Abstract
Host cellular endoplasmic reticulum α-glucosidases I and II are essential for the maturation of viral glycosylated envelope proteins that use the calnexin mediated folding pathway. Inhibition of these glycan processing enzymes leads to the misfolding and degradation of these viral glycoproteins and subsequent reduction in virion secretion. We previously reported that, CM-10-18, an imino sugar α-glucosidase inhibitor, efficiently protected the lethality of dengue virus infection of mice. In the current study, through an extensive structure-activity relationship study, we have identified three CM-10-18 derivatives that demonstrated superior in vitro antiviral activity against representative viruses from four viral families causing hemorrhagic fever. Moreover, the three novel imino sugars significantly reduced the mortality of two of the most pathogenic hemorrhagic fever viruses, Marburg virus and Ebola virus, in mice. Our study thus proves the concept that imino sugars are promising drug candidates for the management of viral hemorrhagic fever caused by variety of viruses.
1. Introduction
Viral hemorrhagic fever (VHF) designates a group of diseases caused by enveloped, single-stranded RNA viruses belonging to four different families of viruses, that include the Arenaviridae, Bunyaviridae, Filoviridae and Flaviviridae. VHFs are usually acquired through contact with infected animals or bites from infected arthropods (Bray, 2005; Geisbert and Jahrling, 2004). Multiple members in each of the four viral families, such as Arenaviridae members Junin virus (JUNV) and Lassa fever virus (LASV), Bunyaviridae member Rift Valley fever virus (RVFV), Filoviridae members Ebola virus (EBOV) and Marburg virus (MARV) or Flaviviridae member Dengue virus (DENV), have been classified by NIAID as category A priority pathogens with bioterrorism potential (Borio et al., 2002; Bray, 2005; LeDuc, 1989; Mahanty and Bray, 2004) due to the high mortality rate in human associated with the infection of these viruses. Currently no therapeutics and vaccines against these dangerous viruses are available for human use, with the only exception being Candid #1 vaccine developed for JUNV (Ambrosio et al., 2011; Bray, 2005; Geisbert and Jahrling, 2004; Kortepeter, Bausch, and Bray, 2011).
Because VHFs caused by different viral agents usually present as a non-specific febrile illness, etiological diagnosis at the early stage of the infection, particularly in the case of naturally occurring infections, is difficult to achieve (Geisbert and Jahrling, 2004). It is, therefore, important to develop antiviral drugs that are broadly active against all or most of the viruses that cause VHFs. As stated above, although the viruses causing VHFs are virologically distinct, one characteristic in common is that they all have virions with viral glycoprotein(s) as envelope components that appear to require a glucosidase trimming event of their N-linked glycans for proper protein folding and/or maturation. These viruses do not encode their own carbohydrate-modifying enzymes. Therefore, like many other enveloped viruses, these VHF viruses rely on the host cellular glycosylation machinery to modify their envelope proteins (Dwek et al., 2002; Helenius and Aebi, 2004).
Endoplasmic reticulum (ER) α-glucosidases I and II sequentially remove the three glucose residues from the high-mannose N-linked glycans attached to nascent glycoproteins (Helenius and Aebi, 2004), a step that is critical for the subsequent interaction between the glycoproteins and ER chaperones, calnexin and calreticulin. It has been shown that such interaction is required for the correct folding and sorting of some, but not all the glycoproteins (Dwek et al., 2002; Helenius and Aebi, 2004). Due to the highly dynamic nature of the viral replication, it is conceivable that inhibition of ER α-glucosidases might differentially disturb the maturation and function of viral envelope glycoproteins, which consequentially inhibit viral particle assembly and/or secretion. Indeed, we and others have validated α-glucosidases as antiviral targets for multiple enveloped viruses (Chang et al., 2011a; Chang et al., 2009; Qu et al., 2011; Sessions et al., 2009; Yu et al., 2012). Moreover, the antiviral effects of glucosidase inhibitors, such as imino sugar compound deoxynorjirimycin (DNJ) and its derivatives, have been observed in many types of enveloped viruses, including hepatitis B virus, human immunodeficiency virus, herpes simplex virus-1, influenza viruses, parainfluenza virus, measles virus as well as several members of Flaviviridae family, such as bovine viral diarrhea virus (BVDV), DENV, West Nile virus, Japanese encephalitis virus and hepatitis C virus (Block and Jordan, 2001; Block et al., 1994; Bridges et al., 1995; Chang et al., 2011a; Chang et al., 2009; Datema et al., 1984; Dwek et al., 2002; Gu et al., 2007; Jordan et al., 2002; Malvoisin and Wild, 1994; Qu et al., 2011; Steinmann et al., 2007; Taylor et al., 1991; Zitzmann et al., 1999).
Imino sugar DNJ and its derivatives are glucose mimics with a nitrogen atom in place of a oxygen which can serve as competitive substrate and inhibit ER α-glucosidases I and II (Dwek et al., 2002). We reported previously a tertiary hydroxyl DNJ, CM-10-18, with in vitro and in vivo inhibitory activity against ER glucosidases I and II (Chang et al., 2011a; Chang et al., 2009). Moreover, we have demonstrated its in vivo efficacy against lethal DENV infection in mouse models (Chang et al., 2011b). The studies reported herein have been focused on the modification of CM-10-18 to further improve its antiviral potency and spectrum through rational designed chemical modification (Yu et al., 2012). Three novel imino sugars (IHVR11029, 17028 and 19029), identified through an extensive Structure-Activity Relationship (SAR) study of 120 derivatives of CM-10-18, demonstrated broad-spectrum in vitro antiviral activities against representative viruses from all the four viral families causing VHFs and significantly reduced the mortality of MARV and EBOV infection in mice.
2. Materials and methods
2.1 Cell lines, viruses and compounds
Madin-Darby bovine kidney cells (MDBK) were cultured in Dulbecco's modified Eagle's medium (DMEM)/F-12 (1:1) (Invitrogen) supplemented with 10% horse serum (Gibco). Human hepatoma Huh7.5 cells, Baby hamster kidney cells (BHK), Vero and HL60 cells were maintained in DMEM supplemented with 10% fetal bovine serum (Gibco).
Bovine viral diarrhea virus (BVDV) (NADL strain), Tacaribe virus (TCRV) (11573 strain) were obtained from ATCC. DENV (serotype 2, New Guinea C) was obtained from Dr. Nigel Bourne, University of Texas Medical Branch. RVFV (MP12) was provided by Dr. Sina Bavari, U.S. Army Medical Research Institute of Infectious Diseases.
CM-10-18, IHVR11029, IHVR17028 and IHVR19029 were synthesized in house with >95% purity. For in vitro studies, compounds were dissolved in DMSO at 100 mM. For in vivo studies, CM-10-18, IHVR17028 and 19029 were formulated in Phosphate Buffered Saline (PBS, pH 7.4), and IHVR11029 was formulated in PBS with 10% solutol, each at 20mM concentration.
