Abstract
About 1 million per second is the number of white blood cells the adult human body produces. However, only a small fraction of them will survive as the majority is eliminated through a genetically controlled form of cell death referred to as apoptosis. This review places into perspective recent studies pertaining to the BCL-2 family of proteins as critical regulators of the development and function of the immune system, with particular attention on B cell and T cell biology. Here we discuss how elegant murine model systems have revealed the major contributions of the BCL-2 family in establishing an effective immune system. Moreover, we highlight some key regulatory pathways that influence the expression, function, and stability of individual BCL-2 family members, and discuss their role in immunity. From deadly methods to more gentle manners, the final portion of the review discusses the non-apoptotic functions of the BCL-2 family and how they pertain to the control of immunity.
Keywords: apoptosis, BCL-2 family, immunity, mitochondria
Introduction
Apoptosis, also known as type I programmed cell death, plays a critical role in a wide range of tissue functions and occurs during embryonic development to establish the architecture and function of tissues and organs.[1] Post-developmental apoptosis is also required for the maintenance of homeostasis, which involves among other things, the control of immunity. Importantly, two distinct signaling cascades engage apoptosis: the extrinsic pathway, which responds to the activation of the surface death receptors, and the intrinsic pathway, triggered by cellular stresses. While the BCL-2 (B-cell CLL/lymphoma-2) family mainly regulates the intrinsic pathway, evidence of regulatory crosstalk between the pathways has been described following death receptor ligation. A detailed overview of the extrinsic and intrinsic apoptotic pathways is presented in Figure 1.
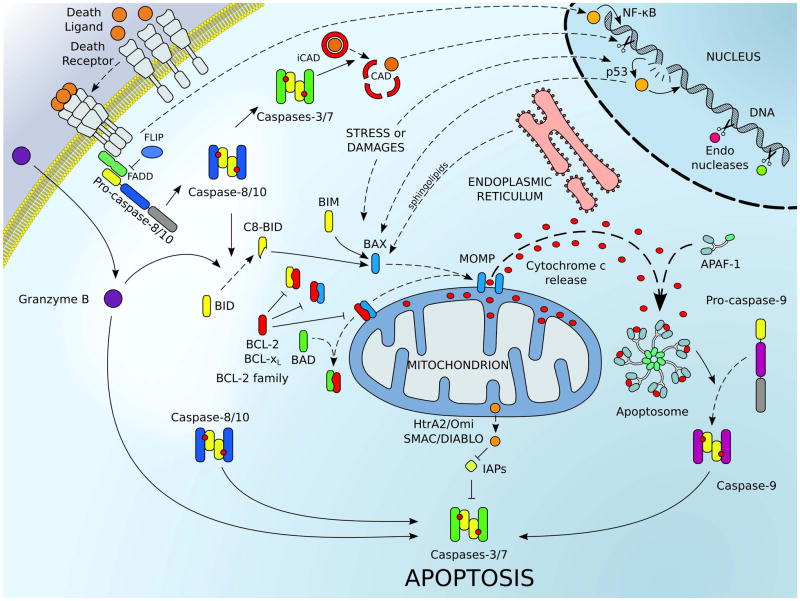
Figure 1.
The major signaling pathways leading to cellular apoptosis. The extrinsic pathway of apoptosis (upper left corner) is engaged by plasma membrane associated death receptors belonging to the TNF-R (tumor necrosis factor receptor) superfamily (e.g., TNF-R1/2, CD95/FAS). Upon engagement of these receptors by their respective ligands (e.g., TNF-α, FASL), conformation changes within the trimerized receptor/ligand complexes recruits adaptor proteins (e.g., FADD) and caspase-8 (and/or caspase-10 in human; represented in blue) to assemble a death inducing signaling complex, referred to as the DISC. Assembly of the DISC promotes caspase-8 activation; cleavage and activation of executioner caspases-3, -6 or -7 (represented in green), and cell death.[165] The intrinsic pathway (also called mitochondrial pathway of apoptosis) responds to cellular stresses like DNA damage (through p53), viral infection, protein misfolding, and oxidative-stress. These signals converge to activate the pro-apoptotic proteins of the BCL-2 family. Pro-apoptotic effectors (e.g., BAX, in blue) are able to target mitochondria and induce mitochondrial outer membrane permeabilization (MOMP); in which numerous pro-apoptotic proteins of the intermembrane space (e.g., cytochrome c, the second mitochondrial-derived activator of caspases SMAC/DIABLO, and HtrA2/Omi) are released into the cytosol. Direct activator BH3-only proteins (e.g., BID and BIM, in yellow) and other signals (e.g., p53 or sphingolipids from the ER) facilitate the activation of BAX and BAK. Sensitizers/de-repressors (e.g., BAD and Noxa, in green) interact with the anti-apoptotic members (e.g., BCL-2 and BCL-xL, in red) to lower the cell death threshold. Once in the cytosol, cytochrome c interacts with the adaptor protein apoptotic protease activating factor 1 (APAF-1) to form the apoptosome, which triggers the recruitment and the activation of the caspase-9 (represented in purple). Inhibitors of apoptosis (IAPs) inhibit caspase activation, and SMAC/DIABLO relieves this inhibition. Once initiator caspases (e.g., caspases-8 and -9) are activated, they trigger downstream activation of effector caspases-3 and -7, which cleave numerous cellular substrates including the inhibitor to the caspase activated DNAse (iCAD). Additionally, granzyme B, which is released by cytotoxic T cells, can also directly trigger effector caspase activation to promote cell death. The extrinsic pathway can also engage the intrinsic pathway via caspase-8-mediated cleavage of BID (in yellow) to amplify pro-apoptotic signaling.
Apoptosis plays a critical role in both the development of immune cells and the execution of an immune response. Throughout the development and maturation of immune cells, many progenitors are produced but not all are suitable candidates to participate in immunity, and apoptosis is required to fulfill a highly selective triage — we will discuss these pathways in the following sections. Furthermore, during an adaptive immune response, a rapid increase within an immune cell population is required to oppose an invading pathogen. Once the pathogen is recognized and eliminated, clearance of the expanded immune cell population occurs through apoptosis, leaving a few remaining cells to ensure durable future responses[2]. Indeed, we will discuss the mechanisms that control the clearance of these dynamic cellular populations.
T cells and B cells are lymphocytes, white blood cells that participate in the adaptive immune response. T cells mediate the cellular immune response (i.e., production of cytotoxic T cells, release of cytokines, antigen presentation, activation of macrophages and natural killer cells), and B cells mediate the humoral immune response (i.e., production of antibodies).[3] Although acting through different mechanisms, T cells and B cells share a common feature: they are able to specifically recognize antigens on pathogens or invading cells. This is achieved by the generation of specialized receptors located on the plasma membrane of the lymphocyte that bind to an antigen and trigger an immune response[4]. The generation of these receptors is a complex process, as the receptors must recognize exogenous antigens but not self-antigens.[5] Because of this requirement, the initial number of lymphocytes far exceeds the actual number of mature cells as unreactive and autoreactive cells are eliminated by apoptosis, as described in the following paragraph.
Looking closer at the T cell maturation process reveals the requirement for both apoptosis and survival mechanisms to modulate cell populations and fulfill their selection. In the early stages of thymocyte development (double negative stage, or CD4−CD8−), the presence of survival signals (e.g. the cytokine IL-7) is needed to prevent apoptosis. These signals control both the population of progenitors and T cell receptor (TCR) differentiation.[6] Later, thymocytes undergo the first step of selection in the thymus cortex by binding their TCR to major histocompatibility complex (MHC) molecules of the surrounding epithelial cells. Cells which fail to interact do not receive the signals required for their survival (e.g., the expression of anti-apoptotic proteins) and are therefore eliminated.[7] This process, termed positive selection, is necessary to ensure that T cells will be able to further participate in the immune response. In contrast, T cells bearing receptors which have too high affinity for MHC are dangerous for an organism as they have the potential to trigger the elimination of cells in healthy, functional tissues. Consequently, these highly reactive cells are also eliminated by apoptosis. This constitutes the negative selection process. Similarly, B cell development and maturation involves positive and negative selection; and the early B cell populations are also dependent on survival cytokines such as IL-7[8]. However, the development of B-cells continues in the bone marrow and the selection signals are received through a different class of receptors, the B cell receptors (BCR).[9]
Independent of the death signal, apoptotic cells are eliminated through regulated cellular disassembly and engulfment mechanisms that prevent inflammation-induced stress of the local environment. Common cellular hallmarks associated with apoptosis include caspase (cysteine-dependent aspartate-directed proteases) activation, and subsequent DNA cleavage, chromatin condensation, and cellular contraction.[10] In parallel, apoptotic cells externalize phosphatidylserine on the plasma membrane in a caspase-dependent manner, which contributes to an “eat me signal” for phagocytic cells to recognize and eliminate the stressed cell; therefore preserving tissue integrity from the dumping of cellular contents into the environment leading to inflammation.[11]
Next, we will discuss the BCL-2 family in the specific context of immune cell development and function. We will first provide a detailed introduction into the structures, functions, and mechanisms of the BCL-2 family members, and then bring into focus their control of the immune system, much of which has been identified by animal models.
The BCL-2 family
BCL-2, the founding member of the family, was identified in human B cell follicular lymphoma in which the chromosomal translocation t(14;18)(q32;q21) induces BCL-2 gene deregulation and over-expression.[12] Soon after its identification, the role of BCL-2 was linked to B cell tumorigenesis using a murine model of follicular B cell lymphoma. In this model, oncogenic c-myc is over-expressed using Eμ, the immunoglobulin heavy chain enhancer (Eμ-myc). While Eμ-myc animals develop lymphoma within a few months, the presence of transgenic bcl-2 markedly decreased tumor-free survival[13]. The mechanism behind this observation is that c-myc promotes both pro-survival and pro-death signals and the presence of transgenic bcl-2 allows for the silencing of the pro-death signal to promote rapid both transformation and apoptotic resistance of the precursor B cells. The Eμ-myc model has been used extensively in the BCL-2 family literature, and further studies indicate that the pro-apoptotic signal induced by Eμ-myc is the transcriptional induction of bim[14,15]. Interestingly, BCL-2 was the first oncogene identified that exerted its tumor promoting function by inhibiting pro-apoptotic signaling,[13] rather than directly promoting cellular proliferation[16].
After the initial studies with BCL-2, nearly all the other members of the BCL-2 family were identified and defined based on their shared function, conserved alpha (α) helical BCL-2 homology (BH) domain composition, and/or structural similarities (see Fig. 2). Based on these functional and structural homologies, the BCL-2 family has been subdivided into two functional groups: the anti-apoptotic and the pro-apoptotic proteins.
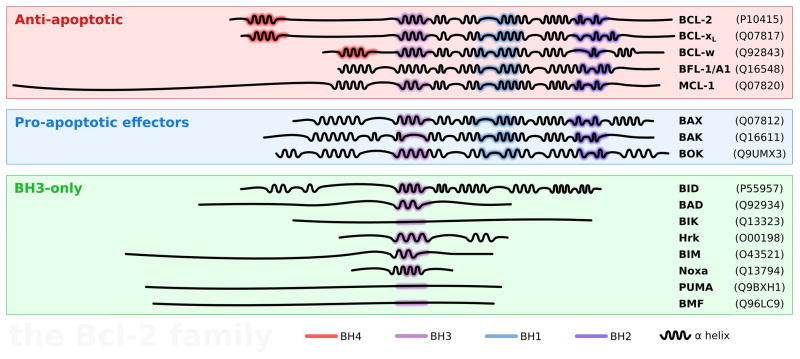
Figure 2.
The BCL-2 family: primary structure, domains, and hierarchy. The anti-apoptotic members of the BCL-2 family are represented in the red frame. The pro-apototic effectors and the BH3-only are in the blue and green frames, respectively. The BCL-2 homology (BH) domains and the secondary structure (α helices) are represented for each member and the UniProt[166] identifier is indicated between parentheses. Anti-apoptotics and pro-apototic effectors have up to four BH domains. The BH1–3 domains (in purple and blue) are spatially close to each other and form a hydrophobic groove which is important for the interaction with the members of the family. Most of the BH3-only proteins are intrinsically unstructured in solution, and acquire a secondary structure upon interaction with other members of the family.[167,168] An exception is BID, which is phylogenically[169] and structurally[169,170] most similar to the folded members than to the other BH3-only proteins and natively structured.
