Abstract
The osteocyte network is crucial for the response of bone to mechanical force. Within this network, connexin43 (Cx43) is thought to mediate the communication of osteocytes and osteoblasts among themselves and the exchange of small molecules with the extracellular milieu. Despite recent advances in understanding Cx43 role for the response of bone cells to mechanical stimulation, the contribution of Cx43 specifically in osteocytes to mechanotransduction in vivo is not well-known. We examined the anabolic response to ulnar axial loading of mice lacking Cx43 in osteocytes (Cx43ΔOt). Loading induced a greater increase in periosteal bone formation rate in Cx43ΔOt mice compared to control littermates, resulting from higher mineralizing surface and enhanced mineral apposition rate. Expression of β-catenin protein, a molecule implicated in mechanotransduction, was higher in bones from Cx43ΔOt mice, compared to littermate controls. In addition, MLO-Y4 osteocytic cells knocked-down for Cx43 exhibited higher β-catenin protein expression and enhanced response to mechanical stimulation. These findings suggest that osteocytes lacking Cx43 are “primed” to respond to mechanical stimulation and that absence of Cx43 in osteocytes unleashes bone formation, by a mechanism that might involve accumulation of β-catenin.
Keywords: connexin43, bone formation, mechanical loading, osteocyte, β-catenin
INTRODUCTION
Bone adapts to the mechanical environment by regulating its architecture through the concerted action of osteoclasts and osteoblasts. Osteocytes are anatomically placed to detect mechanical forces and translate them into signals to promote the recruitment and maturation of osteoblasts on the bone surfaces, where matrix apposition takes place.1 Activation of the Wnt/β-catenin signaling pathway is crucial in the skeletal response to mechanical force.1 Loading increases the expression of Wnt target genes2,3 and downregulates the Wnt inhibitor SOST/sclerostin,4 a pre-requisite for the anabolic response to loading.3 It has been shown that a major effect of signaling downstream of β-catenin in osteoblastic cells is to inhibit osteoclast development and function.5-7 Thus, stabilization of β-catenin in these cells increases osteoprotegerin (OPG) expression and leads to low bone resorption, whereas genetic deletion of β-catenin from osteoblastic cells results in increased resorption.5,7 Moreover, removal of β-catenin primarily in osteocytes also results in reduced OPG expression and high bone resorption.6 β-catenin expression in osteoblasts and osteocytes may also control bone formation, as evidenced by the impaired maturation of osteoblastic cells lacking β-catenin.7 In addition, recent preliminary reports show that β-catenin expression in osteocytes is required for the bone gain exhibited by mice lacking the bone formation inhibitor sclerostin.8 Moreover, β-catenin haploinsufficiency in osteocytes abolishes the osteogenic effect of mechanical loading in vivo.9
Osteocytes are embedded in the bone matrix and highly connected to each other and to cells on bone surfaces. Intercellular communication is mediated at least in part by gap junction channels and hemichannels made of connexin43 (Cx43) in osteoblasts and osteocytes. In osteoblasts, Cx43 regulates the maturation and function and its ubiquitous removal disrupts osteoblast differentiation.10 In cultured osteocytes, hemichannels made of Cx43 open in response to fluid flow shear stress11 and mediate the release of the bone anabolic factor prostaglandin E2 (PGE2).12,13 However, the relevance of Cx43 expression in osteocytes for loading-induced bone anabolism in vivo remained to be determined. Deletion of Cx43 from mature osteoblasts and osteocytes increases periosteal bone formation upon mechanical stimulation of the tibia,14 but this mouse model does not discern the role of Cx43 specifically in osteocytes. We demonstrate here that mice lacking Cx43 selectively in osteocytes (Cx43ΔOt mice) exhibit enhanced periosteal bone formation induced by ulnae loading. Moreover, in the absence of Cx43, osteocytes express higher levels of β-catenin, providing a potential explanation for the increased anabolic response to mechanical signals in these mice. We conclude that Cx43 expression in osteocytes restrains loading-induced bone formation likely by reducing β-catenin levels in osteocytes.
