discrepancies emerged among the selected compounds between their ROS buffering capacity and their activity against 4-HPR-mediated cytotoxicity.
Role of reactive oxygen species in the antitumoral activity of 4-HPR
As mentioned above, prevention from 4-HPR-mediated ROS accumulation was significant but partial with vitamin E (Fig. 5A) while this antioxidant showed sustained capacity to block cell death induced by 4-HPR (Fig. 5B–C). Of note, opposite results were obtained with ascorbic acid (Fig. A–C). CM-H2DCFDA mediated analysis of ROS provides information regarding several types of reactive species including hydrogen peroxide and peroxyl radicals. Nevertheless, a more detailed study of the contribution of specific reactive species required the utilization of alternative approaches.
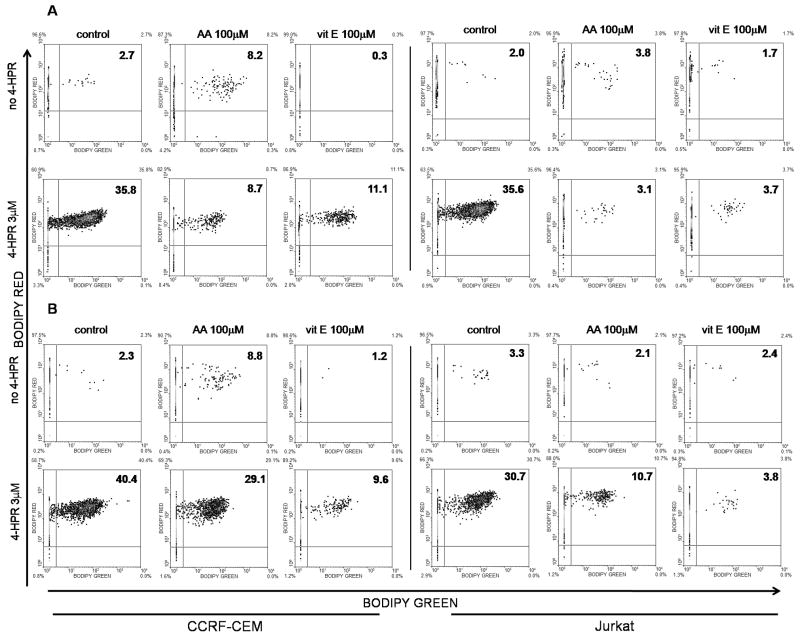
One of the distinctive characteristics of vitamin E (α tocopherol in this study) is that due to its hydrophobic nature, vitamin E is especially potent against oxidative damage to lipidic environments as it plays a role as a peroxyl radical scavenger that terminates chain reactions (Traber & Atkinson, 2007). Of note, lipoxygenase-mediated reactions are an important source of peroxyl radicals (Kühn & Borchert, 2002). Lipoxygenases (LOX), and more specifically 12-LOX have been implicated on 4-HPR-driven cell death in neuroblastoma cells (Lovat et al., 2002). Therefore, we analyzed the capacity of 4-HPR to induce lipid peroxidation as well as the capacity of vitamin E and ascorbic acid to modulate the mentioned reactive species. 4-HPR treatment generated a clear dose and time-dependent increase on lipid peroxidation on both cell lines (Supplementary Figure 1E). As shown in Fig. 6A, ascorbic acid and vitamin E were both able to prevent 4-HPR-(6 h) mediated lipid peroxidation. Nonetheless, only vitamin E retained the capacity to buffer lipid peroxidation after 24 h 4-HPR exposure (Fig. 6B). These data suggested a role for lipid peroxides in the cytotoxicity driven by 4-HPR.
Figure 6. Effect of ascorbic acid and vitamin E on lipid peroxidation following 4-HPR treatment.
CCRF-CEM and Jurkat cells were incubated for 2 h with 100 μM of ascorbic acid (AA) or vitamin E (vit E) and co-incubated with 4-HPR (3 μM) for another 6 h (A) or 24 h (B). Lipid peroxidation was determined by the exogenously added fluorescent undecanoic acid (BODIPY 581/591 C11) as determined in Materials and Methods. Data on 24 h 4-HPR exposure are representative of 2 independent experiments.
To further explore the possible implication of lipoxygenases in cell death, we selected two inhibitors of lipoxygenases: baicalein (12-LOX inhibitor) and nordihydroguaiaretic acid (NDGA) (dose-dependent inhibitor of 5-, 12- and 15-LOX). Based on literature, at least 5-LOX is expressed in the selected leukemia model (Jurkat cells) (Cook-Moreau et al. 2007).
