Abstract
Pre-clinical and clinical evidence from megakaryocyte (MK) related diseases suggest that MKs play a significant role in maintaining bone homeostasis. Findings from our laboratories reveal that MKs significantly increase osteoblast (OB) number through direct MK-OB contact and the activation of integrins. We therefore examined the role of Pyk2, a tyrosine kinase known to be regulated downstream of integrins, in the MK-mediated enhancement of OBs. When OBs were co-cultured with MKs, total Pyk2 levels in OBs were significantly enhanced primarily due to increased Pyk2 gene transcription. Additionally, p53 and Mdm2 were both decreased in OBs upon MK stimulation, which would be permissive of cell cycle entry. We then demonstrated that OB number was markedly reduced when Pyk2−/− OBs, as opposed to wild-type (WT) OBs, were co-cultured with MKs. We also determined that MKs inhibit OB differentiation in the presence and absence of Pyk2 expression. Finally, given that MK replete spleen cells from GATA-1 deficient mice can robustly stimulate OB proliferation and bone formation in WT mice, we adoptively transferred spleen cells from these mice into Pyk2−/− recipient mice. Importantly, GATA-1 deficient spleen cells failed to stimulate an increase in bone formation in Pyk2−/− mice, suggesting in vivo the important role of Pyk2 in the MK-induced increase in bone volume. Further understanding of the signaling pathways involved in the MK-mediated enhancement of OB number and bone formation will facilitate the development of novel anabolic therapies to treat bone loss diseases.
INTRODUCTION
In the last decade, platelet producing MKs have been shown to play a role in regulating bone mass. Myeloproliferative diseases in which increases in MKs are accompanied by osteosclerosis have been reported1–3 and several mouse models have been described in which increased numbers of MKs correlate with increased bone mass. These mouse models, as well as relevant in vitro data were recently reviewed.4 Three key findings from these data provide rationale for our current studies. First, MKs stimulate OB proliferation and bone formation in vivo. This was demonstrated in four distinct mouse models in which MK number was increased and a significant increase in bone volume was observed.5–12 This was further illustrated by adoptive transfer studies in which irradiated WT mice were reconstituted with spleen cells from mice lacking either NF-E2 or GATA-1, transcription factors required for normal MK differentiation. NF-E2 and GATA-1 deficient mice have significantly increased numbers of MKs, reduced platelet numbers, and a 2–3-fold increase in bone mass.10,11,13 Both the hematologic phenotype and the high bone mass phenotype can be adoptively transferred, suggesting a role for hematopoietic cells, most likely MKs, in this mechanism.11 Second, recent studies by Dominici et al,14 described how, in mice treated with lethal total body irradiation, surviving MKs migrated to endosteal bone surfaces (in close contact with OBs) and stimulated a 2-fold increase in OB number. Third, in vitro studies demonstrate that MKs enhanced OB proliferation up to 6-fold by a mechanism that required direct MK-OB cell-cell contact and the engagement of integrins.10,15–18 Taken together, these observations suggest that MKs, via a cell-cell contact mechanism mediated in part by integrins, stimulate an increase in OB number, which in turn results in an increase in bone formation.
The primary goal of our study was to determine the cellular mechanisms by which MKs regulate OBs proliferation. We show for the first time an important role for proline-rich tyrosine kinase 2 (Pyk2), a tyrosine kinase involved in signaling downstream of activated integrins and other key signaling pathways in OBs, in regulating MK-mediated enhancement of OB number, and the importance of Pyk2 expression in regulating MK-mediated bone formation in vivo.
METHODS
Animals
C57BL/6 mice were originally obtained from Jackson Laboratories. GATA-1 deficient mice were generously provided by Dr. Stuart Orkin. Generation and breeding of GATA-1 deficient mice was described previously.13,19 These mice have a selective loss of MK GATA-1 expression but with sufficient erythroid cells to avoid lethal anemia.19 GATA-1 deficient mice have been backcrossed onto the C57BL/6 background for more than 7 generations. Pyk2−/− mice were described previously20 and were generously provided by Pfizer (Groton, CT). Pyk2 mice were backcrossed for greater that 7 generations and maintained on a C57BL/6 background. All procedures were approved by the Institutional Animal Care and Use Committee of the Indiana University School of Medicine and followed NIH guidelines.
Preparation of Neonatal Calvarial Cells (OBs)
Murine calvarial cells were prepared as previously described.15,21 Our technique was a modification of the basic method described by Wong and Cohn.22 Briefly, calvaria from mice less than 48 h old were pretreated with EDTA in PBS for 30 min. The calvaria were then subjected to sequential collagenase digestions. Cells were collected following incubation in collagenase. Fractions 3–5 were used as the starting population. Although heterogeneous, cells collected from these fractions are enriched for osteogenic potential.23 These cells were ~90% OBs or OB precursors by a variety of criteria21,24–26 and are referred to as OBs throughout this manuscript. We recently published the characterization of these cells.24 Here we provide new flow cytometric evidence that 83±2% of 2 day calvarial OBs were CD45-CD31-Ter119-Sca1-27 and of these cells, 90±4% were also OPN+28 (data are presented as the average ± standard deviation of the mean from 4 separate experiments each with 3 replicate samples). OBs were seeded at 2×104 cells/ml in both 6 well plates (3 ml/well) and 10 cm dishes (10 ml/well) (optimal pre-tested).
Preparation of Fetal Liver Derived MKs
Murine MKs were prepared as previously described.10,29 In brief, fetuses were dissected from pregnant mice at E13–15. The livers were removed and single cell suspensions made by forcing cells through sequentially smaller gauge needles (18G, 20G, 23G). Cells were washed 2X with DMEM+10% FCS and then seeded (5 fetal livers/100 mm dish) in 100ml culture dishes, in DMEM+10% FCS+1% murine TPO.7 After 3–5 days, when the cells became confluent, MKs were separated from lymphocytes and other cells using a one-step albumin gradient to obtain a 95% pure MK population.30 The bottom layer was 3% albumin in PBS (Bovine Albumin, protease free, fatty acid poor, Serologicals Proteins Inc., Kankakee, IL), the middle layer was 1.5% albumin in PBS, and the top layer was media containing the cells to be separated. All of the cells sedimented through the layers at 1 g for approximately 40 minutes at room temperature. The MK fraction was collected from the bottom of the tube. MKs were seeded at 1×105 cells/well in 6 well plates and 2×105 cells/dish in 10 cm dishes (optimal pre-tested).
Preparation of Bone Marrow (BM) Stromal Cells
BM was isolated from the tibiae and femurs of 6–10 week-old C57BL/6 or Pyk2−/− mice and seeded at 5×106 cells/well into a 24-well plate and MKs were titrated into wells. To provide osteogenic conditions, cells were cultured in αMEM supplemented with 10% FCS which was further supplemented with ascorbic acid (50μg/ml added on day 0 and at all medium changes) and β-glycerophosphate (5mM added starting on day 7 and all subsequent medium changes). On day 14, cultures were stopped and OB function was analyzed as detailed below.
Differentiation Studies
OBs (2×105 OBs/ml) were cultured in the presence or absence of MKs (2.5×105 MKs/ml) for 14 days. Alternatively, OBs were cultured with MKs for the first 4 hrs of co-culture and then MKs were removed by washing. Co-cultures were incubated in osteogenic media as described above. The media was changed at day 7 and at this time point fresh MKs were added as non-adherent MKs were removed. On day 14, cultures were assessed for calcium deposition (Alizarin Red S staining) as a functional measure of OB differentiation and mineralization (as previously described15,24,31) and mRNA expression of alkaline phosphatase, type I collagen, and osteocalcin was assessed by real-time PCR as detailed below.
Preparation of Cell Lysates for Western Blot Analysis
Cell lysates were prepared by one of two methods. In the first method, OBs were cultured alone or co-cultured with MKs for 5 days. Following respective treatments, MKs were removed by washing (4 washes with PBS) and cells were lysed in a nonionic detergent buffer (40 mM Tris, 150 mM NaCl, 1% Igepal) supplemented with protease inhibitors and sodium orthovanadate. Protein concentrations were measured using the Bradford Reagent (Biorad Laboratories). Lysates were boiled in laemmli buffer for 10 minutes, fractionated using SDS-PAGE and transferred to PVDF membrane (GE Healthcare). Western blots were performed with antibodies to Mdm2 (2A10, 4B11) and GAPDH (6C5) (Calbiochem), and p53 (FL-393) and Vinculin (N-19) (Santa Cruz Biotechnology).
The second method was used for examining expression of Pyk2. In brief, OBs were cultured alone, co-cultured with MKs for 5 days, or co-cultured with MKs for 4 hrs and then MKs removed by washing (4 washes with PBS) and OBs remained in culture for the remaining 5 day period. Alternatively, for short stimulation studies, OBs were cultured until they were ~70% confluent, serum starved overnight (0.1% FCS), and then stimulated for 1, 2 or 4 hrs with MKs, where appropriate. For all cultures, MKs were removed by washing (4 washes with PBS). Cell lysates were collected in SDS-PAGE lysis buffer containing urea. Protein concentrations were measured using the amido black method. The proteins were fractionated via SDS-PAGE and transferred to nitrocellulose. They were then probed for Pyk2 (BD Transduction Labs, 610548) using the Fujifilm LAS-3000 imager. The HRP-tagged secondary antibodies were purchased from Jackson Immunoresearch. The blots were stripped and reprobed for vinculin (Sigma, V 4505) to demonstrate equal protein loading.
