Abstract
Many species are critically dependent on olfaction for survival. In the main olfactory system of mammals, odours are detected by sensory neurons which express a large repertoire of canonical odorant receptors (ORs) and a much smaller repertoire of Trace Amine-Associated Receptors (TAARs)1–4. Odours are encoded in a combinatorial fashion across glomeruli in the main olfactory bulb, with each glomerulus corresponding to a different receptor5–7. The degree to which individual receptor genes contribute to odour perception is unclear. Here we show that genetic deletion of the olfactory TAAR gene family, or even a single TAAR gene, eliminates aversion that mice display to low concentrations of volatile amines and to the odour of predator urine. Our findings identify a role for the TAARs in olfaction, namely in the high-sensitivity detection of innately aversive odours. In addition, our data reveal that aversive amines are represented in a non-redundant fashion, and that individual main olfactory receptor genes can contribute significantly to odour perception.
There are 15 TAAR genes in the mouse, 14 of which are expressed in the main olfactory pathway and serve a chemosensory function8. All of the TAAR genes are located in a single gene cluster on mouse chromosome 10 with no interspersed genes (Fig. 1a)9. To determine how the TAARs contribute to odour perception, we used in vivo trans-allelic recombination to generate a mouse strain (“ΔT2-9”) in which all 14 olfactory TAAR genes (Taar2 through Taar9) are deleted (Fig. 1a). Homozygous ΔT2-9 mice breed normally, show no apparent health issues or behavioural deficits, and exhibit the same weight and locomotor activity as wild-type littermates (Supplementary Fig. 1).
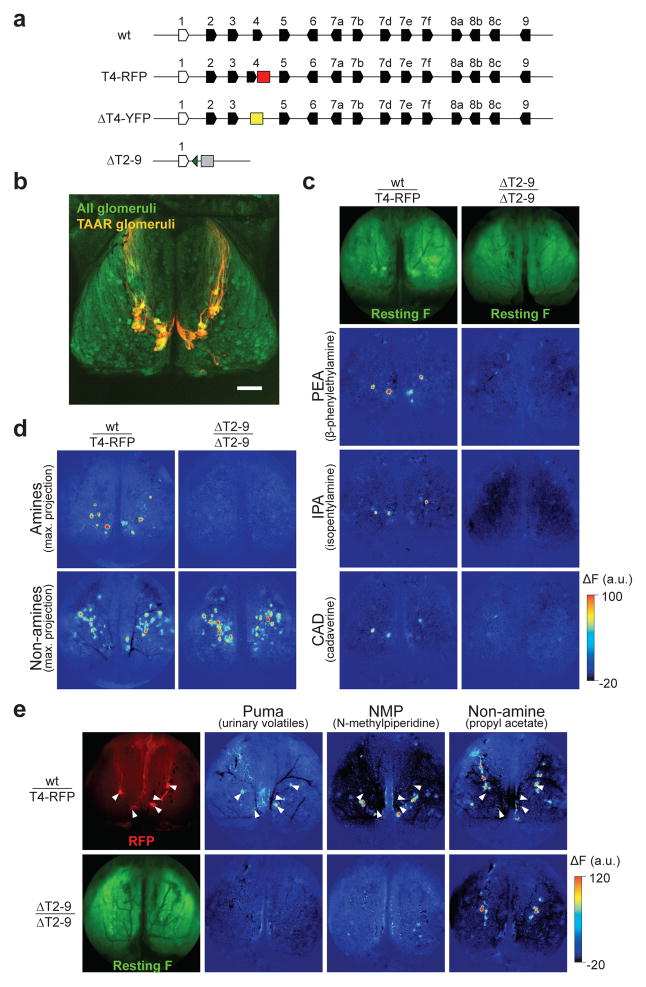
Figure 1. Deleting the olfactory TAARs abolishes high-sensitivity amine and predator odour responses in the dorsal olfactory bulb.
a. Diagram of the TAAR gene cluster and targeted alleles. Olfactory TAARs (black) and non-olfactory Taar1 (white) are shown (polygons reflect gene orientation). T4-RFP: the tau::mCherry marker (red) is inserted downstream of Taar4. ΔT4-YFP: the Taar4 coding sequence is replaced with Venus YFP (yellow). ΔT2-9: all olfactory TAAR genes are deleted and the Taar9 coding sequence is replaced with the OR S50 or CFP (grey box; green triangle indicates loxP site; see Full Methods).