The anti-apoptotic group is comprised of (listed by date of identification): BCL-2, BCL-xL (BCL2L1 long isoform), BCL-w (BCL2L2), MCL-1 (myeloid cell leukemia-1, BCL2L3), and Bfl-1/A1 (BCL2L5). These members share up to four BH domains and a carboxyl terminal transmembrane domain (except for A1) and, in general, are localized to the outer mitochondrial membrane (OMM). Importantly, all the anti-apoptotic proteins share a structural fold that is comprised of five or six α helices that span the BH1–3 domains. This structural fold forms a hydrophobic groove that is primarily responsible for interacting with pro-apoptotic members of the BCL-2 family. In the majority of cells, over-expression of individual anti-apoptotic BCL-2 proteins suppresses pro-apoptotic signaling by directly sequestering pro-apoptotic BCL-2 members;[17] this is frequently associated with resistance to apoptosis, and can lead to immune disorders and tumorigenesis.[18]
The pro-apoptotic group is further subdivided in two classes of proteins based on their structure and associated function. Members of the first class, the pro-apoptotic effector proteins, e.g., BAX (BCL-2 Associated X protein) and BAK (BCL-2 Antagonist Killer 1), are comprised of three BH domains (BH1–3) and oligomerize into proteolipid pores within the OMM. The formation of these pores and the subsequent release of proteins from the mitochondrial intermembrane space[19,20] leads to mitochondrial outer membrane permeabilization (MOMP), which is considered to be the most significant biochemical event in the initiation of the mitochondrial pathway of apoptosis. The diverse signaling pathways that lead to MOMP, along with the extraordinary number of regulatory mechanisms, cannot be summarized here, but it should be kept in mind that BAK- and/or BAX-dependent MOMP is often defined as the “point of no return” in the cellular commitment to apoptosis.[21] In order for BAK and BAX to form pores within the OMM, BAK and BAX must undergo activation, which is often initiated by interactions with a subset of BH3-only proteins in a particular lipid environment.[22,23] In brief, activation is also associated with conformational rearrangements within monomeric species of BAK and BAX that cause stable association and insertion into the OMM, along with oligomerization at the OMM (while BOK ((BCL-2 ovarian killer)) is often considered to be an effector protein, there is minimal biochemical evidence directly implicating BOK in MOMP).[24]
The second class of pro-apoptotic BCL-2 family members is comprised of proteins sharing only the BH3 domain and is referred to as the BH3-only proteins. Examples of this class include: BAD (BCL-2 antagonist of cell death), BID (BH3 interacting-domain death agonist), BIK (BCL-2 interacting killer), BIM (BCL-2 interacting mediator of cell death), BMF (BCL-2 modifying factor), Hrk (Harakiri), Noxa, and PUMA (p53 up-regulated mediator of apoptosis). The BH3-only proteins have key functions to directly bind and regulate both the anti-apoptotic and pro-apoptotic effector proteins, and their mechanisms of action are summarized in Figure 3. Each BH3-only protein demonstrates a unique set of interactions within the BCL-2 family, and these interactions reveal each BH3-only protein’s contribution to BAK/BAK activation and apoptosis. A detailed description of these interactions and subsequent consequences are also presented in Figure 3.
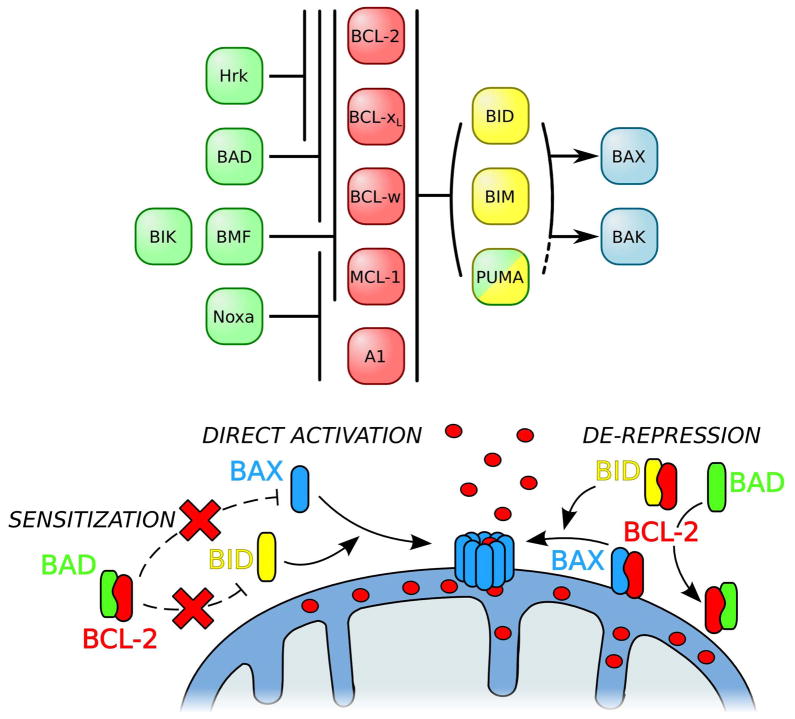
Figure 3.
Interactions and mechanisms of the BH3-only proteins. BH3-only proteins are subdivided in two groups. Sensitizers (in green) selectively interact with anti-apoptotic BCL-2 proteins (in red); as an example, BAD binds to BCL-2, BCL-xL, and BCL-w; whereas Noxa binds to MCL-1 and A1. By binding to the anti-apoptotic BCL-2 proteins, sensitizers prevent the sequestration of the pro-apoptotics and prime for BAX/BAK activation. Direct activators (in yellow) have a broad interaction range within the BCL-2 family. They are able to interact directly with and activate BAX and BAK to induce MOMP. On the other hand they can also counteract the anti-apoptotics. When pro-apoptotics are sequestered by anti-apoptotics, de-repressor BH3-onlys are able to disrupt these complexes. BID and BIM are well characterized direct activators BH3-only proteins[171,172], however the role of PUMA as direct activator remains controversial.[173–175] Lines with stops and arrows indicate an inhibition and an activation, respectively.
It is important to note that the majority of interactions within the BCL-2 family occur mainly through direct binding of the BH3 domain of one protein into the hydrophobic groove formed by the BH1–3 domains of the partner (e.g., the BIM BH3 domain binding into the groove of BCL-2).[25] These interactions represent the classical protein-protein interactions that establish the cellular apoptotic threshold through sensitization and de-repression mechanisms (Fig. 3).
Recently, a new set of interactions has been described that leads to BIM-mediated direct activation of BAX. A helical version of the BIM BH3 domain peptide has been shown to directly interact with the α1 and α2 helices of BAX, which, relative to the BAX hydrophobic groove, are located on the opposite side of the protein.[26] This observation revealed a major development in the study of the role of the BH3-only proteins, especially since BIM is considered one of the most important potent and critical among the BH3-only proteins; these features of BIM will be expanded upon in several upcoming sections.
The final class of interactions within the BCL-2 family is the homo-oligomerization of the effector proteins, which is required for pore formation leading to MOMP. Homo-oligomerization of BAX was shown to be dependent not on only on the BH3 domain but also on the BH1 domain,[27] and BAK homo-oligomerization similarly involves both the BH3 domain and a region close to the BH1 domain (i.e., α6 helix)[28,29]. Directed mutagenesis in the BH1–3 domains is often used as a tool to examine the importance of these interactions;[30,31] in hematopoietic malignancies, spontaneous mutations within the BAX BH1 and BH3 domains are described to promote apoptotic resistance.[32]
With the above summary of definitions, basic interactions, and apoptotic functions of the individual members of the BCL-2 family, we will now present recent mouse models that highlight a role for these proteins in the immune system. In particular, we will focus on the contribution of the BCL-2 proteins in the development and function of lymphocytes. To aid in summarizing this significant literature, Figure 4 recapitulates some of the major checkpoints regulated by the BCL-2 family. Towards the end of the discussion, we will also touch upon the non-apoptotic functions of the BCL-2 family in regulating immune responses.
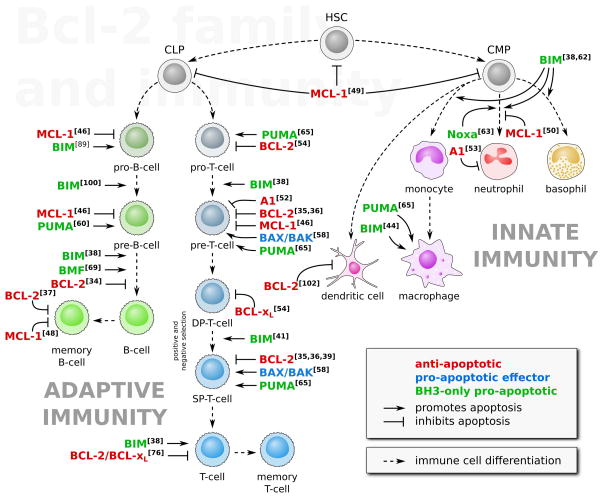
Figure 4.
The BCL-2 family in hematopoiesis. The proteins of the BCL-2 family contribute to numerous steps of both the innate and adaptive immune system development. The adaptive immune system originates from common lymphoid progenitors (CLP) and is comprised of B cells and T cells. The BCL-2 family is a critical regulator of most of the lymphocyte differentiation stages (e.g., the positive and negative selections, in which double positive (DP) cells are maturated in single positive (SP) cells, or the differentiation into memory lymphocytes). The innate immune system originates from common myeloid progenitors (CMP) and, similarly, differentiation of the myeloid cells is controlled by the BCL-2 family. Numbers in brackets refer to the bibliography.
What have we learned using mouse models?
BCL-2
The first member of the BCL-2 family to be intensively studied in murine models of immunity and cancer was BCL-2. Early observations reported that BCL-2 over-expression enhanced the survival of T cells[33], and when introduced into the Eμ-myc background (described previously), BCL-2 promoted enhanced survival of B cells.[34] Furthermore, transgenic bcl-2 caused an amplification of an IgM and IgG antibody-driven immune response and sensitized the mice to an autolymphoproliferative syndrome phenotype.[34]
Further observations describe that despite completing their development, bcl2−/− mice display several defects, including smaller size, polycystic kidneys, and smaller thymus, due to an increase in apoptosis. The bcl2−/− mice have an impaired development of the immune system and display an abnormal cellularity: the number of double positive (CD4+CD8+) and single positive (CD4+ or CD8+) T cells are reduced, whereas the number of double negative cells (CD4−CD8−) is increased.[35] This was confirmed by studying BCL-2 expression levels over time. As described in the introduction, the maturation of T cells requires the population to be dynamic in number due to proliferation, selection and apoptosis. It has been demonstrated that the expression levels of BCL-2 varies accordingly. As an example, BCL-2 is expressed in double negative cells and decreases as they mature into double positive cells; finally, T cells begin to express BCL-2 again when they reach the single positive stage.[36] Another critical role of BCL-2 was discovered in the maintenance of memory B cells. Memory B cells are produced following the interaction with an antigen and constitute a mechanism of adaptation which allows for a more efficient responses to reencounter with antigen. Mice over-expressing BCL-2 display greater secondary immune responses and extended survival of memory B cells.[37]
BIM
BIM plays a critical role during hematopoiesis and the in vivo function of this protein has received the most attention among the pro-apoptotic BCL-2 members. Notably, BIM participates in the elimination of auto-reactive lymphocytes.
Lymphoid cells derived from bim deficient mice display resistance to numerous inducers of the mitochondrial pathway of apoptosis (e.g., γ-irradiation and dexamethasone), which results in an increase of both lymphoid and myeloid cells in vivo.[38] As previously described, bcl-2−/ − mice show a dramatic decrease of T cells and B cells;[35,39] eliminating bim in the bcl-2−/ − background, however, restores the wild-type phenotype.[40] Furthermore, thymocytes derived from bim deficient animals have been to be resistant to pro-apoptotic signaling via TCR–CD3 stimulation during negative selection;[41] a similar negative selection phenotype was observed in B cells. These observations demonstrate the critical role for the pro-apoptotic function of BIM in the selection of non auto-reactive lymphocytes, and implicate BIM in the regulation of autoimmune diseases.[38] While the above data suggest that the pro-apoptotic function of BIM is required for numerous aspects of immune system development, the molecular mechanisms directly impacted by bim expression remained undefined. Is BIM required to cause the direct activation of BAK/BAX or is the inhibitory affect of BIM on numerous anti-apoptotic BCL-2 family members sufficient to promote proper T cell and B cell development and function?
