Abstract
Background
Depression is common during and after breast cancer treatment. However, the role of specific therapeutic modalities and related biological mechanisms remains unclear. Radiation is an essential component of breast conserving therapy and may contribute to depression in breast cancer patients through activation of inflammatory pathways.
Methods
Depressive symptoms and inflammatory mediators including nuclear factor kappa B (NF-kB) were assessed at baseline (before radiation), during, and 6 weeks after radiation in 64 women with Stage 0–IIIA breast cancer.
Results
No significant increases in depressive symptoms occurred during or after radiation, although a number of patients exhibited moderate-to-severe depression throughout the study. Multivariate analyses of baseline factors predictive of depression revealed that educational status, perceived stress, prior chemotherapy and peripheral blood NF-kB DNA binding were all independent predictors of persistent depressive symptoms following radiation (all p<0.05). Of these factors, only prior chemotherapy was associated with inflammatory mediators including NF-kB DNA binding, soluble tumor necrosis factor-alpha receptor 2 and interleukin-6, which in univariate analyses predicted depressive symptoms following radiation (all p<0.05). Chemotherapy-treated patients also exhibited an overrepresentation of gene transcripts regulated by NF-KB.
Conclusions
Radiation was not associated with increased depressive symptoms, but of disease and treatment-related factors, prior chemotherapy predicted significant depression following radiation. Longitudinal studies are warranted to investigate the relationship among prior chemotherapy, inflammation, and persistent depression following breast cancer treatment.
Keywords: Breast Cancer, Depression, Radiation, Chemotherapy, Inflammation, Nuclear Factor Kappa B
INTRODUCTION
Depression is a common acute and potentially long-term debilitating behavioral toxicity of breast cancer and its treatment, occurring in up to 30% of women with breast cancer.1–6 Depression has also been associated with increased breast cancer mortality,7,8 and clinical trials that reduced depressive symptoms have been shown to increase survival in women with metastatic disease.7,9 Many factors may contribute to depression including age at diagnosis, tumor stage, surgery, and chemotherapy.1,3,5,6 However, there is limited data on depression in breast cancer patients undergoing radiation.
Recent data indicate that radiation provides a significant survival advantage to patients following breast conserving surgery.10 However, predictors of depression during and after radiation remain largely unexplored as studies are limited by small patient numbers, cross-sectional designs, and retrospective, secondary analyses.2,4,6,11 Furthermore, among the few longitudinal studies of depression during radiation, subjects have been treated in a highly varied manner (lumpectomy versus (vs.) mastectomy, +/− chemotherapy, and varying radiation doses), and within this literature, there are a number of inconsistencies regarding symptom trajectory and severity as well as clinical and psychosocial predictors of depression.1–3 Clinical factors associated with increased depression during radiation include advanced cancer stage, mastectomy, prior chemotherapy, higher radiation duration/dose, younger age, and increased body mass index (BMI).2–4,12 However, data also suggest that depression is related to baseline psychosocial characteristics including education level, relationship status, and anxiety and distress.13,14 Collectively, these reports suggest the need for prospective, longitudinal studies of patients uniformly treated with standardized surgery and radiation controlling for relevant clinical and psychosocial characteristics to clarify primary risk factors for depression, especially depression that persists after treatment.
One proposed mechanism linking depression to cancer treatments including radiation is inflammation. Increased inflammatory markers are found in patients with depression, and administration of inflammatory cytokines leads to depressive symptoms.15 Recent data also suggest that blockade of inflammatory cytokines reduces depressive symptoms, specifically fatigue, in cancer patients.16
Although radiation is known to cause tissue injury and induce a subsequent inflammatory response, only one study has evaluated the relationship between depression and inflammation in women undergoing breast radiation.6 In this study, soluble interleukin-6 receptor (sIL-6R) was found to be significantly elevated in patients with high vs. low depression.6 Many more studies have examined inflammatory mediators of fatigue, which is included in the diagnostic criteria for depression.3,11,15,17–20 Nevertheless, results from these studies have been inconsistent possibly due to varying strategies for measuring cytokines and lack of longitudinal data.11,18,19 Fatigue during radiation has been associated with increased IL-6, IL-1 receptor antagonist (IL-1ra), and C-reactive protein (CRP).17,18 However, other investigators have not found this relationship after controlling for factors including BMI.3,6,19
Although the data suggest a potential relationship among depression, inflammation, and radiation, the inflammatory signaling pathways have not been explored. One candidate pathway involves nuclear factor kappa B (NF-kB). NF-kB is a lynchpin signaling molecule in the inflammatory cascade and is implicated in cancer development and treatment resistance.20,21 Fatigued breast cancer survivors have increased activation of NF-kB-regulated genes.22 Because radiation increases NF-kB pathway activity in breast cancer cells,21 NF-kB activation may extend beyond the breast to peripheral tissues as a general response to tissue injury, and ultimately contribute to behavioral morbidities including depression. Of note, chemotherapy has been associated with NF-kB activation within breast cancer tissue and in peripheral blood.16,23
To further explore clinical and inflammatory factors associated with depression in women undergoing radiation, a longitudinal study was conducted before, during, and after a standardized course of radiation. The primary objective was to determine which factors were predictive of persistent depressive symptoms following radiation. In addition, we examined the relationship between the clinical factors predictive of depression and inflammation including circulating inflammatory biomarkers and inflammatory gene transcripts. Special emphasis was placed on the potential role of NF-kB, and its downstream mediators, TNF (tumor necrosis factor), IL-1, and IL-6.
