Abstract

A robust and efficient protocol for the introduction of the dioxolanylethyl moiety onto various aryl and heteroaryl halides has been developed, providing cross-coupling yields up to 93%. Copper-catalyzed borylation of 2-(2-bromoethyl)-1,3-dioxolane with bis(pinacolato)diboron followed by treatment with potassium bifluoride provides the key organotrifluoroborate reagent.
The dioxolanylethyl fragment represents a conveniently protected synthetic equivalent for the propanal substructure.1 Surprisingly, the installation of such a motif on aryl and heteroaryl systems via cross-coupling has not been extensively studied. Among the principle methods encountered, the Heck reaction between aryl iodides and allyl alcohol leads directly to the aldehyde,2 and the Negishi cross-coupling between dioxolanylethylzinc bromide and aryl iodides also provides access to the target structures.3 Finally, the cobalt-catalyzed cross-coupling between aryl Grignard reagents and 2-(2-bromoethyl)-1,3-dioxolane has also been reported (Scheme 1).4
Scheme 1.


Synthetic Methods Leading to Arylpropanal and the Corresponding 1,3-Dioxolane Species.
These previously reported methods suffer from several non-negligible drawbacks, such as the requisite use of aryl iodides and high boiling solvents in the Heck reaction (Scheme 1, eq 1), and the employment of reactive reagents such as alkylzinc bromide or arylmagnesium bromide for the other two methods (Scheme 1, eqs 2 and 3), thus limiting the substrate scope and versatility. Additionally, these transformations all lack atom economy in that they require the use of a several fold excess of reagents. The combination of these features lowers the general appeal and subsequent application of these methods.
A strategy based on the use of the Suzuki-Miyaura cross-coupling between a dioxolanylethylboron species and aryl electrophiles has, to our knowledge, never been reported, and would provide a mild and versatile alternative pathway.5 Moreover, the use of the organotrifluoroborate technology would also be beneficial, allowing the use of stable and robust reagents in near stoichiometric ratios. Organotrifluoroborates have been developed as useful boron-containing reagents for various chemical transformations, and more notably as surrogates for boronic acids in Suzuki-Miyaura coupling reactions.6
To our knowledge, the hydroboraton of 2-ethenyl-1,3-dioxolane appears in a single report.7 In that effort, 9-BBN was utilized to hydroborate the alkene with high regioselectivity for terminal hydroboration, but the resulting air-sensitive organoborane was simply oxidized to the alcohol, and no further chemistry has ever appeared on these or related organoboron intermediates.
We chose another convenient route to access the requisite boron reagents, permitting access to shelf-stable organotrifluoroborates. We recently reported the preparation of potassium β-alkoxyethyltrifluoroborates8 using an adaptation of Marder and Liu's conditions,9 wherein a Cu(I)-catalyzed borylation of the corresponding primary bromides was followed by treatment with KHF2 to afford the target structures. The resulting organotrifluoroborates were subsequently used in Suzuki-Miyaura cross-coupling reactions. Based on the success of this approach, we studied an extension of this method to prepare potassium dioxolanylethyltrifluoroborates. Borylation of the commercially available 2-(2-bromoethyl)-1,3-dioxolane with bis(pinacolato)diboron using CuI and polymer-bound triphenylphosphine (PSPPh3) as the catalytic system afforded the desired product in 69-75% yield (Scheme 2).
Scheme 2.

