Abstract

The enantioselective hydroboration of racemic allenylsilane (±)-4 with (dIpc)2BH proceeds via enantiodivergent pathways to give vinylborane 11 and crotylborane intermediate (S)-E-5. Subsequent crotylboration of aldehyde substrates with (S)-E-5 at −78 °C provides (E)-δ-silyl-anti-homoallylic alcohols in 71–89% yield and with 93–96% ee.
A prevailing approach to the synthesis of chiral, nonracemic molecules focuses on introducing chirality in reactions of prochiral substrates using chiral reagents or chiral catalysts. 1 Resolution of racemates, however, remains a valuable tool to access highly enantioenriched molecules. Among resolution strategies, kinetic resolution2 and dynamic kinetic resolution3 have received considerable attention. The enantiodivergent transformation of a racemate, introduced by Kagan, 4 represents another attractive approach to prepare chiral, nonracemic molecules from racemic starting materials. In contrast to kinetic resolution and dynamic kinetic resolution, the enantiodivergent transformation of a racemate involves distinct reactions of each enantiomer of a racemic mixture with a single enantiomer of a chiral reagent or catalyst that proceed at comparable reaction rates (KR ≈ KS) to generate two products that are not enantiomers (Scheme 1). Several elegant transformations utilizing this strategy have been reported. 5 In our continuing efforts to expand the scope of allene hydroboration for the synthesis of functionalized allylboranes, 6 we describe here an enantiodivergent hydroboration of a racemic allenylsilane with the chiral, nonracemic borane reagent, diisopinocampheylborane [(dIpc)2BH], by which the two enantiomers of the racemic allene react efficiently to give two different, non-equilibrating intermediates. Subsequent reaction of one of the two intermediates with aldehydes provides homoallylic alcohols in high yields and with excellent enantioselectivity.
Scheme 1.
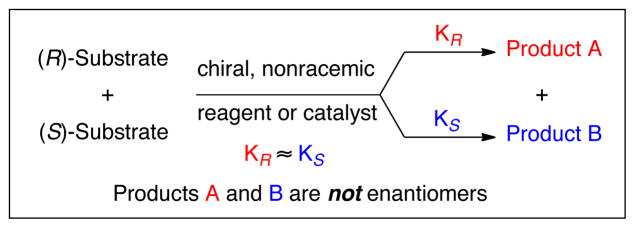
Enantiodivergent Transformation of a Racemate.
We recently reported an enantioselective synthesis of (E)-δ-stannyl-anti-homoallylic alcohols from racemic allenylstannane (±)-1 via an enantioconvergent hydroboration-crotylboration reaction sequence 7 As illustrated in Scheme 2, hydroboration of racemic allene (±)-1 with (dIpc)2BH converted both enantiomers of allenylstannane (±)-1 into the same crotylborane intermediate, (S)-E-2. Subsequent crotylboration of aldehydes with (S)-E-2 gave homoallylic alcohols 3 in good yields and with excellent enantioselectivities. We envisioned that an analogous enantioconvergent reaction sequence might be applicable to an environmentally benign alternative, racemic allenylsilane (±)-4,8 which would then allow the access to highly enantioenriched (E)-δ-silyl-anti-homoallylic alcohols 6. The vinylsilane motif of 6 is as useful for many subsequent transformations as is the vinylstannane unit of 3.
Scheme 2.

