Abstract
Improved neonatal medical care and renal replacement technology has improved the long term survival of patients with autosomal recessive polycystic kidney disease (ARPKD). Ten year survival of those surviving the first year of life is reported to be 82% and is continuing to improve further. However, despite increases in overall survival and improved treatment of systemic hypertension and other complications of their renal disease, nearly 50% of survivors will develop end stage renal disease (ESRD) within the first decade of life 1, 2.
In addition to renal pathology, patients with ARPKD develop ductal plate malformations with cystic dilation of intra- and extra-hepatic bile ducts resulting in congenital hepatic fibrosis (CHF) and Caroli syndrome. Many patients with CHF will develop portal hypertension with resulting esophageal varices, splenomegaly, hypersplenism, protein loosing enteropathy and gastrointestinal bleeding3,4,5. Management of portal hypertension may require endoscopic band ligation of esophageal varices or porto-systemic shunting. Complications of hepatic involvement can include ascending cholangitis, cholestasis with malabsorption of fat soluble vitamins, and rarely benign or malignant liver tumors.
Patients with ARPKD who eventually reach end-stage renal disease, and ultimately require kidney transplantation, present a unique set of complications related to their underlying hepato-biliary disease. In this review, we focus on new approaches to these challenging patients, including the indications for liver transplantation in ARPKD patients with severe chronic kidney disease awaiting kidney transplant.
While survival in patients with ARPKD and isolated kidney transplant is comparable to that of age-matched pediatric patients who have received kidney transplants due to other primary renal diseases, 64–80% of the mortality occurring in ARPKD kidney transplant patients is attributed to cholangitis/sepsis which is related to their hepato-biliary disease6,7 Recent data demonstrate that surgical mortality among pediatric liver transplant recipients is decreased to less than 10% at 1 year8,9. The immunosuppressive regimen used for kidney transplant recipients is adequate for most liver transplant recipients. We therefore suggest that in a select group of ARPKD patients with recurrent cholangitis or complications of portal hypertension, combined liver-kidney transplant is a viable option. Although further study is necessary to confirm our approach, we believe that combined liver-kidney transplantation can potentially decrease overall mortality and morbidity in carefully selected ARPKD patients with ESRD and clinically significant CHF.
Keywords: Autosomal recessive polycystic kidney disease, congenital hepatic fibrosis, Caroli disease/syndrome, kidney transplant, liver transplant, portal hypertension, ascending cholangitis, end stage renal disease
INTRODUCTION
Autosomal Recessive Polycystic Kidney Disease (ARPKD) is a severe form of polycystic kidney disease (PKD) and a significant cause of renal and liver-related morbidity and mortality in children. ARPKD is characterized by: 1) non-obstructive fusiform dilatation of the renal collecting tubules (CT) (following an early, transient phase of proximal renal tubular cyst formation10; and 2) a ductal plate malformation of the liver ultimately resulting in congenital hepatic fibrosis (CHF). Estimates of the prevalence of ARPKD vary (often due to referral bias), but the most consistent data suggest an overall frequency of 1 in 20,000 live births and a heterozygous carrier rate of 1:7011,12.
Despite the recent findings of Adeva et al, that ARPKD/CHF may present in individuals older than age 213, the clinical descriptions of ARPKD provided in most pediatric series are accurate. Most patients present with : 1) enlarged echogenic kidneys, with severe renal and/or combined severe renal/biliary disease that often presents in the youngest patients (including prenatal diagnosis via molecular analysis or imaging) ; and 2) a prominent hepatobiliary clinical phenotype, which may be present in young infants but generally presents in older children and young adults.12,13. The most severe presentation is that of fetuses with enlarged echogenic kidneys and oligohydramnios caused by poor fetal urine production. These signs are potentially detectable clinically in utero, but often not until late in the third trimester11. Improved overall neonatal critical care (including early pediatric nephrology involvement), and referral to tertiary pediatric centers with prenatal disease recognition have significantly increased survival of affected newborns, but death still occurs in 25% to 30% of newborns with ARPKD due to pulmonary hypoplasia, with various manifestations of a severe intrauterine compression syndrome (“Potter’s Syndrome”)13. Individuals with a severe neonatal presentation who survive the first year of life exhibit a spectrum of clinical problems of variable severity as a consequence of their dual organ pathophysiology13. Massive kidney enlargement is often accompanied by systemic hypertension and varying degrees of progressive renal dysfunction. More than 50% of affected children progress to end stage renal disease, usually in the first decade of life.
Liver involvement is always present, and in some cases can be a predominant clinical feature. The most common complication is portal hypertension, a consequence of periportal fibrosis. The fibrosis develops around ectatic bile ducts, and is consistent feature of the basic biliary plate malformation. Other features of hepatobiliary disease include hepatomegaly, malformation of small interlobular bile ducts (congenital hepatic fibrosis), non-obstructive dilation of intrahepatic bile ducts (Caroli syndrome), and dilation of the common bile duct. Ascending cholangitis is a serious and frequent complication, presumably secondary to abnormal antegrade bile flow with ascending infection of pathogenic bowel flora. Abnormal bile flow may also lead to fat malabsorption and decreased absorption of fat-soluble vitamins (A, D, E, K). Despite such abnormalities, liver synthetic and metabolic function remains normal until very late in the disease process.
