Abstract
Background
Prescribed sublingual (SL) buprenorphine is sometimes diverted for intravenous (IV) abuse, but no human pharmacokinetic data are available following high-dose IV buprenorphine.
Methods
Plasma was collected for 72 h after administration of placebo or 2, 4, 8, 12, or 16 mg IV buprenorphine in escalating order (single-blind, double-dummy) in 5 healthy male non-dependent opioid users. Buprenorphine and its primary active metabolite, norbuprenorphine, were quantified by liquid chromatography tandem mass spectrometry with limits of quantitation of 0.1 μg/L.
Results
Maximum buprenorphine concentrations (mean ± SE) were detected 10 min after 2, 4, 8, 12, 16 mg IV: 19.3±1.0, 44.5±4.8, 85.2±7.7, 124.6±16.6, and 137.7±18.8 μg/L, respectively. Maximum norbuprenorphine concentrations occurred 10–15 min (3.7±0.7 μg/L) after 16 mg IV administration.
Conclusions
Buprenorphine concentrations increased in a significantly linear dose-dependent manner up to 12 mg IV buprenorphine. Thus, previously demonstrated pharmacodynamic ceiling effects (over 2–16 mg) are not due to pharmacokinetic adaptations within this range, although they may play a role at doses higher than 12 mg.
Keywords: buprenorphine, norbuprenorphine, intravenous, pharmacokinetics
1. INTRODUCTION
High-dose SL buprenorphine, a partial mu-opioid receptor agonist, has been employed as substitution therapy for opioid addiction in France since 1996 (Gueye et al., 2002; Thirion et al., 2002) and in the US since 2002 (Sporer, 2004). Many early clinical studies utilized an ethanolic buprenorphine solution, while current formulations for buprenorphine opioid agonist maintenance are two types of SL tablets, one containing buprenorphine alone and a second combining buprenorphine with naloxone at a fixed dose ratio of 4:1 (2 mg buprenorphine:0.5 mg naloxone or 8 mg buprenorphine:2 mg naloxone). Oral administration is not employed clinically because of substantial hepatic first pass metabolism (Cone et al., 1984).
Prescribed SL buprenorphine is sometimes diverted for IV misuse, a world-wide problem (Alho et al., 2007; Bazazi et al., 2011; Comer et al., 2010; Moratti et al., 2010; Nordmann et al., 2012;Otiashvili et al., 2010;Vicknasingam et al., 2010). Human laboratory studies suggest that both SL and IV buprenorphine have a high safety margin for respiratory depression and cardiovascular effects because of a ceiling at higher doses (Umbricht et al., 2004; Walsh et al., 1994). One study of ascending SL buprenorphine doses (4–32 mg 30% ethanolic solution or 4–16 mg tablet) found a less than proportional relationship between increasing dose and plasma buprenorphine or norbuprenorphine (its major active metabolite) concentrations, suggesting that the observed plateau for such pharmacodynamic effects might be due to pharmacokinetic factors (Harris et al., 2004). We are unaware of any comparable study with IV buprenorphine.
We evaluated high-dose buprenorphine pharmacokinetics after IV administration to determine if the ceiling phenomenon for cardiorespiratory effects was due to changes in pharmacokinetic parameters rather than to pharmacodynamic adaptations (such as changes in receptor function).
2. METHODS
2.1. Participants
Participants provided written informed consent for this study approved by the National Institute on Drug Abuse Institutional Review Board and were compensated for their time and inconvenience. Prior to admission, participants underwent comprehensive medical and psychological evaluations, and provided a history of self-reported drug use. Volunteers with a history of IV opioid use must have abused opioids for more than two years, having used at least once weekly for a minimum of 12 weeks. History of opiate exposure was verified by a positive urine test. Participants resided on a secure clinical research unit for up to 6 weeks under 24 h medical surveillance to ensure safety and prevent additional drug use.
2.2. Study design
In this dose-ranging study, buprenorphine pharmacokinetics was tested in a double blind, double-dummy design. Participants and nursing staff were unaware of the dosing schedule; the physician monitoring the study was blinded to dose order in the first two sessions, but was aware of dose escalation in other sessions. SL (1mL) 12 mg/mL or placebo buprenorphine was placed under the tongue at the beginning of the session and held for five min, followed immediately by a 4 mL IV injection, administered over 1 min. There were seven experimental sessions separated from one another by at least three days. In the first two sessions, 12 mg SL buprenorphine or placebo were given in random order, followed by IV placebo injections. In sessions 3 through 7, SL placebo solution was given, and due to safety concerns, was followed by increasing IV buprenorphine doses (2, 4, 8, 12, and 16 mg).