To begin to address this question an elegant study made use of genetically altered mice in which the BH3 domain of BIM was replaced with the BH3 domains of other BH3-only members displaying different biochemical activities (i.e., BAD, Noxa, and PUMA). For example, the BIM BH3 domain sequence was genetically replaced with the BAD BH3 domain to generate a chimeric protein that was under the regulation of the endogenous bim promoter and subject to post-translational control of the BIM protein; this allowed for the normal expression and regulation of the BIMBAD chimera, yet afforded the specific evaluation of the BAD BH3 domain in the context of the BIM protein backbone within a cell. The results were intriguing: all the chimeric BIM mutants were less efficient in triggering cell death compared with wild-type BIM, resulting in increased lymphocyte (e.g., thymocytes and B cells) numbers and splenomegaly. The combination of BIMBAD and BIMNoxa alleles, which together target the entire anti-apoptotic BCL-2 repertoire, only partially restored the wild-type phenotype, demonstrating that the function of BIM in vivo extends beyond the inhibition of anti-apoptotic BCL-2 proteins (similar results were also obtained with the BIMPUMA mutant).[42] Interestingly, when expressed in an Eμ-myc background, the BIMBAD or BIMNoxa mutants accelerated the rate of lymphomagenesis either due to incomplete inhibition of the anti-apoptotic proteins or a failure to activate BAK/BAX. In contrast, the BIMPUMA animals displayed no additional susceptibility to the disease, suggesting that the various biochemical activities of BIM may be differentially utilized in developmental verses oncogenic signaling environments.[43]
The role of bim is not restricted to B cells and T cells in the immune system, as the mechanism by which macrophages trigger phagocytosis-induced apoptosis is also dependent on the pro-apoptotic function of BIM. Subsequent to the phogocytosis of pyrogenic bacteria (e.g., Escherichia coli), macrophages are capable of triggering their own death through apoptosis. This pathway, phagocytosis-induced cell death, allows for the clearance of the infected macrophages after the pathogens are removed from the site of infection. Data support that BIM function was up-regulated by bacteria-induced Toll-like receptor (e.g., through TLR2, TLR4, and TLR9) signaling involving the adaptor protein MyD88 and downstream dephosphorylation and activation of BIM. However, although BIM was required for phagocytosis-induced cell death, it is likely that additional signals are required, as the over-expression of bim alone was not sufficient to induce macrophage death.[44]
MCL-1
The requirement for MCL-1 function presents very early during vertebrate development, as mcl1 deletion is pre-implantation lethal.[45] In order to overcome this constraint, the mcl1 gene was flanked with loxP sites allowing for tissue-specific Cre-mediated deletion. The deletion of mcl1 in lymphocytes using Lck-Cre and CD19-Cre demonstrated that MCL-1 is required for the development of CD4+CD8+ T cells and pro-B cells, respectively, by directly antagonizing BIM function. Furthermore, IL-7 signaling during B cell development was shown to promote B cell survival by directly inducing the expression of mcl1.[46] Similar to bim deficiency but to a lesser extent, MCL-1 over-expression also affected the positive selection of thymocytes in several mice models (e.g., the HY-antigen-specific and ovalbumin-specific ((OT-I)) transgenic TCR mice).[47] In latter steps of B cells maturation, MCL-1 was shown to be required for the formation of germinal centers, specialized immune structures that allow rapid B cells proliferation upon T cell dependent immunization and, among others, the differentiation in memory B cells.[48]
A master regulatory role for MCL-1 function in immunity was also demonstrated in the maintenance of hematopoietic stem cells (HSC). As HSCs are the progenitors of most cells involved in immune responses, a decrease in the number and quality of HSCs directly impacts all subsequent lineages. The role of mcl1 in HSCs was shown by conditional deletion using Mx1-Cre, which caused massive apoptosis in HSC progenitors and, subsequently, resulted in a general decrease of derived progenitors (e.g., CLP and CMP, the common lymphoid and myeloid progenitors).[49]
Over the years studies continued to find an important role for mcl1 in protecting cells from development and stress-induced apoptosis. For example, in vivo murine data revealed a major role for mcl1 in both macrophage and neutrophil survival. Interestingly, the absence of mcl1 did not directly affect macrophage survival, due to compensatory over-expression of bcl-2 and bcl-x.[50] More recently, in a model of acute myeloid leukemia (AML), resistance to pharmacologic treatment was demonstrated to be dependent on mcl1 expression inhibiting BIM function; moreover, transformed murine myeloid cells were shown to be sensitive to ex vivo culture in the absence of MCL-1 but not of BCL-xL.[51] Likewise, MCL-1 is an important component for the resistance of cancer cells to ABT-737 treatments.[18]
A1 and BCL-xL
Where BCL-2 and MCL-1 regulate multiple steps of hematopoietic stem cell survival and lymphocyte maturation, the described roles for BCL-xL and A1 are more restricted.
A1 has been shown to be a target of pre-TCR signaling pathway during the transition from double-negative to single-positive T cell maturation; for example, after signaling through the pre-TCR, a1 transcription increases, which leads to decreased activation of caspase-3 in thymocytes. This mechanism correlates with the observed increase of a1 expression in a subset of T cell leukemia patients with deregulated pre-TCR signaling.[52] A1 has also been shown to be important for the maintenance of neutrophils, and a1 deficient mice have high rates of spontaneous neutrophil apoptosis.[53] Despite the fact that no pro-apoptotic BH3-only proteins have been identified as A1 antagonists in this study, one can assume that BIM and Noxa can play this role.
For BCL-xL, the loss of expression in thymocytes was shown to decrease the differentiation of CD4−CD8− to CD4+CD8+ cells, whereas sustained bcl-x expression was observed during the double-positive stage, and was inversely correlated with BCL-2 expression (as reported previously[36]), suggesting a specialized role for BCL-xL in this particular step of T cell differentiation.[54]
BAX and BAK
In the absence of both bak and bax embryos fail to thrive and the phenotype is embryonic lethality; few bak−/−bax−/− double knockout mice have been born.[55] Under stress conditions, BAX is often considered the major effector molecule within the mitochondrial pathway of apoptosis (see Renault and Manon for review[56]); however, bax is dispensable for mammalian development, as the bak−/−bax−/− phenotype can be rescued by bak (and vice versa)[57]. Despite having an outwardly normal phenotype, bax deficient mice display abnormalities in spermatogenesis and in the development of lymphocytes. Bax null mice have an overall higher number of B cells and T cells without a substantial change in the proportion of the different cells, and these cells demonstrated only a mild apoptotic resistance.[57] Double knockout bak−/−bax−/− animals display an increase (three to ten fold) in both myeloid and lymphoid cells compared with either single bak−/− or bax−/− knockouts or with wild-type mice. Similarly, bak−/−bax−/− thymocytes are resistant to γ-irradiation and etoposide-induced apoptosis in vitro (both classical inducers of the mitochondrial pathway).[55] A later study reported an important role of BAX and BAK in the selection process of single-positive and double-positive T cells. Interestingly, bak−/−bax−/− T cells displayed a memory phenotype in terms of surface receptor expression (similar as what is observed for T cells from an aged individual), suggesting a regulatory role of BAX and BAK in the turnover, fate, and/or maintenance of T lymphocytes.[58]
The third member of the effector class of proteins BOK has a secondary structure relatively close to that of BAX and BAK; however, little is known about its function. BOK is expressed in a wide range of tissues[24,59] and, to a lesser extent, in myeloid cells[24]. Bok deficient mice are viable with no major defects; and genetic removal of bak or bax does not appear to regulate bok expression. Most important, there is no marked difference in survival pathways or apoptotic sensitivity within the immune cell populations in bok deficient mice.[24]
Noxa and PUMA
Noxa and PUMA were originally identified as pro-apoptotic regulators of the p53 tumor suppressor pathway but their roles in cell death have recently expanded into several scenarios. Both proteins are transcriptionally induced following genotoxic stress and under conditions of oncogenic signaling, such as deregulated c-myc. Significant effort has been made to determine the biochemical and cellular functions of PUMA because it was demonstrated to play a role in the suppression of c-Myc driven lymphomagenesis. In the Eμ-myc model, the development of lymphoma is not highly penetrant, as over-expression of c-myc promotes BIM-mediated apoptosis.
Likewise, it appears that PUMA mediates the apoptotic response to c-Myc-induced BIM function, as the deletion of a single allele of puma was sufficient to induce pre-B cell and B cell lymphomagenesis; this study also demonstrated a contributory role of noxa.[60] However, the role of noxa may not be directly related to apoptosis, as noxa expression has been shown to regulate a form a cell death induced by oncogenes, independent of caspase function.[61] That said, a role for noxa in mediating neutrophil survival has been shown for spontaneous and cytokine withdrawal–induced apoptosis.[62] It appears that noxa functionally compensates for bim loss to promote apoptosis. Furthermore, the combined deletion of bim and noxa has been shown to result in a complete inhibition of apoptosis under these conditions.[63]
The role of puma in numerous immune cell subtypes has been expanded in recent years by further examination of the puma-null mouse model. PUMA was also shown to promote apoptosis of mast cells[64] in response to DNA damage and to regulate the number of macrophages[65]. In both cases, PUMA was shown to cooperate with BIM to induce cell death. For example, bim−/−puma−/− deficient animals, but not bim or puma single knockout animals, presented abnormal macrophage cellularity. A functional redundancy for the role of BIM and PUMA was demonstrated using primary myeloid cells in culture: the deletion of bim and puma resulted in strong apoptotic resistance upon cytokine withdrawal or ionizing radiation.[64] However, when in vivo depletion of the myeloid populations was performed by carboplatin or γ-irradiation treatment, a non-overlapping function of BIM and PUMA was revealed. Puma-deficient animals survived whereas most bim-deficient animals died within two weeks.[64] The role of PUMA to synergize with BIM in lymphocytes was shown in an additional model: while the deletion of puma alone did not alter the development of B cells and T cells, and puma deficient animals are indistinguishable from wild-type animals,[66] the combination of bim and puma deletion demonstrated a role of PUMA in the generation of T cells, in particular, during the steps of double-negative and single-positive cells. The bim−/−puma−/− knockout animals displayed an accumulation of immature thymocytes in vivo and a resistance to apoptosis in culture.[65] Recently, the cooperation between BIM and PUMA to eliminate auto-reactive T cells was confirmed in a mouse model of thymocyte deletion by peripheral neoantigens.[67] In this model, the combined deletion of bim and puma, but not in either single knockout, impaired the elimination of autoreactive T cells and led to autoimmune reactions in various organs.[67]
Recently, the fate of self-reactive T cells was examined in animals deprived of either BH3-only proteins (i.e., BIM or PUMA) or effector members (i.e., BAX and BAK). The deletion of pro-apoptotic members of the BCL-2 family constitutes a model for auto-immunity by allowing the self-reactive T cells to escape thymic deletion. Animals deleted of bim, puma, or bax and bak presented with high levels of cells with self-reactive TCRs. However self-reactive TCR positive cells also demonstrated an enhanced expression of Foxp3+ (Forkhead box p3), a gene involved in self-tolerance, and a decreased response to TCR engagement. Thus, it appears that over-expression of Foxp3+ could be a mechanism of adaptation to limit the aggressiveness of self-reactive T cells escaping the control exerted by the BCL-2 family.[68]
BMF
Although dispensable for embryonic development, the BH3-only protein BMF has been shown to be involved in the maturation of B cells but has little affect on the normal development of T cells.[69] The most dramatic phenotype in bmf−/− mice is a significant increase of pre-B cells and mature B cells. Likewise, recent work showed that the combined loss of bad and bmf has a limited impact on the number of B cells and on spontaneous apoptosis in thymocytes deprived of glucose. This phenotype may be reconciled as due to BMF, which is suggested to only establish the apoptotic threshold in cells; for example, BMF has been shown to cooperate with BIM in glucocorticoid-induced apoptosis in acute lymphoblastic leukemias.[70] This cooperation suggests a role for BMF that is similar to the one of BAD, which is a de-repressor/sensitizer BH3-only function. Despite the bmf−/− animals having no developmental phenotypes in T cells, the animals were shown to be more susceptible to lymphomagenesis upon γ-irradiation.[69] Furthermore, analysis of bad−/−bmf−/− double knockouts revealed an increased susceptibility for spontaneous tumors (non-Hodgkin lymphomas, carcinomas, and lymphomas) and reduced lifespan.[71] This last example recalls, as for BOK, that not all the members of the BCL-2 family have an absolute role in all apoptotic events, and that original mechanisms and functions may remain to be discovered.
As described throughout the previous section, members of the BCL-2 family control both the development and function of numerous cell types within the immune system, as well as the majority of control centers on setting the cellular threshold leading to apoptosis. The next part of the discussion focuses on how transcriptional and post-transcriptional events regulate the function of individual proteins with the BCL-2 family, and how these events directly affect apoptosis and subsequently, the immune system.