Overall, with neonatal respiratory support and renal replacement therapies, the 10 year survival of children with severe neonatal ARPKD who live beyond the first year of life has improved to 82%. Fifteen year survival of this “neonatal presentation and survival” group is estimated to be 67%–79% 12. Published data regarding survival in children who present as older infants or young adults with a predominant hepatobiliary clinical presentation are not available.
The treatment of dual organ involvement in ARPKD has traditionally focused first on the renal complications (up to, and including, dialysis and transplantation); and then subsequently addressed complications of hepatobiliary disease. However the presence of significant hepatobiliary disease in ARPKD patients has led to the realization that complications of portal hypertension and ascending cholangitis (increased patient morbidity and mortality from ascending cholangitis pre-or post- renal transplantation, and/or variceal bleeding) and problems related to surgical treatment (vascular complications following porto-systemic decompression) can adversely affect outcomes. Recent studies suggest that the “severe renal, severe hepatobiliary” phenotype may include up to 40% of the ARPKD population (M. Gunay-Aygun, personal communication). Coupled with improvements in the outcomes of liver transplantation, it is appropriate to consider new clinical approaches for this significant group of children with ARPKD and dual renal /hepatobiliary disease.
We therefore present a framework, based on published literature, our experience in a unique Multidisciplinary Childhood PKD clinic established in 2006 at our institution, and the authors combined 50 + years of clinical experience in treating patients with ARPKD (including direct involvement in 15 dual liver/kidney transplants) to guide decision-making for ARPKD patients with dual renal and hepatobiliary disease. We also briefly review the potential impact of new therapies emerging from laboratories around the world, and being rapidly being translated into clinical trials and clinical practice.
HEPATOBILIARY DISEASE IN ARPKD
The histological pattern of hepatobiliary disease associated with ARPKD suggests a basic developmental abnormality of the ductal plate14,15 Although histological findings of ductal plate malformation are present at birth, disease may not be clinically apparent in the first year of life16. Laboratory and radiological studies may be normal in newborns. Organomegaly is the most common presenting symptom in infants and children17. At the time of diagnosis the majority of children with ARPKD have significant renal enlargement, and 45% have clinically apparent liver disease18. Hepatic fibrosis in the absence of hepatocellular inflammation is a hallmark of hepatobiliary disease in ARPKD. Molecular pathways leading to fibrosis in CHF are unknown, and remain an exciting area of current investigation in many labs3,19.
Caroli syndrome is characterized by non-obstructive dilation of medium and large size bile ducts. It is generally defined by radiographic criteria, most ideally utilizing MR cholangiography. Presence of multiple irregular bile ducts with cystic ectasia in the absence of alternative diagnosis (such as primary sclerosing cholangitis, or Langerhans histiocytosis) is considered diagnostic. The majority of patients with Caroli disease will have characteristic histological features of ductal plate malformation.
COMPLICATIONS OF LIVER DISEASE IN ARPKD
Synthetic liver failure is unusual in congenital hepatic fibrosis (CHF) and Caroli disease. Both are, however, associated with significant morbidity in patients with more severe hepatobiliary phenotypes. As progressive kidney disease is successfully treated by renal replacement therapy (dialysis and transplantation), the complications of liver disease are likely to present an increasing degree of mortality and morbidity in ARPKD6,7,20,21
Portal Hypertension
Clinical signs of portal hypertension have been documented in 37–44% of patients with ARPKD who have survived the first year of life22. In other studies, 33.2% of patients with CHF had sequelae of portal hypertension (64% of this group had ARPKD)23. Diagnosis of portal hypertension in ARPKD relies on clinical signs such as splenomegaly, hypersplenism or esophageal varices, rather than on radiological features (reversal of the portal flow) or direct measures (wedge pressure or endoscopic ultrasound) of portal pressures. Portal hypertension can lead to the development of esophageal varices and acute variceal bleeding. Ascites, hepato-pulmonary syndrome and/or encephalopathy are uncommon complications of ARPKD/CHF16. Esophageal varices have been reported in 13% of patients with known CHF21. Prevalence of esophageal bleeding is estimated at 30% in patients with isolated CHF and 6% in all patients with ARPKD/CHF 22. In a published series of patients with ARPKD and renal transplantation, clinically significant portal hypertension was present in 78% of patients. GI bleeding was reported in 38% of these patients, and in rare cases led to acute mortality7.
Consequently, we believe that regular (EGD) monitoring to prevent recurrent esophageal bleeding is warranted in all children with ARPKD and clinical signs of portal hypertension. In children, endoscopic band ligation (EBL) is an effective mode of prophylaxis or therapy in patients with varices and esophageal bleeding (secondary prophylaxis). This technique decreases mortality and has a low complication rate24,25,26. Sclerotherapy may be considered as an alternative to EBL, but it is associated with higher complication rates27, 28. Adult studies show that the use of propranolol or other non-selective beta blockers (NSBB) alone or in addition to EBL may decrease mortality of portal hypertension. Use of NSBB in children is controversial26,29 There are no sufficient data to recommend the use NSBB for prevention of esophageal bleeding in children with ARPKD26. Nitrates have been successfully used in adults as a means of secondary prophylaxis, but there are no reports of their use in children.