Proposed Enantioconvergent Reaction of Racemic Allenylsilane (±)-4
In initial experiments, treatment of racemic allenylsilane (±)-4 with (dIpc)2BH (1 equiv) in toluene at 0 °C for 4 h followed by addition of benzaldehyde (1 equiv) at −78 °C provided a 5:1 mixture of anti-homoallylic alcohol 6a and syn isomer 7a in 41% yield with 76% ee, and 7% yield with 58% ee, respectively (entry 1, Table 1). When the hydroboration was carried out at 40 °C for 2 h and the resulting crotylborane was treated with benzaldehyde at −78 °C, a 2:1 mixture of 6a (60% ee) and 7a (50% ee) was obtained in 54% combined yield (entry 2, Table 1). Hydroboration of (±)-4 at higher temperatures (e.g., 60 °C) followed by crotylboration of benzaldehyde at −78 °C also provided a 2:1 mixture of 6a and 7a, but the alcohol products were obtained with much lower enantioselectivity (entry 3, Table 1). When the hydroboration step was performed at −20 °C for 8 h followed by crotylboration of benzaldehyde at −78 °C, a 16:1 mixture of the anti-homoallylic alcohol 6a and syn isomer 7a was obtained in 42% yield and 90% ee (for 6a, entry 4, Table 1). When the hydroboration of (±)-4 was carried out at −40 °C for 10 h followed by addition of benzaldehyde at −78 °C, 6a was obtained as the only product with >95% ee, albeit in diminished yield (31%), owing to incomplete allene hydroboration under these conditions (entry 5, Table 1). Interestingly, a ketone byproduct 8 (ca. 40%) was identified from all of these reactions. Additionally, treatment of racemic allenylsilane (±)-4 with 0.5 equiv of (dIpc)2BH in toluene at −20 °C for 8 h followed by addition of benzaldehyde (0.45 equiv) at −78 °C provided alcohol 6a with 92% ee in 58% yield (based on 0.45 equiv aldehyde) (entry 6, Table 1). Once again, ketone 8 was detected. The recovered allene 4 was nearly racemic (< 10% ee).
Table 1.
Initial Studies of the Hydroboration-Crotylboration of Racemic Allenylsilane (±)-4a

| |||||||
|---|---|---|---|---|---|---|---|
| entry | t (°C) | time (h) | ds | yield (6a) | % eeb | yield (7a) | % eeb |
| 1 | 0 | 4 | 5:1 | 41% | 76 | 7% | 58 |
| 2 | 40 | 2 | 2:1 | 38% | 60 | 16% | 50 |
| 3 | 60 | 2 | 2:1 | 36% | 28 | 16% | 24 |
| 4 | −20 | 8 | 16:1 | 42% | 90 | 2% | ND |
| 5 | −40 | 10 | >20:1 | 31% | >95 | <1% | ND |
| 6c | −20 | 8 | 16:1 | 58% | 92 | <1% | ND |
Reactions were performed by treating (±)-4 with (dIpc)2BH (1 equiv, except for entry 6) in toluene followed by the addition of PhCHO (1 equiv) at −78 °C. The mixture was then allowed to stir at −78 °C for 12 h. The reactions were subjected to a standard workup (NaHCO3, H2O2) at 0 °C prior to product isolation.
Determined by Mosher ester analysis.9
0.5 equiv of (dIpc)2BH was used for the hydroboration and 0.45 equiv of PhCHO was used for the crotylboration. The enantiomeric purity of recovered allene (±)-4 is less than 10% ee.
Gratifyingly, when the hydroboration of racemic allene (±)-4 was performed using (dIpc)2BH (1 equiv) in toluene at −25 °C with warming of the solution to −15 °C, followed by treatment of the resulting crotylborane (not isolated) with benzaldehyde (0.45 equiv) at −78 °C, homoallylic alcohol 6a was obtained in 85% yield and 95% ee (entry 1, Table 2). Application of this procedure to a variety of other representative achiral aldehydes (entries 3–7, Table 2) provided homoallylic alcohols 6b-f in 71–89% yields (based on the amounts of the aldehydes used in the crotylboration reactions) and with 93–96% ee. The absolute stereochemistry of the secondary hydroxyl groups of alcohols 6a-f was assigned by using the modified Mosher ester analysis.9 The olefin geometry of homoallylic alcohols 6a-f was assigned as E based on 1H NMR analysis (JE = 18.8–19.2 Hz).
Table 2.