Keeping the BCL-2 family proteins under control
In the early years of the BCL-2 family literature the majority of efforts focused on understanding how the protein–protein interactions within the family directly affected the balance between life and death. More recently, the mechanisms of regulation within the BCL-2 family have greatly broadened to include numerous points of transcriptional and post-translational control that directly impact on immune function, including transcriptional regulation via transcription factors, alternative splicing, miRNAs, and mRNA stability. In addition, several post-translational effects directly dictate protein-protein interactions, MOMP, and cellular responses.
Transcription factors
Numerous transcription factors regulate the expression of genes encoding the BCL-2 family, and we will highlight just a few recent examples. Several studies show that NF-κB promotes cell survival and plays an important role in the development of lymphocytes[72] and immune response[73]. This may be explained by NF-κB–dependent up-regulation of bcl-2, bcl-x, and a1, along with decreasing expression of bax.[74] The transcriptional regulation of multiple BCL-2 family members by NF-κB has been shown to promote the survival of mature B cells, pre-T cells,[75] or T cells[76] upon TCR engagement.
The transcriptional activity of p53 also regulates the expression of numerous BCL-2 family members. In multiple model systems, the pro-apoptotic activity of p53 has been shown to be related to the transcriptional increases in bax,[77] noxa,[78] puma,[79] bik,[80] and bid[81] expression, along with repression of anti-apoptotic target genes like bcl-2[82]. In addition to direct transcriptional regulation of apoptosis, the p53 protein has been shown to regulate the BCL-2 family proteins in a transcription-independent manner by direct interaction with BAX[83], BAK[84], BCL-2, and BCL-xL[85]. As an example of the control of p53 on the BCL-2 family in immunity, a recent study has shown that p53 is a critical checkpoint in the development of thymocytes. In mice deficient for Rpl22, a ribosomal protein required for the transition of CD4−CD8− T cells to CD4+CD8+ stage, p53 is stabilized and induces puma, bim, bax, and noxa and thereby triggers premature apoptosis and blocks the development of CD4+CD8+ T cells. The death of CD4+CD8+ T cells was shown to be dependent on bim and puma, as gene silencing of the latter was sufficient to inhibit apoptosis leading to the restoration of CD4+CD8+ T cells development.[86]
As we discussed earlier, deregulated c-myc can promote apoptosis, and it is suggested that c-Myc leads to the expression of numerous pro-apoptotic genes, including bax[87] and bim,[14] while also decreasing the apoptotic threshold by negatively regulating the expression of bcl-2 and bcl-x[88]. Furthermore, the concomitant activation of c-myc with transgenic bcl-2 (thereby preventing the suppression of BCL-2 function) is sufficient to promote lymphomagenesis in the Eμ-myc mice.[88] Likewise, the most critical pro-apoptotic protein responsible for the suppression of B-cell lymphomagenesis is bim, and numerous studies provide evidence that c-Myc induced bim expression acts as a tumor suppressor and protects Eμ-myc mice against lymphomagenesis.[14] The transcription of bim was also shown to be up-regulated by the forkhead transcription factor FKHR-L1, leading to apoptosis following cytokine withdrawal in the BaF3 pro-B cell model.[89] Recently, the Zinc-finger protein ASCIZ (Ataxia telangiectasia mutated substrate Chk2-interacting Zn2+ finger protein) was shown to regulate the survival of B cells by the up-regulation of dynlll1 (dynein light chain 1), a protein of the microtubules cytoskeleton. As this will be described further, BIM can associate with microtubules, thus higher levels of DYNLL1 may contribute to the active sequestration of BIM and thus to the inhibition of apoptosis.[90] Finally, the regulation of mcl1 expression by numerous pathways including JAK/STAT, HIF-1α and NF-κB was also demonstrated to influence the lifespan of multiple cell types including neutrophils[91] and B cells[92]. As we will continue to explore, the BCL-2 family is regulated by multiple signaling pathways and transcriptional regulators, so the fate of individual cells is often determined by the overall cellular and signaling environment rather than one particular event.
Alternative splicing
In terms of pro-apoptotic BH3-only protein signaling, the alternative spliced forms of BIM have received the most attention. The presence of multiple BIM isoforms in cells is a commonly observed phenotype, and early data in the BIM literature identified that the different BIM isoforms demonstrate various potencies in pro-apoptotic function. As an example, the shortest isoform of BIM, BIMS, is expressed preferentially upon IL-3 withdrawal and was more potent to mediate cell death.[93] Nineteen BIM isoforms have been described and are organized in six groups: BIMS, BIML, BIMEL, BIMD, BIMDd and BIMEDd.[94,95] The alternative splicing of bim mRNA is believed to be a critical mechanism of its regulation, as it determines the presence of a dynein-binding domain in the protein and thus its interaction with the microtubules (N.B., this interaction is also dependant on BIM phosphorylation). Because the short isoforms of BIM are lacking this regulatory domain and are not actively sequestered, they appear to be more efficient apoptotic inducers than the longer isoforms.
As suggested earlier, other members of the BCL-2 family demonstrate alternative splicing, such as BCL-xL/BCL-xS,[96] BAX-α/BAX-β,[97] and PUMA-α/PUMA-β.[79] While certain isoforms are biochemically classified as anti-apoptotic and pro-apoptotic—which is the case for BCL-xL and BCL-xS, respectively—the isoforms of BAX, PUMA, and BID appear to be functionally equivalent, although they demonstrate potentially unique expression and post-translational mechanisms that may contribute to altered immune response; but this is still not well explored.
mRNA regulation
Nucleolin, a member of the ribonucleoprotein-containing family has been identified as a binding partner of bcl-2 mRNA, promoting its stabilization and BCL-2 protein expression. Nucleolin and BCL-2 upregulation (mRNA and protein) have been hypothesized to be a critical feature in chronic lymphocytic leukemia (CLL); silencing of nucleolin decreased bcl-2 mRNA stability and subsequent BCL-2 protein levels leads to enhanced pro-apoptotic BCL-2 family function, which is likely to be BIM-dependent.[98] Several miRNAs have been implicated in the regulation of the BCL-2 family. MiR-17~92 regulates the development of pro-B cells to pre-B cells by targeting bim mRNA and promoting cell survival (e.g., during the development of pro-B cells to pre-B cells). MiR-17~92 deficient mice possess significantly higher BIM levels that result lethality of the developing B cell repertoire and of the heart and lungs.[99] Bcl-2 mRNA has also been described as the target of two miRNAs, miR-15 and miR-16, the down-regulation of which has been hypothesized to play a role in the development of CLL.[100] This hypothesis was supported by high-throughput profiling of genes involved in CLL, which revealed down-regulation of miR-15 and miR-16, and subsequent upregulation of bcl-2 and mcl-1.[101] Another microRNA, miR-21, often implicated in cancer, is also described to be upregulated following the Bacillus Calmette-Guerin vaccine for tuberculosis, which is associated with bcl-2 mRNA decreases and apoptosis in dendritic cells.[102]
An extensive literature describes the role of miRNAs in immune system development and function[103] and in the regulation of the BCL-2 family.[104] However, not much is currently understood about how these pathways overlap.
Phosphorylation and ubiquitinylation
Phosphorylation and ubiquitinylation are the most commonly described post-translational modification pathways affecting the BCL-2 family. The anti-apoptotic protein BCL-2 possesses multiple residues within a flexible loop region between the BH4 and BH3 domains that are capable of undergoing phosphorylation. One consequence of phosphorylation is enhanced anti-apoptotic function, as demonstrated by the phosphorylation of serine 70 by ERK1/2, which is described to promote BCL-2/BAX interactions in myeloid cells during IL-3 signaling.[105] Multisite phosphorylation of BCL-2 has also been described (e.g., serine 70, serine 87, and threonine 69) in human T cells and in murine B cells that was suggested to weaken BCL-2’s anti-apoptotic function.[106] However, contrasting results were obtained using hematopoietic NSF/N1.H7 cells in which BCL-2 phosphorylation on the same sites resulted in sustained anti-apoptotic activity.[107] The phosphorylation of BCL-2 on serine 87 by ERK1/2 has also been implicated in BCL-2 stability, as mutation of this site resulted in ubiquitin-dependant proteasomal degradation.[108,109] More recently, the role of BCL-2 phosphorylation has also been shown to be required for the hematopoietic differentiation of a murine embryonic stem cell line.[110]
As described previously, the BH3-only protein BIM has numerous critical roles in lymphocytes maturation and is functionally regulated by phosphorylation. Notably, the phosphorylation of serine 69 by ERK1/2 has been well characterized as a trigger of BIMEL ubiquitinylation and subsequent degradation by the proteasome.[111,112] This mechanism was implicated in the survival of anti-CD3/CD28 stimulated T cells and anti-IgM stimulated B cells.[113] Although this feature of cell survival through BIM phosphorylation appears to be important in the normal maintenance of lymphocytes, a recent clinical study implicates BIM phosphorylation in the progression of CLL.[114]
BAD is an additional example of a BH3-only protein that undergoes extensive regulation by phosphorylation. Murine BAD possesses three serines at positions 112, 136, and 155, and their phosphorylation leads to the inhibition of BAD activity. Serine 155 is located within the BH3 domain and its phosphorylation by cAMP-dependant kinase,[115] PKA,[116,117] or RSK1[117] promotes an association with BCL-xL. Serines 112 and 136 are located on the opposite face of the protein; upon phosphorylation of serine 112 by RSK1[117] and PKA[118] or of serine 136 by Akt,[119] BAD was shown to associate with 14-3-3, leading to a decrease in BAD activity.
Other post-translational modifications
Cleavage is another common mechanism of regulating BCL-2 family function. The canonical example is the 22 kDa cytosolic BH3-only protein BID. When a cell receives a death signal through the TNF receptors or CD95/FAS, caspase-8 is activated and cleaves BID at aspartate 60.[120] After cleavage, the amino terminal fragment (p7) remains associated with the carboxyl terminal fragment (p15)[121] until the protein interacts with the mitochondrial outer membrane and the complex dissociates.[122] The removal of the p7 fragment renders the BH3 domain of BID accessible to interact with BAK and BAX, leading to their activation and apoptosis.
The cleavage and activation of BID constitutes a crossroad of different cell death pathways, as other proteases are also able to cleave BID in the same region (Fig. 1). Calpains and cathepsins, lysosomal proteases involved in necrosis and apoptosis.[123] target the glycine 70[124] and arginine 65 and arginine 71,[125,126] respectively. Granzyme B, an effector protease released by cytotoxic T cells and natural killer cells, targets aspartate 75.[127] One further post-translational modification of BID, N-myristoylation, has been proposed to regulate the cellular localization of BID. N-Myristoylation is the addition of a myristoyl group on the N-terminal glycine of a protein, and if often implicated in targeting proteins to membranes. Only the caspase-8–cleaved form of BID, with an exposed glycine 61, can undergo N-myristoylation, and as expected, this modification results in mitochondrial targeting of p15-BID.[121] Importantly, however, the mitochondrial localization of BID should not be considered a pro-apoptotic signal that is sufficient to kill a cell. In order for cell death to proceed, membrane-targeted BID must still directly activate BAK and/or BAX to promote MOMP and apoptosis.
In B cell lymphomas, MCL-1 over-expression is often associated with increased risk of cancer development and chemoresistance; experimentally, this has been reproduced using the Eμ-myc model.[128] A dependency for MCL-1 expression in cells derived from B cell lymphoma has been demonstrated with antisense oligonucleotides targeting mcl-1 mRNA, which leads to decreased protein expression–induced spontaneous apoptotis.[129] In parallel studies, cisplatin treatment of MCL-1–dependent B cell lymphomas was shown to cause caspase-dependent cleavage of MCL-1. Mechanistically, MCL-1 cleavage resulted in the loss of its pro-apoptotic function, and the authors suggested that cleavage converted MCL-1 into a pro-apoptotic molecule, which was sufficient to restore apoptosis.[129] The reported MCL-1 cleavage was dependent on caspase-9 and -3 activation, which requires prerequisite engagement of the mitochondrial pathway of apoptosis, and thus must not be seen as an initiating event; it may be speculated that this mechanism would serve as amplification step within the pro-apoptotic pathway. Similarly, caspase-dependent conversion into a pro-apoptotic form was reported for BCL-2 and BCL-xL.[130–133]
Finally, the enzyme-independent post-translational modification deamidation, which relies on a relatively slow spontaneous reaction dependent on the amino acid environment, will be discussed. Asparagine and glutamine undergo deamidation, and it is believed that this event functions as a molecular timer to change a protein’s function over long periods of time.[134] BCL-xL is described to be regulated by deamidation in several contexts; indeed, BCL-xL possesses a large unstructured loop between the α1 and α2 helices (see Fig. 2), which contains two asparagines. The deamidation of these asparagines (i.e., the transformation into aspartic acid) is thought to inhibit the anti-apoptotic function of BCL-xL by decreasing its affinity to the pro-apoptotic members of the BCL-2 family, e.g., BAX,[135] BIM, and PUMA.[136] This modification has been shown to occur in response to cisplatin or γ-irradiation induced DNA damage in fibroblasts.[135] Because of this resistance, deamidation of BCL-xL and maintenance of its anti-apoptotic function could be an important factor leading to resistance to apoptosis and the initiation of transformation. As an example, thymocytes isolated from a mouse model of T cell lymphoma mediated by the up-regulation of the p56lck tyrosine kinase on a CD45−/− background, present an impaired response to DNA damage due to sustained depletion of BCL-xL deamidation.[137] A very similar resistance mechanism, driven by a loss in BCL-xL deamidation, was observed in primary cells isolated from patients suffering from BCR-ABL and JAK2-dependent chronic myeloid leukemia.[138]
Is the role of the BCL-2 family restricted to apoptosis?