Primary prophylaxis focused on prevention of the first episode of esophageal bleeding has been effective in decreasing mortality in adults with portal hypertension29. EBL and propranolol are recognized modes of therapy for primary prophylaxis in adults30. EBL has been used successfully to obliterate esophageal varices in children31. Due to lack of controlled studies, use of EBL as a primary prophylaxis in children with ARPKD remains controversial, but can be used in selected children with ongoing evaluation of outcomes26. Since 2006, we have performed yearly EGD (with EBL as indicated) in our Childhood PKD Clinic for appropriately selected ARPKD patients. We have seen no acute GI bleeds or complications of the procedure in this high-risk population. Gastric varices and hemorrhoids can also be a source of bleeding secondary to increased portal pressure. Technologies to completely control gastric varices have not been successfully developed. Use of cyanoacrylate glue is a promising technique reported to be successful for treatment of gastric varices in a pediatric pilot study32. Hemorrhoids can be amenable to band ligation, though size limitations may limit this technique in infants.
Splenomegaly and hypersplenism are frequently associated with portal hypertension. Cytopenias resulting from hypersplenism can influence immunosuppression decisions after kidney transplantation and lead to increased risk of rejection or nephrotoxicity. Splenic rupture and subsequent internal bleeding has been described in children with ARPKD and portal hypertension33. This is an uncommon, but catastrophic and potentially lethal complication. An external splenic guard may provide some protection from direct splenic injury for children involved in sports, but no controlled data are available to determine clear evidence of effectiveness.
Portal hypertension in rare cases can be associated with protein losing enteropathy. Subsequent hypoalbuminemia can lead to edema and ascites34.
Neither splenomegaly, hypersplenism nor protein losing enteropathy can be alleviated by EBL. Thus, in some patients with portal hypertension, porto-systemic shunting may be necessary to address symptoms of portal hypertension.
Management of portal hypertension in ARPKD with porto-systemic shunts presents a complex set of variables which directly impact kidney transplantation in these patients. Utilization of portal decompressive surgical shunts are uncommon in pediatric patients. Consequently prospective long-term data are not readily available to assess their utility. The success of portal decompressive shunts in this patient population is intimately associated with the technical skill of the surgeon, the type of shunt performed and the size of the patient. In patients with good long term prognosis regarding liver disease (i.e. normal synthetic function, no cholangitis or cholestasis) surgical porto-systemic shunting is effective26. The use of shunts is not uncommon (19.8%) in ARPKD/CHF patients with portal hypertension23. However, one should consider shunt therapy with caution in patients with end-stage renal dysfunction requiring renal replacement therapy due to reports of terminal encephalopathy after shunt placement in this population35. An additional consideration in ARPKD children is the impact that surgical shunting has on the potential risks and surgical complexity of planned kidney or potential liver transplantation.
Ascending Cholangitis
Patients with CHF and Caroli disease have a high risk of ascending cholangitis (6% for CHF and 65% for Caroli syndrome) 21 and sepsis. Ascending cholangitis can develop due to presence of cystic dilation of the ducts, with bile stasis and retrograde ascent of GI bacteria. It is reasonable to assume that use of immunosuppression in kidney transplant recipients can further increase the risk of ascending cholangitis. Ascending cholangitis and sepsis is major contributor to the mortality in patients with ARPKD who received kidney transplantation6 Some studies have reported 36% overall mortality (7% - 1 year and 14% - 5 year) in patients with a kidney transplant due to ARPKD, with 80% of them related to liver disease (cholangitis, sepsis, GI bleeding)7. Data from the North American Pediatric Renal Transplantation Cooperative Study demonstrated 10% 5 year patient mortality. which was not different than in non-ARPKD kidney transplants. However, 64% of the mortality reported in such patients has been attributed to liver disease (sepsis, cholangitis)6. A new, multi-center study from France emphasized this risk in 14 patients with ARPKD who have undergone renal, or combined renal and liver transplantation21. Of the patients treated, 72% were diagnosed in the prenatal or perinatal period and among the 5 patients with Caroli’s syndrome, 3 experienced recurrent episodes of ascending cholangitis (3–5 episodes each) after renal transplantation. Of the total group of 14 patients, 3 patients (21%) died (2 renal transplant recipients and one renal and liver transplant recipient) - all as a result of ascending cholangitis. Although the number of patients reported is small, this study, combined with previously cited data, suggests that pre-emptive liver transplantation should be considered as a therapeutic option in a subgroup of ARPKD patients with severe liver disease being evaluated for renal transplantation.
Patients with ARPKD and Caroli’s syndrome, who develop high fever and/or signs of sepsis without clear cause, should be assumed to have ascending cholangitis at any time during their clinical course. Aggressive diagnostic evaluation and intervention with parenteral antibiotics is strongly recommended to prevent significant morbidity and mortality. Recurrent cholangitis can cause secondary damage to bile ducts and lead to cholestasis, or in rare cases, end stage liver disease with synthetic failure. At this point there is no consensus regarding routine use of prophylactic antibiotics for such patients1. Similarly, although synthetic bile acids (ursodiol) are sometimes recommended to increase bile flow and decrease the risk of ascending biliary infection, their effectiveness for such indications has never been studied in a controlled fashion.