2.2 Virus yield reduction assay
To determine BVDV titers, MDBK cells were infected with serial 10-fold dilutions of culture media harvested from treated cells and overlaid with medium containing 1% methylcellulose and incubated at 37 °C for 3 days. To determine TCRV, DENV, and RVFV titers, monolayer of Vero cells were infected with 10-fold dilutions of the culture media harvested from treated cells, followed by overlay with media containing 0.5% methylcellulose and incubated at 37 °C for 4 to 5 days. Viral foci were counted after crystal violet staining of the plaques.
2.3 EBOV and LASV pesudotype particle (EBOVpp, LASVpp) packaging assays
pNL4-3.Luc.R-E- is a lentiviral reporter plasmid containing two frameshift mutations in Env and Vpr-coding regions and a firefly luciferase gene inserted into the nef gene of HIV pNL4-3 clone (obtained through the NIH AIDS Research and Reference Reagent Program, from Dr. Nathaniel Landau, The Rockefeller University)(Connor et al., 1995; He et al., 1995). EBOV-G and LASV-G are plasmids expressing EBOV (Zaire strain) and LASV (Josiah strain) glycoprotein, respectively (kindly provided by Dr. Andrea Cuconati). To determine the effects of compounds on the package of EBOV and LASV G protein pseudotyped lentiviral particles, 3×105 of 293T cells seeded in a well of 24-well plates were co-transfected with 0.5 μg EBOV-G or LASV-G expression plasmid, 1 μg of pNL4-3.Luc.R-E- using calcium phosphate precipitation procedure. After 6 h, the cells were replenished with complete DMEM containing concentrations of test compounds. Culture media were harvested at 72 h post transfection and filtered through a 0.45μm pore sized PES filter. The yields of pseudotyped viral particles, in presence and absence of compounds, were determined by infection of Huh7.5 cells grown in 96-well plate with 1:1 diluted media from 293T cells. Luciferase activities in cell lysates of Huh7.5 cells were measured (Steady-glo luciferase assay system, Promega) 72 h post-infection.
2.4 Cytotoxicity Assay
To determine the cell viability, an MTT based assay (Sigma) was performed. Cells were mock treated or treated with concentrations of test compounds under conditions that were identical to that used for each of the antiviral assays, except that cells were not infected. The dose-dependent curves were generated to determine the inhibitory concentration required to inhibit cell viability by 50% (CC50).
2.5 In vitro Adsorption, Distribution, Metabolism and Elimination (ADME) profiling
A standard in vitro ADME profiling study was performed (Absorption Systems), to determine the aqueous solubility in PBS (pH 4.0 and 7.4) at 300μM; plasma protein binding and liver microsome stability in samples of human, rat or mouse origins; inhibition of each of the 5 cytochrome P450 (CYP) isozymes (CYP1A2, 2C9, 2C19, 2D6 and 3A4); and permeability in human epithelial colorectal adenocarcinoma cells Caco-2.
2.6 ER α-glucosidase I inhibition assay
ER α-glucosidase I was isolated and purified from rat liver (Karlsson et al., 1993). Oligosaccharide substrate Glc3Man5GlcNAc1 was obtained and labeled as described previously (Alonzi et al., 2008). Varying concentrations of test compounds were added to the mixture of α-glucosidase I and its substrate for 30 min. Following HPLC separation, the amount of hydrolysis product was quantified using peak area analysis. The 50% inhibitory concentrations (IC50) were calculated based on the dose-dependent enzymatic inhibition curves.
2.7 Pharmacokinetic (PK) and in vivo toxicity analyses
Single dose intravenous (IV) and oral (PO) administration studies were performed for IHVR11029, 17028 and 19029. Additional route of administration, intramuscular (IM) or intraperitoneal (IP), was also included for IHVR19029 (BASi). Three to six male Sprague-Dawley rats per administration group were used to generate PK parameters shown in Table 4. Following each administration, blood samples were collected from each animal at 10, 30 min, and 1.5, 2, 4, and 8 h after administration, with additional samples collected at 12 h for the animals with IM and IP dosing as well as a 17 h sample following PO dosing. Non-compartmental pharmacokinetic analyses were performed for plasma concentrations of each animal in Watson Laboratory Information Management System (v7.3.0.01, Thermo Inc.).
Table 4.
Pharmacokinetic properties in rats
| Dose IV/PO/IM/IP (mg/kg) | AUC IV/PO/IM/IP (μg*h/mL) | C0 IV (μg/mL) | T1/2 IV (h) | CL IV (L/h/kg) | Vd IV (L/kg) | Cmax PO/IM/IP (μg/ml) | Tmax PO/IM/IP (h) | F PO/IM/IP % | |
|---|---|---|---|---|---|---|---|---|---|
| 11029 | 5/25/ND/ND | 1990/9180/ND/ND | 1.62 | 1.44 | 2.54 | 3.1 | 1.34/ND/ND | 4.17/ND/ND | 92/ND/ND |
| 17028 | 5/25/ND/ND | 1540/903/ND/ND | 1.66 | 0.88 | 3.34 | 3.0 | 0.18/ND/ND | 1.56/ND/ND | 12/ND/ND |
| 19029 | 5/75/5/5 | 1383/945/1839/983 | 1.79 | 1.2 | 3.49 | 3.0 | 0.26/1.23/1.33 | 2.1/0.1/ 0.17 | 4.6/71/133 |
AUC, Area under the concentration vs time curve; C0, Initial blood concentration after IV dose; CL, plasma clearance; Vd, Volume of distribution; Cmax, maximum concentration in systemic circulation following dose; Tmax, time of Cmax; T1/2, time for blood concentration to reduce by half; F, percent of dose reaching systemic circulation unchanged. ND, not determined. 3 animals were used for each of the 11029 and 17028 study; 6 animals were used for 19029 study.
In vivo toxicity profiling. A single time oral dose (25, 50, 100 or 200mg/kg) Maximum Tolerated Dose (MTD) study (BASi) for IHVR11029 and 17028 was performed in 10 week-old Sprague-Dawley rats followed by 7-day observation. Each treatment group included two rats. For IHVR19029, single dose (25, 50, 100 or 200mg/kg) MTD study was performed in Balb/c mice following IP or IM administration and 9-day observation. Each treatment group included three mice.
2.8. Antiviral efficacy in MARV and EBOV infected mice
The in vivo efficacy experiments were performed using previously described animal models of MARV and EBOV lethal infection (Warren et al., 2010a). For MARV infection, BALB/c mice (12 week of age, obtained from NCI, Ft. Detrick, MD) were challenged with 1,000 pfu of mouse adapted MARV (Ravn strain) via IP injection. For EBOV infection, C57B1/6 mice (8-12 week of age, obtained from NCI, Ft. Detrick, MD) were challenged with 1,000 pfu of mouse adapted EBOV (Zaire strain) via IP injection. Mice were treated with either vehicle or indicated doses of imino sugar twice daily at 12 h intervals, until 10 days post-infection. Each dosing group contained 10 mice. Animals that survived to day 14 were deemed to be protected.