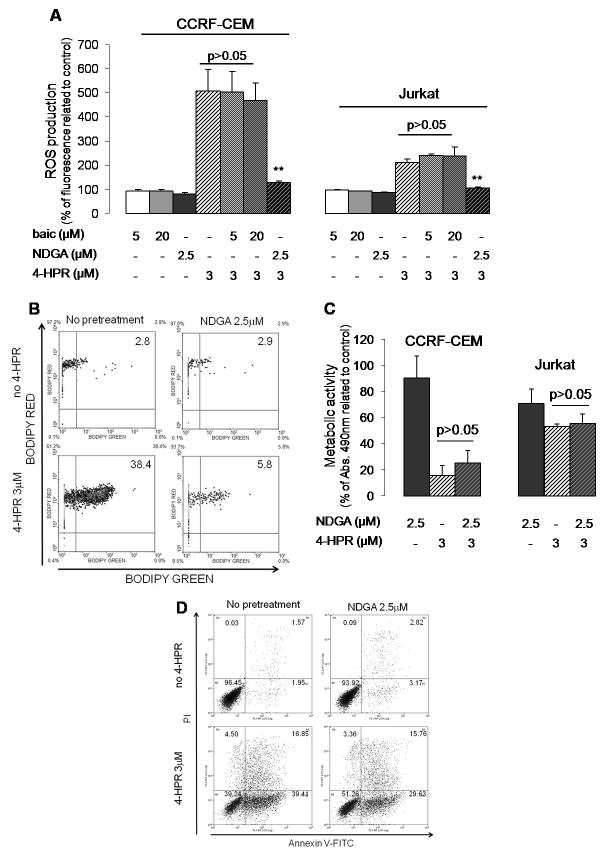
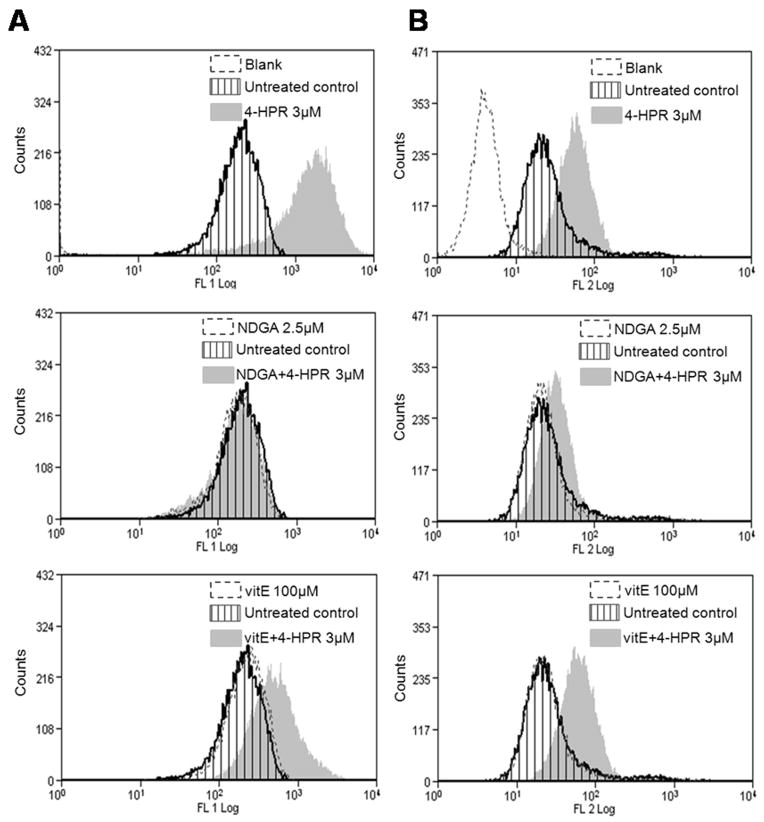
As shown in Fig. 7A, NDGA (but not baicalein) was able to effectively block general oxidative stress (i.e. CM-H2DCFDA oxidation) driven by 4-HPR (3 μM) in both cell lines. In particular, NDGA (but not baicalein) was able to protect from 24 h 4-HPR induced lipid peroxidation (Fig. 7B) as observed with vitamin E (Fig. 6B). Nevertheless, NDGA (as well as baicalein; data not shown) did not prevent either CCRF-CEM or Jurkat cells from dying upon 4-HPR exposure (Fig. 7C–D). Thus, these data suggest a role for lipoxygenases (but not 12-LOX) in 4-HPR-mediated oxidative environment but not in mediating the cytotoxic responses.
Figure 7. Effect of lypoxygenase (LOX) inhibitors on 4-HPR-driven oxidative stress and acute cytotoxicity.
T-ALL cells were incubated for 2 h with indicated LOX inhibitors (baic=baicalein, 12-LOX inhibitor; NDGA, dose dependent 5-LOX>12-LOX, 15-LOX inhibitor) or vitamin E (vitE, 100 μM) prior adding 4-HPR (3 μM). Cellular oxidative stress status (A) was estimated by CM-H2DCFDA oxidation after 30 min 4- HPR treatment while lipid peroxidation (B) was analyzed by BODIPY 581/591 C11 in CCRF-CEM cells after 24 h exposure to 4-HPR. Cytotoxicity of selected treatments was determined by XTT assay (C) and annexin V-FITC/propidium iodide (PI) labeling (D; CCRF-CEM cells) following 24 h 4-HPR exposure. ROS and viability data were related to their corresponding control values. (A, C) Data represent mean±SD of three independent experiments performed in quadruplicates (n=12). **p<0.01 (related to 4-HPR-treated cells); p>0.05 represents lack of statistical difference among indicated groups. (B, D) Representative data of two independent experiments.
In a previous study, we identified mitochondria as an important source of ROS upon 4-HPR treatment in our leukemia model (Asumendi et al., 2002). According to available data, the selected probe for general oxidative stress detection (CM- H2DCFDA) is not specific for mitochondrial superoxide detection. Consequently, the contribution of superoxide would have been ignored and possibly underestimated in the previous assays. At this point, we wondered whether discrepancies among vitamin E and NDGA-mediated antiapoptotic activity could be related to their capacity to buffer mitochondrial superoxide species induced by 4-HPR. To address this question, we performed a side by side analysis of the effect of both compounds on 4-HPR-driven mitochondrial superoxide production. 2 h 4-HPR exposure was selected based on the superoxide production peak observed on CCRF-CEM and Jurkat cells at this time point (Supplementary Fig. 1C–D). As observed upon 30 min 4-HPR exposure (Fig. 5A, 7A) the capacity of NDGA to protect from general oxidative stress (i.e. CM-H2DCFDA oxidation) was sustained and higher than the one exerted by vitamin E (Fig. 8A) even after 6 h 4-HPR treatment (data not shown). Moreover, the data revealed almost complete prevention of 4-HPR-triggered mitochondrial superoxide production with NDGA but a partial or null effect with vitamin E (Fig. 8B). Therefore we conclude that NDGA exerts a more potent antioxidant activity than vitamin E against mitochondrial superoxide accumulation after 4-HPR treatment.