MATERIALS AND METHODS
Subjects
From 03/2010-11/2011, patients were recruited from the Emory University Department of Radiation Oncology. Eligible women were ages 18–75 years with Stage 0–IIIA breast cancer who had undergone breast conserving surgery with or without chemotherapy given neoadjuvantly or adjuvantly.
Subjects were excluded for medical conditions that might directly influence the immune response including pregnancy, autoimmune/inflammatory disorders and uncontrolled cardiovascular, metabolic, pulmonary or renal disease. Subjects with history of major psychiatric disorder including schizophrenia or bipolar disorder or substance abuse/dependence within the past year were also excluded. Patients with depression were not excluded, and the use of antidepressant medications was allowed. Drugs known to affect the immune system (e.g. glucocorticoids, methotrexate) excluding over-the-counter anti-inflammatory medications were not permitted. Caucasians and African Americans were enrolled due to the paucity of other racial groups in the community.
All patients were prescribed 50.0 Gy to whole breast followed by a 10.0–16.0 Gy boost to the lumpectomy cavity given in 2.0 Gy fractions with 6 megavoltage (MV) and/or 18MV photons using standard tangential field technique. Treatment plans were designed according to International Commission on Radiation Units & Measurements (ICRU-50) guidelines.24 Subjects underwent clinical and behavioral assessments and peripheral blood sampling at three time points: baseline (one week before radiation) (T1), week 6 of radiation (T2), and 6 weeks after radiation completion (T3). Study procedures were approved a priori by the Emory University IRB, and all subjects provided written informed consent.
Behavioral Assessments
Depression was assessed using the Inventory of Depressive Symptomatology-Self Report (IDS-SR),25 a 30-item scale measuring all symptom domains used to make a diagnosis of depression included in the Diagnostic and Statistical Manual of Mental Disorders-IV. Higher scores indicate increased severity, and a score of ≥33 points is indicative of moderate-to-severe depression.25 The IDS-SR has been validated in diverse patient populations including cancer patients.26,25 Subjects also completed the 20-item Multidimensional Fatigue Inventory (MFI) to assess fatigue.27 Because previous studies have shown a relationship between baseline distress and depression, distress was assessed by the perceived stress scale (PSS), which has been used in multiple populations including breast cancer patients undergoing radiation.2,28
NF-kB DNA Binding and Downstream Inflammatory Markers
Peripheral blood samples were drawn between 8–11 am (to reduce circadian effects) at all three time points. Plasma was separated and stored at −80°C until subsequent batch assay. Peripheral blood mononuclear cells (PBMCs) were isolated using density gradient centrifugation and stored in freezing serum (90% fetal bovine serum, 10% DMSO) at −80°C until nuclear extraction or mRNA isolation.
DNA-binding of NF-kB in PBMCs was determined by enzyme-linked immunosorbent assay (ELISA) as previously described (Active Motif [Carlsbad, CA]).29 NF-kB DNA binding was performed in 58, 60, and 57 of the 64 subjects at T1, T2, and T3, respectively, based on sample availability/quality. Plasma sTNFR2, IL-1ra, and IL-6 were assayed in duplicate using sandwich ELISA (R & D Systems, Minneapolis, MN). The mean inter- and intra-assay coefficients of variation were 10% or less. CRP was measured by the immunoturbidometric method using the Beckman AU 480 chemistry analyzer and the Ultra WR CRP reagent kit (Sekisui Diagnostics, Framingham, MA). Inter- and intra-assay coefficients of variation were less than 3%.
Microarray Analysis
Total RNA was extracted from PBMCs using RNeasy kits (QIAGEN, Valencia, CA). After extraction, RNA samples were dissolved in RNase-free water, and their concentrations and the A260/280 ratio were determined using the MBA 2000 System (Perkin-Elmer, Shelton, CT, USA). Each sample was linearly amplified by WT-Ovation RNA amplification system (NuGEN) and used for microarray analysis. After hybridization to Illumina HumanHT-12 Expression BeadChips (Illumina, San Diego, CA), BeadChips were scanned on the Illumina BeadArray Reader to determine probe fluorescence intensity. Raw probe intensities were normalized by quantile normalization algorithm.30
Statistics
Wilcoxon rank-sum tests were used to test differences in continuous or ordinal variables between groups defined by binary variables (e.g. chemotherapy- vs. non-chemotherapy-treated patients). Fisher exact tests were used to test association between categorical variables. Spearman correlation coefficients were computed to determine univariate relationships between variables. Multiple linear regression was used to examine associations among relevant variables. Cytokine concentrations were skewed and therefore log-transformed before analyses.