b. Dorsal view of the olfactory bulbs from a double heterozygous ΔT4-YFP; OMP-spH mouse in which all glomeruli express spH (green) and TAAR glomeruli are labelled (yellow). Anterior is up. Scale bar = 500 μm.
c. Imaging of odour evoked activity in the olfactory bulbs of a heterozygous T4-RFP mouse (left panels) and a homozygous ΔT2-9 mouse (right panels). Top panels show resting spH fluorescence. Pseudocolored panels show fluorescence changes in response to β-phenylethylamine (2 nM vapour concentration, v.c.), isopentylamine (24 nM v.c.) and cadaverine (550 nM v.c.). Data are displayed as ΔF in arbitrary units (see Full Methods). Maximum response = 8.3% ΔF/F.
d. Maximum response projection for amines or non-amines in a heterozygous T4-RFP mouse (left) and a homozygousΔT2-9 mouse (right). Amine stimuli: β-phenylethylamine (2 nM v.c.), isopentylamine (24 nM v.c.), cadaverine (550 nM v.c.), N-methylpiperidine (7.5 μM v.c.) and trimethylamine (4 μM v.c.). Non-amine stimuli: propyl acetate (19 μM v.c.), phenetole (860 nM v.c.), 2-heptanone (5.3 μM v.c.) and isopropyl tiglate (1.5 μM v.c.). Maximum response = 7.4%ΔF/F.
e. Responses to urinary volatiles in heterozygous T4-RFP (top) and homozygous ΔT2-9 (bottom) mice. Locations of RFP labelled TAAR4 glomeruli (top left) are indicated (arrowheads). SpH fluorescence is shown in a ΔT2-9 mouse (bottom left). Pseudocolored panels show responses to puma urine (undiluted headspace vapour), N-methylpiperidine (150 nM v.c.) and propyl acetate (19 μM v.c.). Maximum response = 5.4% ΔF/F.
To examine the functional consequences of removing the TAARs, we performed in vivo optical imaging of odour-evoked responses from glomeruli in the olfactory bulbs of anesthetized mice. This was done by crossing ΔT2-9 mice to “OMP-spH” mice in which the genetically encoded activity reporter synaptopHluorin (spH) is expressed in all glomeruli 10. We compared odorant responses in ΔT2-9 homozygous mice with those in control mice that retain two intact TAAR gene clusters.
Glomeruli in the olfactory bulb receive axonal inputs from sensory neurons that express the same OR or TAAR gene, and sensory neurons that express a majority of the TAARs project to a cluster of glomeruli in the dorsal-caudal olfactory bulb (Fig. 1b)11,12. Consistent with our previous observations, low concentrations of structurally diverse amines robustly activated a small subset of dorsal glomeruli in control mice (Fig. 1c–e), and glomeruli with specific response profiles could be recognized across individual animals11,13. Strikingly, all of these high-sensitivity amine responses were abolished in homozygous ΔT2-9 mice, while responses to non-amine odorants persisted (Fig. 1c–e). These results demonstrate that all of the high sensitivity amine responses derive from glomeruli corresponding to TAAR genes.
Urine is a rich source of amines that could be exploited for intra- and interspecific chemical communication. It has been reported that the urine of predator cats contains high concentrations of β-phenylethylamine (PEA), an odorant that specifically activates TAAR4 in cultured cells8,14 and TAAR4 expressing olfactory sensory neurons13. Using our in vivo imaging method, we observe that TAAR4 glomeruli are activated by PEA and by the volatiles from the urine of an adult puma (Fig. 1e). Responses to puma urine in the dorsal bulb were abolished in homozygous ΔT2-9 mice (Fig. 1e). Therefore, the most sensitive amine/urine-responsive glomeruli in the dorsal bulb correspond to the TAARs. We note that it is possible that glomeruli outside of our imaging area (in the ventral bulb) respond to amines at the concentrations tested.
