Abstract
Survivin protein is an attractive candidate for cancer immunotherapy since it is abundantly expressed in most common human cancers and mostly absent in normal adult tissues. Malignant mesothelioma (MM) is a deadly cancer associated with asbestos or erionite exposure for which no successful therapies are currently available. In this study, we evaluated the therapeutic efficacy of a novel survivin-based vaccine by subcutaneous or intraperitoneum injection of BALB/c mice with murine fiber-induced MM tumor cells followed by vaccination with recombinant Fowlpox virus replicons encoding survivin. Vaccination generated significant immune responses in both models, leading to delayed tumor growth and improved animal survival. Flow cytometry and immunofluorescence analyses of tumors from vaccinated mice showed CD8+ T cell infiltration, and real-time PCR demonstrated increased mRNA and protein levels of immunostimulatory cytokines. Analyses of survivin peptide-pulsed spleen and lymph node cells from vaccinated mice using ELISPOT and intracellular cytokine staining confirmed antigen-specific, interferon-γ-producing CD8+ T cell responses. In addition pentamer-based flow cytometry showed that vaccination generated survivin-specific CD8+ T cells. Importantly, vaccination did not affect fertility or induce autoimmune abnormalities in mice. Our results demonstrate that vaccination with recombinant Fowlpox expressing survivin improves T cell responses against aggressive MM tumors and may form the basis for promising clinical applications.
Keywords: Malignant Mesothelioma, Fowlpox, Survivin, Vaccine, Therapy
INTRODUCTION
Malignant mesothelioma (MM) is an aggressive, deadly cancer. It originates from a chronic inflammatory process following asbestos or erionite exposure 1. The median patient survival is 9–12 months from diagnosis and intervention with trimodality therapy that includes chemotherapy, surgical resection, and thoracic radiation only extends survival by an average of 5 months 2. Despite poor prognosis, some MM patients with anti-tumor immune responses have demonstrated improved survival associated characterized by increased CD8+ tumor-infiltrating lymphocytes 3, 4. A better understanding of the underlying biology of MM combined with advances in immunotherapeutic interventions may lead to the design of new treatment modalities specifically targeting tumor-associated antigens (TAAs). Such targets are represented by the members of the family of Inhibitory Apoptotic Proteins (IAPs), which are upregulated in cancer cells and counteract specific apoptosis pathways. Survivin, the smallest member of the IAP family, plays an important role in the control of apoptosis, cell division and cell migration/metastasis 5. Survivin is required for normal fetal development but its expression is generally lost in most adult tissues. However, high expression of survivin is observed in numerous human cancers including MM, in which it is associated with enhanced proliferation, metastasis, poor prognosis and decreased survival 6–9.
Overexpression of survivin in the cytoplasm of tumor cells may lead to increased surface expression of survivin-derived epitopes in association with major histocompatibility complex class I (MHC-I) molecules. These epitopes would be more abundantly presented by MHC-I on tumor cells compared to healthy cells and therefore represent targets for anti-tumor cytotoxic T lymphocytes (CTLs) 10. CTL responses have been detected against survivin-derived T cell epitopes in breast cancer, leukemia, and melanoma patients 11. Successful survivin-based vaccination in a clinical setting was first described in a case involving pancreatic cancer, whereby vaccination with an HLA-A2-restricted survivin peptide immersed in adjuvant led to complete remission of liver metastases 12. Other studies have shown that anti-survivin T cells persist in the periphery for extended periods of time in the absence of clinical manifestations of autoimmunity 13. Currently, survivin-based vaccination trials for different types of cancers are being examined, some of which have been shown to generate survivin-specific CTLs capable of lysing autologous tumor cells 14, 15.
One of the most effective vaccination methods for generating potent specific responses is based on recombinant viral vectors. Fowlpox virus (FP), a member of the avipox family, has been used as a live attenuated vaccine against poultry diseases 16. Recent interest has focused on this virus as a candidate mammalian vaccine due to its large cloning capacity, which can provide sustained expression of heterologous genes combined with an impeccable safety profile 17. A variety of TAAs have been incorporated into FP vectors attempting to elicit effective anti-tumoral immunity. Examples of TAAs inserted into FP include carcinoembyonic antigen, prostate-specific antigen, melanoma antigens and human papillomavirus antigens 18. Our study reveals that a variant of FP expressing the MM-relevant TAA survivin (FP-surv) induced an interferon-γ (IFN-γ) response in mice, mediated by survivin-specific CD8+ T cells. This response exerted a significant antitumor effect against subcutaneous and intraperitoneal experimental MM, without inducing autoimmune reactions.