To identify functional biological processes overrepresented in genes differentially regulated in chemotherapy vs. non-chemotherapy-treated patients (see below), as well as transcriptional regulatory pathways driving observed differences in gene expression, gene ontology (GO) and transcription factor bioinformatic analyses were conducted. These methods are most accurate with relatively large numbers of genes showing large biological differences in expression. Therefore, differentially regulated gene transcripts were identified using an effect size of ≥20% difference (1.2 fold change) corresponding to a <10% false discovery rate15,31 and cutoff of p≤0.05, and then subjected to the stringency of bioinformatic analyses to ensure statistical reliability. The Database for Annotation, Visualization, and Integrated Discovery (DAVID) functional annotation clustering tool was used for GO analysis,32 which employed a modified Fisher Exact test to determine whether a gene list was enriched for genes involved in relevant biological processes. A network-based transcription factor analysis was conducted with MetaCore software (GeneGo, Inc, St. Joseph, MI) using an algorithm designed to query a manually-curated database that has been determined to be both accurate and comprehensive in identifying transcriptional regulatory pathways and target genes, including those regulated by NF-kB.33
RESULTS
Patient/Tumor Characteristics
Sixty-four women with Stage 0–IIIA breast cancer meeting eligibility criteria agreed to participate. Eighteen eligible women refused participation, primarily due to time commitment, blood sampling requirements or lack of interest. No significant differences were found between patients who did or did not agree to participate. One patient dropped out prior to T2 and another dropped out prior to T3. Clinical and psychosocial characteristics of participating subjects are listed in Table 1. The higher percentage of women with stage 0/I (61%) partially accounted for the lower percentage of chemotherapy-treated patients (38%).
Table 1.
Patient Characteristics
| Characteristic | Overall N=64 |
No Chemotherapy N=40 |
Chemotherapy N=24 |
|---|---|---|---|
|
| |||
| Age | |||
| Mean (range) | 56(35–74) | 59(35–74) | 52(35–70)* |
|
| |||
| Race | |||
| Caucasian | 37(58%) | 25(62.5%) | 12(50%) |
| African American | 27(42%) | 15(37.5%) | 12(50%) |
|
| |||
| BMI | |||
| Mean (range) | 28.8(20–54) | 29.4(21–54) | Mean 27.7(20–39) |
| ≥25 | 48(75%) | 30(61%) | 19(79%) |
| ≥30 | 21(33%) | 17(43%) | 7(29%) |
|
| |||
| Stage | |||
| DCIS | 16(25%) | 16(40%) | 0(0%)* |
| I | 23(36%) | 17(42.5%) | 6(25%) |
| II | 21(33%) | 7(17.5%) | 14(58.3%) |
| III | 4(6%) | 0(0%) | 4(16.8%) |
|
| |||
| Baseline Antidepressant Use | 14(21.9%) | 6(15%) | 8(33.3%) |
|
| |||
| Married | |||
| Yes | 33(52%) | 23(57.5%) | 10(41.7%) |
|
| |||
| Income | |||
| <60K | 39(61%) | 16(54.3%) | 15(71.5%) |
| ≥60K | 25(39%) | 19(45.4%) | 6(28.6%) |
|
| |||
| Education | |||
| ≤High School | 24(37.5%) | 12(30%) | 12(50%) |
| ≥College | 40(62.5%) | 28(70%) | 12(50%) |
p<0.05 compared to non-chemotherapy-treated subjects
Depression Symptoms
Compared to baseline, depressive symptoms did not significantly increase during or after radiation (Mean IDSSR score – T1: 15.9+/−11.5; T2: 15.8+/−12.2; and T3: 14.8 +/−12.1; p=NS). Nevertheless, a number of patients exhibited moderate-to-severe depression (IDSSR ≥ 33) throughout the study including 9% at T1, 16% at T2 and 15% at T3. Fatigue severity also did not change during or after radiation. Fatigue and depression scores were highly correlated at all time points (p<0.001).
Multivariate analysis including baseline clinical, psychosocial and inflammatory variables revealed that educational status (p=0.007), perceived stress (p=0.03), chemotherapy (p=0.03), and NF-kB DNA binding (p=0.04) were all independently predictive of persistent depression 6 weeks after radiation. Age, race, initial cancer stage, income, marital status, hemoglobin, BMI, endocrine therapy and antidepressant usage were not associated with depressive symptoms following radiation and did not improve the multivariate model.