Syntheses of (E)-δ-Silyl-anti-homoallylic Alcohols 6a
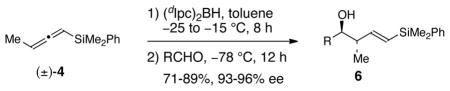
| ||||
|---|---|---|---|---|
| entry | RCHO | product | yieldb | % eec |
| 1 | PhCHO | 6a | 85% | 95 |
| 2d | PhCHO | ent-6a | 82% | 94 |
| 3 | PhCH=CHCHO | 6b | 78% | 95 |
| 4 | Ph(CH2)2CHO | 6c | 89% | 93 |
| 5 | CyCHO | 6d | 71% | 94 |
| 6 | TBSO(CH2)2CHO | 6e | 72% | 95 |
| 7 | BnOCH2CHO | 6f | 75% | 96 |
Reactions were performed by treating (±)-4 with (dIpc)2BH (1 equiv) in toluene at −25 °C and warming to −15 °C over 8 h followed by the addition of RCHO (0.45 equiv) at −78 °C. The mixture was then allowed to stir at −78 °C for 12 h. The reactions were subjected to a standard workup (NaHCO3, H2O2) at 0 °C prior to product isolation.
Based on the amount of the aldehydes used in the crotylboration reaction.
Determined by Mosher ester analysis.9
(lIpc)2BH was used for the hydroboration reaction.
These results indicate that the hydroboration of the two enantiomers of racemic allenylsilane (±)-4 with (dIpc)2BH follows different pathways compared to those for the racemic allenylstannane (±)-1. Based on the efficiency of the reactions of (±)-4 and the observed formation of ketone 8 (Table 1), we speculated that hydroboration of racemic allenylsilane (±)-4 with (dIpc)2BH proceeds in an enantiodivergent manner. As illustrated in Scheme 3, by analogy to the hydroboration of allenylstannane (±)-1,7a hydroboration of the (P)-enantiomer of allenylsilane 4 with (dIpc)2BH is presumed to be a matched case. Hydroboration of (P)-4 with (dIpc)2BH should occur on the re- face (bottom face, as drawn in the first equation of Scheme 3) of the methyl substituted allene carbon of (P)-4, anti to the PhMe2Si– group to give intermediate (R)-Z-9, which can isomerize to crotylborane (S)-E-5 via a reversible boratropic shift. The face selectivity of this hydroboration step is consistent with the known enantioselectivity of hydroboration of (Z)-olefins by (dIpc)2BH.7a,10 Hydroboration of (P)-4 on the allenyl unit adjacent to the PhMe2Si- group leads to the diastereomeric reagent (S)-Z-10 (bottom face hydroboration, as drawn in the second equation of Scheme 3). Crotylboration of benzaldehyde with (S)-Z-10 would give syn-homoallylic alcohol 7a. However, (S)-Z-10 can undergo a sequence of reversible 1,3-boratropic shifts to give the enantiomeric reagent (R)-E-5. Crotylboration of benzaldehyde with (R)-E-5 would give the enantiomeric alcohol product, ent-6a. The hydroboration pathway illustrated in the second equation of Scheme 3 is suppressed when the hydroboration is performed at low temperature (e.g., < −20 °C). However, it becomes much more operational at higher hydroboration temperatures, which corresponds to the reduced diastereoselectivity and reduced enantioselectivity of the reactions summarized in entries 1–3 of Table 1.
Scheme 3.
Proposed Enantioselective and Enantiodivergent Hydroboration-isomerization of the Two Enantiomers of the Racemic Allenylsilane (±)-4 with (dIpc)2BH
On the other hand, hydroboration of the other allene enantiomer. (M)-4, with (dIpc)2BH is likely stereochemically mismatched.7a The three hydroboration pathways illustrated in the 5th line of Scheme 3 are either mismatched with respect to the enantiofacial selectivity of (dIpc)2BH [as determined by the hydroboration of (Z)-olefins7a,10] or mismatched in that hydroboration occurs on the sterically disfavored face of the allene, syn to the PhMe2Si– group. Alternatively, the hydroboration could proceed with opposite regioselectivity, with boron adding to the central allenyl carbon atom of (M)-4, anti to the PhMe2Si– group to give vinylborane 11, the precursor of ketone 8 (as drawn in the 4th line of Scheme 3). The sense of hydroboration in the conversion of (M)-4 to 11 is consistent with the known enantioselectivity of hydroboration of (Z)-olefins by (dIpc)2BH, 7a,10 and is also favored in that the hydroboration occurs on the less hindered side of the allene, anti to the distal PhMe2Si– group. It appears that the rates of hydroboration of the two enantiomers of the racemic allenylsilane (±)-4 with (dIpc)2BH are comparable (1st and 4th lines of Scheme 3), but also that the hydroboration proceeds with different modes of addition to produce two structurally distinct intermediates, (S)-E-5 and 11, respectively.