METHODS
Mice
Mice lacking Cx43 in osteocytes (Cx43ΔOt) were generated by crossing floxed Cx43 (Cx43fl/fl) mice with mice expressing cre recombinase under the control of an 8kb fragment of the murine dentin matrix protein 1 promoter (DMP1-8kb-cre mice), as previously described.15 Mice were all in C57Bl/6 genetic background and were born at the expected Mendelian frequency. Protocols were approved by the IACUC at Indiana University School of Medicine.
Mechanical loading in vivo
The load used to stimulate an osteogenic response in Cx43ΔOt and in Cx43fl/fl mice was determined ex vivo prior to in vivo loading using miniature strain gauges (EA-06-015DJ-120, Vishay Micro-Measurements, Raleigh, NC).16 Right ulnae midshafts from 16 week-old female mice were partially exposed and a single strain gauge was glued to the medial surface of the ulnar midshaft. Bones were loaded at 0.95, 1.40, 1.85, and 2.30 N. Conditioned voltage output from the gauge was converted to strain using a calibration factor derived from measured and calculated (using beam theory) strains collected from an aluminum cantilever of known modulus. The strains were regressed onto applied force in order to derive the load:strain relation within genotypes, which was ~780 με/N in the Cx43fl/fl mice mice and ~640 με/N in the Cx43ΔOt mice. Right ulnae from 17 week-old female mice were loaded for 3 consecutive days at 120 cycles/min once a day, as reported.3,4 Three peak force levels were used (2.3N, 2.5N, and 2.8 N in the Cx43fl/fl mice; and 2.8N, 3.1N, and 3.5N in the Cx43fl/fl mice) and were matched across genotypes based on strain values calculated from the load:strain relation.
Histomorphometric analysis
Mice received calcein (i.p., 30 mg/kg, Sigma Chemical, St. Louis, MO) and alizarin (i.p., 50 mg/kg, Sigma) 11 and 4 days before sacrifice, respectively. Ulnae were fixed in 10% neutral buffered formalin, followed by 70% ethanol and embedded in methyl methacrylate. 100μm cross-sections of the ulnar midshaft were ground down to 30μm. Fluorochrome labels were quantified using OsteoMeasure high resolution digital video system (OsteoMetrics, Inc., Decatur, GA).17,18 A value of 0.1 μm/day was used for mineral apposition rate (MAR) when only single label was present in order to calculate bone formation rate (BFR/BS).19 Terminology and units are by the Histomorphometry Nomenclature Committee of the ASBMR.20
MLO-Y4 cell culture
MLO-Y4 osteocytic cells in which Cx43 expression was silenced by short hairpin (sh)RNA and scramble shRNA controls were generated and cultured as published.21
Reporter assay
MLO-Y4 cells were plated at the density of 2×104 cells/cm2. Twelve hours later a Lef1-luciferase reporter construct (Lef1-Luc)22 was introduced into cells together with a Renilla luciferase plasmid pRL-SV40 by using Lipofectamine Plus (Invitrogen), as published.23 Twenty-four h after transfection, cells were treated with vehicle or 30 nM lithium chloride (LiCl) for 24 h. Luciferase activity was measured in cell lysates using a dual luciferase kit (Promega, Madison, WI) according to the manufacturer's instructions. Lef1 promoter activity was normalized to Renilla activity.
RNA preparation and real-time PCR
Mice ulnae were partially thawed, the ends were removed and the bone marrow was flushed out. Total RNA was purified using Ultraspec (Biotecx Laboratories, Houston, TX) followed by Taqman quantitative RT-PCR, as previously described.21 RNA was isolated from MLO-Y4 cells using Trizol (Invitrogen, Grand Island, NY). Primers and probes for secreted frizzled-related protein 4 (Sfrp4), Axin2, ccdn1/cyclin D1, Smad6, and Cox-2 were designed using the assay design center (Roche Applied Science, Indianapolis, IN). GAPDH was used as housekeeping gene (Applied Biosystems, Foster City, CA), using the ΔCt method.24
Protein lysate preparation and Western blotting
Osteocyte-enriched bone preparations from mouse ulnae were prepared using partially thawed bones. The ends containing trabecular bone were removed, bone marrow cells and osteoblasts lining the endocortical surface were flushed and the periosteum was scraped off. Bones were pulverized, and incubated at room temperature in lysis buffer for 30 min, as published.25 Samples were spun down at 750g for 10 min at 4°C and supernatants were used. Protein extracts from MLO-Y4 cells were obtained following published protocols.26 Membranes were probed with antibodies against murine β-actin (Sigma, A5316), β-catenin (BD Transduction Laboratories, San Jose, CA, 610153), or Cx43 (Sigma, C6219).