2.3. Buprenorphine
Buprenorphine hydrochloride was obtained from Reckitt and Colman Products, Ltd., Hull, UK, (now Reckitt Benckiser Pharmaceuticals, Inc.).. Participants received IV buprenorphine in 4 mL over one minute during each session. The IV solution (buprenorphine hydrochloride, 4 mg/mL) was prepared in sterile water for injection, and diluted to 4 mL for the 2, 4, 8 and 12 mg doses. Sterile water was the placebo IV injection solution. All doses are expressed as buprenorphine hydrochloride.
2.4. Chemicals and reagents
Buprenorphine (for analysis), buprenorphine-d4, and norbuprenorphine were obtained from Cerilliant (Round Rock, TX). Norbuprenorphine-d9 was obtained from Isotec, Inc (Miamisburg, OH). High purity grade n-butyl chloride and acetonitrile were purchased from Baxter Diagnostics, Inc. (Deerfield, IL), and formic acid (88%) from J.T. Baker (Phillipsburg, NJ).
2.5. Specimen collection
In each session, whole blood was collected prior to and up to 72 h after buprenorphine administration, at the following time points; −0.5, 0.17, 0.25, 0.5, 0.75, 1, 2, 3, 5, 7, 12, 24, 48, and 72 h. Whole blood specimens were collected with EDTA, stored on ice and centrifuged within 2 h. Plasma specimens were stored frozen at −20°C until analysis.
2.6. Extraction/quantitative analysis
Plasma buprenorphine and norbuprenorphine specimens were submitted to the Center for Human Toxicology, University of Utah, Salt Lake City, Utah for quantitation employing a previously published method (Moody et al., 2002). Deuterated internal standards (5 ng buprenorphine-d4 and norbuprenorphine-d9) were added to 1 mL calibrators, controls and specimens. Buprenorphine, buprenorphine-d4, norbuprenorphine and norbuprenorphine-d9 were monitored with transitions of m/z 468 to 396, 472 to 400, 414 to 101 and 423 to 110, respectively. Calibration curves (0.1 – 10 μg/L) were established by quadratic equation with a 1/y2 weighting for buprenorphine and a linear curve with 1/y weighting for norbuprenorphine. Limits of quantitation for buprenorphine and norbuprenorphine were 0.1 μg/L. Within and between-run imprecision were calculated at three concentrations across the linear range with coefficients of variation of <12.7%. Bias at the same concentrations (0.25, 1.0, and 5.0 μg/L) was less than ±15.2%.
2.7. Pharmacokinetic and statistical analysis
Buprenorphine and norbuprenorphine plasma data were analyzed with non-linear regression analysis with standard non-compartmental analysis. Calculations were performed with WinNonlin Professional Version 4.0.1. Area under the plasma concentration-time curve (AUC) was calculated by employing the linear trapezoidal rule. Extrapolation of AUC to infinity (∞) was determined by dividing the last observed plasma concentration by the terminal elimination rate constant (ke). The ke was estimated via linear regression of the time versus log concentration curve. The elimination half-life was derived from t1/2 = 0.693/ke. Plasma clearance (CL) after IV administration was calculated with the equation CL = Dose/AUC (0 → ∞).
Results are presented as mean ± standard error (SE) of the mean. Values were compared using one-way analysis of variance, followed by multiple comparison tests using the Bonferroni correction. Comparisons were considered significant if p<0.05. All tests were performed using Prism 3.02 (Graphpad, Inc., La Jolla, CA, USA).
3. RESULTS
3.1. Participants
Data were collected as part of a previously published study documenting buprenorphine pharmacodynamic effects in 6 participants (Umbricht et al., 2004). Five of six participants completed all 7 sessions. One participant experienced severe nausea following the 12 mg IV dose and was unable to complete the study. Therefore, data are only presented for 5 subjects who received all drug administrations. Participants ranged in age from 32 to 39 years (mean 34.6 ± 1.1 years) and weighed between 62.1 and 82.6 kg (mean 74.7 ± 3.2 kg). Although not physically dependent, all were regular users of IV heroin at the time of study entry. Mean (± SE) duration of heroin use was 9.5 ± 2.2 years, with a mean daily amount spent on heroin of $174 ± 46.1 over 11.2 ± 0.9 days in the prior month. No participant showed evidence of opioid or alcohol withdrawal while on the research unit and all were in good health and without psychiatric disturbance other than substance abuse.