MATERIALS AND METHODS
Human mesothelioma specimens
MM tissues were provided by Dr. H. Pass (New York University, New York, NY, USA). Diagnoses of mesothelioma were based on WHO criteria and confirmed in all instances by clinical, morphologic, and immunohistochemical data. Samples consisted of videothoracoscopy biopsy or surgical specimens, fixed in formalin and embedded in paraffin.
Cells
Murine AB12 MM cells were provided by Dr. B. W. Robinson (University of Western Australia, Nedlands, Western Australia, Australia) 19 and human REN MM cells were provided by Dr. A. Albelda (University of Pennsylvania, Philadelphia, PA, USA). Mill, Rob, Con, Phi and Ada human MM cells were characterized and provided by Dr. H. Pass 20. Mouse mastocytoma P815 cells, Hmeso MM cells and HeLa cells were obtained from American Type Culture Collection. Human mesothelial (HM) cultures were obtained from pleural fluids of noncancerous donors and characterized as described 21. CRH5, CRH11, EKKH3, EKKH5, EOH8 and EOH9 murine MM cells were isolated from peritoneal ascites developed in asbestos- or erionite-injected mice in carcinogenesis experiments defined below and characterized as described 22. All cells were cultured in Ham's F12 medium containing 10% FBS and antibiotics. Growth rates were evaluated by MTT assay using Cell titer 96 (Promega, Madison, WI, USA).
Mice
Female BALB/c and C57BL/6 mice at 4–8 weeks of age were purchased from Charles River (Hollister, CA, USA) and maintained in a specific pathogen-free animal facility for at least 1 week before experiments. Procedures were performed in accordance with institutional guidelines approved by University of Hawaii Institutional Animal Care and Use Committee (IACUC).
Production of FP recombinants
A plasmid encoding the human survivin gene (kindly provided by Dr. D. Altieri, Winstar Institute, Philadelphia, PA, USA) was amplified by PCR using primers designed to insert 3' AscI and 5' BamHI restriction sites. The amplicon was cloned into a cassette encoding enhanced GFP (eGFP) in the Transfer Plasmid Green (TPG) 23, 24. A recombinant FP expressing survivin (FP-surv) was then generated by a swapping event between the gene encoding a red fluorescent protein gene in the control acceptor FP (FP-ctrl) and the TPG plasmid cassette coding for both the eGFP and survivin as previously described 24.
Animal experiments
Fiber injection studies were performed in groups of 15 BALB/c mice as previously described 22 by intraperitoneum (i.p.) injection with one of the following fibers: crocidolite asbestos, Turkish erionite or US erionite. Crocidolite was purchased from the Union Internationale Contre le Cancer (UICC, Geneva, Switzerland). Erionites were provided by Dr. U. Dogan (University of Iowa, Iowa City, IA, USA). In immunotherapy studies, subcutaneous (s.c.) and i.p. mouse MM models were evaluated. In the s.c. model, 1×106 AB12, CRH5 or EKKH5 cells were injected in the hind flank in cohorts of 6 BALB/c mice. When tumors became palpable on day 7 (3–4 mm in diameter), mice received an intra-muscular (i.m.) injection of 1 × 107 PFU of FP-ctrl or FP-surv. A second dose of virus was injected 7 days later. Tumor size was measured weekly using a digital caliper until the first death was recorded. Survival was then followed until tumors reached volumes >1,500 mm3 for AB12 and CRH5 tumors and >100 mm3 for slow growing EKKH5 cells. For the i.p. model, AB12 or CRH5 cells (1 × 105 in 100 μl PBS) were injected i.p. in groups of 10 BALB/c mice. FP-ctrl and FP-surv vaccinations were initiated 7 days later, a timepoint resulting from in preliminary experiments that showed tumor nodules of 0.5 mm of diameter in the peritoneum. Mice were monitored weekly and euthanized at signs of morbidity. In immune response studies, groups of BALB/c or C57BL/6 mice were primed with 1 × 107 PFU of FP-ctrl or FP-surv. After 1 week, mice were boosted with the same dose of virus and sacrificed 5 days later to remove spleens and lymph nodes. All animal experiments were repeated at least once.
