Abstract
Background
N-acetylcysteine (NAC) improves transplant-free survival in early coma grade (I-II) patients with non-acetaminophen induced acute liver failure (ALF). We determined whether the clinical benefit was associated with improvements in hepatic function.
Methods
In a prospective, double blind trial, 173 ALF patients without evidence of acetaminophen overdose were stratified by coma grade (I-II vs. III-IV) and randomly assigned to receive either intravenous NAC or dextrose (placebo) for 72 hours, resulting in 4 patient groups. INR, ALT, bilirubin, creatinine, and AST obtained on admission (day 1) and subsequent days (days 2-4) were used for secondary analysis performed by fitting longitudinal logistic regression models to predict death or transplantation or transplantation alone.
Results
Treatment group and day of study in models including bilirubin or ALT were predictors of transplantation or death (maximum p<0.03). Those patients with early coma grade who were treated with NAC showed significant improvement in bilirubin and ALT levels when compared to the other 3 groups (maximum p <0.02 for NAC 1-2 versus the 3 other treatments) when predicting death or transplantation. Treatment group, day of study, and bilirubin were predictors of transplantation (maximum p<0.03) in ALF patients.
Conclusion
The decreased risk of transplantation or death or of transplantation alone with intravenous NAC in early coma grade patients with non-acetaminophen induced ALF was reflected in improvement in parameters related to hepatocyte necrosis and bile excretion: ALT and bilirubin, but not in INR, creatinine, or AST. Hepatic recovery appears hastened by NAC as measured by several important lab values.
Keywords: Acute Liver Failure, ALF, N-acetylcysteine
Introduction
First defined in the 1950's, acute liver failure (ALF) is a catastrophic illness affecting approximately 2000 people in the United States annually.1,2 The forty percent of patients who develop ALF caused by acetaminophen intoxication have a survival rate without transplantation (spontaneous survival-SS) greater than 70%. By contrast, patients with ALF not due to acetaminophen have a SS less than 30% and frequently require transplantation.3 Unfortunately, the latter group is plagued by limited therapeutic options besides transplantation. Acetaminophen intoxication is a common cause of ALF in the United States and Europe; however, employment of N-acetylcysteine (NAC) within 24 hours of ingestion limits liver damage by regeneration of glutathione.4,5 In a recent study from the Acute Liver Failure Study Group (ALFSG), the use of the NAC in non-acetaminophen induced ALF improved transplant-free survival, particularly in patients with early coma grades. NAC appeared ineffective in those with coma grades III or IV on admission to study.6 Patients with coma grade I or II who were treated with NAC had a 52% rate of survival without grafting, while the placebo group only had a 30% survival in this category (p=0.04). The reasons for this apparent benefit are unclear; however, the postulated mechanisms for improved outcomes include improving systemic hemodynamics, tissue oxygen delivery, and hepatic blood flow in patients with ALF.7,8,9 Recent studies have demonstrated that NAC can replete mitochondrial glutathione reserves, restore mitochondrial function, increase mitochondrial subunit expression, and reduce reactive oxidative species generation.10,11 Additionally, toll-like receptor 2 and 4 expression and TNF-alpha expression is decreased when NAC is administered an insult the liver.12 We proposed to evaluate standard laboratory parameters, so-called liver function tests, as markers of resolution of liver injury with NAC, to compare those who recovered from those who died or underwent transplantation, to indicate whether laboratory parameters of injury resolved more rapidly in those demonstrating improved survival.