Figure 8. Side by side comparison of the antioxidant capacity of vitamin E and NDGA against general and mitochondrial increase of ROS.
CCRF-CEM cells were incubated for 2 h with indicated concentrations of vitamin E (vitE) or NDGA and 4-HPR added for additional 2 h incubation in presence of antioxidants. General oxidative status (A) was estimated by CM-H2DCFDA while mitochondrial superoxide production (B) was determined by MitoSOX as described in Materials and Methods. Representative data of 2 (A) or 3 (B) independent experiments.
DISCUSSION
The goal of the current study was to establish the mechanistic relationship between the two earliest events detected after 4-HPR treatment: the increase in the production of reactive oxygen species and the changes in the endogenous profiles of SLs. We also wanted to determine the implication of those two early events in the strong apoptotic effect of 4-HPR in selected T-cells Acute Lymphoblastic Leukemia (TALL) cell lines (CCRF-CEM, Jurkat).
4-HPR has been described to regulate multiple molecular pathways (Lovat et al., 2004; Hail et al., 2006; Tiwari et al. 2006; Kadara et al. 2007) and to induce several cellular processes, including well characterized apoptosis (Delia et al. 1993; Wu et al. 2001; Lovat et al. 2004; Hail et al. 2006), differentiation (Chen et al. 2003) and autophagy (Tiwari et al. 2008). The complexity and abundance of cellular networks affected by 4-HPR makes difficult the identification of key events related to each of the altered cellular processes.
Focused on leukemia, a study by Delia and colleagues (1995) was the first one suggesting a role for oxidative stress in cell death upon 4-HPR treatment. This work also described the capacity of Bcl-2 to delay or to decrease the apoptotic effect of the retinoid also observed in later studies by our group (Morales et al., 2007). Ceramide -mediated cytotoxicity in leukemia cells was first proposed by O’Donnell and colleagues (2002). Later studies have further evaluated the mechanisms following 4-HPR-mediated increase in ROS or ceramide. Mechanistically, several studies describe ROS-mediated cleavage of PKC σ and downstream inactivation of Mcl-1 (Kang et al., 2008; Ruvolo et al., 2010), ROS-mediated dephosphorylation of Akt (Cao et al., 2009) or endoplasmic reticulum stress and proteasome activation (Wang et al., 2009) as important events leading to leukemia cell death. Nevertheless, none of these studied the possibility for antitumor activity in the absence of alterations on SLs and ROS production.
In previous studies, our group demonstrated that in T-ALL cell lines 4-HPR induces intrinsic mitochondrial apoptotic cell death (Asumendi et al. 2002) and that acute cell death was characterized by an early increase on ROS and ceramide levels (Morales et al. 2007). In the present work, additional data prove that 4-HPR exerts therapeutically meaningful antitumor activity (i.e. reduction of clonogenic capacity) at concentrations as low as 0.5 μM which is not necessarily linked to acute toxicity as observed in data obtained from Jurkat cells. Therefore, the data support the potential application of fenretinide as chemotherapeutic agent.
Sphingolipids (especially ceramide and gangliosides) have been largely linked to 4-HPR-driven cytotoxicity (Maurer et al. 1999; Wu et al. 2001; Lovat et al. 2004; Rehman et al. 2004; Darwiche et al. 2005; Hail et al. 2006; Morales et al. 2007). Previous studies based on thin layer chromatography (TLC) pointed out an increase in endogenous ceramide as a major response in 4-HPR mediated cell death (Maurer et al. 1999; Chen et al. 2003; Rehman et al. 2004; Hail et al. 2006; Morales et al. 2007; Tiwari et al. 2008). 4-HPR has been suggested to regulate endogenous ceramide (Cer) levels by activating serine palmitoyltransferase-ceramide synthase (first enzymes of de novo synthesis pathway) (Wang et al. 2001) or acidic-sphingomyelinase (Lovat et al. 2004). Recent studies that applied LC/MS technology provided much more accurate detection of different SL species and revealed a robust accumulation of dhCer (but not Cer) in several cell lines, following 4-HPR treatment (Zheng et al. 2006; Kraveka et al. 2007; Valsecchi et al. 2010). Concomitant accumulation of dhSph and dhCer has been also recently described upon 4-HPR treatment (Wang et al., 2008). Results from the in situ activity assay and LC/MS analysis in our leukemia model demonstrated the rapid inhibitory effect of 4-HPR on cellular DES activity and the resulting time- and dose-dependent dhCer and, to a smaller extent, dhSph and Sph accumulation, in agreement with data obtained by previously mentioned. Similarly, a recent study by Rahmaniyan and colleagues (2011) identified DES as a direct in vitro target of fenretinide.
Despite the large number of studies linking Cer increase to 4-HPR-driven cell death, the current data regarding 4-HPR on SL modulation raises the question about the real implication of SL on observed biological effects. The fact that myriocin inhibited the accumulation of dhCer and free sphingoid bases but did not have any effect on 4-HPR-induced ROS production indicates that in our leukemia model SL modulation is not upstream of observed ROS increase. Moreover, myriocin did not inhibit 4-HPR induced death. Hence, myriocin-based data prove that described accumulation of SLs is not necessarily involved in the observed acute cytotoxicity. Nonetheless, Kraveka and colleagues (2007) linked inhibition of DES to cell cycle arrest. Data presented herein indicate that 4-HPR reduces clonogenic capacity in conditions of significant dhCer accumulation but absence of acute cytotoxicity. Therefore, a role for dhCer not in acute cytotoxicity but in proliferation capacity could be feasible although our data suggest the involvement of a mechanism distinct from cell cycle arrest.