To gain support for this analysis, enantiomerically enriched (≥95% ee) allenylsilanes (P)-4 and (M)-4 were prepared for use in hydroboration-crotylboration studies.8 As illustrated in Scheme 4, hydroboration of (P)-4 with (dIpc)2BH at −20 °C for 8 h followed by addition of benzaldehyde at −78 °C provided homoallylic alcohol 6a in 80% yield and 95% ee (eq. 1). In contrast, when the identical reaction conditions were used for the hydroboration of the enantiomeric allene (M)-4 with (dIpc)2BH, ketone 8 was obtained in 75% yield along with 6% of alcohol 6a, obtained with 72% ee (eq. 2). Thus, the enantioconvergent hydroboration process that is dominant in the enantioselective hydroboration of racemic allenylstannane (±)-1 is only a minor pathway for the enantioselective hydroboration of allenylsilane (M)-4 with (dIpc)2BH that produces crotylborane (S)-E-5 (as drawn in the 3rd line of Scheme 3); the latter reaction is dominated by the enantiodivergent hydroboration pathway that leads to vinylborane 11 (the 4th line of Scheme 3).
Scheme 4.
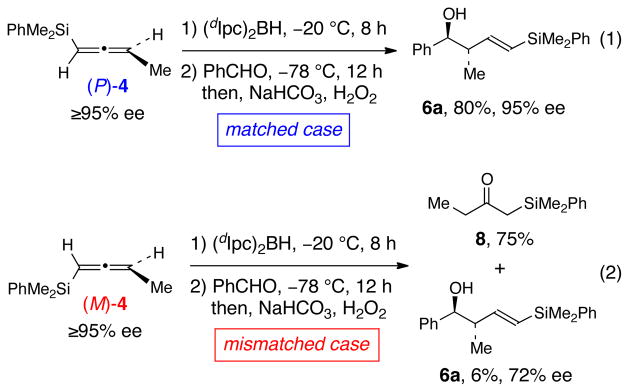
Hydroboration-Crotylboration Studies of Single Enantiomeric Allenylsilanes (P)-4 and (M)-4
Other evidence in support of this analysis was obtained from 1H NMR studies of the hydroboration reactions of (P)-4 or (M)-4 with (dIpc)2BH. Hydroboration of (P)-4 with (dIpc)2BH in d8-toluene produced a major product with two sets of olefinic signals (δ 5.83 ppm, ddq, J = 14.8, 11.2, 1.6 Hz; δ 5.20 ppm, dq, J = 15.2, 6.4 Hz), corresponding to the two (E)-olefinic protons of (S)-E-5. Hydroboration of (M)-4 with (dIpc)2BH, however, produced a major product with a singlet at 5.68 ppm, corresponding to the olefinic proton of vinylborane 11. Weak olefinic signals (<10%) at 5.83 and 5.20 ppm were also observed. In both experiments, 1H NMR signals corresponding to (R)-Z-9 or (S)-Z-10 (Scheme 3) were not observed. These data clearly indicate that the enantioselective hydroboration of the two enantiomers of the racemic allenylsilane (±)-4 proceed with distinct regioselectivities to give different intermediates from each allene enantiomer (P)-4 and (M)-4. These results are fully consistent with the proposed enantiodivergent hydroboration pathways for racemic allenylsilane (±)-4 depicted in Scheme 3. Additionally, the efficiency of the reaction as summarized in Table 1 and the enantiomeric purity (< 10% ee) of the recovered allene (entry 6, Table 1) suggest that the rates of the two hydroboration pathways (equations 1 and 4, Scheme 3) are comparable.