Patients and Methods
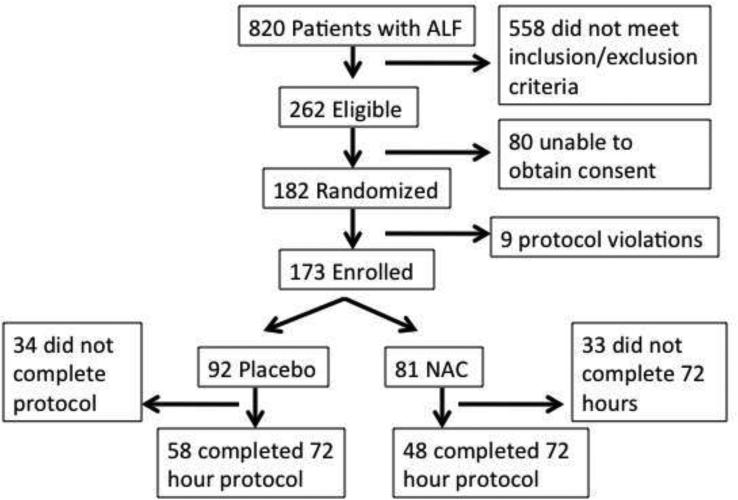
Details of the NAC trial for non-acetaminophen induced ALF have been described previously.6 The overall ALF Study Group registry collected prospective demographic, clinical, biochemical, and pathological data for patients 18 years and older with evidence of ALF based on encephalopathy and coagulopathy caused by an illness during the previous 24 weeks since January 1998. Informed consent was obtained from next of kin for the registry and separately for the clinical trial in light of the presence of encephalopathy in all subjects. Only those diagnosed as having ALF due to causes not related to acetaminophen were eligible for the trial. Additional exclusion criteria were previous NAC treatment, age older than 70, those undergoing immediate transplantation (< 6 hours), and those with evidence of refractory sepsis, cerebral edema or shock. Further, ALF etiologies that were likely to not benefit included ischemia, pregnancy and cancer were also excluded. Patients either received 5% dextrose with NAC as outlined in the main trial paper, or 5% dextrose for 72 hours. A total of 173 patients at 24 study sites were block randomized by coma grade, study site, and treatment group resulting in 92 patients being treated with placebo and 81 patients being treated with NAC (Figure 1).
Figure 1.
Study Patients
The demographic, clinical, and admission to study (Day 1) laboratory data on all 173 patients obtained from the trial registry are shown in Table 1. Daily laboratory values that were available and extracted included INR, bilirubin, creatinine, AST, and ALT on admission to study (day 1) and subsequently (days 2-4). On later days, fewer tests were available because of death or transplantation: these details are in Supplemental Table 1. All sites had approval from their Institutional Review Boards. This study was approved by the Ancillary Studies committee of the ALFSG.
Table 1.
Patient Demographics
| Placebo | NAC | p-value | |
|---|---|---|---|
| Number of Patients | 92 | 81 | |
| Age | 40.5 | 42 | 0.68 |
| Male (%) | 32% | 53% | <0.01 |
| Caucasian (%) | 45% | 44% | 0.99 |
| Coma Grade I/II (%) | 38% | 27% | 0.13 |
| Symptom to Coma (days) | 17 | 15 | 0.37 |
| Jaundice to Coma (days) | 12 | 7 | 0.03 |
| Bilirubin (mg/dL) | 20.3 | 22.3 | 0.65 |
| Creatinine (mg/dL) | 1.0 | 1.3 | 0.34 |
| INR | 2.0 | 2.4 | 0.26 |
| ALT (IU/L) | 756 | 999 | 0.30 |
| AST (IU/L) | 686.5 | 724.0 | 0.84 |
| MELD | 33 | 32 | 0.67 |
| Etiology | 0.10 | ||
| Drug Induced Liver Injury (%) | 28% | 23% | |
| Autoimmune Hepatitis (%) | 16% | 14% | |
| Hepatitis B Virus (%) | 14% | 31% | |
| Indeterminate (%) | 28% | 19% | |
| Other (%) | 13% | 14% | |
| 21 Day Overall Survival | |||
| Coma Grade I-II (%) | 73% | 78% | 0.59 |
| Coma Grade III-IV (%) | 53% | 48% | 0.71 |
| Transplant-free Survival | |||
| Coma Grade I-II (%) | 26% | 48% | 0.02 |
| Coma Grade III-IV (%) | 23% | 14% | 0.50 |
Continuous measures are shown as medians and Mann-Whitney U p-values.
Discrete measures are shown as percentages (%) and χ2 or Fisher's Exact p-values.
Statistical Analysis
Longitudinal logistic regression using SAS V9.2 (Proc GENMOD with Generalized Estimating Equations [GEE]; SAS Institute, Inc., Cary, NC, 2008) was performed to separately examine the outcome measures of transplantation or death (TD) and transplantation alone. The GEE method is used to account for correlated data (repeated lab measurements within each subject). Each logistic regression model was fit to the data using one of the following lab values (INR, ALT, total bilirubin, creatinine, and AST) measured during the first 4 days of admission to the NAC clinical trial. Treatment groups were divided by coma level for analysis (NAC I-II, NAC III-IV, Placebo I-II, and Placebo III-IV). Other covariates included with these lab values were sex and age as well as interactions of covariates; each covariate remained in the final model if significant (p < 0.05). Sex, age, and all interactions were not found to be significant in any of the models. INR, ALT, creatinine, and AST were transformed using log base 10. Using our model, these laboratory values were analyzed to predict transplantation and death, however transplantation is a rescue therapy, which is dependent on organ availability, patient eligibility, and severity of illness.