Liver Tumors
Cholangiocarcinoma is almost universally lethal with 1 year mortality. Most patients with cholangiocarcinoma have been diagnosed in patients with isolated CHF and Caroli disease after 40 years of age21. Thus it is not a significant problem in pediatric patients36,37 However, given the increasingly recognized broad clinical spectrum of ARPKD, including genetic diagnosis being confirmed in adult patients, this remains a concern. Further, given the increased survival of pediatric ARPKD patients, the risk of cholangiocarcinoma should be considered as patients are transitioned to the care of internists. Other liver tumors such as adenomas and hepatocellular carcinoma, as well as adenomatous hyperplasia, have been described. Their relationship to the pathophysiology of the polycystic disorder is uncertain.
Cholestasis
The developmental abnormality of the ductal plate in combination with ascending cholangitis contributes to bile duct damage. Though jaundice is very rare, in selected patients bile duct damage (usually from ascending cholangitis) can lead to clinically apparent cholestasis. Cholestasis leads to fat and fat soluble vitamin malabsorption. This, in combination with anorexia and early satiety resulting from organomegaly and congestive gastropathy, can lead to significant nutritional challenges.
Cholestasis can impact development of osteopenia which can lead to bone fractures with minimal injury. This may exacerbate the bone disease associated with renal failure, and negatively impact quality of life for ARPKD patients.
Intractable pruritus can be a symptom of cholestasis which can severely affect sleep and school performance. Cholestasis and its complications, if severe enough to negatively affect quality of life, may lead to consideration of liver transplantation in ARPKD patients.
LIVER TRANSPLANTATION IN ARPKD
Immunosuppressive therapy is required for kidney transplantation in ARPKD, and therefore, should not be included twice in any calculation of the risk/benefit analysis of combined liver-kidney transplant. Given the immunosuppression required for renal transplantation, the additional risk of liver transplant is lower in ARPKD patients than what is expected in the classic risk/benefit model in end stage liver disease. We therefore believe that one may accept lower PELD/MELD scores to justify the benefit of combined liver-kidney transplant compared to isolated kidney transplant. For isolated liver transplant, survival benefit is apparent for patients with a PELD score greater than 1738. In the standard evaluation of survival benefit, the risk of pre-transplant mortality is compared to risk of transplant related mortality.
In ARPKD patients with indications for kidney transplantation, we assume that PELD scores will still reflect the 3 month risk of liver related mortality (though possibly underestimating risk of death from ascending cholangitis). Post-transplant risk however, is generally considered as the combination of: 1) risk related to immunosuppression; and 2) risk related to liver transplant surgery. For ARPKD patients requiring renal transplantation (and its immunosuppression), only surgical mortality should be considered in assessment of risk-benefit analysis. Liver transplant should not increase risks related to nephrotoxicity, PTLD, and infections. Surgical risk of mortality in liver transplant is below 10% in all recent reports. In 2011, 1 year patient survival for pediatric liver transplant was 95.2%, graft survival 91.7% (Studies of pediatric liver transplantation (SPLIT) registry – unpublished data). Small series of patients with liver transplant in fibrocystic liver disease (including one with combined liver –kidney transplant) had 100% 1 year graft and patient survival39. No direct clinical data exist for the unique situation of liver transplant in patients with ARPKD, renal failure and severe fibrocystic liver disease. Being extremely conservative, it is reasonable to propose that for patients who are accepted for kidney transplant additional liver transplant is likely to provide survival benefit for PELD scores more than 10. Clearly, future studies are necessary to delineate the precise threshold for survival benefit in this unique patient group.
An important, but not insurmountable, challenge for liver transplantation in the ARPKD population is the fact that the PELD/MELD scoring system does not adequately reflect mortality and morbidity of congenital hepatic fibrosis until end-stage parenchymal dysfunction develops. As we acquire more precise data regarding mortality risk secondary to the complications of portal hypertension (bleeding and shunt surgery) or ascending cholangitis in the setting of ARPKD, we can delineate, and then advocate for acceptance of more appropriate PELD/MELD scores for the unique ARPKD population.
Combined liver and kidney transplant from the same donor has been observed to be associated with a significantly lower risk for kidney allograft rejection and lower immunosuppression requirements40,41 It would be anticipated that due to the splenomegaly, hepatomegaly and enlarged bilateral kidneys, that conventional graft size matching for the recipient could be liberalized in patients with ARPKD anticipated to undergo combined liver-kidney transplantation and associated native hepatectomy and bilateral nephrectomies. The opportunity for simultaneous liver-kidney transplant from the same live donor may therefore present a unique opportunity for decreased perioperative mortality and improved patient and graft survival.
Although we lack a predictive model for risk and associated mortality from ascending cholangitis in ARPKD recipients of kidney transplantation, the data presented previously suggest that the Caroli population with recurrent ascending cholangitis prior to ESRD would be at high risk for morbidity and mortality following immunosuppression.