Recent literature has presented increasing evidence for additional, non-apoptotic roles for the BCL-2 family of proteins. In the final part of this review, we discuss how the BCL-2 family of proteins are involved in these processes and their consequences on the regulation of immunity.
Inflammatory responses
For many years BCL-2 and BCL-xL have been suggested to influence inflammation, as an example, BCL-2 over-expression in immortalized macrophages leads to the inhibition of the pro-inflammatory cytokine IL-1β (which mediates the response to infection) maturation and secretion.[139] Whether or not BCL-2 plays a direct role by actively connecting apoptosis to inflammation is still unclear, as BCL-2 binding partners may also regulate these processes. However, an additional study reported a direct role for BCL-2 and BCL-xL on the inhibition of NLRP1, a protein involved in the activation of the pro-inflammatory caspase-1 (i.e, IL-1β converting enzyme, which catalyses the activation of IL-1β). Mechanistically, BCL-2 and BCL-xL were shown to bind NLRP1 through their unstructured loops located between the BH4 and BH3 domains (e.g., residues 71–80 in BCL-2), resulting in the inhibition of ATP binding to NLRP1 and further inhibition of oligomerization-dependent caspase-1 activation.[140]
As mentioned earlier, BCL-2 binding partners may also regulate inflammation, and a recent genome-wide screen for genes regulating NOD1 (nucleotide-binding and oligomerization domain-containing protein 1) signaling led to the identification of BID as critical regulator of inflammation. In an apoptosis-independent manner, BID was shown to participate in NOD2 inflammatory responses by interacting with NOD and the IKK complex in macrophages. BID was further demonstrated to be a required component of the NOD signalosome, as siRNA-induced depletion of BID impaired NF-κB and ERK signaling and downstream production of IL-6.[141]
Autophagy
The BCL-2 family of proteins also regulates autophagy, a catabolic pathway involved in the recycling cellular organelles and the degradation of long-lived proteins; importantly, this process can also be triggered during cellular stress and is a contributor to tumorigenesis.[142,143] This function of the BCL-2 family proteins is also thought to be integral part of the immune system regulation via autophagy, as it plays an important role in intracellular pathogen sensing and lymphocyte development and homeostasis (see Kuballa et al.[144]).
It has been speculated that the BCL-2 family directly influences the autophagic machinery through Beclin-1, a BH3 domain-containing protein. Beclin-1 functions to regulate Vps34, a class III PI-3 kinase involved in the formation of autophagosomes, which function to engulf organelles and proteins destined for degradation as well as intracellular viruses. Curiously, viruses that antagonize autophagy, e.g., hepatitis B, herpes simplex, and influenza A, often directly target Beclin-1 as resistance mechanism.[144] BCL-2[145,146] and BCL-xL[147] have been shown to interact with and sequester Beclin-1, supposedly inhibiting Beclin-1/Vsp34 interaction, although this hypothesis has been debated.[148] In accordance with a direct role of BCL-2 and BCL-xL on Beclin-1 function, BAD and the BAD-BH3 mimetic molecule ABT-737 have been demonstrated to compete with Beclin-1 for the binding to BCL-2 and BCL-xL to induce autophagic responses.[148] More recently, a different role for BCL-2 and BCL-xL has been reported, showing that the two proteins can stimulate autophagy independently of Beclin-1 and, presumably, through another major regulator of autophagy, Atg7.[149]
Mitochondrial dynamics
Mitochondria are dynamic organelles that undergo fusion and fission, which leads to efficient energy production and maintenance of the mitochondrial genome. Mitochondrial fusion is regulated by large dynamin-like GTPases of the outer (MFN1 and MFN2) and inner mitochondrial membranes (OPA1), and by the fission proteins, DRP-1 and Fis1.[150] Given the localization of several BCL-2 members in the mitochondrial outer membrane, an idea emerged years ago that these proteins could also regulate mitochondrial dynamics (see Martinou and Youle[151]). In general, the anti-apoptotic proteins BCL-2 and BCL-xL seem to regulate mitochondrial fission, yet BAX and BAK seem also to be essential to mitochondrial fusion in healthy cells, potentially by inducing the assembly and activation of MFN-2.[152–154] Similar results were obtained with the anti-apoptotic protein BCL-xL, which can interact with MFN-2 and promote mitochondrial fusion.[155] Interestingly, the physiological role of the BCL-2 family on mitochondrial dynamics has been highlighted in several studies using lymphocytes. As an example, the migration of lymphocytes to inflamed tissues requires that these cells become polarized, which has been reported to be dependent on mitochondrial fission. Modulation of the fission/fusion balance by over-expressing or silencing MFN1, OPA1, or DRP-1 was demonstrated to be sufficient to abolish lymphocytes polarization and their subsequent migration.[156] Furthermore, the pro-fusion GTPase DRP-1 has also been implicated in the activation of T cells, as a critical component of T cell receptor assembly.[157]
Metabolism
The role of metabolism in immune responses is beginning to emerge in numerous systems, including T cell activation and B cell lymphomas.[158,159] However, the mechanistic interplay between the BCL-2 family, metabolism, and immunity is still in its infancy. MCL-1 is a master regulator or HSC development and function, and recent studies have demonstrated that MCL-1 may directly influence cellular ATP generation. In many cell types MCL-1 protein is found as both large and small isoforms; the functional differences between these isoforms remained unknown for years. A series of elegant studies have now revealed that the shorter form of MCL-1 can localize to the mitochondrial matrix in a TOM/TIM- (translocase of the outer/inner membrane) dependent manner, leading to enhanced mitochondrial fusion, appropriate structure of inner mitochondrial membrane, and proper assembly of the F1–Fo ATP synthase.[160] Similarly, BCL-2 has been suggested to be a regulator of mitochondrial respiration through an interaction with multiple cytochrome c oxidase subunits. Functionally, these interactions lead to increased mitochondrial respiration in untreated conditions, and decreased cellular respiration during oxidative stress.[161] Additional studies have also reported a protective role for BCL-2 against oxidative stress[162] and cytosolic acidification during ischemia-reperfusion, which may also be due metabolic influences.[163] Furthermore, the BH3-only protein BAD is probably the best-characterized BCL-2 family member regarding its regulation of metabolism, yet BAD’s influence on immunity remains unknown. Thus far, BAD has been shown to significantly influence glucose metabolism; the phenotype of bad deficient mice contains several metabolic deficiencies due to an impairment of glucose-stimulated insulin secretion. The effect of BAD on glucose metabolism was identified to be dependent upon a direct interaction with glucokinase via the BAD BH3 domain, leading to glucokinase activation.[164]
Summary
The members of the BCL-2 family are critical regulators of immune system development and function. Throughout our discussion, we focused on the apoptotic role for these proteins, but the influence of this family of proteins on numerous cellular pathways, including autophagy, metabolism, organelle function, and signal transduction greatly broadens our interest and view on the significance of these proteins. Furthermore, the marked regulation of BCL-2 family function by numerous transcriptional and post-translational pathways suggests that the total cellular signaling environment must be integrated to better understand the influence of BCL-2 proteins on all the pathways in the context of the immune system. Indeed, continuing effort to understand the BCL-2 family promises to further insights into the complexity of life and death cellular fates.
Acknowledgments
We would like to thank everyone in the Chipuk Laboratory for their assistance and support. This work was supported by: NIH CA157740 (to J.E.C.), the JJR Foundation (to J.E.C.), the William A. Spivak Fund (to J.E.C.), and the Fridolin Charitable Trust (to J.E.C.). This work was also supported in part by a Research Grant 5-FY11-74 from the March of Dimes Foundation (to J.E.C.).
Bibliography
- 1.Meier P, Finch A, Evan G. Apoptosis in development. Nature. 2000;407(6805):796–801. doi: 10.1038/35037734. [DOI] [PubMed] [Google Scholar]
- 2.Sprent J, Tough DF. T cell death and memory. Science. 2001;293(5528):245–8. doi: 10.1126/science.1062416. [DOI] [PubMed] [Google Scholar]
- 3.Murphy K. Janeway’s Immunobiology (Immunobiology: The Immune System) 8. Garland Science; 2011. [Google Scholar]
- 4.Huse M. The T-cell-receptor signaling network. J Cell Sci. 2009;122(Pt 9):1269–73. doi: 10.1242/jcs.042762. [DOI] [PubMed] [Google Scholar]
- 5.Zemlin M, Schelonka RL, Bauer K, Schroeder HW., Jr Regulation and chance in the ontogeny of B and T cell antigen receptor repertoires. Immunol Res. 2002;26(1–3):265–78. doi: 10.1385/IR:26:1-3:265. [DOI] [PubMed] [Google Scholar]
- 6.Opferman JT. Life and death during hematopoietic differentiation. Curr Opin Immunol. 2007;19(5):497–502. doi: 10.1016/j.coi.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez JB, Newton RH, Walsh CM. Life and death in the thymus – cell death signaling during T cell development. Curr Opin Cell Biol. 2010;22(6):865–71. doi: 10.1016/j.ceb.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corfe SA, Paige CJ. The many roles of IL-7 in B cell development; mediator of survival, proliferation and differentiation. Semin Immunol. 2012;24(3):198–208. doi: 10.1016/j.smim.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 10.Häcker G. The morphology of apoptosis. Cell Tissue Res. 2000;301(1):5–17. doi: 10.1007/s004410000193. [DOI] [PubMed] [Google Scholar]
- 11.Fadok VA. Clearance: the last and often forgotten stage of apoptosis. J Mammary Gland Biol Neoplasia. 1999;4(2):203–11. doi: 10.1023/a:1011384009787. [DOI] [PubMed] [Google Scholar]
- 12.Tsujimoto Y, Cossman J, Jaffe E, Croce CM. Involvement of the bcl-2 gene in human follicular lymphoma. Science. 1985;228(4706):1440–3. doi: 10.1126/science.3874430. [DOI] [PubMed] [Google Scholar]
- 13.Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335(6189):440–2. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 14.Egle A, Harris AW, Bouillet P, Cory S. Bim is a suppressor of Myc-induced mouse B cell leukemia. Proc Natl Acad Sci US A. 2004;101(16):6164–9. doi: 10.1073/pnas.0401471101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hemann MT, Bric A, Teruya-Feldstein J, Herbst A, Nilsson JA, Cordon-Cardo C, et al. Evasion of the p53 tumour surveillance network by tumour-derived MYC mutants. Nature. 2005;436(7052):807–11. doi: 10.1038/nature03845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hipfner DR, Cohen SM. Connecting proliferation and apoptosis in development and disease. Nature Reviews Molecular Cell Biology. 2004;5(10):805–15. doi: 10.1038/nrm1491. [DOI] [PubMed] [Google Scholar]