2.9. Detection of free oligosaccharides (FOS) in cultured cells and plasma from treated mice
HL60 cells were either mock treated, or treated with concentrations of test compounds for 16h. FOS was isolated and labeled with 2-AA followed by NP-HPLC analysis to separate individual FOS (Alonzi et al., 2008; Mellor et al., 2004). The peak areas of Glc1Man4GlcNAc1 and Glc3Man5GlcNAc1 were measured using Waters Empower software, as marker of ER α-glucosidase II and I inhibition, respectively.
BALB/c mice were treated with vehicle, 75mg/kg of CM-10-18, or IHVR19029 twice daily via IP injection for 7 days. FOS was isolated from 25μl of plasma samples using a procedure described previously (Alonzi et al., 2008; Mellor et al., 2004). The peak areas of two 2-AA-labelled FOS (Glc1Man4GlcNAc1 and Man4GlcNAc1) were measured using Waters Empower software. While Man4GlcNAc1 FOS serves as internal control, Glc1Man4GlcNAc1, a representative FOS of terminal mono- glucose retention, is the indicator of the effect of imino sugar on glucosidases activities in vivo (Alonzi et al., 2008).
2.10. Statistical analysis
For comparing differences in α-glucosidase inhibition, two-tailed student's t-test was performed. For survival analysis, the Mantel-Cox logrank test was used. Differences between experimental groups were considered significant at P values of <0.05.
3. Results and Discussion
3.1 Nomination of three lead imino sugars with potent and broad spectrum antiviral activity
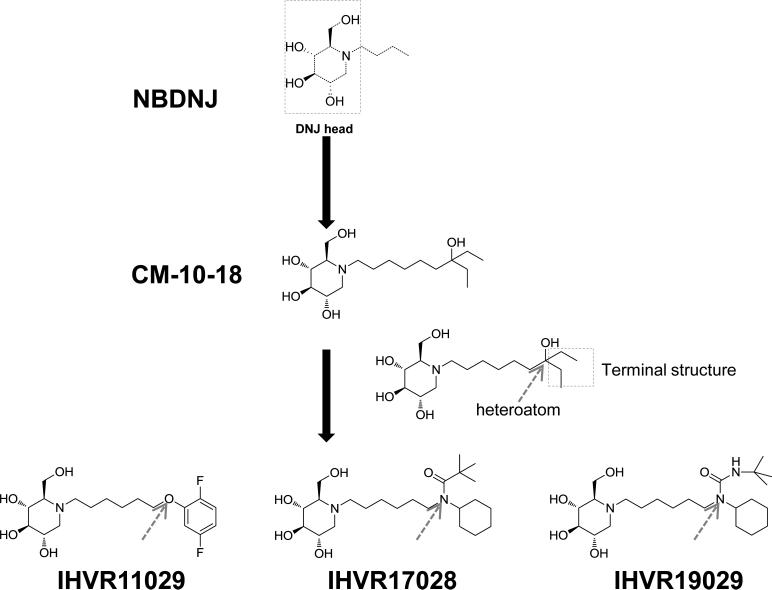
The imino sugars, represented by Zavesca (miglustat or NBDNJ), Precose (acarbose) and Lyset (miglitol), the drugs approved for the treatment of Type II Diabetes and Type 1 Gaucher's disease (EMEA, 2003), consist of a DNJ head group and an alkyl side chain off the nitrogen of the head ring. Although it has been extensively demonstrated that imino sugars inhibited the variety of enveloped viruses in cultured cells, their in vivo antiviral efficacies have thus far only been demonstrated in mice infected with DENV or Japanese encephalitis virus (Schul et al., 2007; Wu et al., 2002). In order to develop imino sugars for the treatment of VHFs, we modified CM-10-18, a pharmacophore with in vitro and in vivo antiviral activities against DENV (Chang et al., 2011a; Chang et al., 2011b; Chang et al., 2009), to further improve its antiviral potency and pharmacological properties. The novel derivatives were synthesized with combinations of heteroatom variations and alterations of terminal structures on the alkyl side chain (Fig. 1).
Figure 1. Structure of imino sugar NBDNJ, CM-10-18 and derivatives.
The DNJ head and terminal structure on N-linked alkyl side chain are indicated in boxes. The position of targeted heteroatom within the N-linked alkyl side chain is indicated by arrows.
Total of 120 derivatives of CM-10-18 were synthesized and screened for their antiviral potency against BVDV and DENV of the Flaviviridae and TCRV of the Arenaviridae as well as cytotoxicity. Twenty-four compounds with superior antiviral activities were selected for an ADME profiling (Yu et al., 2012). Three lead compounds, were nominated based on their structural diversification, antiviral potency, cytotoxicity and ADME profiles (Tables 1 and 2). These are: IHVR11029 ((2R,3R,4R,5S)-1-(6-(2,5-difluorophenoxy)hexyl)-2-(hydroxymethyl)piperidine-3,4,5-triol, phenylether DNJ), IHVR17028 (N-cyclohexyl-N-(6-((2R,3R,4R,5S)-3,4,5-trihydroxy-2-(hydroxymethyl)piperidin-1-yl)hexyl)pivalamide, pivalamide DNJ) and IHVR19029 (3-(tert-butyl)-1-cyclohexyl-1-(6-((2R,3R,4R,5S)-3,4,5-trihydroxy-2-(hydroxymethyl)piperidin-1-yl)hexyl)urea, tert-butyl urea DNJ). Table 1 summaries the antiviral activity against BVDV, TCRV and DENV as determined by virus yield reduction assays, as well as cytotoxicity as determined by MTT assays, all three compounds demonstrated a broad-spectrum antiviral activity in cell cultures and increased potency compared to their parental compound, CM-10-18.
Table 1.
in vitro antiviral activities
| BVDV | TARV | DENV | |||||||
|---|---|---|---|---|---|---|---|---|---|
| compound | EC50 | EC90 | CC50 | EC50 | EC90 | CC50 | EC50 | EC90 | CC50 |
| CM-10-18 | 4.9±1.9 | >100 | >500 | 6.5 | >100 | >500 | 4.5±2.0 | 47.2±27.6 | >500 |
| IHVR11029 | 1.3±0.7 | 16±7.9 | >500 | 3.3±2.7 | 69±37.7 | >500 | 0.75±0.06 | 6.3±3.5 | >500 |
| IHVR17028 | 0.4±0.6 | 16±12 | >500 | 0.26±0.08 | 26.7±20.9 | >500 | 0.3±0.03 | 1.7±0.8 | >500 |
| IHVR19029 | 0.25±0.05 | 16.3±7.8 | >500 | 0.74±0.3 | 52.5±38.9 | >500 | 1.25±1.1 | 22.5±10.6 | >500 |
Cells were either mock treated (0.1% DMSO) or treated with concentrations of compounds ranging from 0.1 to 100 μM;
EC50 and EC90 were determined by virus yield reduction assays;
CC50 values were determined by MTT assay in MDBK, Huh7.5 or BHK cells;
EC50, EC90 and CC50 values are expressed as average ± standard deviation, in μM. Number of experiment: ≥3.