At this time, one cannot advocate for prophylactic liver transplantation in the majority of ARPKD patients, although emerging data suggest that patients with the combination of ARPKD and significant Caroli syndrome are at high risk for morbidity/mortality. We therefore believe that it is reasonable to consider combined organ replacement in patients who have both severe renal disease and congenital hepatic fibrosis with serious complications.
One must emphasize that such therapy can only be considered in a major pediatric organ transplant center: one with experienced transplant surgeons and the full complement of pediatric subspecialists and transplant team specialists. In the case of ARPKD patients, this obviously must include pediatric medical and surgical specialists in nephrology and hepatology.
As disease-specific therapy of ARPKD20, 42, portal hypertension and associated infections develops/improves, the benefit of combined liver-kidney transplant may decrease. On the other hand, improving outcomes of liver transplantation and the development of new artificial liver supportive therapy are likely to decrease risk of this procedure. In this rapidly changing field, clinicians must be aware of the constantly changing balance of the risks and benefits of dual organ transplantation in this unique patient population.
FRAMEWORK FOR DECISION-MAKING
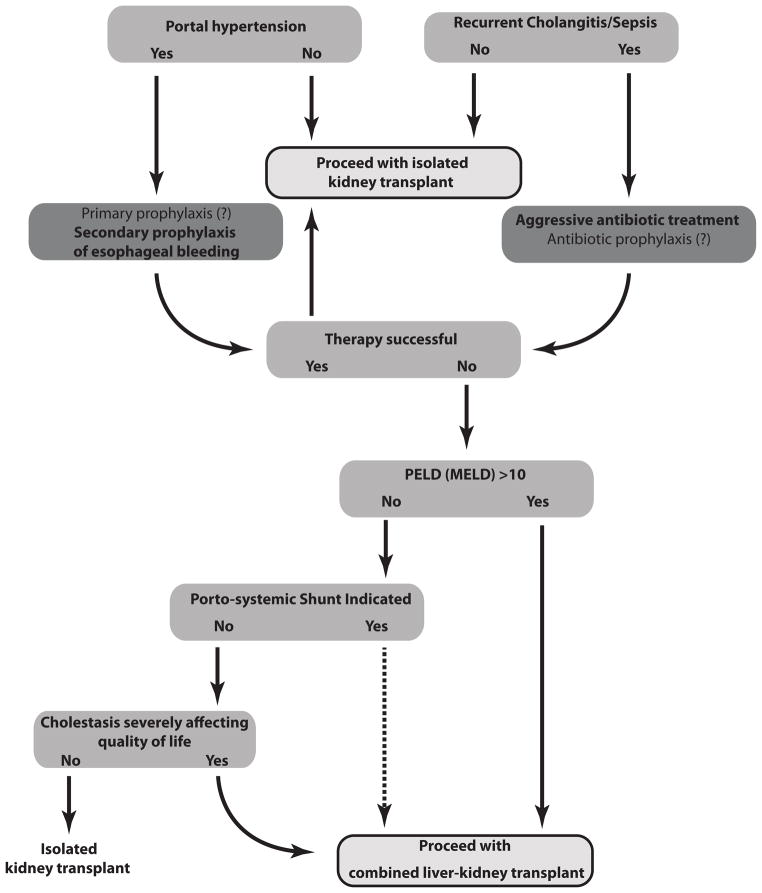
A decision tree considering the risk/benefit analysis of all therapies discussed above needs to be made for each individual ARPKD patient; and all options need to be communicated to the patient and/or their surrogate decision makers. Patient and family need to understand all available options and their risks, based on existing data. We have developed tools to guide step by step decisions, and help our clinical team make difficult decisions. (Table 1/ Figure 1).
Table I.
Risk/benefit considerations in decisions regarding kidney transplant versus combined liver-kidney transplant in ARPKD/CHF patients with dual organ involvement. Rare, uncommon problems in italics.
| Kidney Transplant | Combined Liver-Kidney Transplant |
|---|---|
| Morbidity | |
| Portal hypertension: GI bleeding, hypersplenism, protein loosing enteropathy | Surgical complications of liver transplant: primary non-function, hepatic artery thrombosis, portal vein thrombosis/stenosis, bile duct strictures. Donor complications (if living donor partial hepatectomy) |
| Cholangitis/sepsis | |
| Malignant and benign liver tumors | |
| Cholestasis: failure to thrive, bone disease, intractable pruritus | Liver rejection |
| Complications of immunosuppression: nephrotoxicity, neurotoxicity, bone marrow suppression, hearing deficits, bone disease, infections (viral, bacterial, fungal and parasitic), PTLD, kidney rejection (possible dialysis), lymphomas and other immunosuppression related malignancies. | |
| Mortality | |
| Ascending cholangitis/sepsis | Surgical complications of liver transplant: primary non-function, hepatic artery thrombosis Donor complications (if living donor partial hepatectomy) |
| Gastrointestinal bleeding | |
| Malignant and benign liver tumors | |
| Complications of porto-systemic shunt (if needed) | |
| Complications of immunosuppression: nephrotoxicity, infections (viral, bacterial, fungal and parasitic), PTLD, kidney rejection (and following dialysis), lymphomas and other immunosuppression related malignancies | |
Figure 1.