Synthesis of Potassium Dioxolanylethyltrifluoroborate 1.
Once synthesized, this new trifluoroborate was tested in a Suzuki-Miyaura cross-coupling. Pleasingly, the reaction conditions developed for alkyloxethyltrifluoroborates translated very well with this new substrate. Indeed, PdCl2AtaPhos210 (5 mol %) and Cs2CO3 (3 equiv) in a mixture of toluene/H2O (4 : 1) at 100 °C in the presence of 1.1 equiv of the trifluoroborate for 14 h allowed the formation of 89% of the desired cross-coupled product 2a when using 4-bromoanisole as electrophile (Table 1, entry 1). Halving the catalytic loading to 2.5 mol % still provided 2a in a very acceptable 82% yield.
Table 1.
Suzuki-Miyaura Cross-Coupling of Various Aryl Halides with Dioxolanylethyltrifluoroborate 1 a

| |||
|---|---|---|---|
| entry | electrophile | product | isolated yield (%) |
| 1 |

|

|
89 (Br) 82b (Br) 74 (Cl) |
| 2 |

|

|
56 |
| 3 |

|

|
83 |
| 4 |

|

|
48 (Br) 0c (Cl) |
| 5 |

|

|
70 |
| 6 |

|

|
62 |
| 7 |

|

|
90 |
| 8 |

|

|
38 (Br) 82 (Cl) |
| 9 |

|

|
59 |
| 10 |

|

|
71 (Br) 55 (Cl) |
| 11 |

|

|
50d |
| 12 |

|

|
68d |
| 13 |

|

|
69 |
| 14 |

|

|
38e |
Reaction conditions: Aryl halide (1.0 equiv), organotrifluoroborate (1.1 equiv), PdCl2AtaPhos2 (5 mol %), Cs2CO3 (3.0 equiv), toluene/H2O (4:1, [ ] = 0.25 M), 100 °C, 14 h.
Using 2.5 mol % catalyst.
A mixture of aryl chloride and boronic acid was recovered.
Product contains up to 8 % impurity.
Reaction performed on a 3 mmol scale using a 1 : 1 trifluoroborate/electrophile ratio and only 1 mol % Pd.
4-Chloroanisole was also reactive and resulted in the formation of the desired 2-(4-methoxyphenethyl)-1,3-dioxolane in 74% isolated yield. To examine the scope of the reaction, aryl chlorides became the focus of the investigation, as they tend to be more challenging than their bromide counterparts. However, when the chlorides failed to react, the corresponding bromides were employed as well. During the course of these studies, both electron-rich (Table 1, entries 1-7) and electron-poor (Table 1, entries 8-14) substrates proved to be efficient partners, providing the diversely substituted dioxolanylethylaryls with yields ranging from 38 to 90%. Substituents at the ortho, meta and para-positions were tolerated, as all 3 different anisole isomers reacted to give the desired products 2a-c with good to excellent yields (Table 1, entries 1-3). Although the sterically hindered 2,6-dimethyl-4-methoxy-chlorobenzene did not react, the bromo derivative afforded the cross-coupled product 2d in a promising 48% yield (Table 1, entry 4). A wide array of functional groups proved to be compatible in the process, including free or protected anilines (Table 1, entries 6, 7), nitriles (Table 1, entries 8, 9), aldehydes and ketones (Table 1, entries 11, 12), and fluoro and trifluoromethyl groups (Table 1, entries 13, 14).
Importantly, nitro groups are tolerated as well, providing access to products that were not accessible with the Negishi protocol reported by Cardenas et al.2 Overall,meta-substituted electrophiles (Table 1, entries 3, 5 and 9) provide better yields. Protected amine substrates (entry 7) would appear to be preferred over the free amine counterparts (entry 6). The reaction was also tested on a larger scale (Table 1, entry 14), using a 1:1 trifluoroborate:electrophile ratio and lowering the catalyst loading to 1 mol %. This protocol provided access to the (4-fluorophenethyl)-1,3-dioxolane 2m in a modest 38% yield.
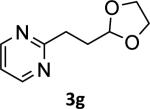
To explore the scope of the method further, a variety of diverse heteroaryl halides were tested using the same set of conditions. Pyridine, quinoline, furan, thiophene and indole systems reacted well, providing the desired products 3a-f with modest to excellent yields ranging from 44 to 93% (Table 2, entries 1-6). Although 2-chloropyrimidine did not react, yielding a mixture of the starting electrophile and the hydrolyzed trifluoroborate, the bromo analog afforded the expected dioxolanylethylpyrimidine 3g in 74% yield (Table 2, entry 7).
Table 2.
Suzuki-Miyaura Cross-Coupling of Various Heteroaryl Halides with Dioxolanylethyltrifluoroborate 1 a