3.2. Pharmacokinetic results
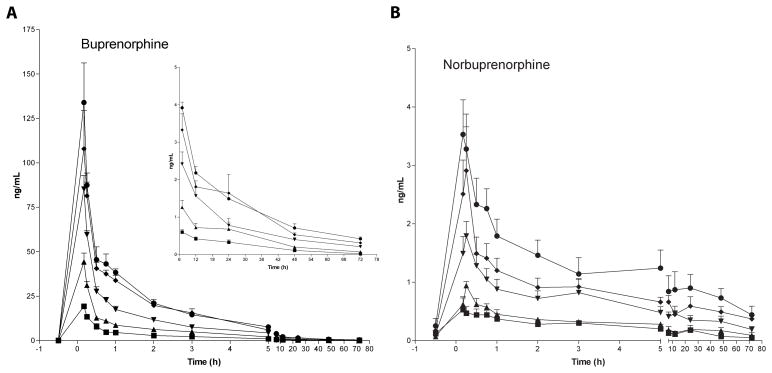
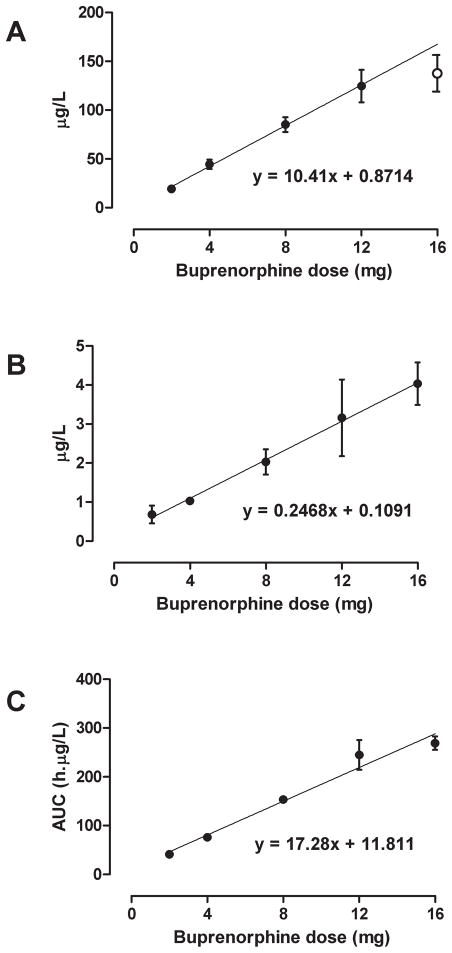
The time course of mean ± SE buprenorphine and norbuprenorphine plasma concentrations after IV administration of 2, 4, 8, 12, and 16 mg buprenorphine is presented in Figure 1A. Maximum buprenorphine concentrations were 19.3±1.0, 44.5±4.8, 85.2±7.7, 124.6±16.6, and 137.7±18.8 μg/L, respectively, and almost always detected in the first specimen, collected 10 min after IV dosing. The dose-maximum concentration relationship was linear over all doses (correlation = 0.984, up to 41.8% deviation from regression line), but deviation from the regression line improved to 11.6% (correlation = 0.999) when the 16 mg dose was omitted from calculations (Figure 2A). Back calculations of dose from mean maximum buprenorphine concentrations based on the regression line were within ± 12% of each target dose.
Figure 1.
Mean ± SE buprenorphine (A) and norbuprenorphine (B) plasma concentrations after IV administration of 2 (■), 4 (▲), 8 (▼), 12 (◆) and 16 (●) mg buprenorphine to five non-dependent IV heroin users. Inset graph (panel A) shows concentrations at later time points at higher resolution.
Figure 2.
Mean ± SE maximum plasma buprenorphine (A) and norbuprenorphine (B) concentrations and area under the curve of buprenorphine concentrations (C) after IV administration of 2, 4, 8, 12 and 16 mg buprenorphine to five non-dependent IV heroin users. Linear regression calculated on all 5 doses for panels B and C and on 2–12 mg (●) for panel A.
Norbuprenorphine was first detected 10 or 15 min after IV buprenorphine (Figure 1B), with maximum concentrations of 3.7±0.7 μg/L after 16 mg buprenorphine. There was a linear dose-maximum concentration relationship over the 2 to 16 mg range (Figure 2B). Mean maximum norbuprenorphine concentrations were within ± 7% of target dose, with correlation coefficients > 0.995.