Quantitative real-time PCR
Following respective treatments, all cultures were washed 4 times with PBS to ensure removal of MKs from MK-OB co-cultures. Total RNA was isolated from OBs using Trizol reagent (Invitrogen Corporation). Total RNA from OBs was treated with DNAse I (Qiagen) and used to generate cDNAs by reverse transcription according to the manufacturer’s instructions (First Strand cDNA Synthesis Kit; Roche Applied Science). QPCR reactions were performed in an MX3000 detection system using SYBR green PCR reagents as described by the manufacturer (Stratagene). For each primer pair, a calibration curve was performed and all oligonucleotides were tested to ensure specificity and sensitivity. For each OB sample, arbitrary units obtained using the standard curve and the expression of GAPDH was used to normalize the amount of the mRNA transcript. The following primer pairs were used:
| GAPDH: | 5′CGTGGGGCTGCCCAGAACAT |
| 5′TCTCCAGGCGGCACGTCAGA | |
| Pyk2: | 5′GGGACACTACCTGGAACGAA |
| 5′CCAGCTTCACACACTCAGGA | |
| Runx2: | 5′CGACAGTCCCAACTTCCTGT |
| 5′CGGTAACCACAGTCCCATCT | |
| Alkaline phosphatase: | 5′GCTGATCATTCCCACGTTTT |
| 5′CTGGGCCTGGTAGTTGTTGT | |
| Type I collagen: | 5′CAGGGAAGCCTCTTTCTCCT |
| 5′ACGTCCTGGTGAAGTTGGTC |
Adoptive Transfer Studies
Ten week-old female C57BL/6 and Pyk2−/− mice were lethally irradiated (1100 cGy split dose) and used as recipient mice. Within 1 hr of the second dose, mice were injected intravenously with 107 spleen cells from either C57BL/6 or GATA-1 deficient mice. It should be noted that identical protocols were followed in 3 separate experiments and all surviving mice were pooled and data analyzed as outlined below (3 experiments, n=3–4/experiment with final n-values 9–12/group, see Tables 1 and 2). Briefly, mice were bled at 4 and 8 weeks post-adoptive transfer and platelet numbers determined using a validated HEMAVET® 950FS Hematology System (Drew Scientific). Mice were sacrificed at 8 weeks, spleens were weighed, and the distal femurs were analyzed by standard micro-computed tomography (μCT; Skyscan 1172) using the nomenclature recommended by Bouxsein et al.32 In brief, the trabecular bone compartment of each femur was sliced into 50 segments from the cortical shell in a region ~0.5 mm below the most distal portion of the growth plate. The x-ray source was set at 60keV and 167 μA over an angular range of 180 degrees (rotational steps of 0.40 degrees) with a 12 μm pixel size, and projection images were reconstructed using standard Skyscan software. Images were binarized (threshold of 100 on a 0–255 scale), and three-dimensional bone volume parameters were calculated: trabecular bone volume fraction (BV/TV), bone surface density (BS/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th), and trabecular separation (Tb.Sp). To convert gray scale values to density (mg/cm3), a phantom standard was scanned and reconstructed using the above parameters. Two densities were assessed: the entire trabecular bone compartment and the bone alone. While the trabecular bone compartment includes the marrow, and thus provides the average density of the bone and marrow, the density of the bone alone provides an index of the degree of bone mineralization. Static histomorphometric analysis of trabecular bone was performed on femurs as previously described using the nomenclature recommended by Parfitt et al.10,11,33
Table 1.
GATA-1 cells adoptively transfer both the hematologic and bone phenotype (μCT) to C57BL/6 recipients but only the hematologic phenotype to Pyk2−/− recipients.
| Recipient Mice | Donor Cells | No. of Mice | Spleen Weight (mg) | Platelets (k/μl) | BV/TV | BS/TV | Tb.Th | Tb.N | Tb.Sp |
|---|---|---|---|---|---|---|---|---|---|
| C57BL/6 | C57BL/6 | 12 | 74 ± 7 | 884 ± 23 | 3.1 ± 0.5 | 3.2 ± 0.3 | 0.042 ± 0.003 | 0.70 ± 0.07 | 0.27 ± 0.01 |
| C57BL/6 | GATA-1 | 9 | 204 ± 5* | 247 ± 14* | 6.5 ± 1.4* | 5.5 ± 1.4* | 0.044 ± 0.003 | 1.43 ± 0.25* | 0.29 ± 0.02 |
| Pyk2−/− | C57BL/6 | 11 | 72 ± 7 | 866 ± 67 | 5.8 ± 0.8 | 5.2± 0.5 | 0.047 ± 0.002 | 1.2 ± 0.1 | 0.23 ± 0.01 |
| Pyk2−/− | GATA-1 | 11 | 207 ± 5* | 239 ± 22* | 6.6 ± 1.2 | 5.4 ± 0.8 | 0.049 ± 0.005 | 1.2 ± 0.2 | 0.28 ± 0.01 |
BV/TV, bone volume fraction; BS/TV, bone surface density; Tb.Th, trabecular thickness; Tb.N, trabecular number; Tb.Sp, trabecular separation.
Results are presented as mean ± SEM;
p < 0.05 compared with like recipient with C57BL/6 donor cells.
Table 2.
Histomorphometric analysis of bone phenotype when C57BL/6 and Pyk2−/− recipient mice are reconstituted with C57BL/6 or GATA-1 cells.
| Recipient Mice | Donor Cells | No. of Mice | BV/TV | N.Ob/T.Ar | N.Oc/T.Ar | N.Ob/BS | Oc.S/BS |
|---|---|---|---|---|---|---|---|
| C57BL/6 | C57BL/6 | 12 | 4.4 ± 0.5 | 42 ± 4 | 28 ± 3 | 1.1 ± 0.5 | 9.9 ± 0.8 |
| C57BL/6 | GATA-1 | 9 | 6.8 ± 0.9* | 66 ± 6* | 53 ± 10* | 0.8 ± 0.2 | 13.1 ± 0.9* |
| Pyk2−/− | C57BL/6 | 11 | 6.9 ± 1.2 | 72 ± 12 | 41 ± 5 | 0.9 ± 0.2 | 11.0 ± 2.3 |
| Pyk2−/− | GATA-1 | 11 | 6.0 ± 0.8 | 71 ± 12 | 49 ± 8 | 0.7 ± 0.1 | 11.3 ± 1.8 |
BV/TV, bone volume/tissue volume; N.Ob/T.Ar, osteoblast number/tissue area; N.Oc/T.Ar, osteoclast number/tissue area; N.Ob/BS, osteoblast number/bone surface; Oc.S/BS, osteoclast surface/bone surface.
Results are presented as mean ± SEM;
p < 0.05 compared with like recipient with C57BL/6 donor cells.
Statistics
Unless otherwise stated, all data are presented as the Mean ± SD and a Student’s t-test was used to determine significant differences, with p<0.05 (Systat 6.0 for Microsoft Windows, SPSS Inc., Chicago). For the adoptive transfer studies a sample size estimate was performed using the standard deviation of the initial Pyk2−/− recipient data sets, an 80% power, and an alpha of 0.05. This yielded a sample size of 10 for each arm of the study. We therefore completed additional experimentation to give an anticipated combined n of 10/experimental group (to account for loss in mice due to death resulting from transplantation failure or anesthetic complications).
RESULTS
MKs Upregulate Pyk2 Expression in OBs
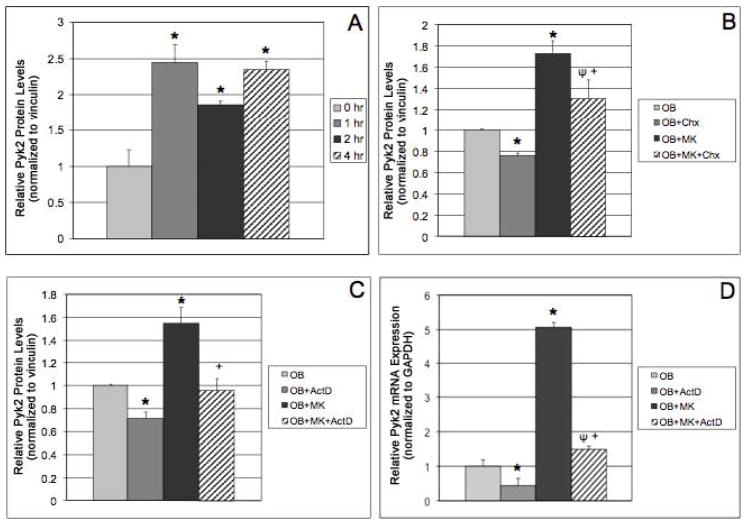
Since we previously demonstrated that MK-induced OB proliferation is mediated in part by α3 and α5 integrins,17 we sought to determine whether Pyk2 was involved in the integrin-mediated regulation of OB proliferation by MKs. As shown using Western blot analysis (Figure 1A), we observed a marked upregulation of Pyk2 expression in OBs following acute stimulation of OBs with MKs (>2-fold increase after 1 hr of stimulation). Pyk2 expression was similarly elevated in OBs co-cultured with MKs for 2 and 4 hrs.
Figure 1.
Data generated from Western blot analysis showing OBs cultured alone, or OBs stimulated with MKs for 1, 2, and 4 hrs. MKs were removed (by washing) and OB lysates collected. A) Pyk2 expression in OBs was significantly increased with just 1 hr of co-culture with MKs and the increase persisted in OBs co-cultured for 2 and 4 hrs. B–D) OBs were pretreated with cycloheximide (Chx; 10 μM, optimal pretested) or actinomycin D (ActD; 5 μg/ml, optimal pretested) for 1 or 3 hrs, respectively. MKs were added to cultures as indicated and cells were incubated for 4 additional hrs (cultures containing inhibitors remained with inhibitors). B&C) Western blot analysis and D) Real-time PCR analysis. n=3/experimental group, representative data are shown from 3 independent experiments conducted (A–D). Pyk2 mRNA expression in OB and OB+MK cultures (real-time PCR data) was completed in more than 10 separate experiments with identical data generated from all of these experiments. *Indicates statistically significant differences (p<0.05) compared to 0 hr (A) or OB (B&C). ΨIndicates statistically significant difference (p<0.05) compared to OB+Chx (B) or OB+ActD (D). +Indicates statistically significant difference (p<0.05) compared to OB+MK (B–D).
