Abstract
Potassium vinyltrifluoroborate was found to be an efficient partner with benzamide derivatives for Rh(III)-catalyzed annulations. 4-Trifluoroboratotetrahydroisoquinolones were generated under mild conditions, affording a regioisomerically complementary substitution pattern to other alkenes in related reactions. These new boron-containing building blocks were derivatized by N-arylations, retaining the boron substituent for further elaboration.
During the last decade, Rh(III)-catalyzed C–H bond functionalizations have emerged as a powerful tool in organic synthesis.1 Their success is due in large part to their atom economy and to selectivity achieved by the use of a directed C–H activation. Indeed, such reactions have been successfully used for the formation of C–C,2 C–N,3 and C–X (X = halogen)4 bonds,5 as well as heterocycles6 through the use of a co-oxidant. Among such transformations, the insertion of unsaturated compounds in benzamide derivatives has received much attention.7,8 Guimond and Fagnou improved the original method by the use of a combined directing group and internal oxidant,9,10 allowing milder reaction conditions for the insertion of unsaturated hydrocarbons in benzamide derivatives (Scheme 1.1).11 Since the emergence of this pioneering work, much attention has been focused on its application to alkynes,12 and with the exception of a study on allenes,13 less effort has been devoted to alkenes. Indeed, whereas a Heck-type reaction with β-hydride elimination usually competes,7,11c,14 Rovis and Cramer have recently reported an asymmetric version of this reaction.15
Scheme 1.
Syntheses of Isoquinolones by Insertion Reactions
Owing to the importance of tetrahydroisoquinolines16 and to the increased demand for new organoboron compounds,17 an annulative approach to these structures utilizing alkenylboron components was sought. Indeed, this would lead to novel boron-containing building blocks, ready for further elaboration. However, such a strategy would meet two main challenges or unknowns: 1) An obvious issue of compatibility between the organoboron species and the co-oxidant required in the desired transformation could arise. 2) The potential participation of the organoboron component in competing Rh(III)-catalyzed reactions would also be of concern.18
To avoid these potential pitfalls and achieve the objective, attention was turned to organotrifluoroborates, which have been shown to be much more stable than the corresponding boronic acids and boronates.19 Thus, it has been demonstrated that the trifluoroborato moiety is capable of remaining intact in a broad range of reactions.20 Additionally, only rare examples of boryl-substituted substrates have been reported in such insertion reactions, and these have all been various alkynylborons.21 In particular, Glorius has reported the insertion of ethynyl-B(MIDA) boronate using Cu(OAc)2 as a co-catalyst with the same regioselectivity as for terminal alkenes (Scheme 1.2).22
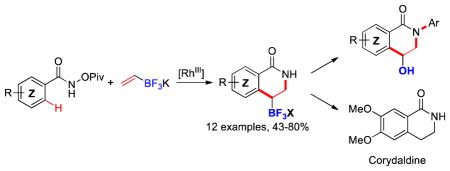
Herein, we report the extension of Rh(III)-catalyzed insertion reactions to potassium vinyltrifluoroborate, leading to a new class of tetrahydroisoquinolones that can subsequently be bidirectionally functionalized (Scheme 1.3).
In the strategy to use alkenyltrifluoroborates in Rh(III)-catalyzed annulations, we were particularly interested in utilizing an internal oxidant because it avoids the use of a co-oxidant, which might be incompatible with an organoboron species. Therefore, investigations were begun using the conditions originally reported for the reaction of benzhydroxamate 1a with potassium vinyltrifluoroborate 2 using a slight excess of the arene substrate to insure complete conversion of the organoboron species. In an initial effort, the desired tetrabutylammonium product 3a was obtained (after salt metathesis to simplify the isolation step),23 but only in 35% yield and as an 85:15 mixture of regioisomers. Of interest is the unusual regiochemistry of the major olefin insertion product, which provides tetrahydroisoquinolones that are regioisomeric to those formed using other alkenes.11,15 Steric effects would not appear to explain this reversal, and thus a substantial and unique electronic effect seen previously in metal-mediated insertion reactions of vinyltrifluoroborate appears to be the major contributing factor.24 Thus, calculations (B3LYP 6-31G*) reveal that the dipole of the C=C bond in vinyltrifluoroborate places enhanced negative charge at the beta position of the double bond, in line with the observed regiochemistry.
An overview of the optimization process can be viewed in the Supporting Information. Importantly, the reaction conditions ultimately settled upon are easily scalable, and the desired product 3a can be obtained in an even better yield as either a tetrabutylammonium salt, or, in a more atom-economical way,25 as a potassium salt by modifying the base and the work-up (Scheme 2).