| |||
|---|---|---|---|
| entry | electrophile | product | isolated yield (%) |
| 1 |

|

|
81 |
| 2 |

|

|
93 |
| 3 |

|

|
66 |
| 4 |

|

|
62 |
| 5 |

|

|
44 |
| 6 |

|

|
77 |
| 7 |

|
|
0 (Cl) 74 (Br) |
Reaction conditions: Heteroaryl halide (1.0 equiv), organotrifluoroborate (1.1 equiv), PdCl2AtaPhos2 (5 mol %), Cs2CO3 (3.0 equiv), toluene/H2O (4:1, [ ] = 0.25 M), 100 °C, 14 h
In conclusion, a new trifluoroborate has been synthesized and successfully cross-coupled with a wide array of aryl and heteroaryl chlorides or bromides, providing easy and straightforward access to a large variety of dioxolanylethylaryl compounds. The method complements previously reported methods leading to the same substructures. This new sp3-sp2 cross-coupling protocol, based on the reliability and versatility of organotrifluoroborates, allows the facile introduction of dioxolanylethyl moieties onto aryl and heteroaryl halides. After hydrolysis of the dioxolanyl moiety to the aldehyde group, further functionalisation is possible, including intramolecular transformations, allowing access to interesting polycyclic structures.11
Supplementary Material
Acknowledgments
This research was supported by the National Science Foundation (GOALI), and the NIGMS (R01 GM-081376). AllyChem is acknowledged for the generous donation of bis(pinacolato)diboron. Dr. Rakesh Kohli (University of Pennsylvania) is acknowledged for obtaining HRMS data.
Footnotes
Supporting Information Available
Experimental procedures, spectral characterization, and copies of 1H, 13C, 19F and 11B NMR spectra for all compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.a Wuts PGM, Greene TW. In: Protecting Groups in Organic Synthesis. 3rd ed. Greene TW, Wuts PGM, editors. John Wiley & Sons, Inc.; New York: 1999. [Google Scholar]; b Kocienski PJ. In: Protecting Groups. 3rd ed. Kocienski PJ, editor. Georg Thieme; Stuttgart: 2005. [Google Scholar]
- 2.a Melpolder JB, Heck RF. J. Org. Chem. 1976;41:265. [Google Scholar]; b Chalk AJ, Magennis SA. J. Org. Chem. 1976;41:273. [Google Scholar]; c Jeffery T. Tetrahedron Lett. 1991;32:2121. [Google Scholar]; d Colbon P, Ruan J, Purdie M, Mulholland K, Xia J. Org. Lett. 2011;13:5456. doi: 10.1021/ol202144z. [DOI] [PubMed] [Google Scholar]; e Pan H, Noel S, Pinel C, Djakovitch L. J. Organomet. Chem. 2008;693:2863. [Google Scholar]
- 3.Phapale VB, Guisán-Ceinos M, Buñuel E, Cárdenas DJ. Chem. Eur. J. 2009;15:12681. doi: 10.1002/chem.200901913. [DOI] [PubMed] [Google Scholar]
- 4.Ohmiya H, Wakabayashi K, Yorimitsu H, Oshima K. Tetrahedron. 2006;62:2207. [Google Scholar]
- 5.For example, it would allow the shortening of reaction pathways where the propanal moiety is directly protected after its installation. See: Quick MP, Fröhlich R, Wünsch B. Tetrahedron: Asymmetry. 2010;21:524.Padaw A, Zanka A, Cassidy MP, Harris JM. Tetrahedron. 2003;59:4939.