Table I. summarizes the elements of the risk benefit analysis in this unique population. We present such data to provide an overview of the factors which must be considered in making clinical decisions for patients with ARPKD. Therefore, although it is impossible to devise a clinical care algorithm for all possible clinical scenarios one may encounter in ARPKD, we consider (Figure 1) the most common clinical decisions in populations of ARPKD patients with dual organ involvement awaiting kidney transplant. Following this paradigm, only a subgroup of patients with ARPKD and severe liver disease would qualify for liver transplant. Though we lack conclusive evidence from prospective clinical studies, we present our risk/benefit analysis based on interpretation of available clinical data. Solid arrows represent decisions supported by risk/benefit analysis, and dashed arrows represent decision points supported by limited data.
We present Table I and Figure I as clinical guidelines “for the perplexed physician” based on our knowledge and impressions in early 2013. We know that the intersecting variables of: a) improved/new therapies based on new data in the molecular and cellular pathophysiology of ARPKD/CHF; and b) improvements in solid organ transplantation based on new data in immunobiology, surgical technique and artificial organ development are constantly changing. Therefore we remind all those who use these guidelines to be aware of new developments in these fields, and “above all, do no harm”.
Acknowledgments
Supported by NIH P50DK079306; and the Children’s Research Institute of the Children’s Hospital Health System of Wisconsin
Footnotes
Author contributions: Grzegorz Telega – Concept/design, drafting article, critical revision
David Cronin - Concept/design, drafting article, critical revision
Ellis D. Avner - Concept/design, drafting article, critical revision
References
- 1.GUNAY-AYGUN M, AVNER ED, BACALLAO RL, et al. Autosomal recessive polycystic kidney disease and congenital hepatic fibrosis: summary statement of a first National Institutes of Health/Office of Rare Diseases conference. J Pediatr. 2006;149(2):159–64. doi: 10.1016/j.jpeds.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ROY S, DILLIN MJ, TROMPETER RS, BARRATT TM. Autosomal recessive polycystic kidney disease: long-term outcome of neonatal survivors. Pediatr Nephrol. 1997;11:302–306. doi: 10.1007/s004670050281. [DOI] [PubMed] [Google Scholar]
- 3.ADEVA M, EL-YOUSSEF M, ROSETTI S, KAMATH PS, KUBLY V, CONSUGAR MB, MILLINER DM, KING BF, TORRES VE, HARRIS PC. Clinical and molecular characterisations defines a broadened spectrum of autosomal recessive polycystic disease (ARPKD) Medicine. 2006;85:1–21. doi: 10.1097/01.md.0000200165.90373.9a. [DOI] [PubMed] [Google Scholar]
- 4.BLYTH H, OCKENDEN BG. Polycystic disease of kidney and liver presenting in childhood. J Med Genet. 1971;8:257–284. doi: 10.1136/jmg.8.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.TURKBEY B, OCAK I, DARYANANI K, FONT-MONTGOMERY E, LUKOSE L, BRYANT J, TUCHMAN M, MOHAN P, HELLER T, GAHL WA, CHOIKE PL, GUNAY-AYGUN M. Autosomal recessive polycystic kidney disease and congenital hepatic fibrosis (ARPKD/CHF) Pediatr Radiol. 2009;39:100–111. doi: 10.1007/s00247-008-1064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DAVIS ID, HO M, HUPERTZ V, AVNER ED. Survival of childhood polycystic kidney disease following renal transplantation: The impact of advanced hepatobiliary disease. 2003;7:364–369. doi: 10.1034/j.1399-3046.2003.00094.x. [DOI] [PubMed] [Google Scholar]
- 7.KHAN K, SCHWARZENBERG SJ, SHARP HL, MATAS AJ, CHAVERS BM. Morbidity from congenital hepatic fibrosis after renal transplantation for autosomal recessive polycystic kidney disease. Am J Transplant. 2002;2:360–5. doi: 10.1034/j.1600-6143.2002.20412.x. [DOI] [PubMed] [Google Scholar]
- 8.MCDIARMID SV, ANAND R, MARTZ K, MILLIS MJ, MAZARIEGOS G. Multivariate Analysis of Pre-, Peri-, and Post-Transplant Factors Affecting Outcome After Pediatric Liver 3. Ann Surg. 2011 Jul;254(1):145–54. doi: 10.1097/SLA.0b013e31821ad86a. [DOI] [PubMed] [Google Scholar]
- 9.BUCUVALAS J. Long-term outcomes in pediatric liver transplantation. Liver Transpl. 2009;15 (Suppl 2):S6–11. doi: 10.1002/lt.21915. [DOI] [PubMed] [Google Scholar]