Of note, many of the factors predictive of depression were inter-related. For example, both lower educational status and prior chemotherapy treatment were associated with higher PSS scores (high school graduates or less: 16.8+/−7.7 vs. college graduates: 12.4+/−6.8, p=0.03; chemotherapy-treated: 17.3+/−6.8 vs. non-chemotherapy treated: 12.1+/−7.2, p=0.008). However, among these factors, only chemotherapy was associated with inflammatory mediators including NF-kB DNA binding, sTNFR2 and IL-6 (see below).
Chemotherapy
As indicated above, prior chemotherapy was the only disease or treatment–related factor that was associated with depression following radiation. As shown in Figure 1, although neither chemotherapy nor non-chemotherapy (radiation alone)-treated patients exhibited an increase in depressive symptoms during or after radiation, chemotherapy-treated patients had significantly higher scores of depression at all time points compared with non-chemotherapy-treated patients (all p<0.01). Similar results were found with fatigue. In addition, before radiation, 25% of chemotherapy-treated vs. 0% of non-chemotherapy-treated patients exhibited moderate-to-severe depression (p<0.005), and after radiation, 29% of chemotherapy-treated patients exhibited moderate-to-severe depression vs. 5% in non-chemotherapy treated subjects (p=0.02). Median time between last cycle of chemotherapy and first day of radiation was 11.9 weeks (range 3.6–21.9). No difference in depression or fatigue severity was found at any time point between subjects receiving neoadjuvant (n=17) vs. adjuvant (n=7) chemotherapy or between subjects receiving anthracyline (n=12) vs. non-anthracycline (n=12) based chemotherapy (all p=ns). Interestingly, after radiation, significantly more chemotherapy-treated subjects were on antidepressants than non-chemotherapy-treated patients [8 (33.3%) vs. 3 (7.5%), respectively, p=0.01]. Chemotherapy-treated patients were more likely to be below age 50 (46% vs. 13%, p<0.01), have Stage II/III disease (75% vs. 18%, p<0.001), and have tumors that were either hormone receptor negative or Her2+ (p<0.01) (see Table 1).
Figure 1. Depression and fatigue before, during, and after radiation in breast cancer patients treated with or without chemotherapy.
Prior chemotherapy-treated patients exhibited significantly higher depression and fatigue scores at all time points compared to non-chemotherapy-treated patients. IDS-SR-Inventory of Depressive Symptoms-Self Report; MFI-Multidimensional Fatigue Inventory. *p<0.01, **p<0.001 compared to non-chemotherapy-treated subjects.
NF-kB DNA Binding and Downstream Inflammatory Mediators
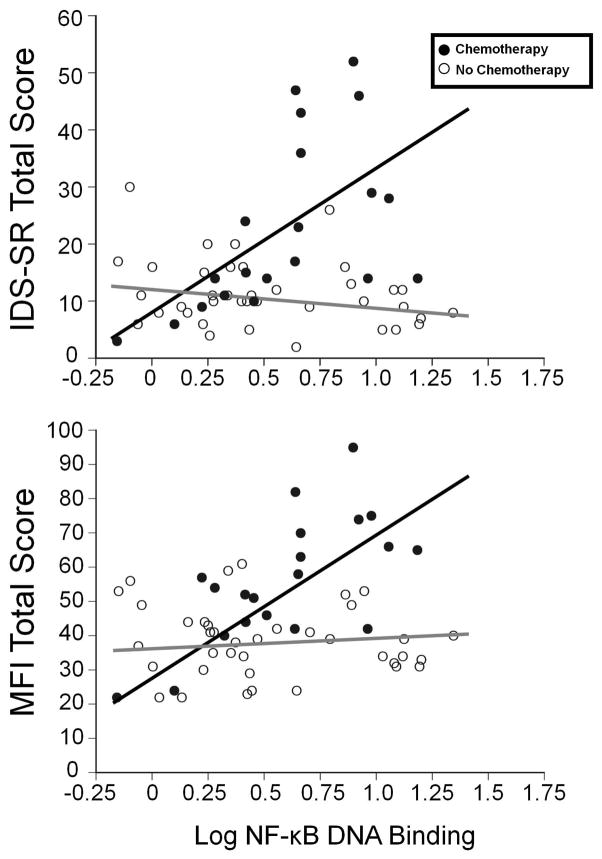
As previously indicated, multivariate analysis revealed that NF-kB DNA-binding at baseline was associated with depressive symptoms following radiation. Univariate analyses revealed that baseline NF-kB DNA binding was also significantly correlated with depressive symptoms at baseline and during radiation, but only in patients who were previously treated with chemotherapy (r=0.64, p<0.005 and r=0.63, p=0.03, respectively) (Figure 2). NF-kB DNA-binding was also correlated with fatigue in chemotherapy-treated but not non-chemotherapy-treated patients throughout the study (all p<0.05)
Figure 2. NF-kB DNA binding and depression and fatigue before radiation in breast cancer patients treated with or without chemotherapy.
Peripheral blood NF-kB DNA binding positively correlated with depression and fatigue in chemotherapy-treated (n=20) but not non-chemotherapy-treated (n=38) women (both p<0.01).