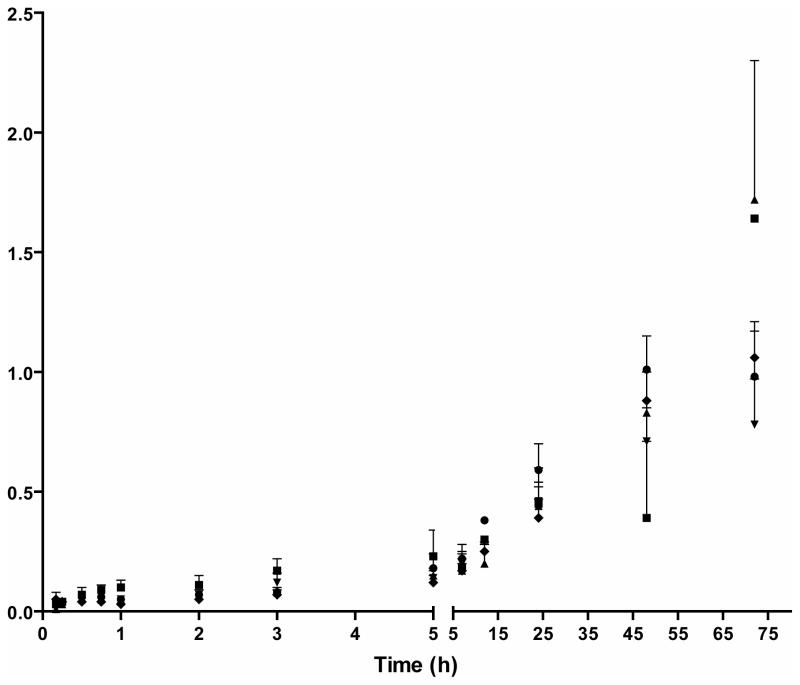
Norbuprenorphine/buprenorphine ratios were less than 0.05 for up to 12 h after administration of 2, 4, 8, 12, and 16 mg IV buprenorphine. Norbuprenorphine/buprenorphine ratios increased over time (Figure 3). There were marked increases in norbuprenorphine/buprenorphine ratios 24 h after 12 and 16 mg IV buprenorphine, and 48 h after the 2, 4, and 8 mg IV buprenorphine doses. Norbuprenorphine/buprenorphine ratios remained below 2 [0.01–1.6] up to 72 hours after administration.
Figure 3.
Mean ± SE norbuprenorphine/buprenorphine ratio of plasma concentrations after IV administration of 2 (■), 4 (▲), 8 (▼), 12 (◆) and 16 (●) mg buprenorphine to five non-dependent IV heroin users.
Buprenorphine pharmacokinetic parameters are shown in Table 1. Mean buprenorphine area under the curve ranged from 41.4 to 269.1 h*μg/L after IV doses (Figure 2C). There was a linear dose-concentration relationship for IV buprenorphine area under the curve (AUC) over all doses (correlation = 0.986, 14.5% deviation from regression line; Figure 2C), which improved only slightly with omission of the 16 mg dose (correlation = 0.998, 10.1% deviation). Mean ± SE buprenorphine clearance, half-life, and volume of distribution were 54.0 ± 1.7 L/h, 25.0 ± 1.3 h, 806.4 ± 38.2 L, respectively, across all doses. There were no significant differences in these parameters across the five IV buprenorphine doses (all P>0.05).
Table 1.
Buprenorphine and norbuprenorphine pharmacokinetic parameters after administration of 2, 4, 8, 12 or 16 mg intravenous buprenorphine to five non-dependent intravenous heroin users.