Many amines share a characteristic, offensive odour. In fact, two primary amines, PEA and isopentylamine (IPA), have been reported to elicit innate aversion in mice14,15. We therefore tested whether the amines that activate TAAR glomeruli are aversive and whether the TAARs mediate this aversion. Wild-type, heterozygous, and homozygous ΔT2-9 littermates (n=504 mice) were tested in a two-chamber place preference assay where they could choose to occupy an odorized or a non-odorized compartment (Fig. 2a). Odorants were diluted in water and presented in partially enclosed dishes so that mice could smell the stimuli without direct contact with the odour source. Under these conditions, all mice strongly avoided the well-characterized aversive odorant trimethylthiazoline (TMT, 2% in water), which is derived from the anal gland of the red fox15–17. In contrast, negative control odours, water, ethyl vanillin and peanut butter oil, did not elicit aversion (Fig. 2b).
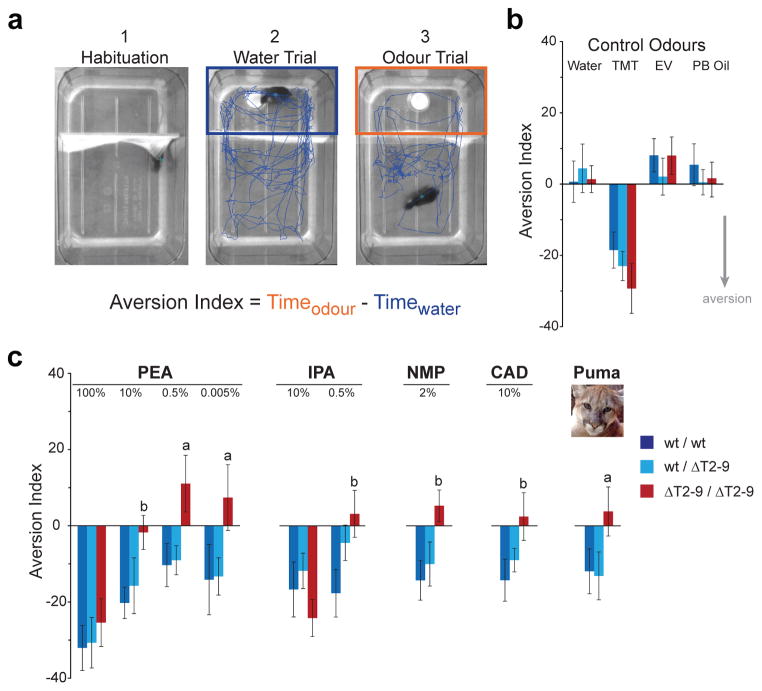
Figure 2. Deletion of all olfactory TAAR genes abolishes aversion to low concentrations of structurally diverse amines and predator urine.
a. Video images of the behavioural testing chamber. Mice move between odorized (top) and non-odorized compartments separated by a curtain. Blue traces show the location of the mouse during single three-minute trials. Panels represent three stages of one experiment— habituation to the chamber, exposure to water, and exposure to a test stimulus (odour or water).
b. Aversion index values for wild-type, heterozygous and homozygous ΔT2-9 cluster deletion mice. Negative values indicate avoidance. Odorants are 2% trimethylthiazoline (TMT), 0.5% ethyl vanillin (EV), and undiluted peanut butter oil (PB Oil). Data are mean ± SE (n=20–25 mice per genotype, per odorant).
c. Aversion index values for β-phenylethylamine (PEA), isopentylamine (IPA), N-methylpiperidine (NMP), cadaverine (CAD) and puma urine. Concentrations are given as percent dilution of pure odorant in water. Puma urine was undiluted. Data are mean ± SE (n=20–25 mice per genotype, per odorant). Statistical significances for pairwise comparisons are indicated: “a”, homozygous mice differ from wild-type and heterozygous, “b”, homozygous mice differ from wild-type (p<0.05, generalized linear mixed model). Wild-type and heterozygous mice did not differ statistically for any odour, and the aversion response did not differ with sex (p=0.669).
Using this assay, we observe that wild-type and heterozygous ΔT2-9 mice exhibit aversion to structurally diverse amines including PEA, IPA, N-methylpiperidine (NMP) and cadaverine (CAD) when tested at multiple concentrations. Notably, the aversion elicited by low concentrations of amines was TAAR-dependent as it was abolished in homozygous ΔT2-9 mice (Fig. 2c). We note that concentrated amines (100% PEA and 10% IPA), which are highly pungent to humans, were aversive to mice regardless of genotype (Fig 2c). Thus, mice are averse to certain amines, and aversion to low concentrations of amines is dependent on the TAARs.