Western blotting
Cells were grown in 2% FBS for 24 h and immunoblotting was performed as previously described 25. Primary antibodies included anti-survivin clone 60.11 and anti-MHC-I clone EP1395Y (Abcam, Cambridge, MA, USA).
Immunohistochemistry
Immunohistochemical staining was performed as previously described 26 using anti-survivin antibody diluted 1:100 (clone D8; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-MHC-I 1:900 (clone F3; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or anti-CD8 1:500 (clone YTS169.4; AbCam, Cambridge, MA, USA). For each human specimen, survivin and MHC-I staining intensity (0, no staining; 1+, weak; 2+, moderate; and 3+, strong) was recorded. The staining intensity of tissue sections was calibrated to minimize background signals to improve the recognition of differences between tumor and normal tissue.
Flow cytometry analysis of H2 Kd molecules
Cells were cultured for 24 h with Ham's F12 supplemented with 2% FBS, washed and stained with anti-mouse H2 Kd clone 1.B.548 (Abcam, Cambridge, MA, USA) for 1 h at 4°C. Following incubation with secondary antibody conjugated with PE, cells were analyzed using a FACSAria (BD Biosciences, San Jose, CA, USA) Flow Cytometer and analyzed with FlowJo software.
Cytoplasmic staining of IFN-γ and ELISPOT assay
Spleen and lymph nodes cells of immunized mice (1×106) were stimulated for 24 h with 10 μg/mL survivin peptides in the presence of 5 IU/mL interleukin-2 (IL-2). For detection of IFN-γ in the cytoplasm Golgi-plug (BD Biosciences, San Jose, CA, USA) was added 6 hours before staining. Fixation/permeabilization kits were used in combination with CD3-FITC, CD8-APC, IFN-γ-PE antibodies (eBiosciences, San Diego, CA, USA). Data were collected using the FACScalibur flow cytometer (BD Biosciences, San Jose, CA, USA). Immunospot assay was performed using the mouse IFN-γ ELISPOT kit from R&D System (Minneapolis, MN, USA).
Detection of survivin reactive CD8+ T cells
Spleen and lymph nodes cells were isolated from C57BL/6 immunized mice. PE-labelled H2-Db pentamer (PENT) presenting the survivin20–28 epitope was purchased from Proimmune (Sarasota, FL, USA) and cellular staining protocol performed as recommended by the manufacturer. Briefly, 2 × 106 splenocytes were stained with 10 μL of PENT at room temperature for 10 min, washed and further incubated for 20 min with CD3-FITC, CD8-APC and CD16/32 PE-Cy5 (BD Biosciences). PENT+/CD8+ cells were analyzed on a FACSCalibur Flow Cytometer and analyzed using FlowJo software.
Isolation of tumor-infiltrating lymphocytes and flow cytometer analysis
AB12 and CRH5 tumors were removed 10 and 18 days after the boost vaccination, respectively. At these time points tumors were large enough for analysis but showed regression in response to vaccination. In s.c. tumor models, the entire tumor was excised and subjected to analysis. In the case of i.p. tumors, three to five tumor nodules were collected per mouse and combined for analysis. Tissues were prepared and stained as described 27 using the following antibodies: CD4-FITC, CD11c-PE, CD8-APC, NK1.1-PECy7 (from eBiosciences, San Diego, CA, USA), B220-PETR, CD11b-APCCy7 (from BD Biosciences, San Jose, CA, USA), Gr1-PercpCy5.5 (BioLegend). Live cells were distinguished from debris using LIVE/DEAD® violet cell viability dye (Invitrogen, Grand Island, NY, USA). Cells were then analyzed on a FACSAria (BD Biosciences, San Jose, CA, USA) Flow Cytometer and analyzed with FlowJo software.
Immunofluorescence
Frozen sections from AB12 and CRH5 tumors were blocked for endogenous biotin and then incubated overnight at 4°C with 1:300 rat anti-mouse CD8 antibodies (clone YTS169.4; Pierce, Rockford, IL, USA). Slides were then incubated for 2 hours with 1:200 donkey anti-rat antibodies conjugated with Alexa Fluor® 594. Expression of CD8 was evaluated with an Axioskop 2 plus fluorescent microscope (Zeiss, Thornwood, NY, USA). Percentage of CD8+ cells was evaluated on 10 fields with at least 100 cells in the same slide using ImageJ software.