In summary, we have developed an enantioselective synthesis of (E)-δ-silyl-anti-homoallylic alcohols 6 via an enantiodivergent hydroboration-crotylboration reaction sequence that originates with the hydroboration of racemic allenylsilane (±)-4 with (dIpc)2BH. Under optimized conditions, homoallylic alcohols 611 were obtained in high yields and with excellent enantioselectivities from racemic allenylsilane (±)-4. Thus, the preparation of enantioenriched allenylsilane is not required to produce highly enantioenriched homoallylic alcohols. In addition, the silyl substituted olefin unit embedded in the homoallylic alcohol products is suitable for use in a variety of subsequent transformations. 12,13 Synthetic applications of this methodology will be reported in due course.
Supplementary Material
Acknowledgments
Financial support provided by the National Institutes of Health (GM038436) is gratefully acknowledged. We thank Eli Lilly for a predoctoral fellowship to M. Chen.
Footnotes
Supporting Information Available: Experimental procedures and spectroscopic data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Jacobsen EN, Pfaltz A, Yamamoto H. Comprehensive Asymmetric Catalysis. I-III. Springer; Berlin: 1999. [Google Scholar]; (b) Ojima I. Catalytic Asymmetric Synthesis. 2. Wiley/VCH; New York: 2000. [Google Scholar]
- 2.For selected reviews on kinetic resolution: Kagan HB, Fiaud JC. In: Topics in Stereochemistry. Allinger AL, Eliel E, editors. Vol. 18. Wiley; New York: 1988. p. 249.Vedejs E, Jure M. Angew Chem, Int Ed. 2005;44:3974. doi: 10.1002/anie.200460842.
- 3.(a) Noyori R, Tokunaga M, Kitamura M. Bull Chem Soc Jpn. 1995;68:36. [Google Scholar]; (b) Huerta FF, Minidis ABE, Backvall JE. Chem Soc Rev. 2001:321. [Google Scholar]; (c) Trost BM, Fandrick DR. Aldrichimica Acta. 2007;40:59. [Google Scholar]; (d) Pellissier H. Tetrahedron. 2008;64:1563. [Google Scholar]
- 4.Kagan HB. Tetrahedron. 2001;57:2449. [Google Scholar]
- 5.For selected examples: Chen Y, Deng L. J Am Chem Soc. 2001;123:11302. doi: 10.1021/ja011766h.Bertozzi F, Crotti P, Macchia F, Pineschi M, Feringa BL. Angew Chem, Int Ed. 2001;40:930.Davies HML, Walji AM. Angew Chem, Int Ed. 2005;44:1733. doi: 10.1002/anie.200462227.Tanaka K, Fu GC. J Am Chem Soc. 2005;127:11492. doi: 10.1021/ja011907f.Wu B, Parquette JR, RajanBabu TV. Science. 2009;326:1662. doi: 10.1126/science.1180739.