Scheme 2.

Preparative scale
Attention was next turned to the scope of this reaction (Scheme 3). Pleasingly, the reaction of 4-substituted hydroxamates 1a–e afforded the expected products 3a–e in similarly good yields for both electron-withdrawing and electron-donating groups. In the case of 3-substituted substrates 1f–i, the regioselectivity of the C–H activation is strongly influenced by steric factors, and the less hindered C–H bond is the more reactive (e.g., providing 3g). Interestingly, various types of heterocycles are suitable substrates for these annulations. Indeed, pyridine, thiophene and furan derivatives have been successfully used to provide good yields of the desired products. Whereas almost complete regioselectivity was observed in the formation of 3k, the reaction was found to be less selective for the thiophene system leading to 3l, but blocking the 2-position of furan 2m afforded a single trifluoroborate 3m in good yield. However, as already highlighted by the results above, the present conditions are very sensitive to steric hindrance, and the use of 2-substituted benzhydroxamates only led to traces of the expected compounds (e.g., 3j), and furthermore no product was obtained in the case of substituted alkenyltrifluoroborates.
Scheme 3.
Scope of the Insertion of Potassium Vinyltrifluoroborate 2 in Benzhydroxamate Derivatives
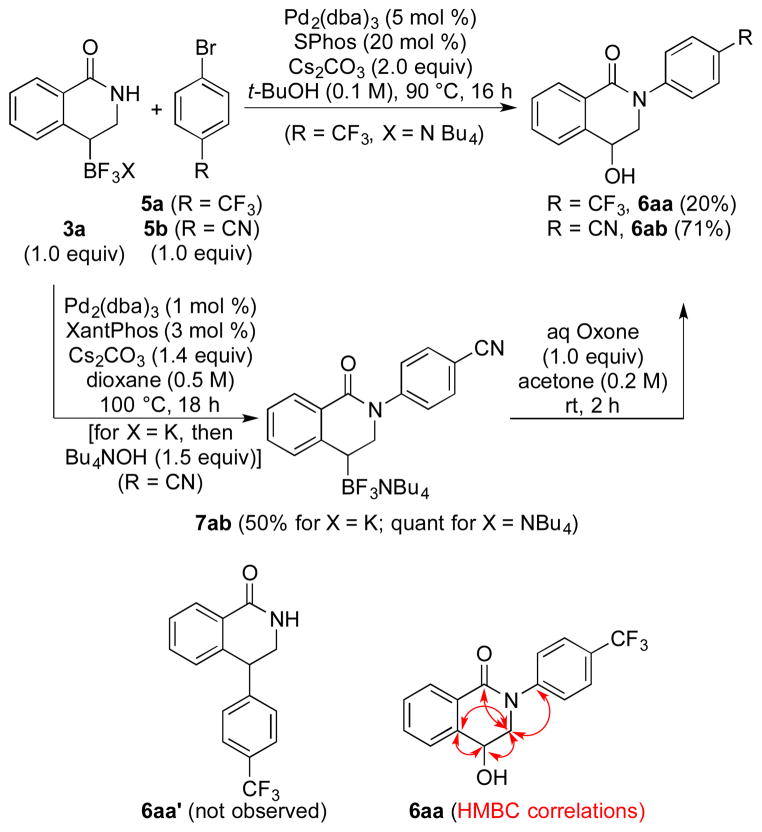
In addition to the regiocomplementary nature of the olefin insertion, the reactivity of these new synthons was found to be somewhat surprising. After unsuccessful attempts to cross-couple the organotrifluoroborates using Crudden’s conditions for benzylic boron species,26 the cross-coupling of 3a with 4-bromobenzotrifluoride 5a under palladium catalysis optimized for secondary alkyl cross-coupling led,27 after purification, to the N-arylated compound 6aa, where the boron had been oxidized (Scheme 4). In a targeted synthesis, 6ab could be prepared by formation of trifluoroborate 7ab via a quantitative arylation, followed by its further oxidation to 6ab.28 This completely chemoselective Buchwald-Hartwig coupling in the presence of a boron derivative clearly demonstrates the low reactivity of these secondary cyclic boron species in cross-coupling,29 and is a rare example of a selective Buchwald-Hartwig coupling in preference to a Suzuki-Miyaura process.30 In these transformations the vinyltrifluoroborate serves as an enol equivalent in the Rh-catalyzed insertion reactions.
Scheme 4.