To better understand how chemotherapy was related to inflammatory mediators downstream of NF-kB, we conducted multivariate analyses controlling for age, BMI, PSS, antidepressant usage, hormonal therapy, initial cancer stage, educational level, income, race, and marital status. Baseline concentrations of IL-6 (p=0.002) and sTNFR2 (p=0.003) were both significantly higher in chemotherapy vs. non-chemotherapy-treated patients (Table 2) (p<0.05). CRP was also increased in chemotherapy-treated patients, but the difference was not statistically significant (p=0.06). Univariate analyses of the relationship between baseline inflammatory mediators and behavioral endpoints revealed a significant correlation between baseline IL-6 and both depression and fatigue at T3 (r=0.32, df=60, p=0.01 and r=0.44, df=61, p<0.01, respectively). Moreover, baseline sTNFR2 and CRP correlated with fatigue (but not depression) at T3 (r=0.31, df=60, p=0.02 and r=0.38, df=61, p=0.002, respectively). No correlations between behavioral endpoints and baseline IL-1ra were found. As noted previously, radiation treatment itself was not associated with increases in depression or fatigue, and no significant increases in peripheral inflammatory markers were found during or after radiation in multivariate analyses controlling for the variables noted above (all p=ns).
Table 2.
Chemotherapy Status and Baseline Inflammatory Biomarkers
| Inflammatory Biomarker Mean(SD) |
Overall N=58 |
No Chemotherapy N=38 |
Chemotherapy N=20 |
|---|---|---|---|
| Interleukin-6 (pg/mL) | 3.1(2.7) | 2.5(2.4) | 4.0(2.9)* |
| Soluble tumor necrosis factor receptor 2 (pg/mL) | 3.2(1.6) | 2.8(0.8) | 4.0(2.7)* |
| Interleukin-1 receptor antagonist (pg/mL) | 736.4(769.1) | 721.6(805.1) | 760.5(723.0) |
| hs-C-reactive protein (mg/L) | 4.9(11.7) | 2.6(2.9) | 8.8(18.4) |
| Nuclear factor kappa B DNA Binding (ng/well) | 5.2(4.9) | 5.3(5.4) | 5.2(3.4) |
p<0.01 compared to non-chemotherapy-treated subjects
Gene Expression
Based on the relationship among chemotherapy, NF-kB DNA binding, and persisting depression found 6 weeks after radiation, we examined the expression of genes in chemotherapy vs. non-chemotherapy-treated patients at T1. 340 gene transcripts were differentially expressed: 128 were up-regulated and 212 were down-regulated in chemotherapy vs. non-chemotherapy-treated patients. As shown in Figure 3, the top two biological processes represented in the GO analysis were the immune and defense response. MetaCore analysis of genes differentially expressed in chemotherapy vs. non-chemotherapy-treated patients revealed an overrepresentation of genes regulated by NF-kB family transcription factors, including RelA, p65 subunit of NF-kB (Z=99.9, p=5.7×10−89) and NF-kB complex (Z=89.4, p=2.3×10−71). Transcription factor analysis of all differentially expressed genes identified 48 NF-kB-regulated genes, corresponding to 60 NF-kB-regulated gene transcripts (Figure 2). Of 60 NF-kB-regulated transcripts, 38 were increased (30.0% of 128 up-regulated gene transcripts) and 22 were decreased (10.4% of 212 down-regulated transcripts) in chemotherapy-treated patients.
Figure 3. Over-represented biological processes and NF-kB-mediated transcription factor pathways in differentially expressed genes in chemotherapy vs. non-chemotherapy-treated breast cancer patients before radiation.
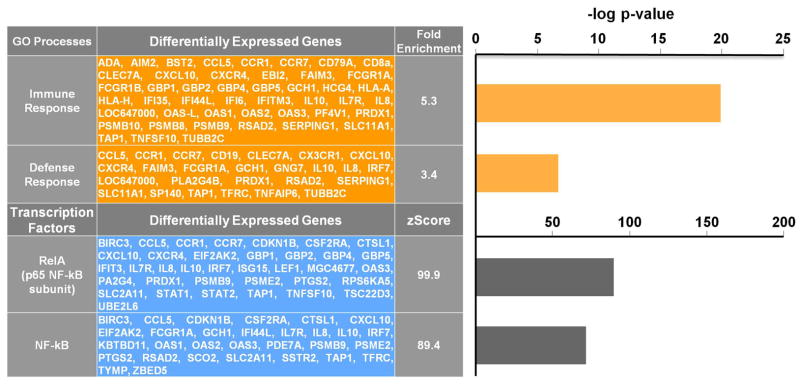
Chemotherapy was associated with an overrepresentation of immune and defense response genes and genes regulated by NF-kB family transcription factors.