- 6.(a) Chen M, Handa M, Roush WR. J Am Chem Soc. 2009;131:14602. doi: 10.1021/ja904599h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kister J, DeBaillie AC, Lira R, Roush WR. J Am Chem Soc. 2009;131:14174. doi: 10.1021/ja905494c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ess DH, Kister J, Chen M, Roush WR. Org Lett. 2009;11:5538. doi: 10.1021/ol902364d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Chen M, Ess DH, Roush WR. J Am Chem Soc. 2010;132:7881. doi: 10.1021/ja103041u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Stewart P, Chen M, Roush WR, Ess D. Org Lett. 2011;13:1478. doi: 10.1021/ol2001599. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Chen M, Roush WR. Org Lett. 2011;13:1992. doi: 10.1021/ol200392u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Chen M, Roush WR. Org Lett. 2012;14:1556. doi: 10.1021/ol300282e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Kister J, Nuhant P, Lira R, Sorg A, Roush WR. Org Lett. 2011;13:1868. doi: 10.1021/ol2003836. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Han JL, Chen M, Roush WR. Org Lett. 2012;14:3028. doi: 10.1021/ol3010968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen M, Roush WR. J Am Chem Soc. 2011;133:5744. doi: 10.1021/ja2010187.For recent synthetic applications of reagent (S)-E-2, see: Sun H, Abbott JR, Roush WR. Org Lett. 2011;13:2734. doi: 10.1021/ol200834p.Yin M, Roush WR. Tetrahedron. 2011;67:10274. doi: 10.1016/j.tet.2011.10.029.Chen M, Roush WR. Org Lett. 2012;14:426. doi: 10.1021/ol203161u.Chen M, Roush WR. Org Lett. 2012;14:1880. doi: 10.1021/ol300476f.Chen M, Roush WR. J Am Chem Soc. 2012;134:3925. doi: 10.1021/ja300472a.Chen M, Roush WR. J Org Chem. 2013;78:3. doi: 10.1021/jo3008226.
- 8.(a) Marshall JA, Maxson K. J Org Chem. 2000;65:630. doi: 10.1021/jo991543y. [DOI] [PubMed] [Google Scholar]; (b) Fleming I, Waterson D. J Chem Soc, Perkin Trans 1. 1984:1809. [Google Scholar]; (c) Fleming I, Newton TW, Roessler F. J Chem Soc, Perkin Trans 1. 1981:2527. [Google Scholar]
- 9.(a) Dale JA, Mosher HS. J Am Chem Soc. 1973;95:512. [Google Scholar]; (b) Ohtani I, Kusumi T, Kashman Y, Kakisawa H. J Am Chem Soc. 1991;113:4092. [Google Scholar]
- 10.(a) Brown HC, Zweifel G. J Am Chem Soc. 1961;83:486. [Google Scholar]; (b) Zweifel G, Brown HC. J Am Chem Soc. 1964;86:397. [Google Scholar]
- 11.For early reports on the syntheses of racemic δ-silyl-homoallylic alcohols: Yamamoto Y, Yatagai H, Maruyama K. J Am Chem Soc. 1981;103:3229.Sato F, Uchiyama H, Iida K, Kobayashi Y, Sato M. J Chem Soc, Chem Commun. 1983:921.Tsai DJ, Matteson DS. Organometallics. 1983;2:236.Fugami K, Nakatsukasa S, Oshima K, Utimoto K, Nozaki H. Chem Lett. 1986:869.Hodgson DM, Wells C. Tetrahedron Lett. 1992;33:4761.Shimizu M, Kitagawa H, Kurahashi T, Hiyama T. Angew Chem, Int Ed. 2001;40:4283. doi: 10.1002/1521-3773(20011119)40:22<4283::AID-ANIE4283>3.0.CO;2-3.Takeda T, Wasa H, Tsubouchi A. Tetrahedron Lett. 2011;52:4575.
- 12.(a) Blumenkopf TA, Overman LE. Chem Rev. 1986;86:857. [Google Scholar]; (b) Fleming I. Org React. 1989;37:57. [Google Scholar]; (c) Fleming I, Barbero A, Walter D. Chem Rev. 1997;97:2063. doi: 10.1021/cr941074u. [DOI] [PubMed] [Google Scholar]
- 13.For select reviews of Hiyama coupling: Denmark SE, Liu JHC. Angew Chem, Int Ed. 2010;49:2978. doi: 10.1002/anie.200905657.Denmark SE, Sweis RF. In: Metal Catalyzed Cross-Coupling Reactions. 2. de Meijere A, Diederich F, editors. Vol. 4 Wiley-VCH; Weinheim: 2004. Hiyama T. In: Metal Catalyzed Cross-Coupling Reactions. Diederich F, Stang PJ, editors. Vol. 10. Wiley-VCH; Weinheim: 1998. and references cited therein.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