- 17.Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, et al. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8(3):705–11. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 18.Certo M, Moore VDG, Nishino M, Wei G, Korsmeyer S, Armstrong SA, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9(5):351–65. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86(1):147–57. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 20.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275(5303):1132–6. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 21.Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18(4):157–64. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, et al. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111(3):331–42. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- 23.Chipuk JE, McStay GP, Bharti A, Kuwana T, Clarke CJ, Siskind LJ, et al. Sphingolipid Metabolism Cooperates with BAK and BAX to Promote the Mitochondrial Pathway of Apoptosis. Cell. 2012;148(5):988–1000. doi: 10.1016/j.cell.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ke F, Voss A, Kerr JB, O’Reilly LA, Tai L, Echeverry N, et al. BCL-2 family member BOK is widely expressed but its loss has only minimal impact in mice. Cell Death Differ. 2012;19(6):915–25. doi: 10.1038/cdd.2011.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, et al. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science. 1997;275(5302):983–6. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 26.Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG, et al. BAX activation is initiated at a novel interaction site. Nature. 2008;455(7216):1076–81. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.George NM, Evans JJD, Luo X. A three-helix homo-oligomerization domain containing BH3 and BH1 is responsible for the apoptotic activity of Bax. Genes Dev. 2007;21(15):1937–48. doi: 10.1101/gad.1553607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dewson G, Kratina T, Sim HW, Puthalakath H, Adams JM, Colman PM, et al. To trigger apoptosis, Bak exposes its BH3 domain and homodimerizes via BH3:groove interactions. Mol Cell. 2008;30(3):369–80. doi: 10.1016/j.molcel.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Dewson G, Kratina T, Czabotar P, Day CL, Adams JM, Kluck RM. Bak activation for apoptosis involves oligomerization of dimers via their alpha6 helices. Mol Cell. 2009;36(4):696–703. doi: 10.1016/j.molcel.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Yin XM, Oltvai ZN, Korsmeyer SJ. BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature. 1994;369(6478):321–3. doi: 10.1038/369321a0. [DOI] [PubMed] [Google Scholar]
- 31.Sedlak TW, Oltvai ZN, Yang E, Wang K, Boise LH, Thompson CB, et al. Multiple Bcl-2 family members demonstrate selective dimerizations with Bax. Proc Natl Acad Sci U S A. 1995;92(17):7834–8. doi: 10.1073/pnas.92.17.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meijerink JP, Mensink EJ, Wang K, Sedlak TW, Slöetjes AW, De Witte T, et al. Hematopoietic malignancies demonstrate loss-of-function mutations of BAX. Blood. 1998;91(8):2991–7. [PubMed] [Google Scholar]
- 33.Strasser A, Harris AW, Cory S. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991;67(5):889–99. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- 34.Strasser A, Whittingham S, Vaux DL, Bath ML, Adams JM, Cory S, et al. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc Natl Acad Sci U S A. 1991;88(19):8661–5. doi: 10.1073/pnas.88.19.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993;75(2):229–40. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- 36.Veis DJ, Sentman CL, Bach EA, Korsmeyer SJ. Expression of the Bcl-2 protein in murine and human thymocytes and in peripheral T lymphocytes. J Immunol. 1993;151(5):2546–54. [PubMed] [Google Scholar]
- 37.Nuñez G, Hockenbery D, McDonnell TJ, Sorensen CM, Korsmeyer SJ. Bcl-2 maintains B cell memory. Nature. 1991;353(6339):71–3. doi: 10.1038/353071a0. [DOI] [PubMed] [Google Scholar]
- 38.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Köntgen F, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286(5445):1735–8. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 39.Nakayama K, Nakayama K, Negishi I, Kuida K, Shinkai Y, Louie MC, et al. Disappearance of the lymphoid system in Bcl-2 homozygous mutant chimeric mice. Science. 1993;261(5128):1584–8. doi: 10.1126/science.8372353. [DOI] [PubMed] [Google Scholar]
- 40.Bouillet P, Cory S, Zhang LC, Strasser A, Adams JM. Degenerative disorders caused by Bcl-2 deficiency prevented by loss of its BH3-only antagonist Bim. Dev Cell. 2001;1(5):645–53. doi: 10.1016/s1534-5807(01)00083-1. [DOI] [PubMed] [Google Scholar]
- 41.Bouillet P, Purton JF, Godfrey DI, Zhang L-C, Coultas L, Puthalakath H, et al. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415(6874):922–6. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- 42.Mérino D, Giam M, Hughes PD, Siggs OM, Heger K, O’Reilly LA, et al. The role of BH3-only protein Bim extends beyond inhibiting Bcl-2–like prosurvival proteins. J Cell Biol. 2009;186(3):355–62. doi: 10.1083/jcb.200905153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mérino D, Bouillet P. The Bcl-2 family in autoimmune and degenerative disorders. Apoptosis. 2009;14(4):570–83. doi: 10.1007/s10495-008-0308-4. [DOI] [PubMed] [Google Scholar]
- 44.Kirschnek S, Ying S, Fischer SF, Häcker H, Villunger A, Hochrein H, et al. Phagocytosis-Induced Apoptosis in Macrophages Is Mediated by Up-Regulation and Activation of the Bcl-2 Homology Domain 3-Only Protein Bim. J Immunol. 2005;174(2):671–9. doi: 10.4049/jimmunol.174.2.671. [DOI] [PubMed] [Google Scholar]
- 45.Rinkenberger JL, Horning S, Klocke B, Roth K, Korsmeyer SJ. Mcl-1 deficiency results in peri-implantation embryonic lethality. Genes Dev. 2000;14(1):23–7. [PMC free article] [PubMed] [Google Scholar]
- 46.Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426(6967):671–6. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- 47.Campbell KJ, Gray DHD, Anstee N, Strasser A, Cory S. Elevated Mcl-1 inhibits thymocyte apoptosis and alters thymic selection. Cell death and differentiation [Internet] 2012 doi: 10.1038/cdd.2012.84. [cited 2012 Sep 11]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/22743995. [DOI] [PMC free article] [PubMed]
- 48.Vikstrom I, Carotta S, Lüthje K, Peperzak V, Jost PJ, Glaser S, et al. Mcl-1 is essential for germinal center formation and B cell memory. Science. 2010;330(6007):1095–9. doi: 10.1126/science.1191793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Opferman JT, Iwasaki H, Ong CC, Suh H, Mizuno S, Akashi K, et al. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science. 2005;307(5712):1101–4. doi: 10.1126/science.1106114. [DOI] [PubMed] [Google Scholar]
- 50.Dzhagalov I, John AS, He Y-W. The antiapoptotic protein Mcl-1 is essential for the survival of neutrophils but not macrophages. Blood. 2007;109(4):1620–6. doi: 10.1182/blood-2006-03-013771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Glaser SP, Lee EF, Trounson E, Bouillet P, Wei A, Fairlie WD, et al. Anti-apoptotic Mcl-1 is essential for the development and sustained growth of acute myeloid leukemia. Genes Dev. 2012;26(2):120–5. doi: 10.1101/gad.182980.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mandal M, Borowski C, Palomero T, Ferrando AA, Oberdoerffer P, Meng F, et al. The BCL2A1 gene as a pre–T cell receptor–induced regulator of thymocyte survival. J Exp Med. 2005;201(4):603–14. doi: 10.1084/jem.20041924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamasaki A, Sendo F, Nakayama K, Ishida N, Negishi I, Nakayama K, et al. Accelerated Neutrophil Apoptosis in Mice Lacking A1-a, a Subtype of the bcl-2–related A1 Gene. J Exp Med. 1998;188(11):1985–92. doi: 10.1084/jem.188.11.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma A, Pena JC, Chang B, Margosian E, Davidson L, Alt FW, et al. Bclx regulates the survival of double-positive thymocytes. Proc Natl Acad Sci US A. 1995;92(11):4763–7. doi: 10.1073/pnas.92.11.4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6(6):1389–99. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Renault TT, Manon S. Bax: Addressed to kill. Biochimie. 2011;93(9):1379–91. doi: 10.1016/j.biochi.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 57.Knudson CM, Tung KS, Tourtellotte WG, Brown GA, Korsmeyer SJ. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995;270(5233):96–9. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- 58.Rathmell JC, Lindsten T, Zong W-X, Cinalli RM, Thompson CB. Deficiency in Bak and Bax perturbs thymic selection and lymphoid homeostasis. Nat Immunol. 2002;3(10):932–9. doi: 10.1038/ni834. [DOI] [PubMed] [Google Scholar]
- 59.Hsu SY, Kaipia A, McGee E, Lomeli M, Hsueh AJW. Bok is a pro-apoptotic Bcl-2 protein with restricted expression in reproductive tissues and heterodimerizes with selective anti-apoptotic Bcl-2 family members. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(23):12401– 12406. doi: 10.1073/pnas.94.23.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Michalak EM, Jansen ES, Happo L, Cragg MS, Tai L, Smyth GK, et al. Puma and to a lesser extent Noxa are suppressors of Myc-induced lymphomagenesis. Cell Death & Differentiation. 2009;16(5):684–96. doi: 10.1038/cdd.2008.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Elgendy M, Sheridan C, Brumatti G, Martin SJ. Oncogenic Ras-induced expression of Noxa and Beclin-1 promotes autophagic cell death and limits clonogenic survival. Mol Cell. 2011;42(1):23–35. doi: 10.1016/j.molcel.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 62.Villunger A, Scott C, Bouillet P, Strasser A. Essential role for the BH3-only protein Bim but redundant roles for Bax, Bcl-2, and Bcl-w in the control of granulocyte survival. Blood. 2003;101(6):2393–400. doi: 10.1182/blood-2002-07-2132. [DOI] [PubMed] [Google Scholar]
- 63.Kirschnek S, Vier J, Gautam S, Frankenberg T, Rangelova S, Eitz-Ferrer P, et al. Molecular analysis of neutrophil spontaneous apoptosis reveals a strong role for the pro-apoptotic BH3-only protein Noxa. Cell Death Differ. 2011;18(11):1805–14. doi: 10.1038/cdd.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garrison SP, Phillips DC, Jeffers JR, Chipuk JE, Parsons MJ, Rehg JE, et al. Genetically defining the mechanism of Puma- and Bim-induced apoptosis. Cell Death Differ. 2012;19(4):642–9. doi: 10.1038/cdd.2011.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Erlacher M, Labi V, Manzl C, Böck G, Tzankov A, Häcker G, et al. Puma cooperates with Bim, the rate-limiting BH3-only protein in cell death during lymphocyte development, in apoptosis induction. J Exp Med. 2006;203(13):2939–51. doi: 10.1084/jem.20061552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Villunger A, Michalak EM, Coultas L, Müllauer F, Böck G, Ausserlechner MJ, et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302(5647):1036–8. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 67.Gray DHD, Kupresanin F, Berzins SP, Herold MJ, O’Reilly LA, Bouillet P, et al. The BH3-Only Proteins Bim and Puma Cooperate to Impose Deletional Tolerance of Organ-Specific Antigens. Immunity [Internet] 2012 doi: 10.1016/j.immuni.2012.05.030. [cited 2012 Sep 17]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/22960223. [DOI] [PMC free article] [PubMed]
- 68.Zhan Y, Zhang Y, Gray D, Carrington EM, Bouillet P, Ko H-J, et al. Defects in the Bcl-2-regulated apoptotic pathway lead to preferential increase of CD25 low Foxp3+ anergic CD4+ T cells. J Immunol. 2011;187(4):1566–77. doi: 10.4049/jimmunol.1100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Labi V, Erlacher M, Kiessling S, Manzl C, Frenzel A, O’Reilly L, et al. Loss of the BH3-only protein Bmf impairs B cell homeostasis and accelerates γ irradiation–induced thymic lymphoma development. J Exp Med. 2008;205(3):641–55. doi: 10.1084/jem.20071658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ploner C, Rainer J, Niederegger H, Eduardoff M, Villunger A, Geley S, et al. The BCL2 rheostat in glucocorticoid-induced apoptosis of acute lymphoblastic leukemia. Leukemia. 2008;22(2):370–7. doi: 10.1038/sj.leu.2405039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baumgartner F, Woess C, Pedit V, Tzankov A, Labi V, Villunger A. Minor cell-death defects but reduced tumor latency in mice lacking the BH3-only proteins Bad and Bmf. Oncogene. 2012 doi: 10.1038/onc.2012.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Siebenlist U, Brown K, Claudio E. Control of lymphocyte development by nuclear factor-kappaB. Nat Rev Immunol. 2005;5(6):435–45. doi: 10.1038/nri1629. [DOI] [PubMed] [Google Scholar]
- 73.Hayden MS, West AP, Ghosh S. NF-kappaB and the immune response. Oncogene. 2006;25(51):6758–80. doi: 10.1038/sj.onc.1209943. [DOI] [PubMed] [Google Scholar]