We next examined whether the elevated Pyk2 protein levels were due changes in Pyk2 gene transcription or protein translation. For these studies, we used the chemical inhibitors actinomycin D (ActD, 5μg/ml, optimal pretested) and cycloheximide (Chx, 10μM, optimal pretested), which inhibit RNA synthesis or mRNA translation, respectively. OBs were pretreated with Chx or ActD for 1 or 3 hrs, respectively, and then cultured in the presence or absence of MKs for 4 hrs (plus inhibitors). Cells were then lysed and proteins were prepared for detection of Pyk2 by Western blotting (Figures 1B&C). Consistent with our previous studies, Pyk2 protein levels increased in untreated OBs co-cultured with MKs compared with untreated OBs cultured alone. We also found that Chx reduced Pyk2 levels in OBs cultured alone or in the presence of MKs, and that the percentage decrease of Pyk2 was similar in both culture conditions (24% and 25%, respectively). ActD treatment also led to a decrease in Pyk2 protein levels in OBs cultured alone or in the presence of MKs. However, while Pyk2 protein levels in OBs were reduced by 29% in the presence of ActD, Pyk2 levels were reduced by 38% in OBs co-cultured with MKs. This finding suggested that the increase in Pyk2 protein levels in response to MKs, was likely due to an increase in transcription of the Pyk2 gene. To confirm the effect of MKs on Pyk2 mRNA levels, we cultured OBs in the presence or absence of MKs as above, isolated RNA from OBs, and then examined Pyk2 mRNA expression via real-time PCR. As illustrated in Figure 1D, Pyk2 mRNA expression was markedly upregulated in OBs co-cultured with MKs. As expected, ActD treatment significantly reduced Pyk2 mRNA expression in OBs as well as in OB+MK cultures. Together, these findings suggest that MKs increase Pyk2 mRNA expression, leading to increased Pyk2 protein levels in OBs.
Pyk2 Expression is necessary for the MK-mediated Increase in OB Number
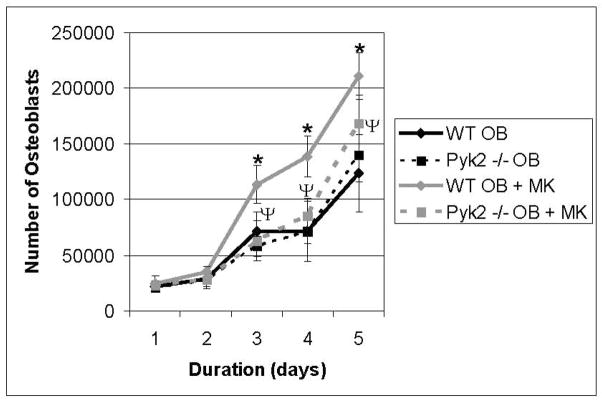
Next, we examined the involvement of Pyk2 in the MK-mediated enhancement of OB number. As illustrated in Figure 2, Pyk2−/− OB numbers were essentially identical to WT OBs at each time point (Pyk2−/− OB vs. WT OB, days 1–5). As would be expected based on our previously published results,10,15–17 by day 3, OB number was significantly increased when WT OBs were co-cultured with MKs compared with cultures in which WT OBs were cultured alone (WT OB+ MK vs. WT OB, p<0.01). In contrast, when Pyk2−/− OBs were co-cultured with MKs, OB number was not significantly different from that measured in cultures containing WT or Pyk2−/− OBs cultured alone, even at day 5, when the greatest increase in OB number in WT OB-MK co-cultures was observed (Pyk2−/− OB + MK vs. WT OB or Pyk2−/− OB). In fact, on day 5, OB number in Pyk2−/− OB cultures containing MKs was significantly lower than that observed in WT OB cultures containing MKs (Pyk2−/− OB + MK vs. WT OB + MK, p=0.01), suggesting that Pyk2 was necessary for OBs to respond maximally to the presence of MKs. We are unable to determine if longer time points would promote Pyk2−/− OB proliferation as the duration of experiments may not be extended past 5 days as OB cultures become confluent. We also examined Annexin V expression as a marker for apoptosis. In all cases, less than 2% of the OBs were Annexin V positive, suggesting that apoptosis was not significant (data not shown). Therefore, our results suggest that Pyk2 is a necessary component of the MK-induced signaling pathway leading to OB proliferation, although additional proteins are also likely to be involved in this process.
Figure 2.
WT and Pyk2−/− OBs were cultured alone or in the presence of MKs. MKs were removed and viable OB number was assessed daily by counting the number of cells excluding trypan blue (n=6/experimental group). We also analyzed these cells for the expression of Annexin V. In all cases, less than 2% of the OBs were Annexin V positive, suggesting that apoptosis was not detected (data not shown). Data reported are representative from 3 experiments conducted. *Indicates statistically significant difference (p<0.05) at the same time point compared to WT OB. ΨIndicates statistically significant difference (p<0.05) at the same time point between WT OB+MK and Pyk2−/− OB+MK.
Role of β1 Integrin Signaling in MK-Mediated Expression of Pyk2 in OBs
We previously demonstrated that the MK-mediated increase in OB proliferation requires direct MK-OB cell-cell contact. We further demonstrated that the response was partially mediated by fibronectin/RGD-binding integrins (α3β1 and αvβ1) since titration of the integrin blocking peptide RGDS into OB-MK co-cultures caused a dose-dependent decrease in MK-mediated OB proliferation without affecting OBs cultured alone. Therefore, in the current study, we determined whether the addition of RGDS to co-cultures would impact Pyk2 expression. Unexpectedly, as shown in Figure 3, treatment with RGDS did not alter Pyk2 levels regardless of the presence or absence of MKs, which indicates that the MK-mediated effects on Pyk2 levels are likely to be regulated by an alternative signaling pathway.34 However, an effect of MKs on the activation of Pyk2’s catalytic activity cannot be ruled out.
Figure 3.
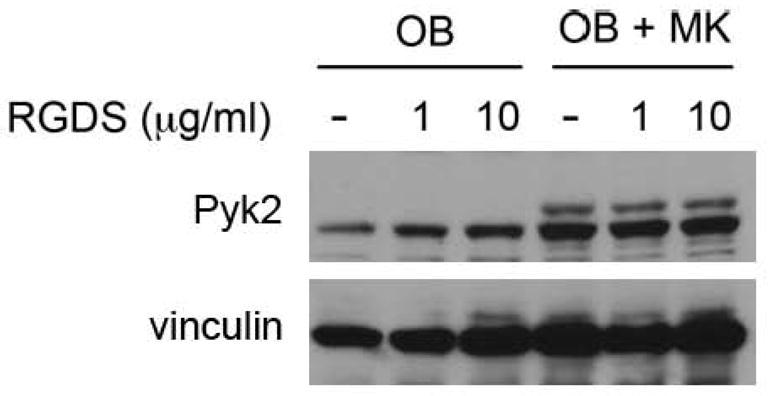
OBs were cultured alone or in the presence of MKs for 5 days. Some cultures were treated with 1 or 10 μl of RGDS (daily), while others remained untreated. MKs were removed by washing and OBs were lysed and resolved by SDS-PAGE. Western blotting shows a significant increase in Pyk2 protein levels in OBs co-cultured with MKs as compared to OBs cultured alone. Treatment of OBs with RGDS did not alter Pyk2 levels compared to untreated OBs. Likewise, treatment of OBs co-cultured with MKs did not alter Pyk2 levels compared to untreated co-cultures. Representative blot from 2 experiments conducted.
Short-Term Priming of OBs with MKs Increases OB Number
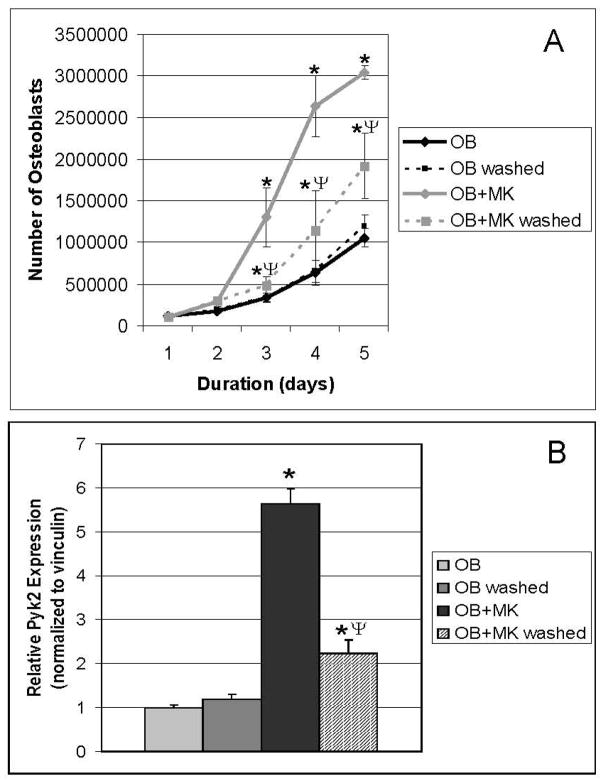
To evaluate at which point the proliferative signal is initiated in OBs in response to MKs, we determined whether short-term stimulation of OBs with MKs impacts OB number long-term. As detailed in Figure 4A, washing had no impact on OB number (OB vs. OB washed). Again, as expected, OB number was significantly elevated in cultures continuously stimulated with MKs for 5 days, compared with OBs cultured alone (OB+MK vs. OB, p<0.001). Interestingly, while continuous stimulation of OBs with MKs resulted in the highest number of OBs, OBs primed with MKs for 4 hrs also exhibited a significant increase in OB number 5 days later, compared with WT OBs cultured alone or washed WT OBs cultured alone (OB+MK washed vs. OB or OB washed, p<0.001 and p<0.01, respectively). These data suggest that priming of OBs with MKs initiates a series of signaling events, which persists for several days, leading to a significant increase in OB number by 5 days.