Selective Buchwald-Hartwig Reactivity
Owing to the importance of N-arylated isoquinolines (and isoquinolones),31,32 the scope of the Buchwald-Hartwig arylation/oxidation sequence was explored. Gratifyingly, under Buchwald’s conditions for the amidation of aryl halides,33 a variety of aryl bromides 5 could be introduced (Scheme 5). Even though a moderate yield was observed in the case of the simple bromobenzene 5c, the reaction tolerated various electron-withdrawing and electron-donating groups in the meta and para positions (compounds 6aa-ae).34 Moreover, the outcome of this sequence is little influenced by the substitution of 3, as both 3c and 3e work equally well. Additionally, 5-bromo-2-trifluoromethylpyridine 5f afforded the desired biheterocyclic compound 6af in 79% yield.
Scheme 5.
Scope of the Buchwald-Hartwig Arylation/Oxidation Sequence
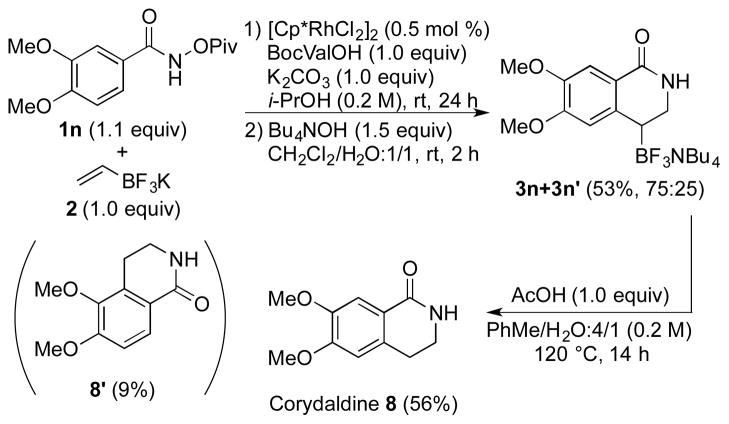
Finally, we briefly studied the protodeborylation of this new type of organotrifluoroborates as an alternative route to the use of gaseous ethylene.11 Therefore, compound 3n has been prepared under our standard conditions from the benzhydroxamate 1n in a 54% yield as a 3:1 mixture in favor of the desired regioisomer 3n′. Protodeborylation of 3n was achieved using AcOH in a toluene/water mixture at 120 °C, affording straightforward access to the natural alkaloid corydaldine 835 in 56% isolated yield (Scheme 6).
Scheme 6.
Synthesis of Corydaldine 8 using a Rh(III)-Catalyzed Annulation/Protodeborylation Strategy
In conclusion, we have developed mild and efficient conditions for the Rh(III)-catalyzed annulations of benzhydroxamates with potassium vinyltrifluoroborate. The vinyltrifluoroborate inserts with high regioselectivity in most cases and in a manner that is regiocomplementary to that of most terminal alkenes. Importantly, the resulting building blocks can be functionalized on both the nitrogen and boron position. In the simplest of these transformations, the vinyltrifluoroborate may serve as the synthetic equivalent of the enol of acetaldehyde or as an ethylene synthon.
Supplementary Material
Acknowledgments
This research was supported by the Neuroscience Medicinal Chemistry Department of Janssen and NIGMS (R01 GM035249). Michel Carpentier (Janssen) is acknowledged for obtaining HRMS data. Dr. Floriane Beaumard and Kaitlin Traister (University of Pennsylvania) are acknowledged for performing HTS experiments and MO calculations, respectively.
Footnotes
Supporting Information Available Experimental details, characterization data, and NMR spectra of all compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Contributor Information
Frederik Rombouts, Email: frombout@its.jnj.com.
Gary A. Molander, Email: gmolandr@sas.upenn.edu.
References
- 1.(a) Colby DA, Bergman RG, Ellman JA. Chem Rev. 2010;110:624. doi: 10.1021/cr900005n. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Colby DA, Tsai AS, Bergman RG, Ellman JA. Acc Chem Res. 2012;45:814. doi: 10.1021/ar200190g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zhu C, Wang R, Falck JR. Chem Asian J. 2012;7:1502. doi: 10.1002/asia.201200035. [DOI] [PubMed] [Google Scholar]; (d) Patureau FW, Wencel-Delord J, Glorius F. Aldrichimica Acta. 2012;45:31. [Google Scholar]
- 2.Recent selected references: for nucleophilic additions, Tsai AS, Tauchert ME, Bergman RG, Ellman JA. J Am Chem Soc. 2011;133:1248. doi: 10.1021/ja109562x.Shi X, Li CJ. Adv Synth Catal. 2012;354:2933.For acylations, Sharma S, Park E, Park J, Kim S. Org Lett. 2012;14:906. doi: 10.1021/ol2034228.Yang Y, Zhou B, Li Y. Adv Synth Catal. 2012;354:2916.For cross-dehydrogenative coupling, Wencel-Delord J, Nimphius C, Wang H, Glorius F. Angew Chem, Int Ed. 2012;51:13001. doi: 10.1002/anie.201205734.and references therein. For insertions in α-diazomalonates Chan W-W, Lo S-F, Zhou Z, Yu W-Y. J Am Chem Soc. 2012;134:13565. doi: 10.1021/ja305771y.