- 74.Kucharczak J, Simmons MJ, Fan Y, Gélinas C. To be, or not to be: NF-kappaB is the answer--role of Rel/NF-kappaB in the regulation of apoptosis. Oncogene. 2003;22(56):8961–82. doi: 10.1038/sj.onc.1207230. [DOI] [PubMed] [Google Scholar]
- 75.Voll RE, Jimi E, Phillips RJ, Barber DF, Rincon M, Hayday AC, et al. NF-kappa B activation by the pre-T cell receptor serves as a selective survival signal in T lymphocyte development. Immunity. 2000;13(5):677–89. doi: 10.1016/s1074-7613(00)00067-4. [DOI] [PubMed] [Google Scholar]
- 76.Zheng Y, Vig M, Lyons J, Parijs LV, Beg AA. Combined Deficiency of p50 and cRel in CD4+ T Cells Reveals an Essential Requirement for Nuclear Factor κB in Regulating Mature T Cell Survival and In Vivo Function. J Exp Med. 2003;197(7):861–74. doi: 10.1084/jem.20021610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80(2):293–9. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 78.Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, et al. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288(5468):1053–8. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- 79.Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7(3):683–94. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 80.Mathai JP, Germain M, Marcellus RC, Shore GC. Induction and endoplasmic reticulum location of BIK/NBK in response to apoptotic signaling by E1A and p53. Oncogene. 2002;21(16):2534–44. doi: 10.1038/sj.onc.1205340. [DOI] [PubMed] [Google Scholar]
- 81.Sax JK, Fei P, Murphy ME, Bernhard E, Korsmeyer SJ, El-Deiry WS. BID regulation by p53 contributes to chemosensitivity. Nat Cell Biol. 2002;4(11):842–9. doi: 10.1038/ncb866. [DOI] [PubMed] [Google Scholar]
- 82.Miyashita T, Krajewski S, Krajewska M, Wang HG, Lin HK, Liebermann DA, et al. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene. 1994;9(6):1799–805. [PubMed] [Google Scholar]
- 83.Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303(5660):1010–4. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 84.Leu JI-J, Dumont P, Hafey M, Murphy ME, George DL. Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex. Nat Cell Biol. 2004;6(5):443–50. doi: 10.1038/ncb1123. [DOI] [PubMed] [Google Scholar]
- 85.Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, et al. p53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003;11(3):577–90. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 86.Stadanlick JE, Zhang Z, Lee S-Y, Hemann M, Biery M, Carleton MO, et al. Developmental Arrest of T Cells in Rpl22-Deficient Mice Is Dependent upon Multiple p53 Effectors. J Immunol. 2011;187(2):664–75. doi: 10.4049/jimmunol.1100029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mitchell KO, Ricci MS, Miyashita T, Dicker DT, Jin Z, Reed JC, et al. Bax is a transcriptional target and mediator of c-myc-induced apoptosis. Cancer Res. 2000;60(22):6318–25. [PubMed] [Google Scholar]
- 88.Eischen CM, Woo D, Roussel MF, Cleveland JL. Apoptosis Triggered by Myc-Induced Suppression of Bcl-XL or Bcl-2 Is Bypassed during Lymphomagenesis. Mol Cell Biol. 2001;21(15):5063–70. doi: 10.1128/MCB.21.15.5063-5070.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dijkers PF, Medema RH, Lammers JW, Koenderman L, Coffer PJ. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr Biol. 2000;10(19):1201–4. doi: 10.1016/s0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- 90.Jurado S, Gleeson K, O’Donnell K, Izon DJ, Walkley CR, Strasser A, et al. The Zinc-finger protein ASCIZ regulates B cell development via DYNLL1 and Bim. J Exp Med. 2012;209(9):1629–39. doi: 10.1084/jem.20120785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Milot E, Filep JG. Regulation of Neutrophil Survival/Apoptosis by Mcl-1. Scientific World Journal. 2011;11:1948–62. doi: 10.1100/2011/131539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Allen JC, Talab F, Zuzel M, Lin K, Slupsky JR. c-Abl regulates Mcl-1 gene expression in chronic lymphocytic leukemia cells. Blood. 2011;117(8):2414–22. doi: 10.1182/blood-2010-08-301176. [DOI] [PubMed] [Google Scholar]
- 93.O’Connor L, Strasser A, O’Reilly LA, Hausmann G, Adams JM, Cory S, et al. Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 1998;17(2):384–95. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Adachi M, Zhao X, Imai K. Nomenclature of dynein light chain-linked BH3-only protein Bim isoforms. Cell Death & Differentiation. 2005;12(2):192–3. doi: 10.1038/sj.cdd.4401529. [DOI] [PubMed] [Google Scholar]
- 95.Liu L, Chen J, Zhang J, Ji C, Zhang X, Gu S, et al. Overexpression of BimSs3, the novel isoform of Bim, can trigger cell apoptosis by inducing cytochrome c release from mitochondria. Acta Biochim Pol. 2007;54(3):603–10. [PubMed] [Google Scholar]
- 96.Boise LH, Gonzalez-Garcia M, Postema CE, Ding L, Lindsten T, Turka LA, et al. Bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74(4):597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 97.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74(4):609–19. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 98.Otake Y, Soundararajan S, Sengupta TK, Kio EA, Smith JC, Pineda-Roman M, et al. Overexpression of nucleolin in chronic lymphocytic leukemia cells induces stabilization of bcl2 mRNA. Blood. 2007;109(7):3069–75. doi: 10.1182/blood-2006-08-043257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ, et al. Targeted deletion reveals essential and overlapping functions of the miR-17~92 family of miRNA clusters. Cell. 2008;132(5):875–86. doi: 10.1016/j.cell.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci U S A. 2005;102(39):13944–9. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Calin GA, Cimmino A, Fabbri M, Ferracin M, Wojcik SE, Shimizu M, et al. MiR-15a and miR-16-1 cluster functions in human leukemia. Proc Natl Acad Sci U S A. 2008;105(13):5166–71. doi: 10.1073/pnas.0800121105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu Z, Lu H, Sheng J, Li L. Inductive microRNA-21 impairs anti-mycobacterial responses by targeting IL-12 and Bcl-2. FEBS Letters [Internet] doi: 10.1016/j.febslet.2012.06.004. [cited 2012 Jul 23];(0). Available from: http://www.sciencedirect.com/science/article/pii/S0014579312004681. [DOI] [PubMed]
- 103.Lindsay MA. microRNAs and the immune response. Trends in Immunology. 2008;29(7):343–51. doi: 10.1016/j.it.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 104.Lima RT, Busacca S, Almeida GM, Gaudino G, Fennell DA, Vasconcelos MH. MicroRNA regulation of core apoptosis pathways in cancer. Eur J Cancer. 2011;47(2):163–74. doi: 10.1016/j.ejca.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 105.Deng X, Ruvolo P, Carr B, May WS. Survival function of ERK1/2 as IL-3-activated, staurosporine-resistant Bcl2 kinases. Proc Natl Acad Sci U S A. 2000;97(4):1578–83. doi: 10.1073/pnas.97.4.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yamamoto K, Ichijo H, Korsmeyer SJ. BCL-2 Is Phosphorylated and Inactivated by an ASK1/Jun N-Terminal Protein Kinase Pathway Normally Activated at G2/M. Mol Cell Biol. 1999;19(12):8469–78. doi: 10.1128/mcb.19.12.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Deng X, Gao F, Flagg T, May WS. Mono- and multisite phosphorylation enhances Bcl2’s antiapoptotic function and inhibition of cell cycle entry functions. Proc Natl Acad Sci U S A. 2004;101(1):153–8. doi: 10.1073/pnas.2533920100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dimmeler S, Breitschopf K, Haendeler J, Zeiher AM. Dephosphorylation Targets Bcl-2 for Ubiquitin-dependent Degradation: A Link between the Apoptosome and the Proteasome Pathway. J Exp Med. 1999;189(11):1815–22. doi: 10.1084/jem.189.11.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Breitschopf K, Haendeler J, Malchow P, Zeiher AM, Dimmeler S. Posttranslational modification of Bcl-2 facilitates its proteasome-dependent degradation: molecular characterization of the involved signaling pathway. Mol Cell Biol. 2000;20(5):1886–96. doi: 10.1128/mcb.20.5.1886-1896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang Y-Y, Deng X, Xu L, Gao F, Flagg T, May WS. Bcl2 enhances induced hematopoietic differentiation of murine embryonic stem cells. Exp Hematol. 2008;36(2):128–39. doi: 10.1016/j.exphem.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Luciano F, Jacquel A, Colosetti P, Herrant M, Cagnol S, Pages G, et al. Phosphorylation of Bim-EL by Erk1/2 on serine 69 promotes its degradation via the proteasome pathway and regulates its proapoptotic function. Oncogene. 2003;22(43):6785–93. doi: 10.1038/sj.onc.1206792. [DOI] [PubMed] [Google Scholar]
- 112.Ley R, Ewings KE, Hadfield K, Howes E, Balmanno K, Cook SJ. Extracellular Signal-regulated Kinases 1/2 Are Serum-stimulated “BimEL Kinases” That Bind to the BH3-only Protein BimEL Causing Its Phosphorylation and Turnover. J Biol Chem. 2004;279(10):8837–47. doi: 10.1074/jbc.M311578200. [DOI] [PubMed] [Google Scholar]
- 113.O’Reilly LA, Kruse EA, Puthalakath H, Kelly PN, Kaufmann T, Huang DC, et al. MEK/ERK-Mediated Phosphorylation of Bim is Required to Ensure Survival of T and B Lymphocytes during Mitogenic Stimulation. J Immunol. 2009;183(1):261–9. doi: 10.4049/jimmunol.0803853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Paterson A, Mockridge CI, Adams JE, Krysov S, Potter KN, Duncombe AS, et al. Mechanisms and clinical significance of BIM phosphorylation in chronic lymphocytic leukemia. Blood. 2012;119(7):1726–36. doi: 10.1182/blood-2011-07-367417. [DOI] [PubMed] [Google Scholar]
- 115.Lizcano JM, Morrice N, Cohen P. Regulation of BAD by cAMP-dependent protein kinase is mediated via phosphorylation of a novel site, Ser155. Biochem J. 2000;349(Pt 2):547–57. doi: 10.1042/0264-6021:3490547. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 116.Virdee K, Parone PA, Tolkovsky AM. Phosphorylation of the pro-apoptotic protein BAD on serine 155, a novel site, contributes to cell survival. Curr Biol. 2000;10(18):1151–4. doi: 10.1016/s0960-9822(00)00702-8. [DOI] [PubMed] [Google Scholar]
- 117.Tan Y, Demeter MR, Ruan H, Comb MJ. BAD Ser-155 phosphorylation regulates BAD/Bcl-XL interaction and cell survival. J Biol Chem. 2000;275(33):25865–9. doi: 10.1074/jbc.M004199200. [DOI] [PubMed] [Google Scholar]
- 118.Harada H, Becknell B, Wilm M, Mann M, Huang LJ, Taylor SS, et al. Phosphorylation and inactivation of BAD by mitochondria-anchored protein kinase A. Mol Cell. 1999;3(4):413–22. doi: 10.1016/s1097-2765(00)80469-4. [DOI] [PubMed] [Google Scholar]
- 119.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, et al. Akt Phosphorylation of BAD Couples Survival Signals to the Cell-Intrinsic Death Machinery. Cell. 1997;91(2):231–41. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 120.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94(4):491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 121.Zha J, Weiler S, Oh KJ, Wei MC, Korsmeyer SJ. Posttranslational N-myristoylation of BID as a molecular switch for targeting mitochondria and apoptosis. Science. 2000;290(5497):1761–5. doi: 10.1126/science.290.5497.1761. [DOI] [PubMed] [Google Scholar]
- 122.Lovell JF, Billen LP, Bindner S, Shamas-Din A, Fradin C, Leber B, et al. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell. 2008;135(6):1074–84. doi: 10.1016/j.cell.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 123.Boya P, Andreau K, Poncet D, Zamzami N, Perfettini J-L, Metivier D, et al. Lysosomal Membrane Permeabilization Induces Cell Death in a Mitochondrion-dependent Fashion. J Exp Med. 2003;197(10):1323–34. doi: 10.1084/jem.20021952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen M, He H, Zhan S, Krajewski S, Reed JC, Gottlieb RA. Bid is cleaved by calpain to an active fragment in vitro and during myocardial ischemia/reperfusion. J Biol Chem. 2001;276(33):30724–8. doi: 10.1074/jbc.M103701200. [DOI] [PubMed] [Google Scholar]
- 125.Stoka V, Turk B, Schendel SL, Kim TH, Cirman T, Snipas SJ, et al. Lysosomal protease pathways to apoptosis. Cleavage of bid, not pro-caspases, is the most likely route. J Biol Chem. 2001;276(5):3149–57. doi: 10.1074/jbc.M008944200. [DOI] [PubMed] [Google Scholar]