Similarly to (dh)Cer accumulation, induction of oxidative stress has been often described as an essential mediator in 4-HPR treatment (Suzuki et al. 1999; Asumendi et al. 2002; Kim et al. 2006; Kadara et al. 2007; Jiang et al. 2011) while the possible cause-effect relationship among 4-HPR modulated ROS and SLs profile has been often discussed (Maurer et al. 1999; Wu et al. 2001; Lovat et al. 2004; Hail et al. 2006; Darwiche et al. 2007; Morales et al. 2007). Rehman and colleagues (2004) described that the effect of 4-HPR on (dh)Cer could be partially reverted by N-acetyl-L-cysteine while Batra and colleagues (2004) did not observe differences between 4-HPR-only and 4-HPR+N-acetyl-L-cysteine treated cell. On the other hand, we have recently shown that oxidative stress inhibits DES activity by an indirect mechanism (Idkowiak-Baldys et al. 2010). As mentioned above, in this study, the effect of myriocin on 4-HPR treatment indicates that ROS production is not downstream of dhCer accumulation in cells. Moreover, results of antioxidants on 4-HPR-mediated SL modulation reveal that endogenous dhCer levels are not regulated (at least directly) by cellular ROS levels; ROS production and DES inhibition may be therefore considered distinct events upon 4-HPR exposure.
Upon detailed analysis of studies focused on 4-HPR triggered oxidative stress, it becomes apparent that the sources and function of ROS in 4-HPR-exerted antitumoral activity are not well defined. 12-LOX (Lovat et al. 2003), NADPH oxidase (Kadara et al. 2008) and mitochondria (Suzuki et al. 1999; Asumendi et al. 2002; Kim et al. 2006; Darwiche et al. 2007; Cuperus et al., 2010) have been proposed as origins for ROS following 4-HPR treatment. In this study we performed an in depth analysis of different types of ROS that brought to light clear discrepancies among the capacity to buffer 4-HPR-mediated oxidative stress and the capacity to protect from 4-HPR-driven cell death. Ascorbic acid, despite being able to completely block early oxidative environment, offered only a transient protection against cell death following 4-HPR treatment. This could be linked to its inability to prevent from later oxidative events. Vitamin E, on the other hand, exerted a sustained protection from lipid peroxidation and limited capacity against mitochondrial superoxide but effectively protected from cytotoxicity mediated by 4-HPR. On the other hand, the lipoxygenase inhibitor NDGA (Salari et al., 1984) demonstrated a clear and sustained antioxidant capacity against all the tested reactive species but had no effect on the cytotoxic response. By contrast, baicalein was neither able to block ROS nor to prevent from 4-HPR-driven leukemia cell death. All together, present data indicate that lipoxygenases (but not 12-LOX) could be the source of the detected increase on ROS. Nevertheless, direct radical scavenger activity by NDGA cannot be excluded as it has been described as a potent in vitro scavenger of a broad range of reactive radical species (Floriano-Sánchez et al. 2006). However, despite its significant capacity to buffer ROS, NDGA was clearly incapable to rescue leukemia cells from 4-HPR-induced cell death. In this regard, Cuperus and colleagues (2010) also observed that Trolox could just partially prevent from 4-HPR-driven loss of viability at conditions previously described to abrogate ROS production (Cuperus et al. 2008). Moreover, ATP production was also decreased in cells with no significant increase on mitochondrial or general ROS production (e.g. FISK neuroblastoma cells) (Cuperus et al. 2010). These observations point out the fact that 4-HPR-driven acute cytotoxicity may occur even in the absence of ROS production, against the generalized idea.
In conclusion, this study provides a clear differentiation between two major early events after 4-HPR treatment, increase in ROS and modulation of endogenous SL levels. It also offers a new view regarding contribution of ROS and SL to the antitumor effect of 4-HPR. We have shown that 4-HPR induces a rapid DES inhibition resulting in a strong dhCer accumulation which is independent of the increase in cellular ROS. Moreover, we have shown that 4-HPR-mediated acute cytotoxicity may occur even in the absence of dhCer or ROS accumulation. Data on dhSph and Sph also keep them apart from 4-HPR-induced ROS or acute cell death in selected leukemia cell models. Characterization of tumor cell death-driving and non driving events following 4-HPR treatment is necessary in order to plan successful therapies based on drug combination or to understand most likely sources for treatment failure.
Supplementary Material
CCRF-CEM (A, C, E) and Jurkat (B, D, E) cells were exposed to indicated concentrations of 4-HPR. General oxidative stress (A, B), mitochondrial superoxide production (C, D) and lipid peroxidation (E) was determined as described in Materials and Methods. (A–D) Data represent mean±SD of three independent experiments performed in quadruplicates (n=12). (E) Single analysis. **p<0.01 and *p<0.05 (related to untreated cells). (A–C) Statistical significance obtained with all the 4-HPR concentrations. (D) Statistical significance obtained just by 3 μM 4-HPR treatment.
CCRF-CEM and Jurkat cells were exposed for 6 h to indicated concentrations of 4-HPR (A) and endogenous dhSph content determined by LC/MS as described in Materials and Methods. Data represent mean±SD of two independent experiments. In order to evaluate the role of the dhSph accumulation on 4-HPR-mediated ROS production and cell death, cells were incubated for 2 h with the SPT inhibitor myriocin (myr) and 4-HPR added for an additional incubation of 6 h (B). Samples for further analysis by LC/MS were collected at indicated time points. Data represent mean±SD of two independent experiments.