Figure 4.
OBs were cultured in the presence or absence of MKs, and viable OB number (A) was determined by counting following trypan blue exclusion (n=6/experimental group) and Pyk2 expression (B) was determine by Western blot (n=3/experimental group). In some groups MKs were removed (by washing) after 4 hrs of co-culture (OB+MK washed). Priming OBs with MKs for 4 hrs is sufficient to significantly enhance OB number and Pyk2 expression 5 days later (OB+MK washed vs. OB washed or OB). Data reported are representative from 3 experiments conducted. *Indicates statistically significant difference (p<0.05) at the same time point compared to OB and OB washed. ΨIndicates statistically significant difference (p<0.05) at the same time point between OB+MK washed and OB+MK.
MKs Enhance Long-Term Pyk2 in OBs
We then determined whether priming OBs by short-term stimulation followed by washing to remove MKs resulted in a persistent increase in Pyk2 in OBs. As detailed in Figure 4B, long-term culture of OBs with MKs resulted in a greater than 5-fold increase in total Pyk2 compared to OBs cultured alone (OB+MK vs. OB, p<0.001). OBs co-cultured with MKs for 4 hrs prior to MK removal also exhibited a ~2-fold increase in Pyk2 compared to OBs cultured alone (OB+MK washed vs. OB or OB washed, p<0.05). Interestingly, the 2-fold increase in Pyk2 levels observed after priming of OBs with MKs for 4 hrs was similar to the increase in Pyk2 observed when OBs were harvested after 1–4 hrs of MK stimulation (Figure 1A). These results suggest that MKs stimulate the persistent upregulation of Pyk2 in OB cultures, which is consistent with transcriptional regulation of the Pyk2 gene that we observed (Figure 1C and 1D). Furthermore, our findings suggest that important signal transduction events are initiated within 4 hrs of MK-OB co-culture, which lead to an increase in Pyk2 expression and to a corresponding increase in OB proliferation.
Pyk2 is Integrated in the p53-Mdm2 Pathway in OBs
Next we examined whether short-term stimulation (priming) of OBs with MKs resulted in alterations in p53 levels, a key protein involved in cell cycle regulation. As shown in Figure 5, OBs co-cultured with MKs for 4 hrs prior to MK removal (OB+MK washed) exhibited a marked reduction in p53 levels compared to OBs cultured alone (OB and OB washed). Co-culture of OBs with MKs for 5 days (OB+MK) also resulted in a marked reduction in p53 levels compared to OBs cultured alone (OB) (Figure 5). These data suggest that both continuous and short-term stimulation of OBs with MKs results in a significant reduction of p53 in OBs after 5 days.
Figure 5.
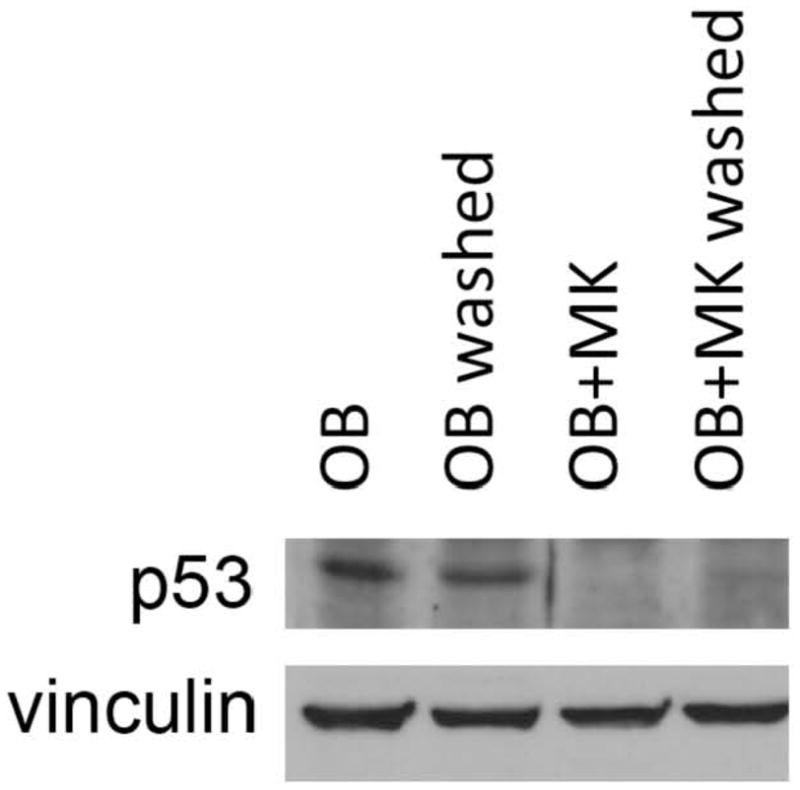
OBs were cultured in the presence or absence of MKs for 5 days. In some groups MKs were removed (by washing) after 4 hrs of co-culture (OB+MK washed). Western blotting demonstrates that p53 expression was significantly reduced in OBs co-cultured with MKs for 5 days (OB vs. OB+MK). Of interest, priming OBs with MKs for 4 hrs (OB washed vs. OB+MK washed) is also sufficient to significantly reduce p53 protein expression. Representative blot from 3 experiments conducted.
To determine whether Pyk2 was obligate in mediating an increase in OB number through Mdm2 and p53 expression, WT and Pyk2−/− OBs were either cultured alone or with WT MKs for 5 days and the levels of Mdm2 and p53 were assessed. As shown in Figure 6, Mdm2 and p53 levels were markedly reduced in WT OBs following incubation with MKs for 5 days, although, both Mdm2 and p53 levels remained largely unchanged in Pyk2−/− OBs co-cultured with MKs. When activated, Mdm2 destabilizes p53 and is then itself degraded. Thus, our data suggests that Pyk2 is involved in the downregulation of Mdm2 and p53, and suggests a novel Pyk2-regulated pathway that is activated in OBs in response to MKs.
Figure 6.
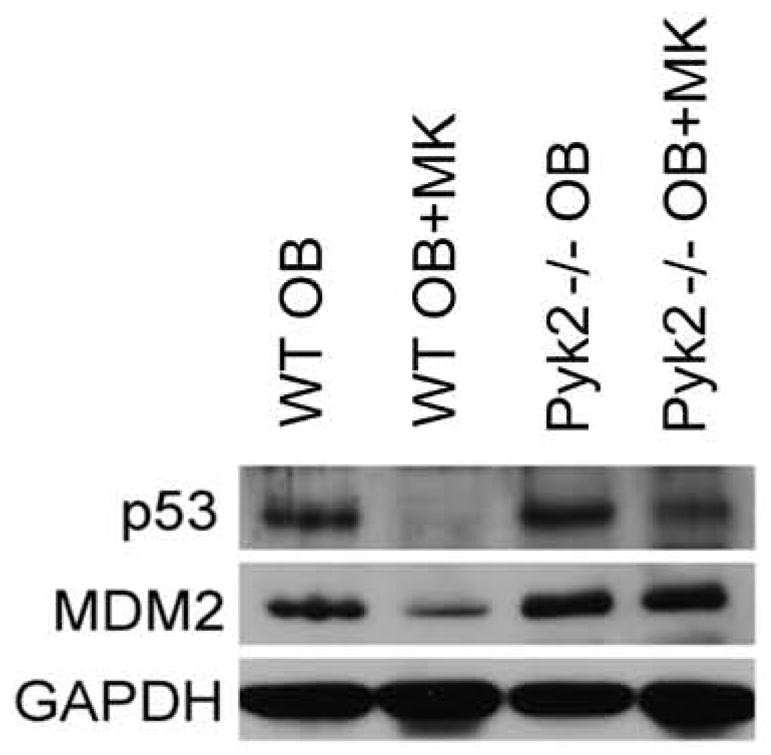
Western blotting demonstrates the importance of osteoblastic Pyk2 expression on Mdm2 and p53 expression. Mdm2 and p53 protein levels were significantly reduced in OBs following co-culture with MKs for 5 days. Pyk2 deficiency ameliorates MK-induced reductions in Mdm2 and p53 expression. Representative blot from 3 experiments conducted.