- 3.(a) Kim JY, Park SH, Ryu J, Cho SH, Kim SH, Chang S. J Am Chem Soc. 2012;134:9110. doi: 10.1021/ja303527m. [DOI] [PubMed] [Google Scholar]; (b) Ryu J, Shin K, Park SH, Kim JY, Chang S. Angew Chem, Int Ed. 2012;51:9904. doi: 10.1002/anie.201205723. [DOI] [PubMed] [Google Scholar]; (c) Grohmann C, Wang H, Glorius F. Org Lett. 2012;14:656. doi: 10.1021/ol203353a. [DOI] [PubMed] [Google Scholar]
- 4.Schröder N, Wencel-Delord J, Glorius F. J Am Chem Soc. 2012;134:8298. doi: 10.1021/ja302631j. [DOI] [PubMed] [Google Scholar]
- 5.For a review, see: Song G, Wang F, Li X. Chem Soc Rev. 2012;41:3651. doi: 10.1039/c2cs15281a.
- 6.Satoh T, Miura M. Chem Eur J. 2010;16:11212. doi: 10.1002/chem.201001363. [DOI] [PubMed] [Google Scholar]
- 7.For a seminal paper with benzoic acids, see: Ueura K, Satoh T, Miura M. Org Lett. 2007;9:1407. doi: 10.1021/ol070406h.
- 8.Hyster TK, Rovis T. J Am Chem Soc. 2010;132:10565. doi: 10.1021/ja103776u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.For a highlight: Patureau FW, Glorius F. Angew Chem, Int Ed. 2011;50:1977. doi: 10.1002/anie.201007241.
- 10.For mechanistic studies, see: Xu L, Zhu Q, Huang G, Cheng B, Xia Y. J Org Chem. 2012;77:3017. doi: 10.1021/jo202431q.
- 11.(a) Guimond N, Gouliaras C, Fagnou K. J Am Chem Soc. 2010;132:6908. doi: 10.1021/ja102571b. [DOI] [PubMed] [Google Scholar]; (b) Guimond N, Gorelsky SI, Fagnou K. J Am Chem Soc. 2011;133:6449. doi: 10.1021/ja201143v. [DOI] [PubMed] [Google Scholar]; (c) Rakshit S, Grohmann C, Besset T, Glorius F. J Am Chem Soc. 2011;133:2350. doi: 10.1021/ja109676d. [DOI] [PubMed] [Google Scholar]
- 12.Most recent publications: Li BJ, Wang HY, Zhu QL, Shi ZJ. Angew Chem, Int Ed. 2012;51:3948. doi: 10.1002/anie.201200271.Xu X, Liu Y, Park CM. Angew Chem, Int Ed. 2012;51:9372. doi: 10.1002/anie.201204970.Pham M, Ye B, Cramer N. Angew Chem, Int Ed. 2012;51:10610. doi: 10.1002/anie.201206191.
- 13.For Rh(III)-catalyzed annulations of allenes, see: Wang H, Glorius F. Angew Chem, Int Ed. 2012;51:7318. doi: 10.1002/anie.201201273.
- 14.Selected references: Ueura K, Satoh T, Miura M. Org Lett. 2007;9:1407. doi: 10.1021/ol070406h.Patureau FW, Glorius F. J Am Chem Soc. 2010;132:9982. doi: 10.1021/ja103834b.Patureau FW, Besset T, Glorius F. Angew Chem, Int Ed. 2010;50:1064. doi: 10.1002/anie.201006222.Tsai AS, Brasse M, Bergman RG, Ellman JA. Org Lett. 2011;13:540. doi: 10.1021/ol102890k.Li G, Ding Z, Xu B. Org Lett. 2012;14:5338. doi: 10.1021/ol302522n.Zhen W, Wang F, Zhao M, Du Z, Li X. Angew Chem, Int Ed. 2012;51:11819. doi: 10.1002/anie.201207204.