Mechanical stimulation of cell cultures
MLO-Y4 cells were plated at the density of 2×104 cells/cm2 on 6-well plates with flexible bottom coated with collagen type I (Flexcell International). After 24 h, cultures at 75-80% confluence were subjected to cycles of 3400 microstrain stretching (FX-4000 Flexercell Strain Unit, Flexcell International) for 5 hours (2Hz, 7200cycle/h), as published.2 mRNA was isolated immediately after stretching.
Statistical analysis
Statistical analysis was performed using SigmaPlot (Systat Software Inc., San Jose, California). Normal distribution of the data was evaluated by the Shapiro-Wilk test. Two-sample paired Student's t-test was used to compare loaded versus non-loaded control arm. The effect of loading was evaluated by two-way ANOVA. Student's t-test or one-way ANOVA followed by Student-Newman-Keuls method were used to evaluate differences in cultured cells.
RESULTS
Cx43ΔOt mice exhibit reduced levels of Cx43 and experience lower strains compared to control mice in response to equal loading force
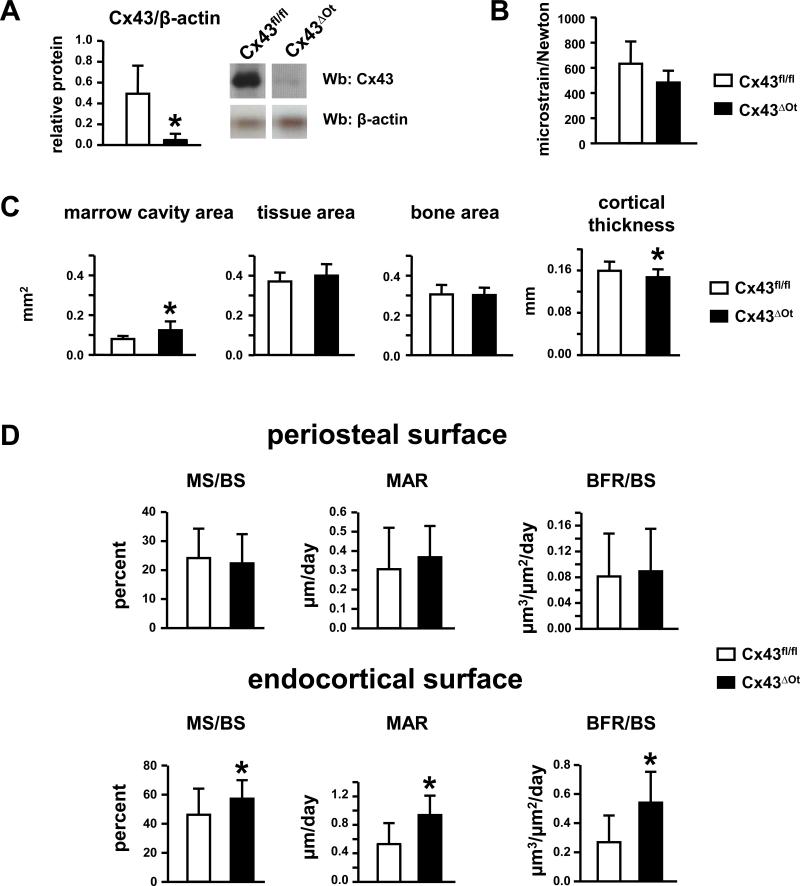
We have recently demonstrated that Cx43 is efficiently deleted only from osteocytes in Cx43ΔOt mice.15 Cx43 mRNA is decreased in osteocyte-enriched cortical bone preparations from Cx43ΔOt mice, compared to Cx43fl/fl littermates. Similarly, Cx43 protein is lower in osteocytes, but not osteoblasts, in bone sections from these Cx43-deficient mice. Consistent with this, protein lysates from ulnar cortical diaphysis from Cx43ΔOt mice showed a 90% decrease in Cx43 compared to Cx43fl/fl mice by Western blotting (Fig. 1A). To determine whether deletion of Cx43 affects the resistance of ulnae to mechanical loading, we evaluated the deformation upon loading ex vivo on ulnae from Cx43ΔOt and control mice by using strain gauges placed on the mid-shaft. We found that ulnae from Cx43ΔOt mice are less compliant and undergo 24% less deformation per unit of load, compared to control mice (Fig. 1B).