CCRF-CEM cells were exposed for 6 h to indicated concentrations of 4-HPR (A) and endogenous Sph content determined by LC/MS as described in Materials and Methods. Data represent mean±SD of two independent experiments. In order to evaluate the role of the Sph accumulation on 4-HPR-mediated ROS production and cell death, cells were incubated for 2 h with the SPT inhibitor myriocin (myr) and 4-HPR added for an additional incubation of 6 h (B.1 and B.2). Samples for further analysis by LC/MS were collected at indicated time points. Figures represent data from individual experiments.
Acknowledgments
Financial support:
FPU Grant ref. AP-2004-6497 from the Spanish Ministry of Science and Innovation
NIH Grant ref. CA97132
Grant ref. IT423-07 from the Basque Government
This work was conducted in the Lipidomics Core Facility at MUSC that is supported by the National Institutes of Health, Grant Number C06 RR018823 from the Extramural Research Facilities Program of the National Center for Research Resources. The work was also partly supported by NIH grant CA087584. Also technical and human support provided by SGIker (UPV/EHU, MICINN, GV/EJ, ERDF, and ESF) is gratefully acknowledged.
ABBREVIATIONS
- 4-HPR
N-(4-hydroxyphenyl)retinamide
- AA
L-Ascorbic Acid
- ATRA
All-Trans Retinoic Acid
- baic
baicalein, 5,6,7-Trihydroxyflavone
- C12-dhCCPS
D-erythro-2-N-[12_-(1_- D-erythro-2-N-[12_-(1_-pyridinium)dodecanoyl]-4,5-dihydrosphingosinebromide; D-erythro-C12-dihydroceramide
- Cer
ceramide
- DES
dihydroceramide desaturase
- dhCer
dihydroceramide
- dhSph
dihydrosphingosine
- dhSL
dihydrosphingolipids
- ER
Endoplasmic Reticulum
- LC/MS
Liquid Chromatography/Mass Spectrometry
- myr
myriocin
- NDGA
Nordihydroguaiaretic acid
- RAR/RXR
Retinoic Acid Receptor
- ROS
Reactive Oxygen Species
- SD
Standard Deviation
- SL
sphingolipid
- Sph
sphingosine
- SPT
Serine-Palmitoyl Transferance
- T:Trolox®
(±)-6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid
- T-ALL
T-cell Acute Lymphoblastic Leukemia
- TLC
Thin Layer Chromatography
- Tm
Trolox methylether® VitE, vitamin E
References
- Asumendi A, Morales MC, Alvarez A, Aréchaga J, Pérez-Yarza G. Implication of mitochondria-derivated ROS and cardiolipin peroxidation in N-(4-hydroxyphenyl) retinamide-induced apoptosis. Br J Cancer. 2002;86(12):1951–56. doi: 10.1038/sj.bjc.6600356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson J, Epand RF, Epand RM. Tocopherols and tocotrienols in membranes: A critical review. Free Rad Biol Med. 2008;44(5):739–64. doi: 10.1016/j.freeradbiomed.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Batra S, Reynolds CP, Maurer BJ. Fenretinide cytotoxicity for Ewing’s sarcoma and primitive neuroectodermal tumor cell lines is decreased by hypoxia and synergistically enhanced by ceramide modulators. Cancer Res. 2004;64(15):5415–24. doi: 10.1158/0008-5472.CAN-04-0377. [DOI] [PubMed] [Google Scholar]
- Bielawski J, Szulc ZM, Hannun YA, Bielawska A. Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods. 2006;39(2):82–91. doi: 10.1016/j.ymeth.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Cao J, Xu D, Wang D, Wu R, Zhang L, Zhu H, He Q, Yang B. ROS-driven Akt dephosphorylation at Ser-473 is involved in 4-HPR-mediated apoptosis in NB4 cells. Free Radic Biol Med. 2009;47(5):536–47. doi: 10.1016/j.freeradbiomed.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Carpinteiro A, Dumitru C, Schenck M, Gulbins E. Ceramide-induced cell death in malignant cells. Cancer Lett. 2008;264(1):1–10. doi: 10.1016/j.canlet.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Chen S, Samuel W, Fariss RN, Duncan T, Kutty RK, Wiggert B. Differentiation of human retinal pigment epithelial cells into neuronal phenotype by N-(4-hydroxyphenyl)retinamide. J Neurochem. 2003;84(5):972–81. doi: 10.1046/j.1471-4159.2003.01608.x. [DOI] [PubMed] [Google Scholar]
- Cook-Moreau JM, El-Makhour Hojeij Y, Barrière G, Rabinovitch-Chable HC, Faucher KS, Sturtz FG, et al. Expression of 5-lipoxygenase (5-LOX) in T lymphocytes. Immunology. 2007;122(2):157–66. doi: 10.1111/j.1365-2567.2007.02621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuperus R, Tytgat GA, Leen R, Brites P, Bras J, Caron HN, et al. Pleiotropic effects of fenretinide in neuroblastoma cell lines and multicellular tumor spheroids. Int J Oncol. 2008;32(5):1011–9. [PubMed] [Google Scholar]
- Cuperus R, Leen R, Tytgat GA, Caron HN, van Kuilenburg AB. Fenretinide induces mitochondrial ROS and inhibits the mitochondrial respiratory chain in neuroblastoma. Cell Mol Life Sci. 2010;67(5):807–16. doi: 10.1007/s00018-009-0212-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwiche N, Abou-Lteif G, Najdi T, Kozhaya L, Abou Tayyoun A, et al. Human T-cell lymphopropic virus type I-transformed T-cells have a partial defect in ceramide synthesis in response to N-(4-hydroxyphenyl)-retinamide. Biochem J. 2005;392(Pt 1):231–39. doi: 10.1042/BJ20050578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwiche N, Abou-Lteif G, Bazarbachi A. Reactive oxygen species mediate N-(4-hydroxyphenyl)retinamide-induced cell death in malignant T cells and are inhibited by the HTLV-1 oncoprotein Tax. Leukemia. 2007;21(2):261–69. doi: 10.1038/sj.leu.2404472. [DOI] [PubMed] [Google Scholar]