DISCUSSION
Prior chemotherapy was associated with significantly higher depression scores before, during and after breast cancer radiation independent of chemotherapy type or whether it was given neoadjuvantly or adjuvantly, underscoring a persisting effect of chemotherapy up to several months after the last cycle of treatment. Only women treated with chemotherapy had increased expression of NF-kB-regulated gene transcripts and increased plasma downstream inflammatory mediators IL-6 and sTNFR2. In addition, baseline NF-kB DNA binding independently predicted depression after radiation controlling for multiple clinical factors including BMI, initial cancer stage, age, endocrine therapy, and anti-depressant usage. Thus, chemotherapy-induced inflammation could be an important mechanism by which breast cancer patients develop depression, with those previously treated with chemotherapy being most at risk for both increased inflammation and depression during and after radiation.
Significant increases in expression of NF-kB-regulated gene transcripts were found in chemotherapy-treated patients, and NF-kB DNA binding was associated with depressive symptoms in chemotherapy-but not non-chemotherapy-treated patients throughout the study. Chemotherapy may activate NF-kB through the destruction of rapidly proliferating malignant and non-malignant cells leading to an inflammatory response. Moreover, chemotherapy can directly activate NF-kB signaling pathways in multiple cell types.34 Relevant to depression, NF-kB activation induces inflammatory cytokines which can access the brain in humans and activate a central inflammatory response associated with altered metabolism of serotonin involved in depression.35 Stress-induced NF-kB activation leads to depressive-like behavior in rodents and inhibits neurogenesis in brain regions involved in depression.15,36 Neurogenesis is an important component of antidepressant action37 and may explain why some patients exhibited significant depressive symptoms after radiation despite antidepressant treatment. In terms of potential mechanisms which may explain the lingering effects of chemotherapy, NF-kB can undergo epigenetic modification leading to persistent activation38 and may explain increased NF-kB gene transcripts reported in fatigued vs. non-fatigued breast cancer survivors several years after completing treatment.22
Previous research has suggested that specific downstream inflammatory mediators of NF-kB are associated with distinct behavioral morbidities of cancer treatment in long-term breast cancer survivors. For example, fatigue but not depression has been associated with elevated levels of sTNFR2, and TNF inhibitors have been shown to reduce fatigue in patients with advanced cancer.16,39 Increases in CRP and IL-1ra have also been correlated with fatigue after controlling for sleep and depression,17 and fatigue has been associated with sIL-6R independent of depression.6 In the current study, fatigue and depression were significantly correlated with each other at all time points. Baseline NF-kB DNA binding activity and IL-6 significantly correlated with depression and fatigue after radiation. Nevertheless, consistent with previous studies, sTNFR2 and CRP correlated with fatigue but not depression after radiation, supporting the notion that activation of fundamental inflammatory signaling pathways such as NF-kB may be common to symptoms of both depression and fatigue, while more nuanced relationships between specific cytokines and specific symptoms may exist, especially following treatment.
Findings from this study indicate that radiation did not contribute to depressive symptoms at the time points measured and did not appear to exacerbate pre-existing depression. Regarding the trajectory of depressive symptoms and inflammation, no significant increases in depression or inflammatory markers were found during or after radiation. In other studies of non-chemotherapy-treated patients, greater increases in depressive symptoms have been observed, although, compared to our study, there was more variability in radiation dose and areas treated.3,17 The results of the current study were unexpected, but are important because previous research has not adequately addressed the effect of multi-modality therapy. Based on our data, the contribution of chemotherapy to persisting depression following radiation far outweighs any contribution from radiation alone.
Regarding clinical relevance, almost 30% of breast cancer patients treated with chemotherapy plus radiation (compared to 5% of patients treated with radiation alone) exhibited depressive symptoms (IDSSR≥33), the severity of which would qualify them for a clinical trial of antidepressant medication. Given the impact of depression on quality of life as well as mortality, these results highlight the importance of psychiatric screening of breast cancer patients who have completed radiation and have been previously treated with chemotherapy.
Several limitations and strengths of the study warrant consideration. Regarding limitations, the relatively small number of participants limits generalizability, although 42% of patients were African American, indicating that our findings may be largely independent of race. In addition, because participants were not assessed before chemotherapy, the chemotherapy effect on depression and trajectory of this symptom during chemotherapy and surgery cannot be determined. It is also possible that the chosen time points missed acute inflammatory or behavioral changes due to radiation treatment. Moreover, factors, such as advanced cancer stage, that contribute to the decision to treat with chemotherapy may be the same factors that are associated with depression, albeit initial cancer stage was not an independent predictor of depressive symptoms and when included in multivariate analyses did not affect results. Furthermore, there may be an interaction between chemotherapy and radiation leading to persistent behavioral changes not seen in patients who forego radiation. A comprehensive prospective longitudinal study tracking patients from diagnosis through treatment with chemotherapy, surgery, with or without radiation is needed to fully address this question. Regarding strengths, all patients underwent lumpectomy and standard radiation treatment. Moreover, the use of the IDS-SR, a validated index with established and defined cut-offs of pathology, allowed determination of the prevalence of moderate-to-severe cases of depression warranting treatment.