Figure 1. Cx43ΔOt mice exhibit reduced Cx43 levels and experience a lower strain compared to control mice in response to equal force.
(A) Cx43 deficiency was confirmed by Western blot analysis on protein lysates prepared from the diaphysis of non-loaded ulnae of Cx43ΔOt and Cx43fl/fl mice. Bars represent mean ± SD, n=3. *: p<0.05 by t-test. (B) Ulnae from Cx43ΔOt mice exhibit 24% lower microstrain per unit load (ε/N) than ulnae from control mice, as measured using ex-vivo strain gauges applied to the ulna diaphysis. Bars represent mean ± SD. n=3, 6. (C) Geometrical properties of ulnar midshafts from Cx43fl/fl (control) and Cx43ΔOt mice assessed by histomorphometry. (D) Dynamic histomorphometric parameters of ulnae mid-diaphysis on the periosteal and endocortical surfaces of non-loaded control and Cx43ΔOt mice. Bars represent mean ± SD, n=17, 23. *: p<0.05 by unpaired t-test.
Histomorphometric analysis of the mid-diaphysis revealed that ulnae from Cx43ΔOt mice have wider marrow cavity area compared to Cx43fl/fl (Fig. 1C). Total tissue and bone areas were not different between genotypes, resulting in significantly thinner cortices in Cx43ΔOt mice. MS/BS, MAR, and BFR/BS were similar between the two genotypes on the periosteal surface (Fig. 1D), but were significantly higher on the endocortical surface of Cx43ΔOt mice than in Cx43fl/fl mice.
The osteogenic response to mechanical loading is enhanced in Cx43ΔOt mice compared to Cx43fl/fl mice
Based on the ex vivo strain measurements, mice were loaded with forces to induce equivalent strain levels in both genotypes. Animals were loaded at low, medium or high magnitude midshaft strains for 1 min/day for 3 consecutive days. Sixteen days after initiating loading, bone formation was assessed on the periosteal surface (Fig. 2A and B).
Figure 2. Deletion of Cx43 from osteocytes results in enhanced response to loading on the ulnae periosteal surface.
(A) Mineralizing surface (MS/BS), mineral apposition rate (MAR) and bone formation rate (BFR/BS) in response to ulnar axial compression at low, medium and high strain. Bars are mean ± SD, *: p<0.05 by paired t-test, n 7-10; #: p<0.05 by two-way ANOVA, n=7-10; (B) Representative images of periosteal bone formation in ulnae of Cx43fl/fl and Cx43ΔOt mice, unloaded and loaded at high magnitude, taken at a 400X magnification. Arrows point at the calcein and alizarin labels.
The response to the low magnitude of strain was comparable between the two genotypes (Fig. 2A). However, in the medium strain magnitude group the response to loading in Cx43ΔOt mice was greater, as evidenced by a significant increase not only in MS/BS, but also in MAR and BFR/BS. Similarly to this, at the high strain magnitude Cx43ΔOt mice showed a statistically greater response than control animals, with a 5-fold increase in BFR/BS upon loading, that resulted from increased MS/BS and MAR. On the other hand, loading did not increase bone formation on the endocortical surface of the ulnae in mice of either phenotype (Fig. 3).
Figure 3. Loading does not affect bone formation on the endocortical ulnae surface.
Mineralizing surface (MS/BS), mineral apposition rate (MAR) and bone formation rate (BFR/BS) in response to ulna compression at low, medium and high strain. Bars represent mean ± SD, n=7-10.