Measurement of intra-tumor cytokine concentration and mRNA
Tumors were collected, weighed and homogenized in PBS. Supernatants were collected by centrifugation and cytokine levels were quantified with the cytometric bead assay mouse inflammation kit using the FACScalibur flow cytometer (BD Biosciences, San Jose, CA, USA). RNA extracted from tumor pellets using RNeasy Mini kit and RNase-free DNase I (all from Qiagen, Germantown, MD, USA) was used for synthesis of cDNA using Superscript III (Invitrogen, Grand Island, NY, USA) and oligo dT primers. For real-time PCR, 1 μL of cDNA was used in 10 μL reactions using Platinum SYBR Green qPCR SuperMix (Invitrogen, Grand Island, NY, USA) carried out in a LightCycler 2.0 thermal cycler (Roche, Indianapolis, IN, USA). Oligonucleotides used for PCR included primers specific for the housekeeping gene hypoxanthine phosphoribosyltransferase (hprt) and murine IL-6, IFN-γ, MCP-1, TNF-α, IL-10 and IL-12. Cycling conditions were used as suggested in the SYBR Green kit instructions and results analyzed using Relative Quantification Software (Roche, Indianapolis, IN, USA).
Safety and fertility evaluation
Histopathological analysis was performed on groups of BALB/c mice (n=5) vaccinated with two doses of FP-surv or FP-ctrl (1 week apart). Fifty days after the first vaccination, mice were euthanized and tissues removed. Paraffin embedded organs were H&E stained and evaluated for inflammation and tissue damage. To evaluate fertility, i) FP-vaccinated female BALB/c mice (n=9) were allowed to cohabitate with males, in a 3:1 breeding ratio; ii) FP-vaccinated male BALB/c mice (n=3) were allowed to cohabitate with females in a 1:3 breeding ratio. The days until parturition and number of pups were noted. The fertility of the same numbers of progeny males and females was also evaluated with the same protocol.
Statistical analyses
Statistical tests were performed using GraphPad Prism (GraphPad 5.0). Means of two groups were compared using two-tailed paired Student's t test. When more than two groups were compared, one-way ANOVA followed by the Bonferroni multiple comparison test was conducted. For survival studies, differences were evaluated using Kaplan-Meier curves with log-rank test. Statistical significance values are indicated in the figures.
RESULTS
Survivin and MHC-I are expressed in human MMs
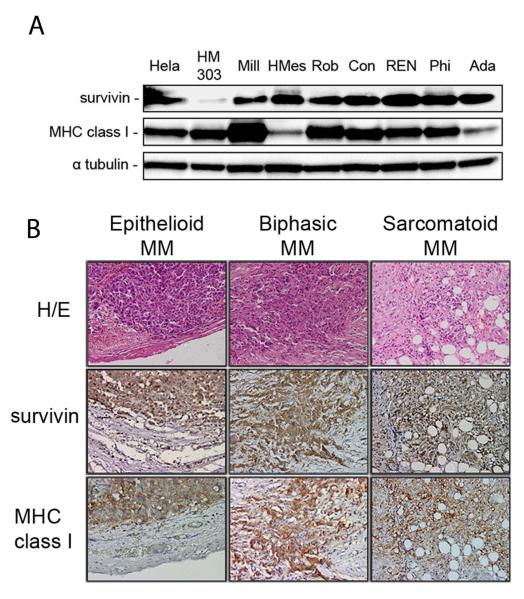
Because downmodulation of MHC-I occurs in some cancer cells as a means of escaping detection by CD8+ T cells, we first assessed whether survivin and MHC-I were both expressed in human MM cells. Immunoblotting of a panel of 7 human MM cell lines showed strong expression of survivin in all tumor cell lines while minor expression was observed in normal human mesothelial cells (HM) (Fig. 1A). Strong MHC-I expression was observed in 5 MM cell lines and in HM, while expression of MHC-I was reduced in the remaining MM cell lines, HMeso and Ada (Fig.1A). Expression of both survivin and MHC-I in MM cells, but not in HM, suggest that these cells may be attractive targets for survivin-specific cytolytic CD8+ T cells induced by vaccination.