To determine whether the TAARs are required for aversion to natural stimuli that contain ethologically relevant concentrations of amines, we tested for avoidance of the odour of predator cat urine, which is enriched in PEA14. Wild-type and heterozygous ΔT2-9 mice were averse to puma urine. The aversion to urine was abolished in homozygous ΔT2-9 mice, which lack the olfactory TAARs (Fig. 2b,c). Taken together, the data indicate that the TAAR family is required for innate aversive responses to volatile amines at naturally occurring concentrations.
Next, we examined the functional impact of removing a single TAAR gene from the receptor repertoire. To do this, we used a gene-targeted mouse strain (ΔT4-YFP) in which the TAAR4 coding sequence is replaced with that of YFP11(Fig. 1a). TAAR4 responds selectively and robustly to PEA and urinary volatiles from predator cats when expressed in cultured cells and in native olfactory sensory neurons8,13,14. Using our in vivo imaging assay, we find that low concentrations of PEA or volatiles from puma urine preferentially activate TAAR4 glomeruli in the dorsal bulb (Fig. 3a). Genetic deletion of TAAR4 specifically eliminated these high-sensitivity responses to PEA and puma urine volatiles without influencing the activation of neighbouring glomeruli by other amines (Fig. 3a).
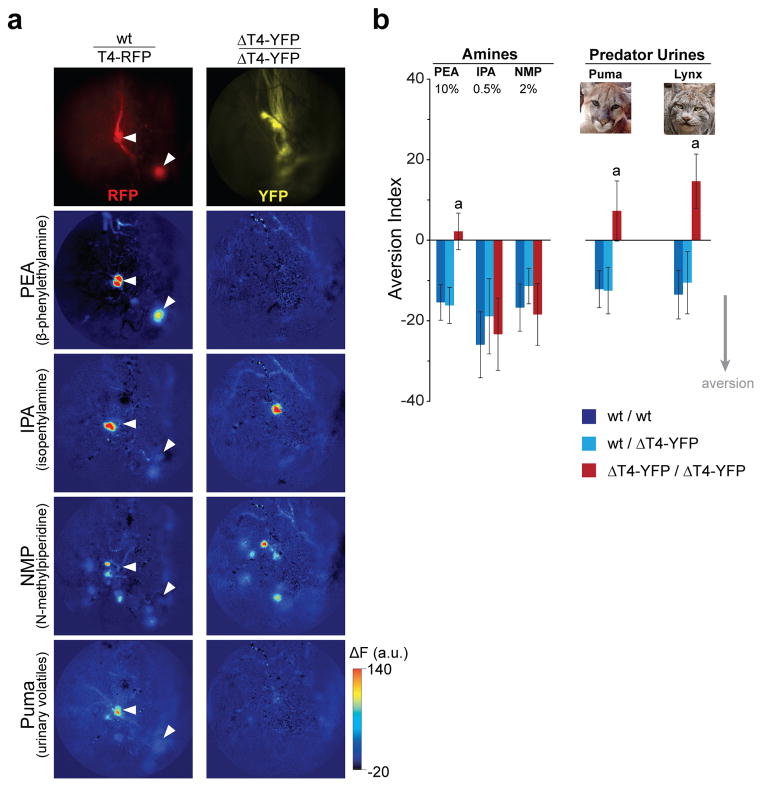
Figure 3. Deletion of a single TAAR gene abolishes aversion to a specific amine and to natural predator odours.
a. In vivo imaging of the left caudomedial olfactory bulb in a heterozygous T4-RFP mouse and a homozygous ΔT4-YFP mouse (anterior is up, medial is right). TAAR4 glomeruli are labelled in T4-RFP mice (top left panel, red) and their locations are indicated with arrowheads in subsequent panels. All dorsal TAAR glomeruli, except for those corresponding to the deleted TAAR4 gene, are labelled in homozygous ΔT4-YFP mice (top right panel, yellow)11. Pseudocolored panels show odour-evoked fluorescence changes in response to β-phenylethylamine (2 nM v.c.), isopentylamine (24 nM v.c.), N-methylpiperidine (150 nM v.c.), and puma urine (undiluted headspace vapour). Response maps are displayed as ΔF in arbitrary units (see Full Methods). Maximum response = 7.5% ΔF/F.