- 126.Cirman T, Oresić K, Mazovec GD, Turk V, Reed JC, Myers RM, et al. Selective disruption of lysosomes in HeLa cells triggers apoptosis mediated by cleavage of Bid by multiple papain-like lysosomal cathepsins. J Biol Chem. 2004;279(5):3578–87. doi: 10.1074/jbc.M308347200. [DOI] [PubMed] [Google Scholar]
- 127.Sutton VR, Davis JE, Cancilla M, Johnstone RW, Ruefli AA, Sedelies K, et al. Initiation of apoptosis by granzyme B requires direct cleavage of bid, but not direct granzyme B-mediated caspase activation. J Exp Med. 2000;192(10):1403–14. doi: 10.1084/jem.192.10.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Campbell KJ, Bath ML, Turner ML, Vandenberg CJ, Bouillet P, Metcalf D, et al. Elevated Mcl-1 perturbs lymphopoiesis, promotes transformation of hematopoietic stem/progenitor cells, and enhances drug resistance. Blood. 2010;116(17):3197–207. doi: 10.1182/blood-2010-04-281071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Michels J, O’Neill JW, Dallman CL, Mouzakiti A, Habens F, Brimmell M, et al. Mcl-1 is required for Akata6 B-lymphoma cell survival and is converted to a cell death molecule by efficient caspase-mediated cleavage. Oncogene. 2004;23(28):4818–27. doi: 10.1038/sj.onc.1207648. [DOI] [PubMed] [Google Scholar]
- 130.Cheng EH, Kirsch DG, Clem RJ, Ravi R, Kastan MB, Bedi A, et al. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science. 1997;278(5345):1966–8. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- 131.Clem RJ, Cheng EH, Karp CL, Kirsch DG, Ueno K, Takahashi A, et al. Modulation of cell death by Bcl-XL through caspase interaction. Proc Natl Acad Sci USA. 1998;95(2):554–9. doi: 10.1073/pnas.95.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kirsch DG, Doseff A, Chau BN, Lim DS, De Souza-Pinto NC, Hansford R, et al. Caspase-3-dependent cleavage of Bcl-2 promotes release of cytochrome c. J Biol Chem. 1999;274(30):21155–61. doi: 10.1074/jbc.274.30.21155. [DOI] [PubMed] [Google Scholar]
- 133.Basañez G, Zhang J, Chau BN, Maksaev GI, Frolov VA, Brandt TA, et al. Pro-apoptotic cleavage products of Bcl-xL form cytochrome c-conducting pores in pure lipid membranes. J Biol Chem. 2001;276(33):31083–91. doi: 10.1074/jbc.M103879200. [DOI] [PubMed] [Google Scholar]
- 134.Robinson NE, Robinson AB. Molecular clocks. Proc Natl Acad Sci U S A. 2001;98(3):944–9. doi: 10.1073/pnas.98.3.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Deverman BE, Cook BL, Manson SR, Niederhoff RA, Langer EM, Rosová I, et al. Bcl-xL deamidation is a critical switch in the regulation of the response to DNA damage. Cell. 2002;111(1):51–62. doi: 10.1016/s0092-8674(02)00972-8. [DOI] [PubMed] [Google Scholar]
- 136.Zhao R, Oxley D, Smith TS, Follows GA, Green AR, Alexander DR. DNA damage-induced Bcl-xL deamidation is mediated by NHE-1 antiport regulated intracellular pH. PLoS Biol. 2007;5(1):e1. doi: 10.1371/journal.pbio.0050001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhao R, Yang FT, Alexander DR. An oncogenic tyrosine kinase inhibits DNA repair and DNA-damage-induced Bcl-xL deamidation in T cell transformation. Cancer Cell. 2004;5(1):37–49. doi: 10.1016/s1535-6108(03)00333-7. [DOI] [PubMed] [Google Scholar]
- 138.Zhao R, Follows GA, Beer PA, Scott LM, Huntly BJP, Green AR, et al. Inhibition of the Bcl-xL deamidation pathway in myeloproliferative disorders. N Engl J Med. 2008;359(26):2778–89. doi: 10.1056/NEJMoa0804953. [DOI] [PubMed] [Google Scholar]
- 139.Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36(3):401–14. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Faustin B, Chen Y, Zhai D, Le Negrate G, Lartigue L, Satterthwait A, et al. Mechanism of Bcl-2 and Bcl-X(L) inhibition of NLRP1 inflammasome: loop domain-dependent suppression of ATP binding and oligomerization. Proc Natl Acad Sci USA. 2009;106(10):3935–40. doi: 10.1073/pnas.0809414106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yeretssian G, Correa RG, Doiron K, Fitzgerald P, Dillon CP, Green DR, et al. Non-apoptotic role of BID in inflammation and innate immunity. Nature. 2011;474(7349):96–9. doi: 10.1038/nature09982. [DOI] [PubMed] [Google Scholar]
- 142.Guo JY, Chen H-Y, Mathew R, Fan J, Strohecker AM, Karsli-Uzunbas G, et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 2011;25(5):460–70. doi: 10.1101/gad.2016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mathew R, White E. Autophagy in Tumorigenesis and Energy Metabolism: Friend by Day, Foe by Night. Curr Opin Genet Dev. 2011;21(1):113–9. doi: 10.1016/j.gde.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kuballa P, Nolte WM, Castoreno AB, Xavier RJ. Autophagy and the immune system. Annu Rev Immunol. 2012;30:611–46. doi: 10.1146/annurev-immunol-020711-074948. [DOI] [PubMed] [Google Scholar]
- 145.Liang XH, Kleeman LK, Jiang HH, Gordon G, Goldman JE, Berry G, et al. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J Virol. 1998;72(11):8586–96. doi: 10.1128/jvi.72.11.8586-8596.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122(6):927–39. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 147.Maiuri MC, Le Toumelin G, Criollo A, Rain J-C, Gautier F, Juin P, et al. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J. 2007;26(10):2527–39. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Maiuri MC, Criollo A, Tasdemir E, Vicencio JM, Tajeddine N, Hickman JA, et al. BH3-only proteins and BH3 mimetics induce autophagy by competitively disrupting the interaction between Beclin 1 and Bcl-2/Bcl-X(L) Autophagy. 2007;3(4):374–6. doi: 10.4161/auto.4237. [DOI] [PubMed] [Google Scholar]
- 149.Priault M, Hue E, Marhuenda F, Pilet P, Oliver L, Vallette FM. Differential dependence on Beclin 1 for the regulation of pro-survival autophagy by Bcl-2 and Bcl-xL in HCT116 colorectal cancer cells. PLoS ONE. 2010;5(1):e8755. doi: 10.1371/journal.pone.0008755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Okamoto K, Shaw JM. Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Annu Rev Genet. 2005;39:503–36. doi: 10.1146/annurev.genet.38.072902.093019. [DOI] [PubMed] [Google Scholar]
- 151.Martinou J-C, Youle RJ. Mitochondria in Apoptosis: Bcl-2 Family Members and Mitochondrial Dynamics. Developmental Cell. 2011;21(1):92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Karbowski M, Norris KL, Cleland MM, Jeong S-Y, Youle RJ. Role of Bax and Bak in mitochondrial morphogenesis. Nature. 2006;443(7112):658–62. doi: 10.1038/nature05111. [DOI] [PubMed] [Google Scholar]
- 153.Cleland MM, Norris KL, Karbowski M, Wang C, Suen D-F, Jiao S, et al. Bcl-2 family interaction with the mitochondrial morphogenesis machinery. Cell Death Differ. 2011;18(2):235–47. doi: 10.1038/cdd.2010.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hoppins S, Edlich F, Cleland MM, Banerjee S, McCaffery JM, Youle RJ, et al. The soluble form of Bax regulates mitochondrial fusion via MFN2 homotypic complexes. Mol Cell. 2011;41(2):150–60. doi: 10.1016/j.molcel.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Delivani P, Adrain C, Taylor RC, Duriez PJ, Martin SJ. Role for CED-9 and Egl-1 as Regulators of Mitochondrial Fission and Fusion Dynamics. Molecular Cell. 2006;21(6):761–73. doi: 10.1016/j.molcel.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 156.Campello S, Lacalle RA, Bettella M, Mañes S, Scorrano L, Viola A. Orchestration of lymphocyte chemotaxis by mitochondrial dynamics. J Exp Med. 2006;203(13):2879–86. doi: 10.1084/jem.20061877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Baixauli F, Martín-Cófreces NB, Morlino G, Carrasco YR, Calabia-Linares C, Veiga E, et al. The mitochondrial fission factor dynamin-related protein 1 modulates T-cell receptor signalling at the immune synapse. EMBO J. 2011;30(7):1238–50. doi: 10.1038/emboj.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35(6):871–82. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Caro P, Kishan AU, Norberg E, Stanley IA, Chapuy B, Ficarro SB, et al. Metabolic signatures uncover distinct targets in molecular subsets of diffuse large B cell lymphoma. Cancer Cell. 2012;22(4):547–60. doi: 10.1016/j.ccr.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Perciavalle RM, Stewart DP, Koss B, Lynch J, Milasta S, Bathina M, et al. Anti-apoptotic MCL-1 localizes to the mitochondrial matrix and couples mitochondrial fusion to respiration. Nat Cell Biol. 2012;14(6):575–83. doi: 10.1038/ncb2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chen ZX, Pervaiz S. Involvement of cytochrome c oxidase subunits Va and Vb in the regulation of cancer cell metabolism by Bcl-2. Cell Death Differ. 2010;17(3):408–20. doi: 10.1038/cdd.2009.132. [DOI] [PubMed] [Google Scholar]
- 162.Hockenbery DM, Oltvai ZN, Yin X-M, Milliman CL, Korsmeyer SJ. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75(2):241–51. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 163.Imahashi K, Schneider MD, Steenbergen C, Murphy E. Transgenic Expression of Bcl-2 Modulates Energy Metabolism, Prevents Cytosolic Acidification During Ischemia, and Reduces Ischemia/Reperfusion Injury. Circulation Research. 2004;95(7):734–41. doi: 10.1161/01.RES.0000143898.67182.4c. [DOI] [PubMed] [Google Scholar]
- 164.Danial NN, Walensky LD, Zhang C-Y, Choi CS, Fisher JK, Molina AJA, et al. Dual role of proapoptotic BAD in insulin secretion and beta cell survival. Nat Med. 2008;14(2):144–53. doi: 10.1038/nm1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Guicciardi ME, Gores GJ. Life and death by death receptors. FASEB J. 2009;23(6):1625–37. doi: 10.1096/fj.08-111005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.The UniProt Consortium. Reorganizing the protein space at the Universal Protein Resource (UniProt) Nucleic Acids Research. 2011;40(D1):D71–D75. doi: 10.1093/nar/gkr981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Petros AM, Nettesheim DG, Wang Y, Olejniczak ET, Meadows RP, Mack J, et al. Rationale for Bcl-xL/Bad peptide complex formation from structure, mutagenesis, and biophysical studies. Protein Sci. 2000;9(12):2528–34. doi: 10.1110/ps.9.12.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hinds MG, Smits C, Fredericks-Short R, Risk JM, Bailey M, Huang DCS, et al. Bim, Bad and Bmf: intrinsically unstructured BH3-only proteins that undergo a localized conformational change upon binding to prosurvival Bcl-2 targets. Cell Death Differ. 2007;14(1):128–36. doi: 10.1038/sj.cdd.4401934. [DOI] [PubMed] [Google Scholar]
- 169.Billen LP, Shamas-Din A, Andrews DW. Bid: a Bax-like BH3 protein. Oncogene. 2009;27(S1):S93–S104. doi: 10.1038/onc.2009.47. [DOI] [PubMed] [Google Scholar]
- 170.Yan N, Shi Y. Mechanisms of Apoptosis Through Structural Biology. Annual Review of Cell and Developmental Biology. 2005;21(1):35–56. doi: 10.1146/annurev.cellbio.21.012704.131040. [DOI] [PubMed] [Google Scholar]
- 171.Wang K, Yin XM, Chao DT, Milliman CL, Korsmeyer SJ. BID: a novel BH3 domain-only death agonist. Genes Dev. 1996;10(22):2859–69. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- 172.Eskes R, Desagher S, Antonsson B, Martinou JC. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol. 2000;20(3):929–35. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cartron P-F, Gallenne T, Bougras G, Gautier F, Manero F, Vusio P, et al. The First [alpha] Helix of Bax Plays a Necessary Role in Its Ligand-Induced Activation by the BH3-Only Proteins Bid and PUMA. Molecular Cell. 2004;16(5):807–18. doi: 10.1016/j.molcel.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 174.Kim H, Tu H-C, Ren D, Takeuchi O, Jeffers JR, Zambetti GP, et al. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol Cell. 2009;36(3):487–99. doi: 10.1016/j.molcel.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jabbour AM, Heraud JE, Daunt CP, Kaufmann T, Sandow J, O’Reilly LA, et al. Puma indirectly activates Bax to cause apoptosis in the absence of Bid or Bim. Cell Death Differ. 2009;16(4):555–63. doi: 10.1038/cdd.2008.179. [DOI] [PubMed] [Google Scholar]