Table 2.
in vitro ADME properties
| Aqueous solubility (μM) | Plasma protein binding (% bound) | CYP inhibition* | Caco-2 permeability | Liver microsome metabolic stability (% remaining) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| pH 4.0 | pH 7.4 | human | rat | mouse | IC50 | Efflux ratio | human | rat | mouse | |
| IHVR11029 | 296 | 292 | 74 | 68 | 77 | >10μM | 4.7 | 91 | 79 | 91 |
| IHVR17028 | 299 | 311 | 88 | 86 | 83 | >10μM | 31.7 | 42 | 27 | 59 |
| IHVR19029 | 309 | 303 | 82 | 54 | 51 | >10μM | 34.2 | 93 | 92 | 87 |
Inhibition of each of the 5 CYP isozymes with 10μM of test compounds.
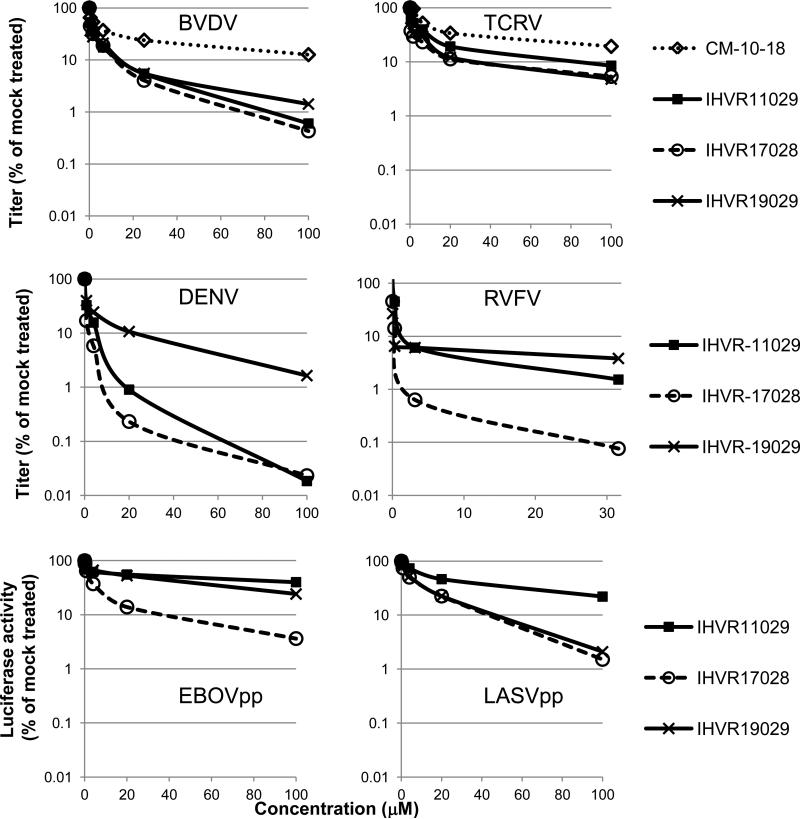
Next, antiviral spectrum and activity of the three lead imino sugars were tested against representative hemorrhagic fever viruses from all four viral families that cause VHFs. As shown in Fig. 2, in addition to surrogate viruses (BVDV and TCRV) and DENV tested in SAR study and lead optimization, these compounds also dose-dependently inhibited RVFV of the Bunyaviridae in a yield reduction assay. Furthermore, the compounds dose-dependently suppressed the assembly/secretion of EBOV and LASV envelope glycoprotein (G) pseudotyped lentiviral particles, suggesting the maturation of the viral glycoproteins was inhibited by the compounds. Taken together, our results convincingly demonstrated the broad-spectrum antiviral activity of the three lead imino sugars against all the representative viruses causing VHFs.
Figure 2. Broad-spectrum antiviral activity of imino sugar derivatives in vitro.
Virus yield reduction assays were performed for BVDV, TCRV, DENV, and RVFV. For BVDV infection, MDBK cells were infected with BVDV at MOI of 1. For TCRV infection, Huh7.5 cells were infected at MOI of 0.01. For DENV and RVFV infection, BHK cells were infected at an MOI of 0.01. Cells were mock treated (0.1% DMSO) or treated with concentrations of test compounds (ranging from 0.1 to 100μM) for 22 h (BVDV) or 48 hours (TCRV, DENV, and RVFV), immediately after incubation with inoculums for 1 h. Relative titers were determined in MDBK cells (BVDV) or Vero cells (TCRV, DENV and RVFV) presented with the mock-treated cells set as 100%. Effects of compounds on EBOV and LASV G-protein pseudotyped lentiviral particle assembly and secretion were assayed in 293T cells and the yields of EBOVpp and LASVpp were titrated in Huh7.5 cells and presented as the percentage of mock-treated controls. Average results (n≥3) were presented.
3.2 The three lead imino sugars have favorable ADME profiles
As shown in Table 2, the three lead compounds did not significantly inhibit the activity of a panel of representative cytochrome P450 enzymes at 10μM concentration. Plasma protein binding of the compounds was 51 % to 88% in the plasma of human, rat or mouse, predicting a favorable serum half life. While IHVR17028 was metabolically un-stable in rat liver microsomes and relatively more stable in human and mouse liver microsomes, both IHVR11029 and 19029 were stable in human, rat or mouse liver microsomes (79% to 93% of drug remained after 60 min). The efflux ratios in Caco2 permeability assay for IHVR17028 and 19029 were both high (31.7 and 34.2, respectively), suggesting a potential lack of efficient transport from gastro-intestinal (GI) lumen toward the bloodstream in vivo, which might influence the bioavailability via oral administration route.
3.3 The lead compounds are more potent α-glucosidase inhibitors
In order to determine if the improved antiviral potency of the lead compounds was due to more potently inhibition of their desired cellular targets, the ER α-glucosidases I and/or II, we at first compared the inhibitory activity of the lead imino sugars and CM-10-18 on α-glucosidase I with an in vitro enzymatic assay. As shown in Table 3, the three imino sugars have IC50 values ranging from 0.09 to 0.48μM. Compared to the parent compound CM-10-18 (IC50 of 0.54μM), IHVR-11029 and IHVR-17028 are more potent in vitro inhibitors of α-glucosidase I.
Table 3.
Inhibition of ER α-glucosidase I in in vitro enzymatic assay
| compound | CM-10-18 | IHVR-11029 | IHVR-17028 | IHVR-19029 |
|---|---|---|---|---|
| IC50 (μM) | 0.54±0.12 | 0.09±0.02* | 0.24±0.08** | 0.48±0.17 |
Enzyme: rat ER α-glucosidase I;
Substrate: Glc3Man5GlcNAc1
IC50s are expressed as average ± standard deviation from triplicate experiments.
P=0.003, compared to CM-10-18
P=0.02, compared to CM-10-18.