b. Aversion index values for wild-type, heterozygous and homozygous ΔT4-YFP mice. Negative values indicate avoidance. Odorants are β-phenylethylamine (PEA), isopentylamine (IPA), N-methylpiperidine (NMP), puma urine and Canadian lynx urine. Concentrations given as percent dilution of pure odorant in water. Predator urines were undiluted. Data are mean ± SE (n=20–25 mice per genotype, per odorant). Statistical significances for pairwise comparisons: “a”, homozygous mice differ from both wild-type and heterozygous mice (p<0.05, generalized linear mixed model). Wild-type and heterozygous mice did not differ statistically for any odour, and the aversion response did not differ with sex (p=0.639).
To determine if behavioural aversion to PEA and predator urine is mediated by TAAR4, we tested wild-type, heterozygous, and homozygous ΔT4-YFP littermates (n=245 mice) in the two-chamber place preference test described above (Fig. 2a). As expected, wild-type and heterozygousΔT4-YFP mice exhibited robust aversion to low concentrations of PEA, IPA, and NMP, as well as to urine from two predator species, puma and Canadian lynx. In contrast, homozygous ΔT4-YFP mice (which lack TAAR4) exhibited no avoidance of PEA or predator urine odours (Fig. 3b). The loss of aversion was odour-specific as homozygous ΔT4-YFP mice still avoided IPA and NMP, amines that activate other TAAR glomeruli (Fig. 3b). Thus, removing a single TAAR gene, Taar4, abolishes aversion to PEA and urinary volatiles.
Taken together, our data reveal that the TAAR gene family contributes significantly to the perception of amines in mice. Moreover, the aversive quality of amines and predator urine is encoded in the olfactory system in a non-redundant manner, since removal of even a single TAAR gene can have a significant impact on the aversive response. The vomeronasal system of mice mediates innate avoidance of proteins found in the urine of predator species18,19, a process that may require direct contact with the stimulus. The TAARs may contribute to predator avoidance over longer distances by mediating aversion to trace concentrations of urinary volatiles. In this regard, it is interesting to note that the response thresholds of TAAR4 sensory neurons and glomeruli to the aversive predator cue, PEA, are the lowest ever observed in the main olfactory system13.
We note that the TAARs may not function solely as detectors of predator-derived and aversive odours. While we show that mouse TAARs contribute to the detection and avoidance of several aversive amines, recent data indicate that TAAR5 mediates attraction to its preferred ligand, trimethylamine—a socially relevant metabolite that is enriched in male mouse urine20. However, it should be noted that this amine elicits robust aversion in rats. The TAAR repertoire is also evolutionarily retained in many vertebrate species, including humans3,9. Aside from their role in aversion or attraction, our view is that the TAARs are retained in many species because they are required more generally for high-sensitivity amine detection. The behavioural response to this input may be context and species specific.
It is generally thought that odour representations in the main olfactory bulb are highly distributed and redundant, with each input channel (glomerulus) making a small contribution to the representation of a given odour21. In this view, single receptor deletions should have little effect at the level of behaviour. Behavioural deficits have been induced by the genetic removal of receptors in specialized olfactory pathways. Mutant mice lacking a subset of vomeronasal receptors, which map to the accessory olfactory bulb, display deficits in aggression and mating22. Mice lacking the gene for guanylyl cyclase D, which is expressed in sensory neurons that project to atypical necklace glomeruli, show deficits in social transmission of food preference23,24. In contrast, the TAARs are mapped to a subset of the ~2,000 typical main olfactory bulb glomeruli that are thought to represent odorants in a combinatorial fashion. In spite of this, removal of even a single TAAR results in a measurable deficit in odour-guided behaviour. Our data suggest that the representations of general odours in the main olfactory system may be less redundant than previously thought, a fact that may shed light on how vertebrates retain large numbers of chemosensory receptor genes over evolutionary time.