Results
Transplantation or Death (TD)
ALT (mean ALT and change across study days) and treatment group were significant predictors of TD (all p<0.003, Table 2). Holding constant all other values, lower ALT was associated with predictions of TD as indicated by the negative estimate for log10(ALT); the influence across study days in predicting TD decreases significantly as noted by the declining estimates as days progressed (maximum pairwise comparisons for study days, p-value=0.007). NAC I-II patients had a significantly lower estimate of predicting TD than any of the other three treatment groups (maximum pairwise p-value=0.020), while the other 3 groups were not significantly different from each other.
Table 2.
GEE parameter estimates for ALT predicting transplantation or death.
| Parameter | Estimate | Odds Ratio | p-value | 95% Confidence Limits for Odds Ratio | |
|---|---|---|---|---|---|
| Placebo 3-4 | 1.22 | 3.37 | 0.0023 | 1.26 | 9.05 |
| NAC 3-4 | 2.30 | 9.99 | 1.90 | 52.36 | |
| Placebo 1-2 | 0.97 | 2.63 | 1.17 | 5.92 | |
| NAC 1-2* | 0.00 | 1.00 | |||
| Day 1 | 0.70 | 2.02 | 0.0005 | 1.40 | 2.93 |
| Day 2 | 0.49 | 1.64 | 1.26 | 2.13 | |
| Day 3 | 0.25 | 1.29 | 1.07 | 1.55 | |
| Day 4** | 0.00 | 1.00 | |||
| Log10(ALT) | -1.23 | 0.29 | 0.0026 | 0.12 | 0.74 |
Using NAC 1-2 as the reference group, all other groups show positive estimate values indicating higher likelihood of death or transplant. Day 4 is the reference day that is then compared with the prior 3 days. The effect is greatest on day 1 and declines over succeeding days.
Reference group for Treatment/Coma combination
Reference group for Days 1-3
Total bilirubin (overall total bilirubin and change across study days) and treatment group were significant predictors of TD (all p<0.030). Holding constant all other values, higher levels of total bilirubin were predictive of TD as reflected by the positive estimate; the influence across study days in predicting TD decreased significantly (maximum pairwise p-value=0.034) except between days 1 and 2 and between days 3 and 4 as days progressed as noted by the decrease in estimates. NAC I-II patients had significantly lower estimates of TD than any of the other three treatment groups (maximum pairwise p-value=0.013), while the other 3 groups were not significantly different from each other (Table 3).
Table 3.
GEE parameter estimates for Total Bilirubin predicting transplantation or death.
| Parameter | Estimate | Odds Ratio | p-value | 95% Confidence Limits for Odds Ratio | |
|---|---|---|---|---|---|
| Placebo 3-4 | 1.32 | 3.75 | 0.0016 | 1.36 | 10.36 |
| NAC 3-4 | 2.28 | 9.74 | 2.03 | 46.61 | |
| Placebo 1-2 | 1.06 | 2.89 | 1.25 | 6.64 | |
| NAC 1-2* | 0.00 | 1.00 | |||
| Day 1 | 0.27 | 1.31 | 0.0290 | 1.09 | 1.58 |
| Day 2 | 0.21 | 1.24 | 1.03 | 1.49 | |
| Day 3 | 0.09 | 1.09 | 0.93 | 1.29 | |
| Day 4** | 0.00 | 1.00 | |||
| Total Bilirubin | 0.06 | 1.06 | 0.0030 | 1.02 | 1.10 |
Using NAC 1-2 as the reference group, all other groups show positive estimate values indicating higher likelihood of death or transplant. Day 4 is the reference day that is then compared with the prior 3 days. The effect is greatest on day 1 and declines over succeeding days.
Reference group for Treatment/Coma combination
Reference group for Days 1-3
When AST, INR and creatinine were analyzed over time for association with TD by treatment and coma group, none of these were significant.
Transplantation
During the first four days of hospitalization, INR (overall [p<0.001] and change across study days [p=0.028]) were significant predictors of transplantation while treatment group was not (p=0.071). Holding constant all other values, higher INR was predictive of transplantation reflected in the positive estimate. The influence across study days in predicting transplantation significantly declined from day 3 to 4 (p=0.010); none of the other pairwise comparisons were significant. There was a significant decrease in predicting transplantation in NAC I-II patients compared to placebo I-II patients (p=0.01, Table 4).