In summary, activation of inflammatory signaling pathways including NF-kB appears to be a potential mechanism by which chemotherapy is linked to depression, thereby identifying a subgroup of patients at high risk for depression and a potential biological pathway for intervention. Future longitudinal studies assessing breast cancer patients from diagnosis through each component of multi-modal treatment are needed to clarify the time sequence linking chemotherapy, inflammation, and the development of depression.
Supplementary Material
Acknowledgments
Funding Source: R21 CA155511
Footnotes
Financial Disclosures: No author has financial interests/conflicts related to this study.
References
- 1.Hopwood P, Sumo G, Mills J, Haviland J, Bliss JM. The course of anxiety and depression over 5 years of follow-up and risk factors in women with early breast cancer: results from the UK Standardisation of Radiotherapy Trials (START) Breast. 2010;19(2):84–91. doi: 10.1016/j.breast.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Kawase E, Karasawa K, Shimotsu S, et al. Estimation of anxiety and depression in patients with early stage breast cancer before and after radiation therapy. Breast Cancer. 2012;19(2):147–152. doi: 10.1007/s12282-010-0220-y. [DOI] [PubMed] [Google Scholar]
- 3.Noal S, Levy C, Hardouin A, et al. One-year longitudinal study of fatigue, cognitive functions, and quality of life after adjuvant radiotherapy for breast cancer. Int J Radiat Oncol Biol Phys. 2011;81(3):795–803. doi: 10.1016/j.ijrobp.2010.06.037. [DOI] [PubMed] [Google Scholar]
- 4.Luutonen S, Vahlberg T, Eloranta S, Hyvari H, Salminen E. Breast cancer patients receiving postoperative radiotherapy: distress, depressive symptoms and unmet needs of psychosocial support. Radiother Oncol. 2011;100(2):299–303. doi: 10.1016/j.radonc.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Khan F, Amatya B, Pallant JF, Rajapaksa I. Factors associated with long-term functional outcomes and psychological sequelae in women after breast cancer. Breast. 2012;21(3):314–320. doi: 10.1016/j.breast.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Courtier N, Gambling T, Enright S, Barrett-Lee P, Abraham J, Mason MD. Psychological and immunological characteristics of fatigued women undergoing radiotherapy for early-stage breast cancer. Support Care Cancer. 2012 doi: 10.1007/s00520-012-1508-6. [DOI] [PubMed] [Google Scholar]
- 7.Giese-Davis J, Collie K, Rancourt KM, Neri E, Kraemer HC, Spiegel D. Decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer: a secondary analysis. J Clin Oncol. 2011;29(4):413–420. doi: 10.1200/JCO.2010.28.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falagas ME, Zarkadoulia EA, Ioannidou EN, Peppas G, Christodoulou C, Rafailidis PI. The effect of psychosocial factors on breast cancer outcome: a systematic review. Breast Cancer Res. 2007;9(4):R44. doi: 10.1186/bcr1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer. 2009;115(22):5349–5361. doi: 10.1002/cncr.24561. [DOI] [PubMed] [Google Scholar]
- 10.Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet. 2011 doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merriman JD, Dodd M, Lee K, et al. Differences in self-reported attentional fatigue between patients with breast and prostate cancer at the initiation of radiation therapy. Cancer Nurs. 2011;34(5):345–353. doi: 10.1097/NCC.0b013e318202520a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hopwood P, Haviland J, Mills J, Sumo G, JMB The impact of age and clinical factors on quality of life in early breast cancer: an analysis of 2208 women recruited to the UK START Trial (Standardisation of Breast Radiotherapy Trial) Breast. 2007;16(3):241–251. doi: 10.1016/j.breast.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Burgess C, Cornelius V, Love S, Graham J, Richards M, Ramirez A. Depression and anxiety in women with early breast cancer: five year observational cohort study. BMJ. 2005;330(7493):702. doi: 10.1136/bmj.38343.670868.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bardwell WA, Natarajan L, Dimsdale JE, et al. Objective cancer-related variables are not associated with depressive symptoms in women treated for early-stage breast cancer. J Clin Oncol. 2006;24(16):2420–2427. doi: 10.1200/JCO.2005.02.0081. [DOI] [PubMed] [Google Scholar]
- 15.Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26(6):971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monk JP, Phillips G, Waite R, et al. Assessment of tumor necrosis factor alpha blockade as an intervention to improve tolerability of dose-intensive chemotherapy in cancer patients. J Clin Oncol. 2006;24(12):1852–1859. doi: 10.1200/JCO.2005.04.2838. [DOI] [PubMed] [Google Scholar]