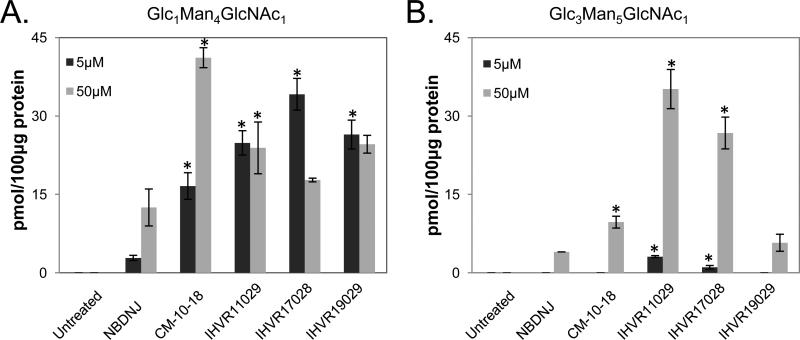
To further determine the inhibitory activity of these compounds against ER α-glucosidases I and II in cultured cells, HL60 cells were treated with the indicated concentrations of the compounds and the accumulation of hyper-glucosylated FOS Glc3Man5GlcNAc1 and Glc1Man4GlcNAc1 were used as markers for inhibition of α-glucosidases I and II, respectively. As shown in Fig.3, in general, the three lead imino sugars demonstrated significantlyincreased activities against one or both enzymes, compared to NBDNJ, and more potent or comparable activity compared to CM-10-18, in this cell-based assay.
Figure 3. In vitro glycan analysis in cells treated with imino sugars.
FOS samples were obtained from HL60 cells treated with indicated doses of imino sugars and quantitatively analyzed for the presence of Glc1Man4GlcNAc1 (A) and Glc3Man5GlcNAc1 (B) as markers of glucosidase II and I inhibition. Values are expressed as pmol in 100μg of total protein and represent average and standard deviation obtained from two independent experiments. P values were calculated using 2-tailed student's t-test. * indicates P<0.05 compared to treatment with NBDNJ.
In summary, the results presented above support the notion that the improved antiviral potency of the three lead compounds is most likely due to their enhanced inhibitory activity against the ER α-glucosidases.
3.4 PK and MTD of the three lead compounds
The PK parameters of IHVR11029 and IHVR17028 were determined in rats following single dose IV and oral dosing. While IHVR11029 demonstrated a superior oral bioavailability (92% vs. 56% for CM-10-18) (Chang et al., 2011a), the bioavailability of IHVR17028 was limited (12.1%) (Table 4), which is consistent with its high efflux ratio in Caco2 assay. Since both IHVR17028 and IHVR19029 have nitrogen heteroatom substitution on alkyl side chain (Fig. 1), and are structurally more closely related, it is not surprising that the efflux ratio of IHVR19029 in the Caco2 assay was also high (Table 2) and oral bioavailability was poor (4.6%). On the contrary, single dosing of IHVR-19029 via IP and IM routes resulted in rapid (Tmax 10 mins) and nearly complete or complete absorption (71% and 133%) (Table 4).
In a standard single oral dose MTD study in rats, all animals treated with IHVR11029 and IHVR17028 at all doses survived, with gain of body weight and normal behavior. However, at 200mg/kg, 50% of the rats dosed with IHVR11029, and 100% of the rats dosed with IHVR17028 had diarrhea. Another adverse event observed was sign of GI stress, such as anogenital staining or soft stool, at lower doses for both compounds (50 and 100mg/kg). A single dose MTD determination study of IHVR19029 was conducted in Balb/c mice following IP or IM administration. No mortality or clinical signs were observed in dose up to 200mg/kg. No significant weight loss and GI alterations (including anogenital staining, soft stool, or diarrhea) were observed.
It is well known that GI stress, as result of off-target inhibition of GI lumen resident α-glucosidases following oral administration, is one of the major side effects of α-glucosidase inhibitors, including imino sugars (Reuser and Wisselaar, 1994). In principle, this side effect can be overcome by parenteral administration or development of prodrugs. Considering IM and IP route of administration demonstrated optimal absorption rates, we initially chose IP administration route for proof-of-principle in vivo efficacy stduies.
3.5 The lead imino sugars inhibited EBOV and MARV infection in mice
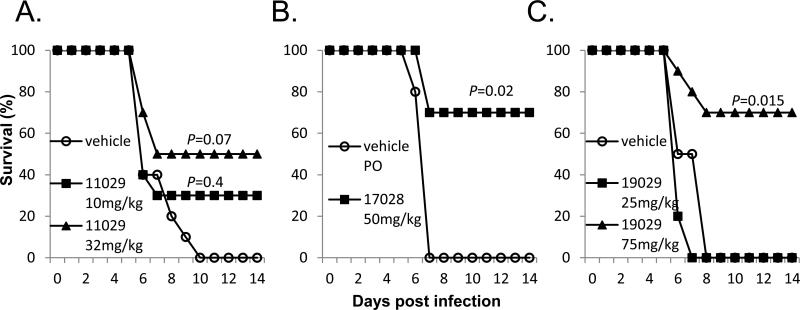
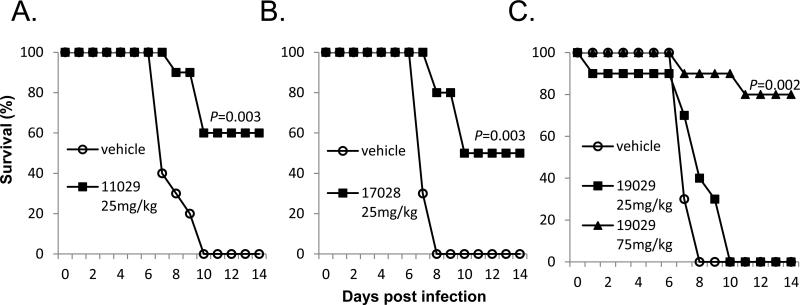
Evaluations of in vivo antiviral activity were conducted in a mouse model of MARV lethal infection. Treatment with IHVR11029 at 32mg/kg, initiated 1 day prior to virus challenging resulted in 50% survival (Fig. 4A). Significant protection (by logrank analysis) of MARV induced death were observed for 50mg/kg of IHVR17028 when the treatment was initiated 1 day prior to virus challenging (Fig. 4B) or 75mg/kg of 19029 starting at 4 h post challenging (Fig. 4C).
Figure 4. Protection of death in mouse model of lethal MARV infection.
BALB/c mice were challenged with 1,000 PFU mouse adapted MARV via IP injection. (A) Mice were treated either with PBS containing 10% solutol or indicated doses of IHVR11029, 1 day prior to challenging with virus. (B) Mice were treated either with PBS or indicated dose of IHVR17028, 1 day prior to challenging with virus. (C) Mice were treated either with PBS or indicated doses of IHVR19029, 4 h post challenging with virus. Unless otherwise indicated in the figure, treatment was given twice daily at 12 h interval by IP injection, for 10 days (or until the animals die). Each group included 10 mice. P values were calculated using Mantel-Cox logrank test.