| Buprenorphine | ||||||
|---|---|---|---|---|---|---|
| Buprenorphine dose (mg) | 2 | 4 | 8 | 12 | 16 | Mean Intravenous |
| Cmax (μg/L) | 21.6 | 56.3 | 110.8 | 164.5 | 174.8 | --- |
| AUCINF (predicted)* (h.μg/L) | 41.4 ±3.0 | 75.9 ± 3.6 | 153.3 ±4.6 | 245.1 ± 27.7 | 269.1 ± 12.9 | --- |
| Cl** (L/h) | 49.8 ± 4.1 | 53.2 ± 2.4 | 52.4 ± 1.6 | 54.7 ± 3.5 | 60.0 ± 2.7 | 54.0 ± 1.7 |
| t1/2 Lambda z*** (h) | 21.8 ± 3.7 | 27.5 ± 4.9 | 28.0 ± 3.0 | 22.3 ± 2.6 | 25.6 ± 1.1 | 25.0 ± 1.3 |
| Vz (predicted)/F**** (L/h) | 743.0 ± 73.5 | 887.5 ± 115.0 | 804.5 ± 69.1 | 702.6 ± 102.0 | 894.5 ± 113.2 | 806.4 ± 38.2 |
| Norbuprenorphine | ||||||
|---|---|---|---|---|---|---|
| Buprenorphine dose (mg) | 2 | 4 | 8 | 12 | 16 | Mean Intravenous |
| Cmax (μg/L) | 1.6 | 1.2 | 2.8 | 6.8 | 5.6 | --- |
| AUClast***** (h.μg/L) | 8.0 ± 3.8 | 11.0 ± 3.4 | 27.6 ± 6.9 | 41.5 ± 9.4 | 61.1 ± 14.6 | --- |
All values are mean ± standard error of the mean.
Abbreviations:
AUCINF (predicted): Extrapolation of the area under the plasma concentration-time curve to infinity.
Cl: Plasma clearance.
t1/2 Lambda z: Elimination half-life.
Vz (predicted)/F: Volume of distribution/bioavailability.
AUClast (predicted): Extrapolation of the area under the plasma concentration-time curve to last.
4. DISCUSSION
This study describes IV buprenorphine pharmacokinetics and its primary active metabolite norbuprenorphine across a higher range of acute doses than previously reported in the literature (Bullingham et al., 1981, 1980, 1982; Ho et al., 1991; Kuhlman et al., 1996; Olley and Tiong, 1988). We previously demonstrated the absence of dose-related increases in physiological and subjective effects from 2–16 mg IV buprenorphine, indicating a ceiling effect of buprenorphine administered by the IV route (Umbricht et al., 2004). That study could not determine whether these observed ceiling effects were due to pharmacodynamic or pharmacokinetic adaptations. A prior study of SL buprenorphine found a less than proportional relationship between increasing dose and plasma buprenorphine or norbuprenorphine concentrations, suggesting that the observed ceiling for such pharmacodynamic effects might be due to pharmacokinetic factors (Harris et al., 2004).
In the present study, while linear increases in mean maximum buprenorphine concentrations and AUC after IV administration were observed up to 16 mg, deviation from the regression line was substantially improved when the 16 mg dose was omitted from the calculations for maximum concentration, suggesting a flattening of linearity at the highest dose (Fig. 2A). These findings document that pharmacokinetic changes in buprenorphine metabolism and/or excretion were not responsible for the observed ceiling effects in physiological and subjective responses at doses up to 12 mg (Umbricht et al., 2004), although pharmacokinetic changes may contribute at higher doses. A possible mechanism for the somewhat lower buprenorphine Cmax observed after 16 mg IV buprenorphine could involve distribution and excretion processes. All doses were administered intravenously, eliminating absorption as a contributing factor (i.e., there was 100% bioavailability). However, distribution could have been affected if buprenorphine concentrations at the highest dose exceeded the capacity of blood transport mechanisms, leaving more buprenorphine available for excretion by the kidney.
In our study, IV buprenorphine half-life (21.8–28.0 h ± 1.1–4.9) was much greater than that observed after 1.2 mg IV buprenorphine (3.2 ± 1.3 h; Kuhlman et al., 1996), possibly because of higher limits of quantification and an inability to quantify low concentrations at later time points. Earlier studies may have underestimated buprenorphine half-life. Inaccurate buprenorphine half-lives can be obtained if insufficient sampling times and insensitive analytical methods are employed that inadequately capture the terminal elimination phase of buprenorphine excretion. The apparent differences in half-lives across buprenorphine doses may be due to plasma concentrations after low doses declining rapidly to the limit of quantification, resulting in underestimation of terminal elimination half-lives.
Half-lives affect variability in reported volumes of distribution and clearance. Mean apparent volume of distribution following IV doses in the present study was 806.4 ± 38.2 L, higher than previously reported (334.9 ± 116.2 L and 188–400 L) after lower IV buprenorphine doses (Bullingham et al., 1983, 1980; Kuhlman et al., 1996). Mean plasma clearance after five IV doses in the present study was 54.0 ± 1.7 L/h, similar to the range of 59 to 77 L/h reported in previous studies (Bullingham et al., 1983, 1980; Kuhlman et al., 1996).