- Das A, Banik NL, Ray SK. N-(4-hydroxyphenyl)retinamide induced both differentiation and apoptosis in human glioblastoma T98G and U87MG cells. Brain Res. 2008;1227:207–15. doi: 10.1016/j.brainres.2008.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delia D, Aiello A, Lombardi L, Pelicci PG, Grignani F, Grignani F, et al. N-(4-hydroxyphenyl)retinamide induces apoptosis of malignant hemopoietic cell lines including those unresponsive to retinoic acid. Cancer Res. 1993;53(24):6036–41. [PubMed] [Google Scholar]
- Delia D, Aiello A, Formelli F, Fontanella E, Costa A, Miyashita T, Reed JC, Pierotti MA. Regulation of apoptosis induced by the retinoid N-(4-hydroxyphenyl) retinamide and effect of deregulated bcl-2. Blood. 1995;85(2):359–67. [PubMed] [Google Scholar]
- Faderl S, Lotan R, Kantarjian HM, Harris D, Van Q, Estrov Z. N-(4-Hydroxylphenyl)retinamide (fenretinide, 4-HPR), a retinoid compound with antileukemic and proapoptotic activity in acute lymphoblastic leukemia (ALL) Leuk Res. 2003;27(3):259–66. doi: 10.1016/s0145-2126(02)00162-5. [DOI] [PubMed] [Google Scholar]
- Floriano-Sánchez E, Villanueva C, Medina-Campos ON, Rocha D, Sánchez-González DJ, Cárdenas-Rodríguez N, et al. 2 acid is a potent in vitro scavenger of peroxynitrite, singlet oxygen, hydroxyl radical, superoxide anion and hypochlorous acid and prevents in vivo ozone-induced tyrosine nitration in lungs. Free Radic Res. 2006;40(5):523–33. doi: 10.1080/10715760500419365. [DOI] [PubMed] [Google Scholar]
- Garaventa A, Luksch R, Lo Piccolo MS, Cavadini E, Montaldo PG, Pizzitola MR, et al. Phase I trial and pharmacokinetics of fenretinide in children with neuroblastoma. Clin Cancer Res. 2003;9(6):2032–39. [PubMed] [Google Scholar]
- Hail N, Jr, Kim HJ, Lotan R. Mechanisms of fenretinide-induced apoptosis. Apoptosis. 2006;11(10):1677–94. doi: 10.1007/s10495-006-9289-3. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9(2):139–50. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- Idkowiak-Baldys J, Apraiz A, Li L, Rahmaniyan M, Clarke CJ, Kraveka JM, et al. Dihydroceramide desaturase activity is modulated by oxidative stress. Biochem J. 2010;427(2):265–74. doi: 10.1042/BJ20091589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Pan X, Chen Y, Wang K, Du Y, Zhang J. Preferential involvement of both ROS and ceramide in fenretinide-induced apoptosis of HL60 rather than NB4 and U937 cells. Biochem Biophys Res Commun. 2011;405(2):314–8. doi: 10.1016/j.bbrc.2011.01.036. [DOI] [PubMed] [Google Scholar]
- Kadara H, Lacroix L, Lotan D, Lotan R. Induction of endoplasmic reticulum stress by the pro-apoptotic retinoid N-(4-hydroxyphenyl)retinamide via a reactive oxygen species-dependent mechanism in human head and neck cancer cells. Cancer Biol Ther. 2007;6(5):705–11. doi: 10.4161/cbt.6.5.3963. [DOI] [PubMed] [Google Scholar]
- Kang MH, Wan Z, Kang YH, Sposto R, Reynolds CP. Mechanism of synergy of N-(4-hydroxyphenyl)retinamide and ABT-737 in acute lymphoblastic leukemia cell lines: Mcl-1 inactivation. J Natl Cancer Inst. 2008;100(8):580–95. doi: 10.1093/jnci/djn076. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Chakravarti N, Oridate N, Choe C, Claret FX, Lotan R. N- (4-hydroxyphenyl)retinamide-induced apoptosis triggered by reactive oxygen species is mediated by activation of MAPKs in head and neck squamous carcinoma cells. Oncogene. 2006;25(19):2785–94. doi: 10.1038/sj.onc.1209303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitareewan S, Spinella MJ, Allopenna J, Reczek PR, Dmitrovsky E. 4HPR triggers apoptosis but not differentiation in retinoid sensitive and resistant human embryonal carcinoma cells through an RARgamma independent pathway. Oncogene. 1999;18 (42):5747–55. doi: 10.1038/sj.onc.1202981. [DOI] [PubMed] [Google Scholar]
- Kitatani K, Idkowiak-Baldys J, Hannun YA. The sphingolipid salvage pathway in ceramide metabolism and signalling. Cell Signal. 2008;20(6):1010–18. doi: 10.1016/j.cellsig.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraveka JM, Li L, Szulc ZM, Bielawski J, Ogretmen B, Hannun YA, et al. Involvement of dihydroceramide desaturase in cell cycle progression in human neuroblastoma cells. J Biol Chem. 2007;282(23):16718–28. doi: 10.1074/jbc.M700647200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn H, Borchert A. Regulation of enzymatic lipid peroxidation: the interplay of peroxidizing and peroxide reducing enzymes. Free Radic Biol Med. 2002;33(2):154–72. doi: 10.1016/s0891-5849(02)00855-9. Review. [DOI] [PubMed] [Google Scholar]