Removal of Cx43 from osteocytes results in increased expression of β-catenin and some Wnt target genes
Loading promotes the transcription of Wnt-target genes in osteocytes27 through the stabilization and cytoplasmic accumulation of the transcriptional activator β-catenin.28,29 Moreover, increased β-catenin levels enhance the sensitivity to mechanical loading, demonstrated by the increased response to mechanical forces in cells treated with GSK3β inhibitors or Wnt3a.2,30 Based on this evidence, we hypothesized that the exaggerated response to loading in Cx43ΔOt mice might result from higher expression or activity of β-catenin, priming osteocytes to respond to mechanical forces. We found that β-catenin protein was more abundant in osteocyte-enriched ulnae bone preparations from Cx43ΔOt mice, compared to controls (Fig. 4A). To test whether removal of Cx43 from osteocytes intrinsically alters the response to loading, we used MLO-Y4 osteocytic cells in which Cx43 was knocked-down (Fig. 4B). Similar to ulnae (Fig. 4A) and femora (not shown) isolated from Cx43ΔOt mice, Cx43-silenced cells exhibited significantly higher basal β-catenin protein compared to cells transfected with scramble shRNA. Moreover, the expression of the Wnt target genes Axin2 (Fig. 4B) and Sfrp4 (Fig. 5) were increased in Cx43-deficient osteocytic cells. On the other hand, silencing of Cx43 did not change the expression of cyclin D1 (Fig. 4) and Smad 6 (Fig. 5), genes which expression has been shown to be also increased by activating Wnt-signaling. In addition, silencing Cx43 did not alter Wnt-mediated transcription, as Lef1-luciferase was similar in scramble- and Cx43-silenced cells under basal conditions or in cells in which Wnt signaling was induced by LiCl (Fig. 4C).
Figure 4. Absence of Cx43 in osteocytes results in increased accumulation of β-catenin in vivo and in vitro.
(A) β-catenin levels were evaluated by Western blotting of protein lysates from cortical bone and normalized to β-actin. Bars represent means ± SD, n=3,4. *: p<0.05 versus Cx43fl/fl mice by t-test. (B) Cx43, Axin2, and cyclin D1 mRNA levels normalized by GAPDH were evaluated by qPCR and β-catenin protein levels normalized by β-actin were evaluated by Western blotting in MLO-Y4 osteocytic cells expressing (scr shRNA) or not (Cx43 shRNA) Cx43. (B) Lef1-mediated trasncription was measured in cells treated with vehicle or 30 nM LiCl for 24h. Relative luciferase units (RLU) were normalized by Renilla activity. Bars represent means ± SD, n=4-6. *: p<0.05 versus cells treated with scrambled shRNA (scr) by t-test (B) or the corresponding vehicle-treated culture by one-way ANOVA (C).
Figure 5. Absence of Cx43 in osteocytes results in increased Wnt signaling in response to mechanical loading in vitro.
Cells were mechanically stimulated by biaxial stretching at 3,400 microstrain for 5 hours. Gene expression was measured by qPCR on RNA isolated from MLO-Y4 cells expressing (scr shRNA) or not (Cx43 shRNA) Cx43 and normalized to GAPDH. Bars represent means ± SD, n=3. * indicates p<0.05 versus the corresponding non-loaded cells by t-test. #: p<0.05 versus non-loaded scr cells by t-test.
Although mRNA expression for Smad6 was not different at the basal level, it increased upon stretching only in Cx43-deficient cells (Fig. 5). Stretching also increased Sfrp4 expression only in Cx43-silenced cells. On the other hand, mRNA for cyclooxygenase-2 (Cox-2), an enzyme that catalyzes PGE2 synthesis was markedly reduced to 10% in Cx43-deficient cells; and stretching increased Cox-2 mRNA expression to a similar extent in control and Cx43-deficient cells.
DISCUSSION
Intercellular communication among cells of the osteocyte network through gap junctions and hemichannels formed by Cx43 is thought to facilitate the transmission of signals perceived within the bone to cells on the bone surface. We report that, unexpectedly, absence of Cx43 in osteocytes enhances the osteogenic response to mechanical stimulation in vivo. This increased anabolic response is associated with elevated levels of β-catenin in vivo and in vitro in osteocytic cells lacking Cx43.