In Figure 2, dose concentration curves had intercepts of 0.87 for buprenorphine, 0.11 for norbuprenorphine and 11.8 for buprenorphine AUC. Although greater than zero, these intercepts are low and acceptable.
The mean ± SE maximum norbuprenorphine concentrations after 2, 4, 8, 12, and 16 IV doses were dose-related, increasing from 0.56–3.68 ± 0.1–0.99 μg/L. Kuhlman et al. reported a 0.6 ± 0.1 μg/L maximum norbuprenorphine concentration following a 1.2 mg IV dose (Kuhlman et al., 1996). Mean Tmax plasma norbuprenorphine concentrations occurred between 0.17 and 0.25 h for IV buprenorphine, consistent with a previously reported Tmax of 0.18 ± 0.2 h (Kuhlman et al., 1996)..
At high doses (3 or 9 mg/kg), norbuprenorphine is more potent than buprenorphine in producing respiratory depression in the rat (Megarbane et al., 2006). Norbuprenorphine concentrations in the present study and in human buprenorphine-attributed fatalities were about 100-fold less than those inducing respiratory depression in rats. The mechanisms responsible for this discrepancy could be differences in buprenorphine toxicokinetics or to inherent differences in norbuprenorphine toxicity in humans and rats (Megarbane et al., 2006). Median plasma buprenorphine and norbuprenorphine concentrations in forensic fatal cases were 4.9 [1.1–17.9] and 1.6 [0.4–14.0] μg/L, respectively, with mean norbuprenorphine/buprenorphine ratios of 0.45 [0.08–2.0]. In 23% of these cases, ratios were >1, but never exceeded 3. Norbuprenorphine concentrations and norbuprenorphine/buprenorphine ratios reported in our study were similar. Our data showed buprenorphine concentrations up to 174.8 μg/L from IV administration of clinically relevant opioid maintenance doses of 2–16 mg, whereas previously, therapeutic buprenorphine blood concentrations were considered to be less than 5 μg/L (Meyer, 1994). These data suggest that toxic buprenorphine blood concentration ranges should be reevaluated and emphasize, as reported previously by Pirnay et al., that prediction of cause of death based on plasma buprenorphine concentrations alone is difficult (Pirnay et al., 2004).
This study has several limitations. First, there was some accumulation of buprenorphine across sessions at higher doses, although concentrations were low. Buprenorphine has a long half-life and was detected more than 72 h after 8, 12, and 16 mg IV buprenorphine at 0.15–0.54 μg/L. These low residual concentrations would have minimally contributed to buprenorphine pharmacokinetics. Second, safety concerns dictated that IV doses be given in ascending rather than randomized order. Third, the number of participants was small. Fourth, all participants were male, limiting the external validity of the study. A recent study found that buprenorphine kinetics differ in men and women, related to differences in lean body mass (Moody et al., 2011).
This study is the first of which we are aware to investigate buprenorphine and its active metabolite norbuprenorphine following high IV doses. These data provide novel information on the pharmacokinetics of IV buprenorphine and norbuprenorphine that are clinically relevant to the IV misuse of diverted SL buprenorphine.
4.1 Conclusion
This study evaluated the pharmacokinetics of a range of IV buprenorphine doses clinically relevant to the diversion of SL buprenorphine prescribed for treatment of opioid dependence. Maximum plasma buprenorphine concentrations increased in a linear dose-related manner up to 12 mg IV buprenorphine, then flattened somewhat at 16 mg. These data document that the previously demonstrated ceiling phenomenon for physiological and subjective effects (Umbricht et al., 2004) are most likely due to pharmacodynamic rather than pharmacokinetic adaptations, at least up to 12 mg.
Acknowledgments
Role of funding sources
This study was supported by the Intramural Research Program, NIH, National Institute on Drug Abuse. The funding source played no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
The authors thank Nelda Snydow, RN for nursing assistance, Nora Chiang, PhD for assistance in obtaining drug supplies, Allan Barnes for assistance with statistical analysis and preparation of figures, and David A. Gorelick, MD, PhD for editorial assistance with the manuscript.
Footnotes
Contributors
Authors Huestis, Cone, Umbricht, and Preston designed the study, wrote the protocol and were responsible for data collection oversight. Author Pirnay performed the data analyses and created the figures. Author Huestis wrote the first draft of the manuscript. All authors reviewed and approved the final manuscript.