- Lovat PE, Oliverio S, Ranalli M, Corazzari M, Rodolfo C, Bernassola F, et al. GADD153 and 12-lipoxygenase mediate fenretinide-induced apoptosis of neuroblastoma. Cancer Res. 2002;62(18):5158–67. [PubMed] [Google Scholar]
- Lovat PE, Di Sano F, Corazzari M, Fazi B, Donnorso RP, Pearson AD, et al. Gangliosides link the acidic sphingomyelinase-mediated induction of ceramide to 12-lipoxygenase-dependent apoptosis of neuroblastoma in response to fenretinide. J Natl Cancer Inst. 2004;96(17):1288–99. doi: 10.1093/jnci/djh254. [DOI] [PubMed] [Google Scholar]
- Maurer BJ, Metelitsa LS, Seeger RC, Cabot MC, Reynolds CP. Increase of ceramide and induction of mixed apoptosis/necrosis by N-(4-hydroxyphenyl)retinamide in neuroblastoma cell lines. J Natl Cancer Inst. 1999;91(13):1138–46. doi: 10.1093/jnci/91.13.1138. [DOI] [PubMed] [Google Scholar]
- Morales MC, Pérez-Yarza G, Nieto-Rementería N, Boyano MD, Jangi M, Atencia R, et al. Intracellular glutathione levels determine cell sensitivity to apoptosis induced by the antineoplasic agent N-(4-hydroxyphenyl)retinamide. Anticancer Res. 2005;25(3B):1945–51. [PubMed] [Google Scholar]
- Morales MC, Pérez-Yarza G, Rementería NN, Boyano MD, Apraiz A, Gómez-Muñoz A, et al. 4-HPR-mediated leukemia cell cytotoxicity is triggered by ceramide-induced mitochondrial oxidative stress and is regulated downstream by Bcl-2. Free Radic Res. 2007;41(5):591–601. doi: 10.1080/10715760701218558. [DOI] [PubMed] [Google Scholar]
- O’Donnell PH, Guo WX, Reynolds CP, Maurer BJ. N-(4-hydroxyphenyl)retinamide increases ceramide and is cytotoxic to acute lymphoblastic leukemia cell lines, but not to non-malignant lymphocytes. Leukemia. 2002;16(5):902–10. doi: 10.1038/sj.leu.2402485. [DOI] [PubMed] [Google Scholar]
- Obeid LM, Linardic CM, Karolak LA, Hannun YA. Programmed cell death induced by ceramide. Science. 1993;259(5102):1769–71. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- Ponzoni M, Bocca P, Chiesa V, Decensi A, Pistoia V, Raffaghello L, et al. Differential effects of N-(4-hydroxyphenyl)retinamide and retinoic acid on neuroblastoma cells: apoptosis versus differentiation. Cancer Res. 1995;55(4):853–61. [PubMed] [Google Scholar]
- Rahmaniyan M, Curley RW, Jr, Obeid LM, Hannun YA, Kraveka JM. Identification of dihydroceramide desaturase as a direct in vitro target for fenretinide. J Biol Chem. 2011;286(28):24754–64. doi: 10.1074/jbc.M111.250779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman F, Shanmugasundaram P, Schrey MP. Fenretinide stimulates redox-sensitive ceramide production in breast cancer cells: potential role in drug-induced cytotoxicity. Br J Cancer. 2004;91(10):1821–28. doi: 10.1038/sj.bjc.6602212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CP, Lemons RS. Retinoid therapy of childhood cancer. Hematol Oncol Clin North Am. 2001;15(5):867–910. doi: 10.1016/s0889-8588(05)70256-2. [DOI] [PubMed] [Google Scholar]
- Rotmensz N, De Palo G, Formelli F, Costa A, Marubini E, Campa T, et al. Long-term tolerability of fenretinide (4-HPR) in breast cancer patients. Eur J Cancer. 1991;27 (9):1127–31. doi: 10.1016/0277-5379(91)90309-2. [DOI] [PubMed] [Google Scholar]
- Ruvolo VR, Karanjeet KB, Schuster TF, Brown R, Deng Y, Hinchcliffe E, Ruvolo PP. Role for PKC δ in Fenretinide-Mediated Apoptosis in Lymphoid Leukemia Cells. J Signal Transduct. 2010;2010:584657. doi: 10.1155/2010/584657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabichi AL, Lerner SP, Atkinson EN, Grossman HB, Caraway NP, Dinney CP, et al. Phase III prevention trial of fenretinide in patients with resected non-muscle-invasive bladder cancer. Clin Cancer Res. 2008;14(1):224–9. doi: 10.1158/1078-0432.CCR-07-0733. [DOI] [PubMed] [Google Scholar]
- Salari H, Braquet P, Borgeat P. Comparative effects of indomethacin, acetylenic acids, 15-HETE, nordihydroguaiaretic acid and BW755C on the metabolism of arachidonic acid in human leukocytes and platelets. Prostaglandins Leukot Med. 1984;13(1):53–60. doi: 10.1016/0262-1746(84)90102-1. [DOI] [PubMed] [Google Scholar]
- Sun S-Y, Li W, Yue P, Lippman SM, Hong WK, Lotan R. Mediation of N-(4-hydroxyphenyl)retinamide-induced apoptosis in human cancer cells by different mechanisms. Cancer Res. 1999;59(10):2493–98. [PubMed] [Google Scholar]
- Suzuki S, Higuchi M, Proske RJ, Oridate N, Hong WK, Lotan R. Implications of mitochondria-derived reactive oxygen species, cytochrome C and caspase-3 in N-(4-hydroxyphenyl)retinamide-induced apoptosis in cervical carcinoma cells. Oncogene. 1999;18(46):6380–87. doi: 10.1038/sj.onc.1203024. [DOI] [PubMed] [Google Scholar]