MKs Reduce OB Differentiation in the Presence or Absence of Pyk2
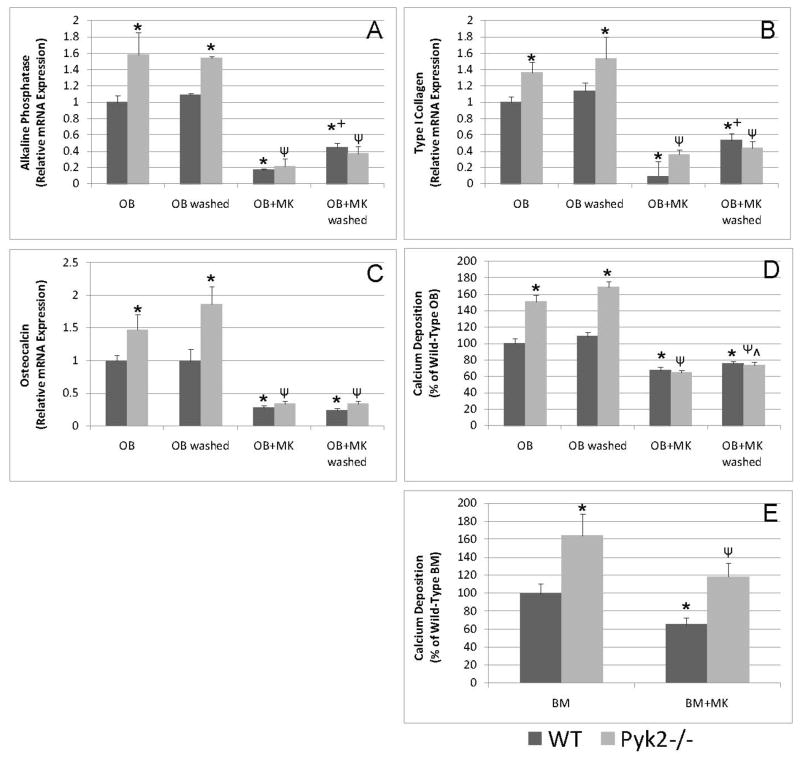
Since we previously demonstrated that MKs inhibit OB differentiation after 14 days of co-culture,15 we next wanted to determine if short-term stimulation of OBs by MKs is sufficient for MK-mediated reductions in OB differentiation. Furthermore, we examined if Pyk2 expression is important in MK-mediated reductions in OB differentiation. The addition of MKs to WT and Pyk2−/− OBs significantly reduced the expression of alkaline phosphatase, type I collagen, osteocalcin, and calcium deposition (Figures 7A–D). These data support the idea that MKs inhibit OB differentiation in the presence or absence of Pyk2, albeit the reduction in OB differentiation markers (with the possible exception of type I collagen) was far greater in Pyk2−/− OBs than WT OBs. Additionally, MKs inhibited OB differentiation when co-cultured with OBs for the entire 14 day period or when co-cultured for the first 4 hrs (Figures 7A–D). Finally, we examined the effects of MKs on the differentiation of stromal-derived osteoprogenitors by culturing BM cells from WT and Pyk2−/− mice under osteogenic conditions in the presence or absence of MKs for 14 days (Figure 7E). As expected, Pyk2−/− BM cultures were significantly more mineralized than WT BM cultures. Similar to our findings with 2 day calvarial OBs, co-culture of both WT and Pyk2−/− BM cells with MKs resulted in a significant reduction in mineralization on day 14, compared to WT and Pyk2−/− BM cells cultured alone, respectively.
Figure 7.
Real-time quantitative PCR and calcium deposition analyses of OB or BM cells cultured under osteogenic conditions in the presence or absence of MKs for 14 days. A–D) WT and Pyk2−/− OBs were cultured alone or in the presence of MKs for 14 days or for the first 4 hrs of culture and then MKs were removed by washing. OB differentiation as determined by mRNA expression and mineralization was higher in Pyk2−/− OBs cultured alone as compared to that observed in WT OBs cultured alone. Additionally, co-culture of OBs with MKs resulted in a dramatic reduction in OB differentiation irrespective of MK culture duration and in the presence and absence of OB Pyk2 expression. E) To test the effect of MKs on osteogenic progenitors BM cells from WT and Pyk2−/− mice were cultured alone or in the presence of MKs and mineralization assessed on day 14. Pyk2−/− BM cultures were more mineralized than were WT cultures. MKs were able to suppress mineralization in both WT and Pyk2−/− BM cultures. n=3–4/experimental group, representative data are shown from 2 (E&F) or 3 (A–D) experiments conducted. *Indicates statistically significant differences (p<0.05) compared to WT OB or WT BM. ΨIndicates statistically significant difference (p<0.05) compared to Pyk2−/− OB or Pyk2−/− BM. +Indicates statistically significant difference (p<0.05) compared to WT OB+MK. ^Indicates statistically significant difference (p<0.05) compared to Pyk2−/− OB+MK.
Pyk2 is Important for MK-Induced Bone Formation In Vivo
While our in vitro findings clearly demonstrate the involvement of Pyk2 in the MK-stimulated increase in OB number, MKs blocked OB differentiation in the presence or absence of Pyk2. To obtain further functional in vivo evidence for the role of Pyk2 in the MK-mediated increase in OB number we employed a method known as adoptive transfer11 which involves the transfer of bone marrow or spleen cells from one mouse into a lethally irradiated recipient mouse. We previously demonstrated that GATA-1 deficient mice have a significant >2-fold increase in bone volume and that the high bone mass phenotype of these mice can be adoptively transferred into C57BL/6 recipient mice with GATA-1 deficient spleen cells suggesting a role for hematopoietic cells in this mechanism, most likely MKs11 (and data not shown). Because the marrow cavity in GATA-1 deficient mice is filled with bone, spleen cells rather than bone marrow cells were used for the adoptive transfer. Spleen cells are an excellent source of immature MKs (up to 100 fold-increase) and hematopoietic progenitor cells due to the extra-medullary hematopoiesis. For the current studies, C57BL/6 or Pyk2−/− mice were lethally irradiated and used as recipient mice. The hematopoietic system of these recipients was reconstituted with 10 million spleen cells derived from either C57BL/6 or GATA-1 deficient mice. Eight weeks post-transfer, mice were bled and platelet numbers determined. As shown in Table 1, all mice reconstituted with GATA-1 deficient cells had significantly fewer platelets as compared to those reconstituted with C57BL/6 cells, which demonstrates the transfer of the hematologic phenotype of GATA-1 deficient mice.11 The transfer of the hematologic phenotype was further confirmed by increased spleen weight 8 weeks post-transfer, whereby mice receiving GATA-1 deficient donor cells had a 3–4 fold increase in spleen weight as compared to those receiving C57BL/6 donor cells.
To investigate differences in the bone phenotypes of recipient mice at 8 weeks post-transfer, the distal femur was analyzed by μCT and histomorphometric analyses. These data sets are reported in Tables 1 and 2, respectively. With respect to μCT analyses as detailed in Table 1 and consistent with our previously published studies,11 reconstituting C57BL/6 mice with GATA-1 deficient spleen cells resulted in a statistically significant, >2-fold increase in bone volume fraction (BV/TV, p<0.01) compared to C57BL/6 mice reconstituted with C57BL/6 cells. The effect size was a robust 1.16. Likewise, C57BL/6 mice reconstituted with GATA-1 deficient spleen cells had significant elevations in bone surface density (BS/TV, p<0.01), and trabecular number (Tb.N, p<0.001) compared to those reconstituted with WT cells. As predicted based on the high bone mass phenotype previously reported in Pyk2−/− mice,20,39 most of the bone parameters measured in Pyk2−/− recipient mice reconstituted with C57BL/6 cells were higher than C57BL/6 recipient mice reconstituted with C57BL/6 cells (BV/TV, p<0.01; BS/TV, p<0.01; Tb.N, p<0.001). Importantly, although some of the bone parameters measured by μCT in Pyk2−/− recipient mice reconstituted with GATA-1 deficient spleen cells were slightly elevated compared to those measured in Pyk2−/− recipients reconstituted with C57BL/6 cells, none of them were significantly different and the effect size was a modest 0.26 (BV/TV). We next conducted more rigorous bone histomorphometric analyses which are recorded in Table 2 (please recall that each femur was first scanned for μCT analysis and the processed for histomorphometric analysis). Like μCT measurements (Table 1), histomorphometric analyses (Table 2) demonstrated that reconstituting C57BL/6 mice with GATA-1 deficient spleen cells resulted in a significant increase in BV/TV (p<0.05). Similarly, Pyk2−/− mice reconstituted with C57BL/6 cells had a significantly higher BV/TV than did C57BL/6 mice reconstituted with C57BL/6 cells (p<0.05). Importantly, similar to our μCT analyses, no difference in BV/TV was observed when Pyk2−/− mice were reconstituted with spleen cells from GATA-1 deficient mice as compared to spleen cells from C57BL/6 control mice (p=0.5). In addition to measuring BV/TV, the numbers of OBs and osteoclasts in the tissue area were also examined. Consistent with our previously published adoptive transfer findings,11 when C57BL/6 mice were reconstituted with GATA-1 deficient cells significantly higher numbers of OBs and osteoclasts were observed, compared to C57BL/6 mice reconstituted with C57BL/6 cells (p=0.001 and p<0.01, respectively). However, when Pyk2−/− mice were reconstituted with GATA-1 deficient cells, no significant differences were detected compared to mice reconstituted with C57BL/6 cells (p=0.6 and 0.4, respectively). Of interest, when OB number was normalized for bone surface rather than the tissue area, no significant differences were detected (p=0.2 for C57BL/6 recipients and p=0.4 Pyk2 recipients). However, analysis of osteoclast surface/bone surface resulted in a similar trend as observed for number of osteoclasts/tissue area. That is, osteoclast surface/bone surface was significantly higher when C57BL/6 mice were reconstituted with GATA-1 cells, as compared to reconstitution with C57BL/6 cells (p<0.01). In addition, no significant difference was detected between Pyk2−/− mice reconstituted with GATA-1 cells or C57BL/6 cells (p=0.9).