Bone cells respond to changes in their strain (deformation), rather than load environment. One of the limitations of our experimental approach was that we measured strain only at one location in the ulnae. Thus, it is possible that genotype-related differences in bone shape and thickness might result in mechanical strain differences (and perhaps fluid movement) in other regions of the tissue that were not accounted for by our measurements. We cannot rule out the possibility that these effects produced some of the bone formation differences found between experimental groups.
It has been previously shown that fluid flow induces opening of Cx43 hemichannels in cultured osteocytic cells, associated with the release of PGE212 and ATP.13 Yet, we did not find reduced bone formation induced by mechanical loading in the absence of osteocytic Cx43 in vivo. Moreover, mice lacking Cox-2, the enzyme responsible for PGE2 synthesis, exhibit normal osteogenic response to loading.16 However, residual Cx43 in Cx43ΔOt mice may still be able to mediate the release of small molecules from osteocytes involved in the anabolic effect of loading.
We have previously shown that Cx43ΔOt mice exhibit increased periosteal bone formation and total tissue area on the femoral midshaft.15 However, these parameters are not changed in the ulnae of Cx43ΔOt mice compared to controls. Whether these differences are due to distinct levels of loading or to intrinsic differences in the osteocytic populations in the hindlimbs versus the forelimbs remains to be determined.
Consistent with our findings, removal of Cx43 from osteoblastic cells enhances the activity of periosteal osteoblasts in all the animal models of Cx43 deletion studied.14,31 These findings suggest that periosteal osteoblasts are intrinsically more sensitive to load in the absence of Cx43. In contrast, we found no effect of Cx43 deletion on bone formation rate in the endocortical surface of the loaded ulna. Civitelli and colleagues showed even a decreased endocortical bone formation induced by loading in the tibia of mice lacking Cx43 in pre-osteoblasts and osteocytes32 and in osteochondroprogenitors31 when compared to control littermates. Thus, the role of Cx43 on the execution of the osteogenic response in osteoblasts on endocortical surfaces should be distinguished from its role in periosteal osteoblasts.
Consistent with the accumulation of β-catenin in osteocytic cells with reduced Cx43 expression, the levels of the β-catenin/Wnt target genes Axin2 and Sfrp2 are increased. However, cyclin D1 and Smad6, which are also β-catenin/Wnt target genes, are not changed by Cx43 deletion. Moreover, Lef1-mediated transcription under basal conditions or stimulated by stabilization of β-catenin by inactivating GSK3β with LiCl is similar in scramble- and Cx43-silenced MLO-Y4 cells. This evidence suggests that accumulation of β-catenin in Cx43-deficient cells does not lead to increase in Wnt-mediated transcription. Similarly, mechanical stimulation induces β-catenin accumulation but not TCF-mediated transcription in MLO-Y4 osteocytic cells.33 This raises the possibility that elevated β-catenin protein itself, independently of Wnt/β-catenin transcription is involved in the higher response to loading in Cx43ΔOt mice. Consistent with this, β-catenin haploinsufficiency in osteocytes results in reduced bone formation in response to loading in mice.9
Cx43 associates with β-catenin in Sertoli cells in the blood-testis barrier,34 in cardiomyocytes,35 and in mammary cells.36 Through this association, Cx43 sequesters β-catenin away from the nuclear compartment in mammary cells, thus limiting its activity.36 Future studies will aim to determine whether a Cx43/β-catenin interact also in osteocytic cells, and in particular, whether this plays a role in restraining the response to loading, which is unleashed in Cx43 deficient cells.
In conclusion, our data show that absence of Cx43 improves osteocyte–mediated mechanotransduction, resulting in enhanced loading-induced osteogenesis, and suggests that the intrinsic function of Cx43 in osteocytes is to reduce β-catenin availability.
ACKNOWLEDGEMENTS
The authors thank Dr. David Burr for insightful discussions, and Dr. Xiaolin Tu and Iraj Hassan for technical support. This research was supported by the NIH (R01-AR053643 to LIP).