Conflict of interest
No conflict declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alho H, Sinclair D, Vuori E, Holopainen A. Abuse liability of buprenorphine–naloxone tablets in untreated intravenous drug users. Drug Alcohol Depend. 2007;88:75–78. doi: 10.1016/j.drugalcdep.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Bazazi AR, Yokell M, Fu JJ, Rich JD, Zaller ND. Illicit Use of Buprenorphine/naloxone among injecting and noninjecting opioid users. J Addict Med. 2011;5:175–180. doi: 10.1097/ADM.0b013e3182034e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullingham RE, McQuay HJ, Moore RA. Clinical pharmacokinetics of narcotic agonist-antagonist drugs. Clin Pharmacokinet. 1983;8:332–343. doi: 10.2165/00003088-198308040-00004. [DOI] [PubMed] [Google Scholar]
- Bullingham RES, Dwyer D, Allen MC, Moore RA, McQuay HJ. SL buprenorphine used postoperatively: clinical observations and preliminary pharmacokinetic analysis. Br J Clin Pharmacol. 1981;12:117–122. doi: 10.1111/j.1365-2125.1981.tb01189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullingham RES, McQuay HJ, Moore A, Bennett MRD. Buprenorphine kinetics. Clin Pharmacol Ther. 1980;28:667–672. doi: 10.1038/clpt.1980.219. [DOI] [PubMed] [Google Scholar]
- Chiang CN, Hawks RL. Pharmacokinetics of the combination tablet of buprenorphine and naloxone. Drug Alcohol Depend. 2003;70:S39–47. doi: 10.1016/s0376-8716(03)00058-9. [DOI] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Vosburg SK, Manubay J, Amass L, Cooper ZD, Saccone P, Kleber HD. Abuse liability of intravenous buprenorphine/naloxone and buprenorphine alone in buprenorphine-maintained intravenous heroin abusers. Addiction. 2010;105:709–718. doi: 10.1111/j.1360-0443.2009.02843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone EJ, Dickerson SL, Darwin WD, Fudala P, Johnson RE. Elevated drug saliva levels suggest a “depot-like” effect in subjects treated with sublingual buprenorphine. NIDA Res Monogr. 1991;105:569. [PubMed] [Google Scholar]
- Cone EJ, Gorodetzky CW, Yousefnejad D, Buchwald WF, Johnson RE. The metabolism and excretion of buprenorphine in humans. Drug Metab Dispos. 1984;12:577–581. [PubMed] [Google Scholar]
- Gueye PN, Megarbane B, Borron SW, Adnet F, Galliot-Guilley M, Ricordel I, Tourneau J, Goldgran-Toledano D, Baud FJ. Trends in opiate and opioid poisonings in addicts in north-east Paris and suburbs, 1995–99. Addiction. 2002;97:1295–1304. doi: 10.1046/j.1360-0443.2002.00189.x. [DOI] [PubMed] [Google Scholar]
- Harris DS, Mendelson JE, Lin ET, Upton RA, Jones RT. Pharmacokinetics and subjective effects of sublingual buprenorphine, alone or in combination with naloxone: lack of dose proportionality. Clin Pharmacokinet. 2004;43:329–340. doi: 10.2165/00003088-200443050-00005. [DOI] [PubMed] [Google Scholar]
- Ho ST, Wang JJ, Ho W, Hu OY. Determination of buprenorphine by high-performance liquid chromatography with fluorescence detection: application to human and rabbit pharmacokinetic studies. J Chromatogr. 1991;570:339–350. doi: 10.1016/0378-4347(91)80537-m. [DOI] [PubMed] [Google Scholar]
- Kuhlman JJ, Lalani S, Magluilo J, Levine B, Darwin WD, Johnson RE, Cone EJ. Human pharmacokinetics of intravenous, sublingual, and buccal buprenorphine. J Anal Toxicol. 1996;20:369–378. doi: 10.1093/jat/20.6.369. [DOI] [PubMed] [Google Scholar]
- McAleer SD, Mills RJ, Polack T, Hussain T, Rolan PE, Gibbs AD, Mullins FG, Hussein Z. Pharmacokinetics of high-dose buprenorphine following single administration of sublingual tablet formulations in opioid naive healthy male volunteers under a naltrexone block. Drug Alcohol Depend. 2003;72:75–83. doi: 10.1016/s0376-8716(03)00188-1. [DOI] [PubMed] [Google Scholar]
- Megarbane B, Hreiche R, Pirnay S, Marie N, Baud FJ. Does high-dose buprenorphine cause respiratory depression? Possible mechanisms and therapeutic consequences. Toxicol Rev. 2006;25:789–85. doi: 10.2165/00139709-200625020-00002. [DOI] [PubMed] [Google Scholar]