Finally, it should be noted that the bone phenotype of non-irradiated C57BL/6 and Pyk2−/− mice was also examined as additional baseline controls. Using μCT, the BV/TV was 4.2±0.3% for C57BL/6 controls and was 17.4±1.0% for Pyk2−/− mice (p<0.001), again confirming the previously reported high bone mass phenotype. 20,39 Comparing these measurements to those in Table 1, there was a significant increase in BV/TV in untreated C57BL/6 mice compared to C57BL/6 mice reconstituted with C57BL/6 cells (35% increase). Likewise there was a dramatic increase in BV/TV in untreated Pyk2−/− mice compared to Pyk2−/− mice reconstituted with C57BL/6 cells (197% increase). The striking decreases in BV/TV in reconstitution studies (likely owing to radiation-induced bone loss) do not lend non-irradiated mice as a useful control for these studies. Of note, virtually identical data were observed by histomorphometric analyses for these baseline control mouse bone phenotypes (data not shown). Taken together, the robust bone phenotype observed when C57BL/6 recipients received GATA-1 deficient cells versus the minimal difference, lacking significance, observed when Pyk2−/− recipients received GATA-1 cells, suggests that Pyk2 expression facilitates the MK-mediated enhancement of bone formation in vivo. However, additional de novo pathways are likely able to help in the maintenance of skeletal homeostasis.
DISCUSSION
Integrins play a central role in regulating tissue homeostasis and our recent work demonstrates that integrins are also important for MK-mediated regulation of OB proliferation.17 While in vitro studies have demonstrated an important interaction between MKs and OBs, the specific details regarding the downstream signaling pathways and the effector molecules involved in this process are unknown. Pyk2 is a non-receptor protein tyrosine kinase, which is closely related to the focal adhesion kinase (FAK) family. FAK and Pyk2 function in signal transduction pathways that are stimulated by integrin activation. Pyk2 is known to be activated in a Ca2+-dependent manner following integrin engagement and has been linked to the proliferation, migration and activity of a variety of mesenchymal, epithelial and hematopoietic cell types.34
Our studies and those of others have reported the pivotal role of Pyk2 in bone mass and bone cell function.35–39 Of importance, Pyk2−/− animals have a significant increase in bone mass and bone formation,20,40 and in vitro studies by Buckbinder et al.,20 which were confirmed here (Figure 7), demonstrated that OBs differentiated from Pyk2−/− mice showed a dramatic increase in alkaline phosphatase activity and mineralization compared to WT controls. Taken together, these data suggest that Pyk2 negatively regulates bone mass in part by reducing osteoblastogenesis.
On the other hand, MKs consistently stimulate WT OB proliferation and we show that MKs stimulate the rapid and sustained increase in total Pyk2 levels by promoting Pyk2 gene transcription in WT OBs (Figure 1). Also, in the absence of Pyk2, OBs are less responsive to MK stimulated proliferation (Figure 2). Furthermore, examination of the role of Pyk2 as an integration point for integrin-mediated signaling in OBs revealed that the MK-mediated increase in Pyk2 levels in OBs was unaltered by treatment of cultures with integrin-blocker RGDS at doses we have previously shown to be efficacious for reducing MK-mediated OB proliferation (Figure 3).17 This finding was unexpected since Pyk2 is known to be activated by phosphorylation downstream of integrin engagement in other cell types, including OCs,34 MKs express and secrete fibronectin,41–43 a β1-binding integrin ligand, and titration of RGDS into MK-OB cultures resulted in a significant reduction in OB proliferation.17 It should be noted that our findings do not, however, exclude the possibility that integrin activation in response to MKs, results in an increase in the phosphorylation and therefore catalytic activity of Pyk2 in OBs. Additionally, it is also important to note that in our previous studies RGDS blocked MK-induced OB proliferation by approximately 50%,17 suggesting that a signaling pathway(s) other than β1 integrin signaling may also be involved in regulating MK-mediated OB proliferation. In support of this, Pyk2 is also known to be regulated by G-protein-coupled receptors, inflammatory cytokines, and stress signal activation.34,44 In other studies we are investigating whether Pyk2 activity in OBs is regulated by MK stimulation.
To begin to further dissect the signaling pathways by which MKs regulate OB proliferation we examined whether stimulation of OBs by MKs required chronic exposure or whether acute exposure (4 hrs) could also enhance OB proliferation. Here we show that Pyk2 expression and OB number are significantly increased in OBs co-cultured with MKs following acute or chronic exposure to MKs. We demonstrated that stimulation of OBs with MKs for 1 hr results in a significant (> 2-fold) increase in total Pyk2 protein levels (Figure 1A). We also found that enhanced Pyk2 levels are sustained and even elevated with long-term MK stimulation (5 days, Figure 4B). Additionally, we showed that priming OBs for 4 hrs with MKs and then removing the MKs still results in a greater than 2-fold increase in total Pyk2 protein expression 5 days later (Figure 4B). Interestingly, we also observed that the increase in Pyk2 levels in OBs stimulated with MKs paralleled the MK-induced increase in OB number. Our data further illustrate the requirement of Pyk2 in the observed reduction of known cell cycle regulator Mdm2 and p53. Thus, the reduction in p53 likely results in the release of cycle arrest mediated by p53 and thereby stimulates proliferation to increase OB number in response to MK. We are currently investigating the mechanism of interaction of Pyk2 with Mdm2 and p53 in OBs and the role of these proteins in OB cell cycle progression.
While a major goal in this study was to determine whether Pyk2 expression was important in the MK-mediated increase in OB proliferation, to conduct a more rigorous study, we also examined the importance of Pyk2 expression on MK-mediated changes in OB differentiation. We previously reported that the co-culture of MKs with OBs for 14 days dramatically inhibited OB differentiation.15 Here we confirm these findings in WT OBs, and extend these data by demonstrating that Pyk2−/− OB differentiation, as assessed by OB expression of type I collagen, osteocalcin, and alkaline phosphatase as well as bound calcium as a measure of mineralization, are all significantly reduced with 14 days of co-culture with MKs (Figure 7A–D). We and others20 have shown that Pyk2 has a negative effect on OB differentiation. Interestingly, although Pyk2−/− OBs have increased alkaline phosphatase and mineralization compared to WT OBs, MKs reduced OB differentiation in Pyk2−/− OBs to the same extent as that observed in WT OBs. This suggests that MKs can suppress OB differentiation in the absence of Pyk2 and irrespective of the differentiation status of OBs. Moreover, our findings that MKs increase Pyk2 levels in OBs, and that Pyk2 expression is important for the MK-mediated increase in OB number, are consistent with the idea that MKs act via Pyk2 to increase OB number. Furthermore, as discussed below, it appears that in vivo the MK-mediated increase in OB number may be able to compensate for the reduction in differentiation, resulting in a high turnover state with a net gain in bone mass.
To address the role of Pyk2 in MK-mediated OB differentiation, we further demonstrate that acute exposure of both WT and Pyk2−/− OBs to MKs results in a dramatic reduction in OB differentiation (Figure 7A–D). In addition to examining markers of OB differentiation in 2 day calvarial OBs cultured in the presence and absence of MKs, we also tested the effects of MKs on osteogenic progenitors. We found that co-culture of both WT and Pyk2−/− BM in the presence of MKs resulted in a significant reduction in mineralization compared to that observed in WT and Pyk2−/− BM cultures alone. Thus, it appears that the effects of MKs on both OB proliferation and differentiation are rapidly initiated upon co-culture and that MKs reduce OB and BM differentiation in co-cultures in the presence or absence of Pyk2. Taken together, our findings that MKs upregulate Pyk2 mRNA and protein levels in OBs soon after cell-cell contact suggests that Pyk2 may play a role in the proliferative effect of MKs on OBs, rather than at later stages of OB mineralization. This finding is also consistent with our recently published study demonstrating that MKs stimulate OB proliferation via Pyk2.45
While our studies begin to unravel the mechanisms by which MKs mediate OB proliferation and differentiation in vitro, it is of clinical importance to determine whether these observations can be replicated in vivo. To address this, we used an adoptive transfer model whereby C57BL/6 and Pyk2−/− recipient mice are reconstituted with C57BL/6 donor cells or GATA-1 deficient donor cells. The GATA-1 deficient mice contain numerous MKs which allows us to assess whether MKs differentially regulate WT and Pyk2−/− bone mass. As detailed in Tables 1 and 2, consistent with our previous findings,11 reconstitution of C57BL/6 mice with GATA-1 deficient cells resulted in transfer of both the hematologic and bone phenotype observed in GATA-1 deficient mice.10,13,19 Also, as detailed in Table 2, and consistent with our previously published studies,10,11 reconstitution of C57BL/6 mice with GATA-1 deficient cells resulted in what appears to be a high turnover state with a significant increase in both OB and osteoclast number resulting in a net gain in bone volume. Further, as shown in Tables 1 and 2, Pyk2−/− recipient mice reconstituted with C57BL/6 donor cells exhibited an increase in bone volume, compared to C57BL/6 recipient mice reconstituted with C57BL/6 donor cells, as would be predicted based on previous reports of a high bone mass phenotype in Pyk2−/− mice.20,40 Of interest, baseline bone measurements of C57BL/6 and Pyk2−/− mice of the same age and gender showed that both mouse strains experienced significant radiation-induced bone loss (compared to C57BL/6 and Pyk2−/− recipient mice, respectively, reconstituted with C57BL/6 cells), but the relative loss was more pronounced for Pyk2−/− mice. This may be a result of the impairment of MK-mediated OB proliferation observed in Pyk2−/− OBs, especially considering the findings of Dominici et al,14 who demonstrated that lethally irradiated mice (as was done for adoptive transfer studies described here), surviving MKs migrated to endosteal bone surfaces (in close contact with OBs) and stimulated a 2-fold increase in OB number. Likewise, when Pyk2−/− mice were reconstituted with GATA-1 deficient cells containing significantly increased numbers of MKs, the MK-mediated OB proliferation resulted in only a marginal increase in bone volume as compared to the significant increase observed when C57BL/6 recipients were reconstituted with GATA-1 deficient cells rather than C57BL/6 cells. These in vivo data suggest that in Pyk2 deficient recipients, MKs are unable to stimulate a significant increase in bone volume like is observed in WT recipient mice. This suggests that Pyk2 plays a significant role in the OB response to MKs. The small, but not significant, rise in BV/TV in the Pyk2−/− recipient mice transplanted with GATA-1 deficient cells, may indicate that other proteins in OBs are also mediated in part by MKs.