Table 4.
GEE parameter estimates for INR predicting Transplantation only
| Parameter | Estimate | Odds Ratio | p-value | 95% Confidence Limits for Odds Ratio | |
|---|---|---|---|---|---|
| Placebo 3-4 | 0.66 | 1.94 | 0.0715 | 0.76 | 4.95 |
| NAC 3-4 | 0.48 | 1.61 | 0.49 | 5.26 | |
| Placebo 1-2 | 1.04 | 2.82 | 1.28 | 6.24 | |
| NAC 1-2* | 0.00 | 1.00 | |||
| Day 1 | 0.15 | 1.16 | 0.0281 | 0.87 | 1.54 |
| Day 2 | 0.26 | 1.30 | 1.00 | 1.68 | |
| Day 3 | 0.26 | 1.30 | 1.06 | 1.58 | |
| Day 4** | 0.00 | 1.00 | |||
| Log10(INR) | 2.13 | 8.38 | 0.0004 | 2.41 | 29.14 |
Using NAC 1-2 as the reference group, all other groups show positive estimate values indicating higher likelihood of transplantation. Day 4 is the reference day that is then compared with the prior 3 days. The effect is greatest on Days 2 and 3 and less so for Day 1 compared to Day 4.
Reference group for Treatment/Coma combination
Reference group for Days 1-3
Total bilirubin (overall total bilirubin and change across study days) and treatment group were significant predictors of transplantation (maximum p<0.028). Holding constant all other values, higher total bilirubin was associated with predictions of transplantation reflected in the positive estimate for total bilirubin. In predicting transplantation, the estimates for study days was found to significantly decline from day 2 to 4 (p=0.006) and day 3 to 4 (p=0.047). The prediction of transplantation in the NAC I-II patients was significantly lower than placebo I-II (p=0.004), however, no other pairwise differences in predicting transplantation were found (Table 5).
Table 5.
GEE parameter estimates for Total Bilirubin predicting Transplant
| Parameter | Estimate | Odds Ratio | p-value | 95% Confidence Limits for Odds Ratio | |
|---|---|---|---|---|---|
| Placebo 3-4 | 0.82 | 2.28 | 0.0266 | 0.90 | 5.75 |
| NAC 3-4 | 0.87 | 2.38 | 0.81 | 6.98 | |
| Placebo 1-2 | 1.20 | 3.31 | 1.48 | 7.41 | |
| NAC 1-2* | 0.00 | 1.00 | |||
| Day 1 | 0.23 | 1.26 | 0.0282 | 1.00 | 1.59 |
| Day 2 | 0.32 | 1.37 | 1.10 | 1.72 | |
| Day 3 | 0.18 | 1.20 | 1.00 | 1.44 | |
| Day 4** | 0.00 | 1.00 | |||
| Total Bilirubin | 0.03 | 1.03 | 0.0227 | 1.00 | 1.07 |
Using NAC 1-2 as the reference group, all other groups show positive estimate values indicating higher likelihood of transplantation. Day 4 is the reference day that is then compared with the prior 3 days. The effect is greatest on Day 2 and less so for Days 1 and 3 compared to Day 4.
Reference group for Treatment/Coma combination
Reference group for Days 1-3
When using AST, ALT, and creatinine longitudinally in combination with treatment/coma grade to predict transplantation, none of these models were significant.
Discussion
When caring for a patient with ALF, clinicians must manage someone with a poor prognosis and few treatment options. As demonstrated in the NAC trial, the clinical course of patients with grade III-IV hepatic encephalopathy is unlikely to be altered by medical management that does not include transplantation. Based on our study, improvements in outcome without transplantation noted in the NAC trial in patients with early (Grade 1-2) encephalopathy reflected greater improvements in ALT and bilirubin during the first four days of hospitalization among NAC-treated patients. Similarly, bilirubin trends in early coma grade ALF patients treated with NAC correlated with improvements (a decrease) in the rate of transplantation. Although the exact mechanism by which NAC benefits patients with non-acetaminophen induced ALF remains unclear, this study provides further evidence that the use of NAC speeds recovery from hepatic insults as reflected by downward trends in ALT and bilirubin.