- 6.a Molander GA, Figueroa R. Aldrichimica Acta. 2005;38:49. [Google Scholar]; b Molander GA, Ellis N. Acc. Chem. Res. 2007;40:275. doi: 10.1021/ar050199q. [DOI] [PubMed] [Google Scholar]; c Stefani HA, Cella R, Adriano S. Tetrahedron. 2007;63:3623. [Google Scholar]; d Darses S, Genet J-P. Chem. Rev. 2008;108:288. doi: 10.1021/cr0509758. [DOI] [PubMed] [Google Scholar]; e Molander GA, Jean-Gérard L. In: Boronic Acids. Hall DG, editor. Vol. 2. Wiley-VCH; Weinheim: 2011. pp. 507–548. [Google Scholar]; f Lennox AJJ, Lloyd-Jones GC. Isr. J. Chem. 2010;50:664. [Google Scholar]
- 7.Brown HC, Chen JC. J. Org. Chem. 1981;46:3978. [Google Scholar]
- 8.Fleury-Brégeot N, Presset M, Beaumard F, Colombel V, Oehrlich D, Rombouts F, Molander GA. J. Org. Chem. 2012;77:10399. doi: 10.1021/jo3021665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang C-T, Zhang Z-Q, Tajuddin H, Wu C-C, Liang J, Liu J-H, Fu Y, Czyzewska M, Steel PG, Marder TB, Liu L. Angew. Chem., Int. Ed. 2012;51:528. doi: 10.1002/anie.201106299. For other metal catalyzed borylation procedures of alkyl halides see: Yi J, Liu J-H, Liang J, Dai J-J, Yang C-T, Fu Y, Liu L. Adv. Syn. Catal. 2012;354:1685.Ito H, Kubota K. Org. Lett. 2012;14:890. doi: 10.1021/ol203413w.Dudnik AS, Fu GC. J. Am. Chem. Soc. 2012;134:10693. doi: 10.1021/ja304068t.Joshi-Pangu A, Ma X, Diane M, Iqbal S, Kribs RJ, Huang R, Wang C-Y, Biscoe MR. J. Org. Chem. 2012;77:6629. doi: 10.1021/jo301156e.
- 10.a Guram AS, King AO, Allen JG, Wang X, Schenkel LB, Chan J, Bunel EE, Faul MM, Larsen RD, Martinelli MJ, Reider P. J. Org. Lett. 2006;8:1787. doi: 10.1021/ol060268g. [DOI] [PubMed] [Google Scholar]; b Colacot TJ, Carole WA, Neide BA, Harad A. Organometallics. 2008;27:5605. [Google Scholar]; c Krasovskiy A, Duplais C, Lipshutz BH. J. Am. Chem. Soc. 2009;131:15592. doi: 10.1021/ja906803t. [DOI] [PMC free article] [PubMed] [Google Scholar]; d He A, Falck JR. J. Am. Chem. Soc. 2010;132:2524. doi: 10.1021/ja910582n. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Krasovskiy A;, Lipshutz BH. Org. Lett. 2011;13:3822. doi: 10.1021/ol201307y. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Pudasaini B, Janesko BG. Organometallics. 2012;31:4610. [Google Scholar]; g Li H, Seechurn C, Colacot TJ. ACS Catal. 2012;2:1147. [Google Scholar]; h Pu X, Li H, Colacot TJ. J. Org. Chem. 2013;78:568. doi: 10.1021/jo302195y. [DOI] [PubMed] [Google Scholar]; i Colacot TJ. Encyclopedia of Reagents for Organic Synthesis www.mrw.interscience.wiley.com/erosDOI:10.1002/047084289X.rn009 68. [Google Scholar]
- 11.Bunce RA, Herron DM, Johnson LB, Kotturi SV. J. Org. Chem. 2001;66:2822. doi: 10.1021/jo001761n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