In a murine protection-of-death model of EBOV infection, significant survival (by logrank analysis) were observed for 25mg/kg of IHVR11029 or IHVR17028 (Fig. 5A&B), as well as 75mg/kg of IHVR19029 (Fig. 5C), when the treatment was initiated 4 h post virus challenging. A 4 h post challenging schedule was chosen to represent our initial effort to evaluate the efficacy of compounds in post exposure treatment.
Figure 5. Protection of death in mouse model of lethal EBOV infection.
C57B1/6 mice were challenged with 1,000 PFU mouse adapted EBOV via IP injection. Treatment with IHVR11029 (A), 17028 (B), or 19029 (C) was given at indicated doses twice daily at 12 h interval by IP injection, starting 4 h post infection for 10 days (or until the animals die). Control mice were given vehicle as described in Fig. 4. Each group included 10 mice. P values were calculated using Mantel-Cox logrank test.
These studies thus provided the preliminary evidence that all the three lead imino sugars are active against lethal infections of EBOV and MARV in mice. However, more detailed PK profiling, toxicity assessment, and in vivo efficacy studies are necessary to further optimize the dose, dosing frequency, route of administration for each of these three compounds. Considering the many potential obstacles during the future development, having multiple candidates, with diversified structure, should provide greater assurance of the likelihood of success.
3.6 Imino sugars efficiently inhibited ER a-glucosidases in mice
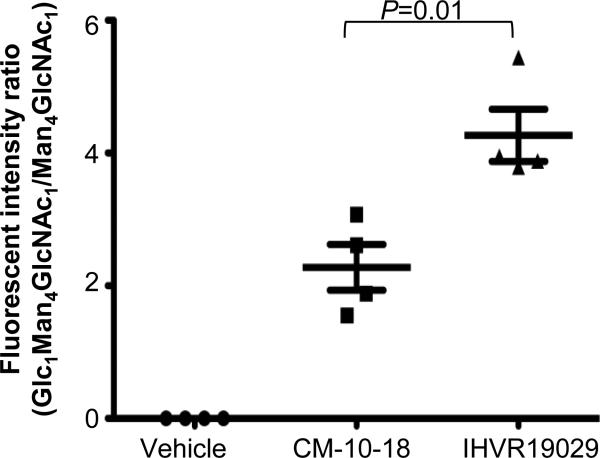
We have performed studies to confirm the inhibition of α-glucosidases in vivo in animals treated with test compounds. Glycan analysis result indicated that the hyper-glucosylated FOS (Glc1Man4GlcNac1) was observed in the sera of mice treated with either CM-10-18 or IHVR19029 and there was over 2 times as much Glc1Man4GlcNac1 glycan in the sera of mice treated with IHVR19029 compared to CM-10-18, as judged by the ratio of Glc1Man4GlcNac1/Man4GlcNac1 (Man4GlcNac1 serves as internal control) (Fig. 6). This result indicated that IHVR19029 indeed inhibited the target enzymes in vivo, and supported the notion that the antiviral effect is likely through the proposed antiviral mechanism in vivo.
Figure 6. In vivo glycan analysis in mice treated with imino sugars.
Serum samples were obtained from mice treated with vehicle (PBS) or indicated imino sugars at 75mg/kg twice daily for 7 days (4 mice per group). FOS was analyzed for the presence of Glc1Man4GlcNAc1 as representative of glucosidase inhibition. A corresponding FOS Man4GlcNAc1 was used as internal control. Quantitation of the peak areas of the two FOS was performed and expressed as ratio of Glc1Man4GlcNAc1 vs Man4GlcNAc1. Values represent average and standard deviation obtained from samples collected from 4 animals/experimental group. P value was calculated using 2-tailed student's t-test.
3.7 Concluding remarks
The studies reported herein identified three lead imino sugars with potent and broad spectrum antiviral activity against representative HFVs from 4 different viral families. We also provided compelling evidence suggesting that the improved antiviral efficacy of the three lead compounds is likely due to their enhanced inhibitory activity against their intended cellular targets, the ER resident α-glucosidases I and II. More importantly, we showed that the lead imino sugars are active against MARV and EBOV in vivo in lethal mouse models, suggesting they could be further developed, after modification of treatment protocol and test in non human primate models, for treatment of not only filoviruses, but also other viruses causing hemorrhagic fever.
There are currently 4 known clinically relevant species of EBOV (Towner et al., 2008) and a single species of MARV (Kortepeter, Bausch, and Bray, 2011). Outbreaks are associated with high mortality in humans. Death occurs in up to 90% of the infections (Kortepeter, Bausch, and Bray, 2011). Recent work with entry inhibitors (Cote et al., 2011), S-adenosine homocystein hydrolase (SAHS) inhibitors (Huggins, Zhang, and Bray, 1999), as well as small molecule (Warren et al., 2010a), antisense oligonucleotides (Warren et al., 2010b) and immuno-adhesion approaches (Radoshitzky et al., 2011) have been reported. Although some of the EBOV or MARV drugs are found to be efficacious in animal models, the pipeline for candidate filovirus therapeutics is still limited, since all of these are in early stages of development. The imino sugars reported herein, with known targets (host ER α-glucosidases) and mechanism-of-action, would be complementary to these approaches.
While our antiviral drug platform is based on analogs of imino sugar NBDNJ, an FDA-approved therapeutic for Gaucher's disease, other α-glucosidase inhibitors such as celgosivir, a prodrug of a natural product castanospermine, has been tested in phase II clinical trial for HCV infection (Durantel, 2009). In 2012, a clinical trial was initiated to treat DENV patients with celgosivir in Singapore, based on the efficacious results obtained in DEVN infected mouse model (Rathore et al., 2011; Schul et al., 2007; Watanabe et al., 2012). Given the lethality of the hemorrhagic fever viruses, and by analogy to management of other infectious diseases, successful control of filovirus infection will benefit from, having more than one drug for use and being broadly active against other hemorrhagic fever virus (Block, Guo, and Guo, 2007; Geffen, 2009).
Highlights.
Three imino sugars (IHVR11029, 17028 and 19029), were identified through SAR study of 120 derivatives
These three imino sugars demonstrated broad antiviral activities against HFVs in vitro, and in vivo.
All three compounds inhibited ER α-glucosidases in vitro and in vivo.