- 15.(a) Hyster TK, Knörr L, Ward TR, Rovis T. Science. 2012;338:500. doi: 10.1126/science.1226132. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ye B, Cramer N. Science. 2012;338:504. doi: 10.1126/science.1226938. [DOI] [PubMed] [Google Scholar]
- 16.Lovering F, Bikker J, Humblet C. J Med Chem. 2009;52:6752. doi: 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]
- 17.Hall DG, editor. Boronic Acids. Wiley-VCH; Weinheim: 2011. [Google Scholar]
- 18.Karthikeyan J, Haridharan R, Cheng C-H. Angew Chem, Int Ed. 2012;51:12343. doi: 10.1002/anie.201206890.For Rh(III)-catalyzed oxidative coupling of arylboronic acids and alkynes, see: Fukutani T, Hirano K, Satoh T, Miura M. Org Lett. 2009;11:5198. doi: 10.1021/ol9021172.
- 19.(a) Molander GA, Figueroa R. Aldrichimica Acta. 2005;38:49. [Google Scholar]; (b) Darses S, Genet JP. Chem Rev. 2008;108:288. doi: 10.1021/cr0509758. [DOI] [PubMed] [Google Scholar]
- 20.(a) Molander GA, Ellis N. Acc Chem Res. 2007;40:275. doi: 10.1021/ar050199q. [DOI] [PubMed] [Google Scholar]; (b) Molander GA, Canturk B. Angew Chem, Int Ed. 2009;48:9240. doi: 10.1002/anie.200904306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.(a) Miura T, Yamauchi M, Murakami M. Org Lett. 2008;10:3085. doi: 10.1021/ol8010826. [DOI] [PubMed] [Google Scholar]; (b) Geny A, Leboeuf D, Rouquié G, Vollhardt KPC, Malacria M, Gandon V, Aubert C. Chem Eur J. 2007;13:5408. doi: 10.1002/chem.200700337. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Grohmann C, Nimphius C, Glorius F. J Am Chem Soc. 2012;134:19592. doi: 10.1021/ja310153v. [DOI] [PubMed] [Google Scholar]
- 23.Batey RA, Quach TD. Tetrahedron Lett. 2001;42:9099. [Google Scholar]
- 24.Molander GA, Sandrock DL. J Am Chem Soc. 2008;130:15792. doi: 10.1021/ja807076d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trost BM. Science. 1991;254:1471. doi: 10.1126/science.1962206. [DOI] [PubMed] [Google Scholar]
- 26.Imao D, Glasspoole BW, Laberge VS, Crudden CM. J Am Chem Soc. 2009;131:5024. doi: 10.1021/ja8094075. [DOI] [PubMed] [Google Scholar]
- 27.These conditions for cross-coupling have been found by High Throughput Experimentation, see: Dreher SD, Dormer PG, Sandrock DL, Molander GA. J Am Chem Soc. 2008;130:9257. doi: 10.1021/ja8031423.
- 28.Molander GA, Cavalcanti LN. J Org Chem. 2011;76:623. doi: 10.1021/jo102208d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.No traces of Suzuki-Miyaura coupling product was observed with N-methylated 3a.
- 30.For Buchwald-Hartwig couplings of inactive azaborine in Suzuki- Miyaura couplings, see: Agou T, Kojima T, Kobayashi J, Kawashima T. Org Lett. 2009;11:3534. doi: 10.1021/ol901035d.The reverse selectivity is usually observed: see, for example, Sandrock DL, Jean-Gérard L, Chen C, Dreher SD, Molander GA. J Am Chem Soc. 2010;132:17108. doi: 10.1021/ja108949w.Treu M, Zahn SK, Amouzegh P, Lecci C, Tye H. 2012085126. WO. 2011
- 31.Buckley BR, Christie SDR, Elsegood MRJ, Gillings CM, Bulman Page PC, Pardoe WJM. Synlett. 2010:939.and references therein.
- 32.Komoda M, Kakuta H, Takahashi H, Fujimoto Y, Kadoya S, Kato F, Hashimoto Y. Bioorg Med Chem. 2001;9:121. doi: 10.1016/s0968-0896(00)00231-5. [DOI] [PubMed] [Google Scholar]
- 33.Yin J, Buchwald SL. J Am Chem Soc. 2002;124:6043. doi: 10.1021/ja012610k. [DOI] [PubMed] [Google Scholar]
- 34.As reported by Buchwald and Yin, no reaction was observed with ortho-substituted aryl bromides under these conditions.
- 35.For a recent synthesis, see: Oliva-Madrid M-J, Garcia-Lopez J-A, Saura-Llamas I, Bautista D, Vicente J. Organometallics. 2012;31:3647. doi: 10.1021/acs.organomet.0c00787.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.