Although in the current studies we did not conduct dynamic histomorphometry nor measure biochemical markers of bone turnover, our previous investigations demonstrated significant increases in all bone formation indices measured, including an increase in bone formation rate, mineral apposition rate, and osteocalcin levels in the bone and serum following adoptive transfer. Additionally, we observed no differences in urinary Dpd.10,11 Thus, there appears to be a paradox between the high bone mass phenotype of Pyk2−/− mice and our findings that MKs stimulate a rapid increase in Pyk2 expression, OB proliferation, and bone formation in vivo. However, it must be remembered that global deletion of Pyk2 results in a significant impairment in osteoclast activity along with enhancement of bone formation.20,40. In the current study, we investigated the specific MK-mediated increases in OB Pyk2 expression, and we demonstrate using both in vitro and in vivo approaches, that Pyk2−/− OBs are less responsive to MK stimulation than WT OBs.
In conclusion, we demonstrate that Pyk2 expression is important for MK-mediated OB proliferation. We also show that Pyk2 regulates Mdm2 and p53, which are known regulators of the cell cycle. Finally, we demonstrate that Pyk2 expression is positively associated with the MK-induced increase in bone mass. Although in normal BM MKs tend to be localized away from endosteal surfaces, it is known that OBs stimulate megakaryopoiesis by expressing cytokines and growth factors important for MK proliferation, including membrane bound stem cell factor,46–48 which is suggestive of paracrine effects. Furthermore, there are a number of human diseases characterized by abnormally elevated numbers and/or distribution of MKs, in which osteosclerosis is evident,1–3 and in which closer proximity of MKs to endosteal surfaces is observed. Perhaps the most convincing evidence of the proximity of MKs to endosteal surfaces is seen in recent radiation studies in which MKs were observed to migrate within close proximity of endosteal surfaces and stimulate a 2-fold increase in OB number, thus augmenting the so-called endosteal hematopoietic stem cell niches. This in turn enhances hematopoietic stem cell recovery.14 Thus, the MK-stimulated increase in OB proliferation could be of clinical importance not only for radiation-induced bone loss and bone loss diseases such as osteoporosis, but also for cytotoxic regimens required for bone marrow transplantation. Of importance, these studies have begun to dissect the mechanism by which MKs regulate OB proliferation and bone formation and may provide valuable insights into therapeutic interventions in which OBs could be stimulated via a mechanism similar to that mediated by MKs.
Acknowledgments
This work was sponsored in part by the Department of Orthopaedic Surgery at Indiana University School of Medicine, the Department of Oral Biology at Indiana University School of Dentistry, a Biomedical Research Grant and Pilot Funding for Research Use of Core Facilities Award both from Indiana University School of Medicine (MAK), and by the following NIH grants: R03 AR055269 (MAK), R01 AR060332 (MAK, AB), R01 HL55716 (EFS), R01 AR052682 (FMP), and R01 CA109262 (LDM). We would sincerely like to thank Dr. Charles H. Turner (posthumously) for helpful discussions and Pfizer for providing us with the Pyk2 deficient mice. We would also like to thank Drs. Stuart Orkin and Ramesh Shivdasani for providing us with the GATA-1 deficient mice. We are thankful to Dr. Matthew Allen for training us in μCT analyses. Finally, we would like to thank the operators of the Indiana University Melvin and Bren Simon Cancer Center Flow Cytometry Resource Facility for their technical help and support. The FCRF is partially funded by P30 CA082709.
Footnotes
Authors’ roles: Study conception and design: COO, EFS, LDM, FMP, AB, and MAK. Acquisition of data: Y-HC, RAH, KN, RG-O, DLW, BRC, TEM, HLC, APP, and MAK. Analysis and Interpretation of data: Y-HC, RAH, RG-O, DLW, BRC, TEM, EFS, LDM, FMP, AB, and MAK. Drafting of manuscript and revising it critically for important intellectual content: Y-HC, RAH, KN, RG-O, DLW, BRC, TEM, HLC, APP, COO, EFS, LDM, FMP, AB, and MAK. Approving final version of manuscript: Y-HC, RAH, KN, RG-O, DLW, BRC, TEM, HLC, APP, COO, EFS, LDM, FMP, AB, and MAK. MAK and AB take responsibility for the integrity of the data analysis.
References
- 1.Thiele J, Kvasnicka HM, Fischer R. Histochemistry and morphometry on bone marrow biopsies in chronic myeloproliferative disorders - aids to diagnosis and classification. Ann Hematol. 1999;78:495–506. doi: 10.1007/s002770050546. [DOI] [PubMed] [Google Scholar]
- 2.Lennert K, Nagai K, Schwarze EW. Patho-anatomical features of the bone marrow. Clin Haematol. 1975;4:331–351. [PubMed] [Google Scholar]
- 3.Chagraoui H, Wendling F, Vainchenker W. Pathogenesis of myelofibrosis with myeloid metaplasia: Insight from mouse models. Best Pract Res Clin Haematol. 2006;19:399–412. doi: 10.1016/j.beha.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Kacena MA, Ciovacco WA. Megakaryocyte-bone cell interactions. Adv Exp Med Biol. 2010;658:31–41. doi: 10.1007/978-1-4419-1050-9_4. [DOI] [PubMed] [Google Scholar]
- 5.Yan XQ, Lacey D, Fletcher F, Hartley C, McElroy P, Sun Y, Xia M, Mu S, Saris C, Hill D, Hawley RG, McNiece IK. Chronic exposure to retroviral vector encoded MGDF (mpl-ligand) induces lineage-specific growth and differentiation of megakaryocytes in mice. Blood. 1995;86:4025–4033. [PubMed] [Google Scholar]
- 6.Yan XQ, Lacey D, Hill D, Chen Y, Fletcher F, Hawley RG, McNiece IK. A model of myelofibrosis and osteosclerosis in mice induced by overexpressing thrombopoietin (mpl ligand): reversal of disease by bone marrow transplantation. Blood. 1996;88:402–409. [PubMed] [Google Scholar]
- 7.Villeval JL, Cohen-Solal K, Tulliez M, Giraudier S, Guichard J, Burstein SA, Cramer EM, Vainchenker W, Wendling F. High thrombopoietin production by hematopoietic cells induces a fatal myeloproliferative syndrome in mice. Blood. 1997;90:4369–4383. [PubMed] [Google Scholar]
- 8.Frey BM, Rafii S, Crystal RG, Moore MA. Adenovirus long-term expression of thrombopoietin in vivo: a new model for myeloproliferative syndrome and osteomyelofibrosis. Schweiz Med Wochenschr. 1998;128:1587–1592. [PubMed] [Google Scholar]
- 9.Frey BM, Rafii S, Teterson M, Eaton D, Crystal RG, Moore MA. Adenovector-mediated expression of human thrombopoietin cDNA in immune-compromised mice: insights into the pathophysiology of osteomyelofibrosis. J Immunol. 1998;160:691–699. [PubMed] [Google Scholar]
- 10.Kacena MA, Shivdasani RA, Wilson K, Xi Y, Troiano N, Nazarian A, Gundberg CM, Bouxsein ML, Lorenzo JA, Horowitz MC. Megakaryocyte-osteoblast interaction revealed in mice deficient in transcription factors GATA-1 and NF-E2. J Bone Miner Res. 2004;19:652–660. doi: 10.1359/JBMR.0301254. [DOI] [PubMed] [Google Scholar]
- 11.Kacena MA, Gundberg CM, Nelson T, Horowitz MC. Loss of the transcription factor p45 NF-E2 results in a developmental arrest of megakaryocyte differentiation and the onset of a high bone mass phenotype. Bone. 2005;36:215–223. doi: 10.1016/j.bone.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 12.Suva LJ, Hartman E, Dilley JD, Russell S, Akel NS, Skinner RA, Hogue WR, Budde U, Varughese KI, Kanaji T, Ware J. Platelet dysfunction and a high bone mass phenotype in a murine model of platelet-type von Willebrand disease. Am J Pathol. 2008;172:430–439. doi: 10.2353/ajpath.2008.070417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shivdasani RA, Fujiwara Y, McDevitt MA, Orkin SH. A lineage-selective knockout establishes the critical role of transcription factor GATA-1 in megakaryocyte growth and platelet development. EMBO J. 1997;16:3965–3973. doi: 10.1093/emboj/16.13.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dominici M, Rasini V, Bussolari R, Chen X, Hofmann TJ, Spano C, Bernabei D, Veronesi E, Bertoni F, Paolucci P, Conte P, Horwitz EM. Restoration and reversible expansion of the osteoblastic hematopoietic stem cell niche after marrow radioablation. Blood. 2009;114:2333–2343. doi: 10.1182/blood-2008-10-183459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciovacco WA, Goldberg CG, Taylor AF, Lemieux JM, Horowitz MC, Donahue HJ, Kacena MA. The role of gap junctions in megakaryocyte-mediated osteoblast proliferation and differentiation. Bone. 2009;44:80–86. doi: 10.1016/j.bone.2008.08.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciovacco WA, Cheng YH, Horowitz MC, Kacena MA. Immature and mature megakaryocytes enhance osteoblast proliferation and inhibit osteoclast formation. J Cell Biochem. 2010;109:774–781. doi: 10.1002/jcb.22456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemieux JM, Horowitz MC, Kacena MA. Involvement of integrins alpha(3)beta(1) and alpha(5)beta(1) and glycoprotein IIb in megakaryocyte-induced osteoblast proliferation. J Cell Biochem. 2010;109:927–932. doi: 10.1002/jcb.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miao D, Murant S, Scutt N, Genever P, Scutt A. Megakaryocyte-bone marrow stromal cell aggregates demonstrate increased colony formation and alkaline phosphatase expression in vitro. Tissue Eng. 2004;10:807–817. doi: 10.1089/1076327041348473. [DOI] [PubMed] [Google Scholar]