- Szulc ZM, Bielawski J, Gracz H, Gustilo M, Mayroo N, Hannun YA, et al. Tailoring structure-function and targeting properties of ceramides by site-specific cationization. Bioorg Med Chem. 2006;14(21):7083–104. doi: 10.1016/j.bmc.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Taha TA, Mullen TD, Obeid LM. A house divided: ceramide, sphingosine, and sphinosine-1-phosphate in programmed cell death. Biochim Biophys Acta. 2006;1758(12):2007–36. doi: 10.1016/j.bbamem.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari M, Kumar A, Sinha RA, Shrivastava A, Balapure AK, Sharma R, et al. Mechanism of 4-HPR-induced apoptosis in glioma cells: evidences suggesting role of mitochondrial-mediated pathway and endoplasmic reticulum stress. Carcinogenesis. 2006;27(10):2047–58. doi: 10.1093/carcin/bgl051. [DOI] [PubMed] [Google Scholar]
- Tiwari M, Bajpai VK, Sahasrabuddhe AA, Kumar A, Sinha RA, Behari S, et al. Inhibition of N-(4-hydroxyphenyl)retinamide-induced autophagy at a lower dose enhances cell death in malignant glioma cells. Carcinogenesis. 2008;29(3):600–9. doi: 10.1093/carcin/bgm264. [DOI] [PubMed] [Google Scholar]
- Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic Biol Med. 2007;43(1):4–15. doi: 10.1016/j.freeradbiomed.2007.03.024. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valsecchi M, Aureli M, Mauri L, Illuzzi G, Chigorno V, Prinetti A, et al. Sphingolipidomics of A2780 human ovarian carcinoma cells treated with synthetic retinoids. J Lipid Res. 2010;51(7):1832–40. doi: 10.1194/jlr.M004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Veldhoven PP, Bell RM. Effect of harvesting methods, growth conditions and growth phase on diacylglycerol levels in cultured human adherent cells. Biochim Biophys Acta. 1988;959(2):185–96. doi: 10.1016/0005-2760(88)90030-6. [DOI] [PubMed] [Google Scholar]
- Veronesi U, Mariani L, Decensi A, Formelli F, Camerini T, Miceli R, et al. Fifteen-years results of a randomized phase III trial of fenretinide to prevente second breast cancer. Ann Oncol. 2006;17(7):1065–71. doi: 10.1093/annonc/mdl047. [DOI] [PubMed] [Google Scholar]
- Wang H, Maurer BJ, Reynolds CP, Cabot MC. N-(4-hydroxylphenyl)retinamide elevates ceramide in neuroblastoma cell lines by coordinate activation of serine palmitoyltransferase and ceramide synthase. Cancer Res. 2001;61 (13):5102–05. [PubMed] [Google Scholar]
- Wang H, Maurer BJ, Liu YY, Wang E, Allegood JC, Kelly S, et al. N-(4-Hydroxyphenyl)retinamide increases dihydroceramide and synergizes with dimethylsphingosine to enhance cancer cell killing. Mol Cancer Ther. 2008;7(9):2967–76. doi: 10.1158/1535-7163.MCT-08-0549. [DOI] [PubMed] [Google Scholar]
- Wang K, Fang H, Xiao D, Zhu X, He M, Pan X, Shi J, Zhang H, Jia X, Du Y, Zhang J. Converting redox signaling to apoptotic activities by stress-responsive regulators HSF1 and NRF2 in fenretinide treated cancer cells. PLoS One. 2009;4(10):e7538. doi: 10.1371/journal.pone.0007538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JM, DiPietrantonio AM, Hsieh TC. Mechanism of fenretinide (4- HPR)-induced cell death. Apoptosis. 2001;6(5):377–88. doi: 10.1023/a:1011342220621. [DOI] [PubMed] [Google Scholar]
- Zheng W, Kollmeyer J, Symolon H, Momin A, Munter E, Wang E, et al. Ceramides and other bioactive sphingolipid backbones in health and disease: lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochim Biophys Acta. 2006;1758(12):1864–84. doi: 10.1016/j.bbamem.2006.08.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CCRF-CEM (A, C, E) and Jurkat (B, D, E) cells were exposed to indicated concentrations of 4-HPR. General oxidative stress (A, B), mitochondrial superoxide production (C, D) and lipid peroxidation (E) was determined as described in Materials and Methods. (A–D) Data represent mean±SD of three independent experiments performed in quadruplicates (n=12). (E) Single analysis. **p<0.01 and *p<0.05 (related to untreated cells). (A–C) Statistical significance obtained with all the 4-HPR concentrations. (D) Statistical significance obtained just by 3 μM 4-HPR treatment.
CCRF-CEM and Jurkat cells were exposed for 6 h to indicated concentrations of 4-HPR (A) and endogenous dhSph content determined by LC/MS as described in Materials and Methods. Data represent mean±SD of two independent experiments. In order to evaluate the role of the dhSph accumulation on 4-HPR-mediated ROS production and cell death, cells were incubated for 2 h with the SPT inhibitor myriocin (myr) and 4-HPR added for an additional incubation of 6 h (B). Samples for further analysis by LC/MS were collected at indicated time points. Data represent mean±SD of two independent experiments.
CCRF-CEM cells were exposed for 6 h to indicated concentrations of 4-HPR (A) and endogenous Sph content determined by LC/MS as described in Materials and Methods. Data represent mean±SD of two independent experiments. In order to evaluate the role of the Sph accumulation on 4-HPR-mediated ROS production and cell death, cells were incubated for 2 h with the SPT inhibitor myriocin (myr) and 4-HPR added for an additional incubation of 6 h (B.1 and B.2). Samples for further analysis by LC/MS were collected at indicated time points. Figures represent data from individual experiments.