- Mendelson J, Upton RA, Everhart ET, Jacob P, III, Jones RT. Bioavailability of sublingual buprenorphine. J Clin Pharmacol. 1997;37:31–37. doi: 10.1177/009127009703700106. [DOI] [PubMed] [Google Scholar]
- Meyer FP. Indicative therapeutic and toxic drug concentrations in plasma: a tabulation. Int J Clin Pharmacol Ther. 1994;32:71–81. [PubMed] [Google Scholar]
- Moody DE, Slawson MH, Strain EC, Laycock JD, Spanbauer AC, Foltz RL. A liquid chromatographic-electrospray ionization-tandem mass spectrometric method for determination of buprenorphine, its metabolite, norbuprenorphine, and a coformulant, naloxone, that is suitable for in vivo and in vitro metabolism studies. Analytical Biochem. 2002;306:31–39. doi: 10.1006/abio.2002.5673. [DOI] [PubMed] [Google Scholar]
- Moratti E, Kashanpour H, Lombardelli T, Maisto M. Intravenous misuse of buprenorphine: characteristics and extent among patients undergoing drug maintenance therapy. Clin Drug Investig. 2010;30(Suppl 1):3–11. doi: 10.2165/11536020-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Nordmann S, Frauger E, Pauly V, Orleans V, Pradel V, Mallaret M, Thirion X, Micallef J. Misuse of buprenorphine maintenance treatment since introduction of its generic forms: OPPIDUM survey. Pharmacoepidemiol Drug Saf. 2012;21:184–190. doi: 10.1002/pds.2263. [DOI] [PubMed] [Google Scholar]
- Moody DE, Fang WB, Morrison J, McCance-Katz E. Gender differences in pharmacokinetics of maintenance dosed buprenorphine. Drug Alcohol Depend. 2011;118:479–83. doi: 10.1016/j.drugalcdep.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olley JE, Tiong GKL. Plasma levels of opioid material in man following sublingual and intravenous administration of buprenorphine: exogenous/endogenous opioid interaction? J Pharm Pharmacol. 1988;40:666–667. doi: 10.1111/j.2042-7158.1988.tb05336.x. [DOI] [PubMed] [Google Scholar]
- Otiashvili D, Zabransky T, Kirtadze I, Piralishvili G, Chavchanidze M, Miovsky M. Why do the clients of Georgian needle exchange programmes inject buprenorphine? Eur Addict Res. 2010;16:1–8. doi: 10.1159/000253858. [DOI] [PubMed] [Google Scholar]
- Pirnay S, Borron SW, Giudicelli CP, Tourneau J, Baud FJ, Ricordel I. A critical review of the causes of death among post-mortem toxicological investigations: analysis of 34 buprenorphine-associated and 35 methadone-associated deaths. Addiction. 2004;99:978–988. doi: 10.1111/j.1360-0443.2004.00790.x. [DOI] [PubMed] [Google Scholar]
- Sporer KA. Buprenorphine: a primer for emergency physicians. Ann Emerg Med. 2004;43:580–584. doi: 10.1016/j.annemergmed.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Strain EC, Moody DE, Stoller KB, Walsh SL, Bigelow GE. Relative bioavailability of different buprenorphine formulations under chronic dosing conditions. Drug Alcohol Depend. 2004;74:37–43. doi: 10.1016/j.drugalcdep.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Thirion X, Lapierre V, Micallef J, Ronfle E, Masut A, Pradel V, Coudert C, Mabriez JC, Sanmarco JL. Buprenorphine prescription by general practitioners in a French region. Drug Alcohol Depend. 2002;65:197–204. doi: 10.1016/s0376-8716(01)00161-2. [DOI] [PubMed] [Google Scholar]
- Umbricht A, Huestis MA, Cone EJ, Preston KL. Effects of high-dose intravenous buprenorphine in experienced opioid abusers. J Clin Psychopharmacol. 2004;24:479–487. doi: 10.1097/01.jcp.0000138766.15858.c6. [DOI] [PubMed] [Google Scholar]
- Vicknasingam B, Mazlan M, Schottenfeld RS, Chawarski MC. Injection of buprenorphine and buprenorphine/naloxone tablets in Malaysia. Drug Alcohol Depend. 2010;111:44–49. doi: 10.1016/j.drugalcdep.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569–580. doi: 10.1038/clpt.1994.71. [DOI] [PubMed] [Google Scholar]