- 10.NAKANISHI K, SWEENEY WE, JR , ZERRES K, GUAY-WOODFORD LM, AVNER ED. Proximal tubular cysts in fetal human autosomal recessive polycystic kidney disease. J Am Soc Nephrol. 2000;11:760–763. doi: 10.1681/ASN.V114760. [DOI] [PubMed] [Google Scholar]
- 11.ZERRES K, MUCHER G, BECKER J, et al. Prenatal diagnosis of autosomal recessive polycystic kidney disease (ARPKD): molecular genetics, clinical experience, and fetal morphology. Am J Med Genet. 1998;76:137–144. [PubMed] [Google Scholar]
- 12.DELL KM, AVNER ED. Autosomal recessive polycystic kidney disease: GeneClinics: Clinical Genetic Information Resource [database online] Copyright. University of Washington; Seattle: Available at http://wwwgeneclinicsorg. Initial posting July 2001 updated July 2011. [Google Scholar]
- 13.DELL KM, SWEENEY WE, AVNER ED. Polycystic Kidney Disease. In: Avner ED, Harmon WE, Niaudet P, editors. Pediatric Nephrology. 6. Berlin, Heidelberg: Springer-Verlag; 2009. pp. 849–887. [Google Scholar]
- 14.KAMATH BM, PICCOLI DA. Heritable disorders of the bile ducts. Gastroenterol Clin North Am. 2003;32:857–75. doi: 10.1016/s0889-8553(03)00054-2. [DOI] [PubMed] [Google Scholar]
- 15.BERGMANN C, FRANK V, KUPPER, KAMITZ D, HANTEN J, BERGES P, MAGER S, MOSER M, KIRFEL J, BUTTNER R, SENDEREK J, ZERRES K. Diagnosis, pathogenesis, and treatment prospects in cystic kidney disease. Molecular Diagnosis & Therapy. 2006;10(3):163–74. doi: 10.1007/BF03256455. [DOI] [PubMed] [Google Scholar]
- 16.SCHNEIDER BL, MAGID MS. Liver disease in autosomal recessive polycystic kidney disease. Pediatr Transplant. 2005;9:634–9. doi: 10.1111/j.1399-3046.2005.00342.x. [DOI] [PubMed] [Google Scholar]
- 17.ROY S, DILLON MJ, TROMPETER RS, BARRATT TM. Autosomal recessive polycystic kidney disease: long-term outcome of neonatal survivors. Pediatr Nephrol. 1997;11:302–6. doi: 10.1007/s004670050281. [DOI] [PubMed] [Google Scholar]
- 18.ZERRES K, RUDNIK-SCHONEBORN S, DEGET F, HOLTKAMP U, BRODEHL J, GEISERT J, SCHARER K. Autosomal recessive polycystic kidney disease. J Mol Med. 1998b;76:303–9. doi: 10.1007/s001090050221. [DOI] [PubMed] [Google Scholar]
- 19.WEN J. Congenital Hepatic Fibrosis In Autosomal Recessive Polycystic Kidney Disease. Clin Transl Sci. 2011;4:460–465. doi: 10.1111/j.1752-8062.2011.00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.SWEENEY WE, Jr, AVNER ED. Diagnosis and management of childhood polycystic kidney disease. Pediatr Nephrol. 2011;26:675–92. doi: 10.1007/s00467-010-1656-1. [DOI] [PubMed] [Google Scholar]
- 21.CHAPAL M, DEBOOT A, DUFAY A, SALOMON R, ROUSSEY G, BURTEY S, LAUNAY EA, VIGNEAU C, BLANCHO G, LOIRAT C, HOURMANT M, FAKHOURI F. Kidney and liver transplantation in patients with autosomal recessive polycystic kidney disease: a multicentric study. Nephrol Dial Transplant. 2012;27:2083–2088. doi: 10.1093/ndt/gfr588. [DOI] [PubMed] [Google Scholar]
- 22.GUAY-WOODFORD LM, DESMOND RA. Autosomal recessive polycystic kidney disease and congenital hepatic fibrosis. Pediatrics. 2003;111:1072–80. doi: 10.1542/peds.111.5.1072. [DOI] [PubMed] [Google Scholar]
- 23.SRINATH A, SHNEIDER BL. Congenital hepatic fibrosis and autosomal recessive polycystic kidney disease. Journal of Pediatric Gastroenterology & Nutrition. 2012;54(5):580–7. doi: 10.1097/MPG.0b013e31824711b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.FOX VL, CARR-LOCKE DL, CONNORS PJ, LEICHTNER A. Endoscopic ligation of esophageal varices in children. J Pediatr Gastroenterol Nutr. 1995;20 (2):202–8. doi: 10.1097/00005176-199502000-00011. [DOI] [PubMed] [Google Scholar]
- 25.REINOSO MA, SHARP HL, RANK J. Endoscopic variceal ligation in pediatric patients with portal hypertension secondary to liver cirrhosis. Gastrointest Endosc. 1997;46(3):244–6. doi: 10.1016/s0016-5107(97)70094-4. [DOI] [PubMed] [Google Scholar]
- 26.SHNEIDER BL, BOSCH J, DE FRANCHIS R, EMRE SH, GROSZMANN RJ, LING SC, LORENZ JM, SQUIRES RH, SUPERINA RA, THOMPSON AE, MAZARIEGOS GV. Portal Hypertension in Children: Expert pediatric opinion on the report of Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. Pediatric Transplantation. 2012;16:426–437. doi: 10.1111/j.1399-3046.2012.01652.x. [DOI] [PubMed] [Google Scholar]