METHODS
Gene targeting
The TAAR cluster deletion allele ΔT2-9CFP was generated by Cre-mediated trans-allelic recombination in vivo25. We employed two targeted alleles that introduce loxP sites into the 5′ and 3′ ends of the cluster—au1::Taar1-loxP-IRES-tau::Venus (aT1-YFP) in which a loxP site is inserted just downstream of the Taar1 coding sequence, and Cerulean→Taar9-loxP (ΔT9-CFP) in which the Taar9 coding sequence is replaced with that of Cerulean CFP followed by loxP. An HPRT-Cre strain (129S1/Sv-Hprttm1(cre)Mnn/J; Jax 004302;27) was used to mediate recombination in aT1-YFP/ΔT9-CFP compound heterozygotes as described11.
In vivo imaging
For glomerular imaging, mice were anesthetized with sodium pentobarbital as described11, or with urethane (1g/kg IP; Sigma) and chlorprothixene hydrochloride (10 mg/kg), and given atropine sulfate (5.4 mg/kg; Med-Pharmex). The bone overlying the bulbs was thinned using a dental drill. Glomeruli were imaged using a custom Nikon epifluorescence microscope and a 4x (0.2 NA) objective. Light excitation was provided using a 200 W metal-halide lamp (Prior Scientific) attenuated by neutral density filters and standard filter sets for mCherry (49008; Chroma), YFP (86001 JP3, Chroma), or GFP (96343, Nikon).
Odorants were applied using a custom-made, flow dilution olfactometer and controller (LASOM, RPMetrix). Amines were diluted in water and subsequently by flow dilution. Predator urine volatiles were applied from the undiluted headspace concentration. Images were acquired at 25 Hz over 20 s (encompassing a 4 s pre-stimulus period and a 4 s odorant pulse) using a NeuroCCD-SM256 camera and Neuroplex software (RedShirtImaging, Decatur, GA). Blank trials were subtracted from odour trials prior to analysis to compensate for photobleaching. Response maps were obtained by subtracting a 3 s temporal average preceding the stimulus from a 3 s temporal average encompassing the response peak. Responses are expressed as ΔF to account for the fact that the background spH fluorescence is not correlated with the pool of indicator that reports neuronal activity10,26. Stimuli were presented at least twice in a given experiment. Images were processed and analysed in Neuroplex (RedShirtImaging) and Image J (http://imagej.nih.gov/ij) software. Vapour concentrations were estimated using published vapour pressures (US EPA, Estimation Programs Interface Suite, v 4.0).
Behavioural Analysis
ΔT4-YFP and ΔT2-9CFP littermates were housed in same-sex groups of 2–5 individuals. All animals were maintained in a reverse 12/12 hr light - dark cycle and provided with food and water ad libitum. Cages were changed daily to prevent adaptation to amines that are present in mouse urine. Mice of all genotypes were tested between 5–7 weeks of age under low intensity red light during the nocturnal phase.
The experimental protocol consisted of three parts: handling (2 days), pre-trials (2 days), and experimental trials (1–3 days). Handling habituated the mice to the experimenter and consisted of placing each mouse individually onto the experimenter’s cupped, gloved hands for five minutes and allowing them to roam this small area freely. Pre-trials were identical to experimental trials (see below) except that no odorants were used. Pre-trials functioned to eliminate the novelty of the odour delivery and experimental chamber. Experiments were performed in clean, autoclaved 30 × 18 × 12 cm cages. A disposable curtain isolated 1/3 of the cage with minimal air transfer between sections. This smaller section, or “odorized” compartment, was topped with a thin piece of clear acrylic, which functioned to minimize the loss of odour. Each mouse was introduced to the larger section of the cage and allowed to habituate for 3 minutes. At the end of this time period, a 3.5 cm covered petri dish containing 20 μl of water on filter paper was introduced to the odorized compartment. The top of the petri dish was perforated to allow odorants to escape, but to prevent direct contact with the stimulus. Mice were allowed to interact with this petri dish for 3 minutes. The petri dish was then removed and another identical petri dish with 20 μl of an odorant on filter paper was added. The experiment was terminated after another 3 minutes.