- 17.Bower JE, Ganz PA, Tao ML, et al. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin Cancer Res. 2009;15(17):5534–5540. doi: 10.1158/1078-0432.CCR-08-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wratten C, Kilmurray J, Nash S, et al. Fatigue during breast radiotherapy and its relationship to biological factors. Int J Radiat Oncol Biol Phys. 2004;59(1):160–167. doi: 10.1016/j.ijrobp.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Geinitz H, Zimmermann FB, Stoll P, et al. Fatigue, serum cytokine levels, and blood cell counts during radiotherapy of patients with breast cancer. Int J Radiat Oncol Biol Phys. 2001;51(3):691–698. doi: 10.1016/s0360-3016(01)01657-1. [DOI] [PubMed] [Google Scholar]
- 20.DiDonato JA, Mercurio F, Karin M. NF-kappaB and the link between inflammation and cancer. Immunol Rev. 2012;246(1):379–400. doi: 10.1111/j.1600-065X.2012.01099.x. [DOI] [PubMed] [Google Scholar]
- 21.Braunstein S, Formenti SC, Schneider RJ. Acquisition of stable inducible up-regulation of nuclear factor-kappaB by tumor necrosis factor exposure confers increased radiation resistance without increased transformation in breast cancer cells. Mol Cancer Res. 2008;6(1):78–88. doi: 10.1158/1541-7786.MCR-07-0339. [DOI] [PubMed] [Google Scholar]
- 22.Bower JE, Ganz PA, Irwin MR, Arevalo JM, Cole SW. Fatigue and gene expression in human leukocytes: increased NF-kappaB and decreased glucocorticoid signaling in breast cancer survivors with persistent fatigue. Brain Behav Immun. 2011;25(1):147–150. doi: 10.1016/j.bbi.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernandez-Vargas H, Rodriguez-Pinilla SM, Julian-Tendero M, et al. Gene expression profiling of breast cancer cells in response to gemcitabine: NF-kappaB pathway activation as a potential mechanism of resistance. Breast Cancer Res Treat. 2007;102(2):157–172. doi: 10.1007/s10549-006-9322-9. [DOI] [PubMed] [Google Scholar]
- 24.Measurements ICoRUa, editor. Prescribing, Recording and Reporting Photon Beam Therapy. Bethesda, MD: 1993. ICRU Report No.50. [Google Scholar]
- 25.Nezu AM. Practitioner’s guide to empirically based measures of depression. New York: Kluwer Academic / Plenum Publishers; 2000. [Google Scholar]
- 26.Jenkins C, Carmody TJ, Rush AJ. Depression in radiation oncology patients: a preliminary evaluation. J Affect Disord. 1998;50(1):17–21. doi: 10.1016/s0165-0327(98)00039-1. [DOI] [PubMed] [Google Scholar]
- 27.Purcell A, Fleming J, Bennett S, Burmeister B, Haines T. Determining the minimal clinically important difference criteria for the Multidimensional Fatigue Inventory in a radiotherapy population. Support Care Cancer. 2010;18(3):307–315. doi: 10.1007/s00520-009-0653-z. [DOI] [PubMed] [Google Scholar]
- 28.Albuquerque K, Tell D, Lobo P, Millbrandt L, Mathews H, Witek-Janusek L. Impact of partial versus whole breast radiation therapy on fatigue, perceived stress, quality of life and natural killer cell activity in women with breast cancer. BMC Cancer. 2012;12(1):251. doi: 10.1186/1471-2407-12-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pace TW, Mletzko TC, Alagbe O, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163(9):1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- 30.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19(2):185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 31.Cole SW, Galic Z, Zack JA. Controlling false-negative errors in microarray differential expression analysis: a PRIM approach. Bioinformatics. 2003;19(14):1808–1816. doi: 10.1093/bioinformatics/btg242. [DOI] [PubMed] [Google Scholar]
- 32.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 33.Shmelkov E, Tang Z, Aifantis I, Statnikov A. Assessing quality and completeness of human transcriptional regulatory pathways on a genome-wide scale. Biol Direct. 2011;6:15. doi: 10.1186/1745-6150-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Das KC, White CW. Activation of NF-kappaB by antineoplastic agents. Role of protein kinase C. J Biol Chem. 1997;272(23):14914–14920. doi: 10.1074/jbc.272.23.14914. [DOI] [PubMed] [Google Scholar]
- 35.Raison CL, Borisov AS, Majer M, et al. Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biol Psychiatry. 2009;65(4):296–303. doi: 10.1016/j.biopsych.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci U S A. 2010;107(6):2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perera TD, Lu D, Thirumangalakudi L, et al. Correlations between hippocampal neurogenesis and metabolic indices in adult nonhuman primates. Neural Plast. 2011;2011:1–6. doi: 10.1155/2011/875307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamamoto M, Ito T, Shimizu T, et al. Epigenetic alteration of the NF-kappaB-inducing kinase (NIK) gene is involved in enhanced NIK expression in basal-like breast cancer. Cancer Sci. 2010;101(11):2391–2397. doi: 10.1111/j.1349-7006.2010.01685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol. 2011;29(26):3517–3522. doi: 10.1200/JCO.2011.36.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.