- 27.HOU MC, LIN HC, KUO BIT. Comparison of endoscopic variceal injection sclerotherapy and ligation for the treatment of esophageal variceal hemorrhage: A prospective randomized trial. Hepatology. 1995;21(6):1517–1522. [PubMed] [Google Scholar]
- 28.ZARGAR SA, JAVID G, KHAN BA, YATTOO GN, SHAH AH, GULZAR GM, SINGH J, REHMAN BU, DIN Z. Endoscopic ligation compared with sclerotherapy for bleeding esophageal varices in children with extrahepatic portal venous obstruction. Hepatology. 2002;36(3):666–72. doi: 10.1053/jhep.2002.35278. [DOI] [PubMed] [Google Scholar]
- 29.SCHREIBER RA. Propranolol and portal hypertension: should kids be on the block? J Pediatr Gastroenterol Nutr. 1999;29(1):10–1. doi: 10.1097/00005176-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 30.IMPERIALE TF, CHALASANI N. A meta-analysis of endoscopic variceal ligation for primary prophylaxis of esophageal variceal bleeding. Hepatology. 2001 Apr;33(4):802–7. doi: 10.1053/jhep.2001.23054. [DOI] [PubMed] [Google Scholar]
- 31.CELIŃSKA-CEDRO D, TEISSEYRE M, WOYNAROWSKI M, SOCHA P, SOCHA J, RYZKO J. Endoscopic ligation of esophageal varices for prophylaxis of first bleeding in children and adolescents with portal hypertension: preliminary results of a prospective study. J Pediatr Surg. 2003;38(7):1008–11. doi: 10.1016/s0022-3468(03)00181-7. [DOI] [PubMed] [Google Scholar]
- 32.RIVET C, ROBLES-MEDRANDA C, DUMORTIER J, LE GALL C, PONCHON T, LACHAUX A. Endoscopic treatment of gastroesophageal varices in young infants with cyanoacrylate glue: a pilot study. Gastrointest Endosc. 2009;69(6):1034–8. doi: 10.1016/j.gie.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 33.KISEWETTER WB, PATRICK DB. Childhood splenectomy: indications for and results from. American Surgeon. 1971;37(3):135–9. [PubMed] [Google Scholar]
- 34.ALKHOURI N, CARTER-KENT C, MAYACY S, HUPERTZ V, EGHTESAD B, QUINTINI C, FUNG J, RADHAKRISHNAN K. Reversal of Protein-losing Enteropathy after Liver Transplantation in a Child with Idiopathic Familial Neonatal Hepatitis. Liver Transplantation. 2009;15:1894–1896. doi: 10.1002/lt.21856. [DOI] [PubMed] [Google Scholar]
- 35.TSIMARATOS M, CLOAREC S, ROQUELAURE B, RETORNAZ K, PICON G, CHABROL B, GUYS JM, SARLES J, NIVET H. Chronic renal failure and portal hypertension--is portosystemic shunt indicated? Pediatr Nephrol. 2000;14(8–9):856–8. doi: 10.1007/s004679900268. [DOI] [PubMed] [Google Scholar]
- 36.DHALLUIN-VENIER V, FABRE M, JACQUEMIN E, RANGHEARD A-S, PELLETIER G, BUFFET C. Liver cell adenomas and portosystemic shunt. Gastroenterologie Clinique et Biologique. 2008;32(2):164–6. doi: 10.1016/j.gcb.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 37.JAIN D, NAYAK NC, SAIGAL S. Hepatocellular carcinoma arising in association with von-Meyenburg’s complexes: an incidental finding or precursor lesions? A clinicopatholigic study of 4 cases. Annals of Diagnostic Pathology. 2010;14(5):317–20. doi: 10.1016/j.anndiagpath.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 38.BARSHES NR, LEE TC, UDELL IW, O’MAHONEY CA, KARPEN SJ, CARTER BA, GOSS JA. The pediatric end-stage liver disease (PELD) model as a predictor of survival benefit and posttransplant survival in pediatric liver transplant recipients. Liver Transplantation. 2006;12(3):475–80. doi: 10.1002/lt.20703. [DOI] [PubMed] [Google Scholar]
- 39.KO J, YI NJ, SUH KS, SEO JK. Pediatric liver transplantation for fibrocystic liver disease. Pediatric Transplantation. 2012;16:195–200. doi: 10.1111/j.1399-3046.2012.01661.x. [DOI] [PubMed] [Google Scholar]
- 40.BAAN CC, WEIMAR W. How does auxiliary liver transplantation regulate alloreactivity in sensitized kidney transplant patients? Transplantation. 2011;91(8):823–4. doi: 10.1097/TP.0b013e3182100f9a. [DOI] [PubMed] [Google Scholar]
- 41.CHAVA SP, SINGH B, STANGOU A, BATTULA N, BOWLES M, O’GRADY J, RELA M, HEATON ND. Simultaneous combined liver and kidney transplantation: a single center experience. Clinical Transplantation. 2010;24(3):E62–8. doi: 10.1111/j.1399-0012.2010.01168.x. [DOI] [PubMed] [Google Scholar]
- 42.SWEENEY WE, JR, VON VIGIER RO, FROST P, AVNER ED. Src Inhibition Ameliorates Polycystic Kidney Disease. J Am Soc Nephrol. 2008;7:1331–1341. doi: 10.1681/ASN.2007060665. [DOI] [PMC free article] [PubMed] [Google Scholar]