The main strength of this study is the diversity in patients in regards to race, gender, geography, and underlying etiology; thus, the results would appear to be generalizable to a broad range of patients with non-acetaminophen related ALF. Additionally, the availability of clinical and laboratory data over time allowed the use of longitudinal logistic regression to demonstrate the possible effect of NAC. The study was limited by the small number of patients with coma grade III-IV encephalopathy (34%) at time of randomization. Since these late coma stage patients were more likely to either die or require early transplantation, the study's analysis of laboratory data during the first 96 hours is even more limited. Because of this, we were unable to determine if the improvement in serological markers is unique to patients with early coma grades or if the analysis is unable to detect a difference in late coma stage patients because of the small numbers. Furthermore, transplantation is a rescue therapy dependent on organ availability, patient eligibility, and illness severity. This creates challenges when performing research within the area of liver transplantation and acute liver failure.
In summary, NAC appears to benefit patients via a reduction in need for transplantation and a greater degree of spontaneous recovery. This is confirmed by greater improvements in several but not all parameters of liver injury.
Supplementary Material
Acknowledgment
The Acute Liver Failure Study Group is supported by DK-058369 and similar grants since 1998. Additional funding was provided by the Rollin and Mary Ella King Fund of the Southwestern Medical Foundation. We recognize the patients and their families as well as the coordinators and investigators who participated in this network registry.
Contributor Information
Sundeep Singh, Division of Gastroenterology and Hepatology, Stanford University 300 Pasteur Drive, Always Building, M211, Stanford, CA 94305-5187 sundeeps@stanford.edu
Linda S. Hynan, Division of Biostatistics, University of Texas Southwestern Medical Center 5323 Harry Hines Boulevard, Dallas, TX 75390 Linda.Hynan@utsouthwestern.edu
William M. Lee, Division of Digestive and Liver Diseases University of Texas Southwestern Medical Center 5959 Harry Hines Boulevard, Dallas, TX 75390 William.lee@utsouthwestern.edu
References
- 1.Trey C, Davidson CS. The management of fulminant hepatic failure. In: Popper H, Schaffner F, editors. Progress in Liver Diseases. Grune & Stratton; New York: 1970. pp. 282–298. [PubMed] [Google Scholar]
- 2.Hoofnagle JH, Carithers RL, Shapiro C, Ascher N. Fulminant hepatic failure: summary of a workship. Hepatology. 1995;21:240–252. [PubMed] [Google Scholar]
- 3.Ostapowicz G, Fontanta RJ, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137(12):947–955. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 4.Prescott LF, Critchley JA. The treatment of acetaminophen poisoning. Ann Rev Pharmacol Toxicol. 1983;23:87–101. doi: 10.1146/annurev.pa.23.040183.000511. [DOI] [PubMed] [Google Scholar]
- 5.Hamlyn AN, Douglas AP, James O. The spectrum of paracetamol (acetaminophen) overdose: clinical and epidemiological studies. Postgrad Med J. 1978;54:400–404. doi: 10.1136/pgmj.54.632.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee WM, Hynan LS, et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137:856–864. doi: 10.1053/j.gastro.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrison PM, Wendon JA, et al. Improvement by acetylcysteine of hemodynamics and oxygen transport in fulminant hepatic failure. N Engl J Med. 1991;324:1852–1857. doi: 10.1056/NEJM199106273242604. [DOI] [PubMed] [Google Scholar]
- 8.Walsh TS, Hopton P, et al. The effect of N-acetylcysteine on oxygen transport and uptake in patients with fulminant hepatic failure. Hepatology. 1998;27:1332–1340. doi: 10.1002/hep.510270520. [DOI] [PubMed] [Google Scholar]
- 9.Rank N, Michel C, Lenhart A, et al. N-acetylcysteine increases blood flow and improves liver function in septic shock patients: results of a prospective, randomized, double-blind study. Crit Care Med. 2000;28:3799–807. doi: 10.1097/00003246-200012000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Johansson E, Yang Y, et al. Oral N-acetylcysteine rescues lethality of hepatocyte-specific GcIc-knockout mice, providing a model for hepatic cirrhosis. J Hepatol. 2010;53:1085–1094. doi: 10.1016/j.jhep.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez R, Ferrin G, Hidalgo AB, et al. N-acetylcysteine, coenzyme Q10 and superoxide dismutase mimetic prevent mitochondrial cell dysfunction and cell death induced by d-galactosamine in primary culture of human hepatocytes. Chem Biol Interact. 2009;181:95–106. doi: 10.1016/j.cbi.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Jin X, Wang L, Wu HS, et al. N-acetylcysteine inhibits activation of toll-like receptor 2 and 4 gene expression in the liver and lung after partial hepatic ischemia-reperfusion injury in mice. Hepatobiliary Pancreat Dis Int. 2007;6:284–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.