Figure 1.
Survivin and MHC-I expression in human MM. (A) Immunoblotting with survivin and MHC-I antibodies on human mesothelial cells (HM) and 7 human MM cell lines. HM served as a normal tissue negative control and positive controls included HeLa cells known to express both survivin and MHC-I. (B) Immunohistochemical staining for survivin and MHC-I in different histological subtypes of human MM. Original magnification: 200x. Representative sections of epithelioid, biphasic and sarcomatoid MM tumors stained with H/E, survivin and MHC-I.
In view of these results, we evaluated the expression of survivin and MHC-I in MM and healthy adjacent tissues by immunohistochemistry. MM tissues analyzed from the three different histological types (epithelioid, biphasic and sarcomatoid) expressed survivin and MHC-I (Fig. 1B). Importantly, surrounding normal tissues had lower expression for survivin and negligible MHC-I, as detected by immunohistochemistry (see Material and Methods). Analysis of 17 MM specimens showed moderate to strong expression of survivin and MHC-I in tumor tissues as shown in Supplementary Table 1.
Survivin is expressed in mouse MMs
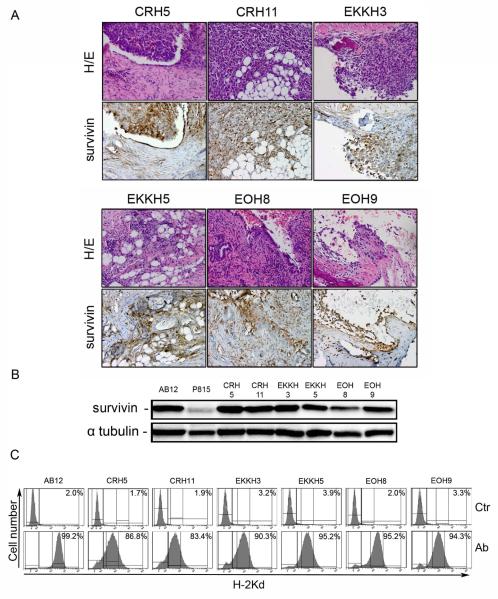
We investigated the expression of survivin in MM tumors arising in mice following asbestos or erionite inoculation. BALB/c mice were injected i.p. with a total of 4 mg of crocidolite asbestos, U.S. erionite or Turkish erionite. Mice started to develop MM 4 months after the first injection and within 2 years about 70% of mice injected with fibers died of MM, whereas no tumors were observed in control mice. Strong survivin expression was detected by immunohistochemistry in MM tumors excised from 6 mice injected with the different fibers: crocidolite (CRH5 and CRH11), Turkish erionite (EKKH3 and EKKH5), US erionite (EOH8 and EOH9) (Fig.2A). In all specimens normal surrounding tissues stained negative for survivin. Cells isolated from peritoneal ascites of these mice were used to establish 6 different MM cell lines. Immunoblotting on extracts from all cell lines revealed strong survivin expression, comparable with that of AB12 MM cells (Fig.2B). All cells established from mouse MM also expressed high levels of MHC-I (H-2Kd) on the cell surface as determined by flow cytometry (Fig.2C).
Figure 2.
Survivin and MHC-I expression in MM tumors induced in mice by i.p. injection of carcinogenic fibers. BALB/c mice were i.p. injected with PBS (control) or 4 mg of fibers: crocidolite, Turkish erionite or U.S. erionite. (A) Immunohistochemical staining for survivin in MM tumors from six different mice i.p. injected with crocidolite (CRH5, CRH11), Turkish erionite (EKKH3, EKKH5) or US erionite (EOH8, EOH9). Original magnification: 200x. H/E staining was performed to localize malignant cells, and sections from the same specimens were stained using survivin antibodies. (B) Immunoblotting with survivin antibodies of MM cells isolated from peritoneal fluid of fiber-injected mice. MM cells were isolated as described in Materials and Methods, placed in culture for 5 passages and then lysed. Positive and negative controls included AB12 mouse MM cell line and P815 mouse mastocytoma cell line, respectively, with α-tubulin used as loading control. (C) Frequency (as percentage of cell population) of H-2Kd expressing MM cells isolated from fiber-injected mice determined by flow cytometric analysis.