References
- 1.Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42:606–615. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, et al. WNT/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem. 2006;281:31720–31728. doi: 10.1074/jbc.M602308200. [DOI] [PubMed] [Google Scholar]
- 3.Tu X, Rhee Y, Condon KW, et al. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone. 2012;50:209–217. doi: 10.1016/j.bone.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robling AG, Niziolek PJ, Baldridge LA, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283:5866–5875. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 5.Glass DA, Bialek P, Ahn JD, et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8:751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Kramer I, Halleux C, Keller H, et al. Osteocyte Wnt/beta-catenin signaling is required for normal bone homeostasis. Mol Cell Biol. 2010;30:3071–3085. doi: 10.1128/MCB.01428-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmen SL, Zylstra CR, Mukherjee A, et al. Essential role of beta-catenin in postnatal bone acquisition. J Biol Chem. 2005;280:21162–21168. doi: 10.1074/jbc.M501900200. [DOI] [PubMed] [Google Scholar]
- 8.Chang M, Kramer I, Kneissel M. Sclerostin deficiency does not induce bone gain in mice lacking osteocyte ß-catenin. J Bone Miner Res. 2011;26:S13. [Google Scholar]
- 9.Javaheri B, Dallas M, Zhao H, et al. ß-catenin haploinsufficiency in osteocytes abolishes the osteogenic effect of mechanical loading in vivo. J Bone Miner Res. 2011;26:S24. [Google Scholar]
- 10.Lecanda F, Warlow PM, Sheikh S, et al. Connexin43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction. J Cell Biol. 2000;151:931–944. doi: 10.1083/jcb.151.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang JX, Cherian PP. Hemichannels formed by connexin 43 play an important role in the release of prostaglandin E(2) by osteocytes in response to mechanical strain. Cell Commun Adhes. 2003;10:259–264. doi: 10.1080/cac.10.4-6.259.264. [DOI] [PubMed] [Google Scholar]
- 12.Siller-Jackson AJ, Burra S, Gu S, et al. Adaptation of connexin 43-hemichannel prostaglandin release to mechanical loading. J Biol Chem. 2008;283:26374–26382. doi: 10.1074/jbc.M803136200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Genetos DC, Kephart CJ, Zhang Y, et al. Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J Cell Physiol. 2007;212:207–214. doi: 10.1002/jcp.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Paul EM, Sathyendra V, et al. Enhanced osteoclastic resorption and responsiveness to mechanical load in gap junction deficient bone. PLoS ONE. 2011;6:e23516. doi: 10.1371/journal.pone.0023516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bivi N, Condon KW, Allen MR, et al. Cell autonomous requirement of connexin 43 for osteocyte survival: consequences for endocortical resorption and periosteal bone formation. J Bone Min Res. 2012;27:374–389. doi: 10.1002/jbmr.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alam I, Warden SJ, Robling AG, Turner CH. Mechanotransduction in bone does not require a functional cyclooxygenase-2 (COX-2) gene. J Bone Miner Res. 2005;20:438–446. doi: 10.1359/JBMR.041124. [DOI] [PubMed] [Google Scholar]
- 17.Allen MR, Reinwald S, Burr DB. Alendronate reduces bone toughness of ribs without significantly increasing microdamage accumulation in dogs following 3 years of daily treatment. Calcif Tissue Int. 2008;82:354–360. doi: 10.1007/s00223-008-9131-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plotkin LI, Bivi N, Bellido T. A bisphosphonate that does not affect osteoclasts prevents osteoblast and osteocyte apoptosis and the loss of bone strength induced by glucocorticoids in mice. Bone. 2011;49:122–127. doi: 10.1016/j.bone.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hauge E, Mosekilde L, Melsen F. Missing observations in bone histomorphometry on osteoporosis: implications and suggestions for an approach. Bone. 1999;25:389–395. doi: 10.1016/s8756-3282(99)00194-5. [DOI] [PubMed] [Google Scholar]