Acknowledgement
We thank Matthew Campagna for technical support. This project was supported by Transformational Medical Technologies program contract [HDTRA1-09-CHEM-BIOBAA] from the Department of Defense Chemical and Biological Defense program through the Defense Threat Reduction Agency (DTRA), NIH grants (AI061441 and AI084267-0109) and by the Hepatitis B Foundation through an appropriation from the Commonwealth of Pennsylvania. DAS and TDB thank the Glycobiology Institute for support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonzi DS, Neville DC, Lachmann RH, Dwek RA, Butters TD. Glucosylated free oligosaccharides are biomarkers of endoplasmic-reticulum alpha-glucosidase inhibition. Biochem J. 2008;409(2):571–80. doi: 10.1042/BJ20070748. [DOI] [PubMed] [Google Scholar]
- Ambrosio A, Saavedra M, Mariani M, Gamboa G, Maiza A. Argentine hemorrhagic fever vaccines. Hum Vaccin. 2011;7(6):694–700. doi: 10.4161/hv.7.6.15198. [DOI] [PubMed] [Google Scholar]
- Block TM, Guo H, Guo JT. Molecular virology of hepatitis B virus for clinicians. Clin Liver Dis. 2007;11(4):685–706. vii. doi: 10.1016/j.cld.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block TM, Jordan R. Iminosugars as possible broad spectrum anti hepatitis virus agents: the glucovirs and alkovirs. Antivir Chem Chemother. 2001;12(6):317–25. doi: 10.1177/095632020101200601. [DOI] [PubMed] [Google Scholar]
- Block TM, Lu X, Platt FM, Foster GR, Gerlich WH, Blumberg BS, Dwek RA. Secretion of human hepatitis B virus is inhibited by the imino sugar N-butyldeoxynojirimycin. Proc Natl Acad Sci U S A. 1994;91(6):2235–9. doi: 10.1073/pnas.91.6.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borio L, Inglesby T, Peters CJ, Schmaljohn AL, Hughes JM, Jahrling PB, Ksiazek T, Johnson KM, Meyerhoff A, O'Toole T, Ascher MS, Bartlett J, Breman JG, Eitzen EM, Jr., Hamburg M, Hauer J, Henderson DA, Johnson RT, Kwik G, Layton M, Lillibridge S, Nabel GJ, Osterholm MT, Perl TM, Russell P, Tonat K. Hemorrhagic fever viruses as biological weapons: medical and public health management. JAMA. 2002;287(18):2391–405. doi: 10.1001/jama.287.18.2391. [DOI] [PubMed] [Google Scholar]
- Bray M. Pathogenesis of viral hemorrhagic fever. Curr Opin Immunol. 2005;17(4):399–403. doi: 10.1016/j.coi.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Bridges CG, Ahmed SP, Kang MS, Nash RJ, Porter EA, Tyms AS. The effect of oral treatment with 6-O-butanoyl castanospermine (MDL 28,574) in the murine zosteriform model of HSV-1 infection. Glycobiology. 1995;5(2):249–53. doi: 10.1093/glycob/5.2.249. [DOI] [PubMed] [Google Scholar]
- Chang J, Schul W, Butters TD, Yip A, Liu B, Goh A, Lakshminarayana SB, Alonzi D, Reinkensmeier G, Pan X, Qu X, Weidner JM, Wang L, Yu W, Borune N, Kinch MA, Rayahin JE, Moriarty R, Xu X, Shi PY, Guo JT, Block TM. Combination of alpha-glucosidase inhibitor and ribavirin for the treatment of dengue virus infection in vitro and in vivo. Antiviral Res. 2011a;89(1):26–34. doi: 10.1016/j.antiviral.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Schul W, Yip A, Xu X, Guo JT, Block TM. Competitive inhibitor of cellular alpha-glucosidases protects mice from lethal dengue virus infection. Antiviral Res. 2011b;92(2):369–71. doi: 10.1016/j.antiviral.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Wang L, Ma D, Qu X, Guo H, Xu X, Mason PM, Bourne N, Moriarty R, Gu B, Guo JT, Block TM. Novel imino sugar derivatives demonstrate potent antiviral activity against flaviviruses. Antimicrob Agents Chemother. 2009;53(4):1501–8. doi: 10.1128/AAC.01457-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor RI, Chen BK, Choe S, Landau NR. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology. 1995;206(2):935–44. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- Cote M, Misasi J, Ren T, Bruchez A, Lee K, Filone CM, Hensley L, Li Q, Ory D, Chandran K, Cunningham J. Small molecule inhibitors reveal Niemann-Pick C1 is essential for Ebola virus infection. Nature. 2011;477(7364):344–8. doi: 10.1038/nature10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datema R, Romero PA, Rott R, Schwarz RT. On the role of oligosaccharide trimming in the maturation of Sindbis and influenza virus. Arch Virol. 1984;81(1-2):25–39. doi: 10.1007/BF01309294. [DOI] [PubMed] [Google Scholar]
- Durantel D. Celgosivir, an alpha-glucosidase I inhibitor for the potential treatment of HCV infection. Curr Opin Investig Drugs. 2009;10(8):860–70. [PubMed] [Google Scholar]
- Dwek RA, Butters TD, Platt FM, Zitzmann N. Targeting glycosylation as a therapeutic approach. Nat Rev Drug Discov. 2002;1(1):65–75. doi: 10.1038/nrd708. [DOI] [PubMed] [Google Scholar]
- EMEA Scientific Discussion -- Zavesca. 2003.
- Geffen N. Beyond HAART: scientists and activists need to work together. Lancet. 2009;374(9693):860–1. doi: 10.1016/S0140-6736(09)61260-5. [DOI] [PubMed] [Google Scholar]
- Geisbert TW, Jahrling PB. Exotic emerging viral diseases: progress and challenges. Nat Med. 2004;10(12 Suppl):S110–21. doi: 10.1038/nm1142. [DOI] [PubMed] [Google Scholar]
- Gu B, Mason P, Wang L, Norton P, Bourne N, Moriarty R, Mehta A, Despande M, Shah R, Block T. Antiviral profiles of novel iminocyclitol compounds against bovine viral diarrhea virus, West Nile virus, dengue virus and hepatitis B virus. Antivir Chem Chemother. 2007;18(1):49–59. doi: 10.1177/095632020701800105. [DOI] [PubMed] [Google Scholar]
- He J, Choe S, Walker R, Di Marzio P, Morgan DO, Landau NR. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J Virol. 1995;69(11):6705–11. doi: 10.1128/jvi.69.11.6705-6711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A, Aebi M. Roles of N-linked glycans in the endoplasmic reticulum. Annu Rev Biochem. 2004;73:1019–49. doi: 10.1146/annurev.biochem.73.011303.073752. [DOI] [PubMed] [Google Scholar]
- Huggins J, Zhang ZX, Bray M. Antiviral drug therapy of filovirus infections: S-adenosylhomocysteine hydrolase inhibitors inhibit Ebola virus in vitro and in a lethal mouse model. J Infect Dis. 1999;179(Suppl 1):S240–7. doi: 10.1086/514316. [DOI] [PubMed] [Google Scholar]
- Jordan R, Nikolaeva OV, Wang L, Conyers B, Mehta A, Dwek RA, Block TM. Inhibition of host ER glucosidase activity prevents Golgi processing of virion-associated bovine viral diarrhea virus E2 glycoproteins and reduces infectivity of secreted virions. Virology. 2002;295(1):10–9. doi: 10.1006/viro.2002.1370. [DOI] [PubMed] [Google Scholar]