The acrylic top was cleaned with 70% isopropyl alcohol and the cages were washed and autoclaved. The mice were video recorded and their location tracked using Limelight 3.0 software (Actimetrics). The aversion index was calculated as the difference between the time spent in the odorized chamber when an odour (or water) was present and the time spent in the odorized chamber when water was present. Data from mice that showed a very strong preference for either chamber (i.e. spent < 2% or > 98% of the trial duration in the odorized chamber) during the initial water trial were discarded.
Mice were naïve to each stimulus and were tested only once for a given odour. Monomolecular stimuli consisted of three control odorants, water, trimethylthiazoline (2% in water) and a saturated solution of ethyl vanillin (0.5% w/v, in water), as well as 4 amines that activate TAAR glomeruli, β-phenylethylamine (100%, 10%, 0.5% and 0.005%), isopentylamine (10% and 0.5%), N-methyl piperidine (2%) and cadaverine (10%). Complex odorants were undiluted peanut butter oil, puma urine (from P. concolor) and lynx urine (from L. canadensis). Predator urines were collected at the Philadelphia Zoo, shipped frozen and stored at −80 °C. Odour concentrations represent what was placed in the petri dish and are expressed as percent dilution in water. The saturated vapour concentrations (maximum possible odorant concentration) for the stimuli are as follows: 100% phenylethylamine=22 μM; 10% isopentylamine=243 μM; 10% cadaverine = 5 μM; 2% N-methylpiperidine=30 μM. The actual stimulus concentrations in the odorized chamber are likely much lower than these theoretical maxima. We note that the concentrations in the behavioural and imaging experiments are difficult to compare given the differences in odour presentation methods.
Supplementary Material
Acknowledgments
We thank Tammy Schmidt, Chris Waldron, Lynn Tunmer, Vik Dewan and the staff of the Philadelphia Zoo for collecting predator urine and for providing images of the animals. We thank David Ferster for help with video tracking, Dillon Cawley, Alan Ge and Tammy Bozza for help analysing behavioural data, the Northwestern University Center for Comparative Medicine for behavioural space, and the Northwestern University Biostatistics Collaboration Center for advice on statistical analyses. This work was supported by grants from the NIH/NIDCD (R01DC009640 to TB and F32DC012004 to AD), The Whitehall Foundation and The Brain Research Foundation (TB).
References
- 1.Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- 2.Liberles SD. Trace amine-associated receptors are olfactory receptors in vertebrates. Ann N Y Acad Sci. 2009;1170:168–172. doi: 10.1111/j.1749-6632.2009.04014.x. [DOI] [PubMed] [Google Scholar]
- 3.Nei M, Niimura Y, Nozawa M. The evolution of animal chemosensory receptor gene repertoires: roles of chance and necessity. Nat Rev Genet. 2008;9:951–963. doi: 10.1038/nrg2480. [DOI] [PubMed] [Google Scholar]
- 4.Touhara K, Vosshall LB. Sensing odorants and pheromones with chemosensory receptors. Annu Rev Physiol. 2009;71:307–332. doi: 10.1146/annurev.physiol.010908.163209. [DOI] [PubMed] [Google Scholar]
- 5.Wilson RI, Mainen ZF. Early events in olfactory processing. Annu Rev Neurosci. 2006;29:163–201. doi: 10.1146/annurev.neuro.29.051605.112950. [DOI] [PubMed] [Google Scholar]
- 6.Kauer JS, White J. Imaging and coding in the olfactory system. Annu Rev Neurosci. 2001;24:963–979. doi: 10.1146/annurev.neuro.24.1.963. [DOI] [PubMed] [Google Scholar]
- 7.Hildebrand JG, Shepherd GM. Mechanisms of olfactory discrimination: converging evidence for common principles across phyla. Annu Rev Neurosci. 1997;20:595–631. doi: 10.1146/annurev.neuro.20.1.595. [DOI] [PubMed] [Google Scholar]
- 8.Liberles SD, Buck LB. A second class of chemosensory receptors in the olfactory epithelium. Nature. 2006;442:645–650. doi: 10.1038/nature05066. [DOI] [PubMed] [Google Scholar]