- 20.Dempster DW, Compston JE, Drezner MK, et al. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28:2–17. doi: 10.1002/jbmr.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plotkin LI, Lezcano V, Thostenson J, et al. Connexin 43 is required for the anti-apoptotic effect of bisphosphonates on osteocytes and osteoblasts in vivo. J Bone Miner Res. 2008;23:1712–1721. doi: 10.1359/JBMR.080617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tu X, Joeng KS, Nakayama KI, et al. Noncanonical Wnt signaling through G protein-linked PKCdelta activation promotes bone formation. Dev Cell. 2007;12:113–127. doi: 10.1016/j.devcel.2006.11.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plotkin LI, Aguirre JI, Kousteni S, et al. Bisphosphonates and estrogens inhibit osteocyte apoptosis via distinct molecular mechanisms downstream of ERK activation. J Biol Chem. 2005;280:7317–7325. doi: 10.1074/jbc.M412817200. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Bellido T, Ali AA, Plotkin LI, et al. Proteasomal degradation of Runx2 shortens parathyroid hormone-induced anti-apoptotic signaling in osteoblasts. A putative explanation for why intermittent administration is needed for bone anabolism. J Biol Chem. 2003;278:50259–50272. doi: 10.1074/jbc.M307444200. [DOI] [PubMed] [Google Scholar]
- 26.Plotkin LI, Weinstein RS, Parfitt AM, et al. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest. 1999;104:1363–1374. doi: 10.1172/JCI6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santos A, Bakker AD, Zandieh-Doulabi B, et al. Pulsating fluid flow modulates gene expression of proteins involved in Wnt signaling pathways in osteocytes. J Orthop Res. 2009;27:1280–1287. doi: 10.1002/jor.20888. [DOI] [PubMed] [Google Scholar]
- 28.Santos A, Bakker AD, Zandieh-Doulabi B, et al. Early activation of the beta-catenin pathway in osteocytes is mediated by nitric oxide, phosphatidyl inositol-3 kinase/akt, and focal adhesion kinase. Biochem Biophys Res Commun. 2010;391:364–369. doi: 10.1016/j.bbrc.2009.11.064. [DOI] [PubMed] [Google Scholar]
- 29.Xia X, Batra N, Shi Q, et al. Prostaglandin Promotion of Osteocyte Gap Junction Function through Transcriptional Regulation of Connexin 43 by GSK-3-{beta}-catenin Signaling. Mol Cell Biol. 2010;30:206–219. doi: 10.1128/MCB.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Case N, Ma M, Sen B, et al. Beta-catenin levels influence rapid mechanical responses in osteoblasts. J Biol Chem. 2008;283:29196–29205. doi: 10.1074/jbc.M801907200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grimston SK, Watkins MP, Brodt MD, et al. Enhanced periosteal and endocortical responses to axial tibial compression loading in conditional connexin43 deficient mice. PLoS ONE. 2012;7:e44222. doi: 10.1371/journal.pone.0044222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grimston SK, Brodt MD, Silva MJ, Civitelli R. Attenuated response to in vivo mechanical loading in mice with conditional osteoblast ablation of the Connexin43 gene (Gja1). J Bone Miner Res. 2008;23:879–886. doi: 10.1359/JBMR.080222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gortazar AR, Martin-Millan M, Bravo B, et al. Crosstalk between caveolin-1/ERKs and ß-catenin survival pathways in osteocyte mechanotransduction. J Biol Chem. 2013 doi: 10.1074/jbc.M112.437921. doi: 10.1074/jbc.M112.437921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li MW, Mruk DD, Lee WM, Cheng CY. Connexin 43 and plakophilin-2 as a protein complex that regulates blood-testis barrier dynamics. Proc Natl Acad Sci U S A. 2009;106:10213–10218. doi: 10.1073/pnas.0901700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ai Z, Fischer A, Spray DC, et al. Wnt-1 regulation of connexin43 in cardiac myocytes. J Clin Invest. 2000;105:161–171. doi: 10.1172/JCI7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Talhouk RS, Mroue R, Mokalled M, et al. Heterocellular interaction enhances recruitment of alpha and beta-catenins and ZO-2 into functional gap-junction complexes and induces gap junction-dependant differentiation of mammary epithelial cells. Exp Cell Res. 2008;314:3275–3291. doi: 10.1016/j.yexcr.2008.07.030. [DOI] [PubMed] [Google Scholar]