- Karlsson GB, Butters TD, Dwek RA, Platt FM. Effects of the imino sugar N-butyldeoxynojirimycin on the N-glycosylation of recombinant gp120. J Biol Chem. 1993;268(1):570–6. [PubMed] [Google Scholar]
- Kortepeter MG, Bausch DG, Bray M. Basic clinical and laboratory features of filoviral hemorrhagic fever. J Infect Dis. 2011;204(Suppl 3):S810–6. doi: 10.1093/infdis/jir299. [DOI] [PubMed] [Google Scholar]
- LeDuc JW. Epidemiology of hemorrhagic fever viruses. Rev Infect Dis. 1989;11(Suppl 4):S730–5. doi: 10.1093/clinids/11.supplement_4.s730. [DOI] [PubMed] [Google Scholar]
- Mahanty S, Bray M. Pathogenesis of filoviral haemorrhagic fevers. Lancet Infect Dis. 2004;4(8):487–98. doi: 10.1016/S1473-3099(04)01103-X. [DOI] [PubMed] [Google Scholar]
- Malvoisin E, Wild F. The role of N-glycosylation in cell fusion induced by a vaccinia recombinant virus expressing both measles virus glycoproteins. Virology. 1994;200(1):11–20. doi: 10.1006/viro.1994.1157. [DOI] [PubMed] [Google Scholar]
- Mellor HR, Neville DC, Harvey DJ, Platt FM, Dwek RA, Butters TD. Cellular effects of deoxynojirimycin analogues: inhibition of N-linked oligosaccharide processing and generation of free glucosylated oligosaccharides. Biochem J. 2004;381(Pt 3):867–75. doi: 10.1042/BJ20031824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X, Pan X, Weidner J, Yu W, Alonzi D, Xu X, Butters T, Block T, Guo JT, Chang J. Inhibitors of Endoplasmic Reticulum {alpha}-Glucosidases Potently Suppress Hepatitis C Virus Virion Assembly and Release. Antimicrob Agents Chemother. 2011;55(3):1036–44. doi: 10.1128/AAC.01319-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radoshitzky SR, Warfield KL, Chi X, Dong L, Kota K, Bradfute SB, Gearhart JD, Retterer C, Kranzusch PJ, Misasi JN, Hogenbirk MA, Wahl-Jensen V, Volchkov VE, Cunningham JM, Jahrling PB, Aman MJ, Bavari S, Farzan M, Kuhn JH. Ebolavirus delta-peptide immunoadhesins inhibit marburgvirus and ebolavirus cell entry. J Virol. 2011;85(17):8502–13. doi: 10.1128/JVI.02600-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathore AP, Paradkar PN, Watanabe S, Tan KH, Sung C, Connolly JE, Low J, Ooi EE, Vasudevan SG. Celgosivir treatment misfolds dengue virus NS1 protein, induces cellular pro-survival genes and protects against lethal challenge mouse model. Antiviral Res. 2011;92(3):453–60. doi: 10.1016/j.antiviral.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Reuser AJ, Wisselaar HA. An evaluation of the potential side-effects of alpha-glucosidase inhibitors used for the management of diabetes mellitus. Eur J Clin Invest. 1994;24(Suppl 3):19–24. doi: 10.1111/j.1365-2362.1994.tb02251.x. [DOI] [PubMed] [Google Scholar]
- Schul W, Liu W, Xu HY, Flamand M, Vasudevan SG. A dengue fever viremia model in mice shows reduction in viral replication and suppression of the inflammatory response after treatment with antiviral drugs. J Infect Dis. 2007;195(5):665–74. doi: 10.1086/511310. [DOI] [PubMed] [Google Scholar]
- Sessions OM, Barrows NJ, Souza-Neto JA, Robinson TJ, Hershey CL, Rodgers MA, Ramirez JL, Dimopoulos G, Yang PL, Pearson JL, Garcia-Blanco MA. Discovery of insect and human dengue virus host factors. Nature. 2009;458(7241):1047–50. doi: 10.1038/nature07967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann E, Whitfield T, Kallis S, Dwek RA, Zitzmann N, Pietschmann T, Bartenschlager R. Antiviral effects of amantadine and iminosugar derivatives against hepatitis C virus. Hepatology. 2007;46(2):330–8. doi: 10.1002/hep.21686. [DOI] [PubMed] [Google Scholar]
- Taylor DL, Sunkara PS, Liu PS, Kang MS, Bowlin TL, Tyms AS. 6-0-butanoylcastanospermine (MDL 28,574) inhibits glycoprotein processing and the growth of HIVs. AIDS. 1991;5(6):693–8. doi: 10.1097/00002030-199106000-00008. [DOI] [PubMed] [Google Scholar]
- Towner JS, Sealy TK, Khristova ML, Albarino CG, Conlan S, Reeder SA, Quan PL, Lipkin WI, Downing R, Tappero JW, Okware S, Lutwama J, Bakamutumaho B, Kayiwa J, Comer JA, Rollin PE, Ksiazek TG, Nichol ST. Newly discovered ebola virus associated with hemorrhagic fever outbreak in Uganda. PLoS Pathog. 2008;4(11):e1000212. doi: 10.1371/journal.ppat.1000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren TK, Warfield KL, Wells J, Enterlein S, Smith M, Ruthel G, Yunus AS, Kinch MS, Goldblatt M, Aman MJ, Bavari S. Antiviral activity of a small-molecule inhibitor of filovirus infection. Antimicrob Agents Chemother. 2010a;54(5):2152–9. doi: 10.1128/AAC.01315-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren TK, Warfield KL, Wells J, Swenson DL, Donner KS, Van Tongeren SA, Garza NL, Dong L, Mourich DV, Crumley S, Nichols DK, Iversen PL, Bavari S. Advanced antisense therapies for postexposure protection against lethal filovirus infections. Nat Med. 2010b;16(9):991–4. doi: 10.1038/nm.2202. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Rathore AP, Sung C, Lu F, Khoo YM, Connolly J, Low J, Ooi EE, Lee HS, Vasudevan SG. Dose- and schedule-dependent protective efficacy of celgosivir in a lethal mouse model for dengue virus infection informs dosing regimen for a proof of concept clinical trial. Antiviral Res. 2012;96(1):32–5. doi: 10.1016/j.antiviral.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Wu SF, Lee CJ, Liao CL, Dwek RA, Zitzmann N, Lin YL. Antiviral effects of an iminosugar derivative on flavivirus infections. J Virol. 2002;76(8):3596–604. doi: 10.1128/JVI.76.8.3596-3604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Gill T, Wang L, Du Y, Ye H, Qu X, Guo JT, Cuconati A, Zhao K, Block TM, Xu X, Chang J. Design, Synthesis, and Biological Evaluation of N-Alkylated Deoxynojirimycin (DNJ) Derivatives for the Treatment of Dengue Virus Infection. J Med Chem. 2012;55(13):6061–75. doi: 10.1021/jm300171v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitzmann N, Mehta AS, Carrouee S, Butters TD, Platt FM, McCauley J, Blumberg BS, Dwek RA, Block TM. Imino sugars inhibit the formation and secretion of bovine viral diarrhea virus, a pestivirus model of hepatitis C virus: implications for the development of broad spectrum anti-hepatitis virus agents. Proc Natl Acad Sci U S A. 1999;96(21):11878–82. doi: 10.1073/pnas.96.21.11878. [DOI] [PMC free article] [PubMed] [Google Scholar]