- 9.Lindemann L, et al. Trace amine-associated receptors form structurally and functionally distinct subfamilies of novel G protein-coupled receptors. Genomics. 2005;85:372–385. doi: 10.1016/j.ygeno.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Bozza T, McGann JP, Mombaerts P, Wachowiak M. In vivo imaging of neuronal activity by targeted expression of a genetically encoded probe in the mouse. Neuron. 2004;42:9–21. doi: 10.1016/s0896-6273(04)00144-8. [DOI] [PubMed] [Google Scholar]
- 11.Pacifico R, Dewan A, Cawley D, Guo C, Bozza T. An Olfactory Subsystem that Mediates High-Sensitivity Detection of Volatile Amines. Cell Rep. 2012;2:76–88. doi: 10.1016/j.celrep.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson MA, et al. Neurons expressing trace amine-associated receptors project to discrete glomeruli and constitute an olfactory subsystem. Proc Natl Acad Sci U S A. 2012;109:13410–13415. doi: 10.1073/pnas.1206724109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J, Pacifico R, Cawley D, Feinstein P, Bozza T. Ultrasensitive detection of amines by a trace amine associated receptor. J Neurosci. 2013;33:3228–3239. doi: 10.1523/JNEUROSCI.4299-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrero DM, et al. Detection and avoidance of a carnivore odor by prey. Proc Natl Acad Sci U S A. 2011;108:11235–11240. doi: 10.1073/pnas.1103317108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayakawa K, et al. Innate versus learned odour processing in the mouse olfactory bulb. Nature. 2007;450:503–508. doi: 10.1038/nature06281. [DOI] [PubMed] [Google Scholar]
- 16.Vernet-Maury E, Polak EH, Demael A. Structure-activity relationship of stress-inducing odorants in the rat. J Chem Ecol. 1984;10:1007–1018. doi: 10.1007/BF00987509. [DOI] [PubMed] [Google Scholar]
- 17.Fendt M, Endres T, Lowry CA, Apfelbach R, McGregor IS. TMT-induced autonomic and behavioral changes and the neural basis of its processing. Neurosci Biobehav Rev. 2005;29:1145–1156. doi: 10.1016/j.neubiorev.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 18.Papes F, Logan DW, Stowers L. The vomeronasal organ mediates interspecies defensive behaviors through detection of protein pheromone homologs. Cell. 2010;141:692–703. doi: 10.1016/j.cell.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ben-Shaul Y, Katz LC, Mooney R, Dulac C. In vivo vomeronasal stimulation reveals sensory encoding of conspecific and allospecific cues by the mouse accessory olfactory bulb. Proc Natl Acad Sci U S A. 2010;107:5172–5177. doi: 10.1073/pnas.0915147107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Q, et al. Synchronous evolution of an odor biosynthesis pathway and behavioral response. Curr Biol. 2013;23:11–20. doi: 10.1016/j.cub.2012.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kauer JS. Contributions of topography and parallel processing to odor coding in the vertebrate olfactory pathway. Trends Neurosci. 1991;14:79–85. doi: 10.1016/0166-2236(91)90025-p. [DOI] [PubMed] [Google Scholar]
- 22.Del Punta K, et al. Deficient pheromone responses in mice lacking a cluster of vomeronasal receptor genes. Nature. 2002;419:70–74. doi: 10.1038/nature00955. [DOI] [PubMed] [Google Scholar]
- 23.Munger SD, et al. An olfactory subsystem that detects carbon disulfide and mediates food-related social learning. Curr Biol. 2010;20:1438–1444. doi: 10.1016/j.cub.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munger SD, Leinders-Zufall T, Zufall F. Subsystem organization of the mammalian sense of smell. Ann Rev Physiol. 2009;71:115–140. doi: 10.1146/annurev.physiol.70.113006.100608. [DOI] [PubMed] [Google Scholar]
- 25.Wu S, Ying G, Wu Q, Capecchi MR. Toward simpler and faster genome-wide mutagenesis in mice. Nat Genet. 2007;39:922–930. doi: 10.1038/ng2060. [DOI] [PubMed] [Google Scholar]
- 26.McGann JP, et al. Odorant representations are modulated by intra- but not interglomerular presynaptic inhibition of olfactory sensory neurons. Neuron. 2005;48:1039–1053. doi: 10.1016/j.neuron.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 27.Tang SH, Silva FJ, Tsark WM, Mann JR. A Cre/loxP-deleter transgenic line in mouse strain 129S1/SvImJ. Genesis. 2002;32:199–202. doi: 10.1002/gene.10030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.