- 19.McDevitt MA, Shivdasani RA, Fujiwara Y, Yang H, Orkin SH. A “knockdown” mutation created by cis-element gene targeting reveals the dependence of erythroid cell maturation on the level of transcription factor GATA-1. Proc Natl Acad Sci U S A. 1997;94:6781–6785. doi: 10.1073/pnas.94.13.6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buckbinder L, Crawford DT, Qi H, Ke HZ, Olson LM, Long KR, Bonnette PC, Baumann AP, Hambor JE, Grasser WA, 3rd, Pan LC, Owen TA, Luzzio MJ, Hulford CA, Gebhard DF, Paralkar VM, Simmons HA, Kath JC, Roberts WG, Smock SL, Guzman-Perez A, Brown TA, Li M. Proline-rich tyrosine kinase 2 regulates osteoprogenitor cells and bone formation, and offers an anabolic treatment approach for osteoporosis. Proc Natl Acad Sci U S A. 2007;104:10619–10624. doi: 10.1073/pnas.0701421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horowitz MC, Fields A, DeMeo D, Qian HY, Bothwell AL, Trepman E. Expression and regulation of Ly-6 differentiation antigens by murine osteoblasts. Endocrinology. 1994;135:1032–1043. doi: 10.1210/endo.135.3.7520861. [DOI] [PubMed] [Google Scholar]
- 22.Wong GL, Cohn DV. Target cells in bone for parathormone and calcitonin are different: enrichment for each cell type by sequential digestion of mouse calvaria and selective adhesion to polymeric surfaces. Proc Natl Acad Sci U S A. 1975;72:3167–3171. doi: 10.1073/pnas.72.8.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Towler DA, St Arnaud R. Use of Cultured Osteoblastic Cells to Identify and Characterize Transcriptional Regulatory Complexes. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of Bone Biology. 2. Vol. 2. Academic Press; San Diego, CA: 2002. pp. 1503–1527. [Google Scholar]
- 24.Cheng YH, Chitteti BR, Streicher DA, Morgan JA, Rodriguez-Rodriguez S, Carlesso N, Srour EF, Kacena MA. Impact of maturational status on the ability of osteoblasts to enhance the hematopoietic function of stem and progenitor cells. J Bone Miner Res. 2011;26:1111–1121. doi: 10.1002/jbmr.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simmons DJ, Kent GN, Jilka RL, Scott DM, Fallon M, Cohn DV. Formation of bone by isolated, cultured osteoblasts in millipore diffusion chambers. Calcif Tissue Int. 1982;34:291–294. doi: 10.1007/BF02411253. [DOI] [PubMed] [Google Scholar]
- 26.Jilka RL, Cohn DV. Role of phosphodiesterase in the parathormone-stimulated adenosine 3′,5′-monophosphate response in bone cell populations enriched in osteoclasts and osteoblasts. Endocrinology. 1981;109:743–747. doi: 10.1210/endo-109-3-743. [DOI] [PubMed] [Google Scholar]
- 27.Arai F, Nakamura Y, Gomei Y, Suda T. Characterization of the niche complex molecules in bone marrow. Exp Hematol. 2008;36:S25. [Google Scholar]
- 28.Mayack SR, Wagers AJ. Osteolineage niche cells initiate hematopoietic stem cell mobilization. Blood. 2008;112:519–531. doi: 10.1182/blood-2008-01-133710. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Kacena MA, Nelson T, Clough ME, Lee SK, Lorenzo JA, Gundberg CM, Horowitz MC. Megakaryocyte-mediated inhibition of osteoclast development. Bone. 2006;39:991–999. doi: 10.1016/j.bone.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Drachman JG, Sabath DF, Fox NE, Kaushansky K. Thrombopoietin signal transduction in purified murine megakaryocytes. Blood. 1997;89:483–492. [PubMed] [Google Scholar]
- 31.Stanford CM, Jacobson PA, Eanes ED, Lembke LA, Midura RJ. Rapidly forming apatitic mineral in an osteoblastic cell line (UMR 106-01 BSP) J Biol Chem. 1995;270:9420–9428. doi: 10.1074/jbc.270.16.9420. [DOI] [PubMed] [Google Scholar]
- 32.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 33.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 34.Avraham H, Park SY, Schinkmann K, Avraham S. RAFTK/Pyk2-mediated cellular signalling. Cell Signal. 2000;12:123–133. doi: 10.1016/s0898-6568(99)00076-5. [DOI] [PubMed] [Google Scholar]
- 35.Duong LT, Rodan GA. Integrin-mediated signaling in the regulation of osteoclast adhesion and activation. Front Biosci. 1998;3:d757–68. doi: 10.2741/A319. [DOI] [PubMed] [Google Scholar]
- 36.Duong LT, Nakamura I, Lakkakorpi PT, Lipfert L, Bett AJ, Rodan GA. Inhibition of osteoclast function by adenovirus expressing antisense protein-tyrosine kinase 2. J Biol Chem. 2001;276:7484–7492. doi: 10.1074/jbc.M008368200. [DOI] [PubMed] [Google Scholar]
- 37.Lakkakorpi PT, Bett AJ, Lipfert L, Rodan GA, Duongle T. PYK2 autophosphorylation, but not kinase activity, is necessary for adhesion-induced association with c-Src, osteoclast spreading, and bone resorption. J Biol Chem. 2003;278:11502–11512. doi: 10.1074/jbc.M206579200. [DOI] [PubMed] [Google Scholar]
- 38.Wang Q, Xie Y, Du QS, Wu XJ, Feng X, Mei L, McDonald JM, Xiong WC. Regulation of the formation of osteoclastic actin rings by proline-rich tyrosine kinase 2 interacting with gelsolin. J Cell Biol. 2003;160:565–575. doi: 10.1083/jcb.200207036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bruzzaniti A, Neff L, Sandoval A, Du L, Horne WC, Baron R. Dynamin reduces Pyk2 Y402 phosphorylation and SRC binding in osteoclasts. Mol Cell Biol. 2009;29:3644–3656. doi: 10.1128/MCB.00851-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gil-Henn H, Destaing O, Sims NA, Aoki K, Alles N, Neff L, Sanjay A, Bruzzaniti A, De Camilli P, Baron R, Schlessinger J. Defective microtubule-dependent podosome organization in osteoclasts leads to increased bone density in Pyk2(−/−) mice. J Cell Biol. 2007;178:1053–1064. doi: 10.1083/jcb.200701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schick PK, Wojenski CM, Bennett VD, Ivanova T. The synthesis and localization of alternatively spliced fibronectin EIIIB in resting and thrombin-treated megakaryocytes. Blood. 1996;87:1817–1823. [PubMed] [Google Scholar]
- 42.Schick PK, Wojensk CM, Bennett V, Denisova L. Fibronectin isoforms in megakaryocytes. Stem Cells. 1996;14(Suppl 1):212–219. doi: 10.1002/stem.5530140728. [DOI] [PubMed] [Google Scholar]
- 43.Schick PK, Walker J, Profeta B, Denisova L, Bennett V. Synthesis and secretion of von Willebrand factor and fibronectin in megakaryocytes at different phases of maturation. Arterioscler Thromb Vasc Biol. 1997;17:797–801. doi: 10.1161/01.atv.17.4.797. [DOI] [PubMed] [Google Scholar]
- 44.Xiong W, Parsons JT. Induction of apoptosis after expression of PYK2, a tyrosine kinase structurally related to focal adhesion kinase. J Cell Biol. 1997;139:529–539. doi: 10.1083/jcb.139.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kacena MA, Eleniste PP, Cheng YH, Huang S, Shivanna M, Meijome TE, Mayo LD, Bruzzaniti A. Megakaryocytes Regulate Expression of Pyk2 Isoforms and Caspase-mediated Cleavage of Actin in Osteoblasts. J Biol Chem. 2012;287:17257–17268. doi: 10.1074/jbc.M111.309880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang EJ, Nocka KH, Buck J, Besmer P. Differential expression and processing of two cell associated forms of the kit-ligand: KL-1 and KL-2. Mol Biol Cell. 1992;3:349–362. doi: 10.1091/mbc.3.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blair HC, Julian BA, Cao X, Jordan SE, Dong SS. Parathyroid hormone-regulated production of stem cell factor in human osteoblasts and osteoblast-like cells. Biochem Biophys Res Commun. 1999;255:778–784. doi: 10.1006/bbrc.1999.0260. [DOI] [PubMed] [Google Scholar]
- 48.Blair HC, Dong SS, Julian BA. Expression of stem cell factor by osteoblasts in normal and hyperparathyroid bone: relation to ectopic mast cell differentiation. Virchows Arch. 1999;435:50–57. doi: 10.1007/s004280050394. [DOI] [PubMed] [Google Scholar]