Vaccination with FP-surv elicits a potent anti-tumor response in subcutaneous mouse models of MM
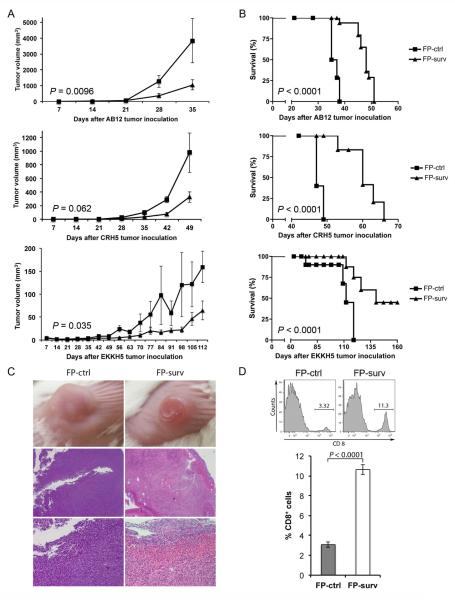
The high expression of survivin and MHC-I observed in MM prompted us to verify the potential anti-tumor effect of survivin vaccination. BALB/c mice were inoculated s.c. with AB12, or CRH5, or EKKH5. When tumors reached 3–4 mm in diameter, mice were immunized i.m. with 1×107 FP-surv or FP-ctrl (priming) followed by boost injections 7 days later. In control mice, tumors grew at rates that varied in a manner that correlated with the growth rates of the MM cells used to inoculate the mice. In particular, AB12 displayed a more rapid growth compared to the intermediate growth rate exhibited by CRH5 and the slowest tumor progression exhibited by EKKH5. These data were consistent with the relative in vitro proliferation rates (Supplementary Figure 1). Prime-boost vaccination with FP-surv resulted in a significantly reduced growth of the tumors compared to unvaccinated controls for all three MM cells (Fig.3A and Supplementary Figure 2). Survival analyses revealed that FP-surv immunized mice, carrying AB12 or CRH5 tumors, had significantly prolonged median survival compared with the controls, even in the cases where no complete tumor regression was observed. FP-surv vaccination of mice injected with EKKH5 cells led to increased survival (Fig. 3B), with complete tumor regression in 2 of 6 animals (Supplementary Figure 2C). Histological analyses of tumors from FP-surv vaccinated mice showed intra-tumor necrosis with a considerable abundance of infiltrating lymphoid cells. In contrast, tumors from control mice were highly vascularized and densely packed with no signs of necrosis (Fig.3C and Supplementary Figure 3). Flow cytometer analysis of AB12 tumor-derived cells revealed a significantly higher number of CD8+ T cells in FP-surv vaccinated mice compared with controls (Fig.3D).
Figure 3.
Survivin vaccination with Fowlpox vectors delays tumor growth and improves survival in s.c. mouse models of MM. BALB/c mice bearing palpable tumors (n = 6 for each group) were vaccinated with two i.m. injections of FP-surv or FP-ctrl (one week apart). Tumor volume (A) and animal survival (B) are shown for injection with three different cell lines: AB12 (Top), CRH5 (Middle) and EKKH5 (Bottom). Tumors were measured weekly and a Student's t test was used to compare means of each group. For survival, mice were followed until tumors reached volumes of 1,500 mm3 (AB12 and CRH5) or 100 mm3 (EKKH5) w. (C) Tumors from survivin-vaccinated mice exhibited intra-tumor necrosis with the presence of lymphoid cells. AB12 tumors from FP-ctrl and FP-surv vaccinated mice displayed different macroscopic appearance after 17 days from the first immunization (Top). Tumors were excised, paraffin embedded and stained with H/E. Representative AB12 tumor tissues at 20x (Middle) and 200x (Bottom) magnification. (D) Tumors were collected and enzyme-digested as described in the Materials and Methods section and stained with anti-CD8-APC. Live cells were distinguished from dead cells and debris using LIVE/DEAD® cell viability dye. Top: Flow cytometry analysis of representative data from AB12 tumors in survivin-vaccinated and control mice. Bottom: CD8+ T cells were counted in AB12 subcutaneous tumors. Results represent mean ± S.E. and statistical significance was determined by Student's t test (N = 5 mice/group).
Fp-surv vaccination induces antigen-specific T cell responses
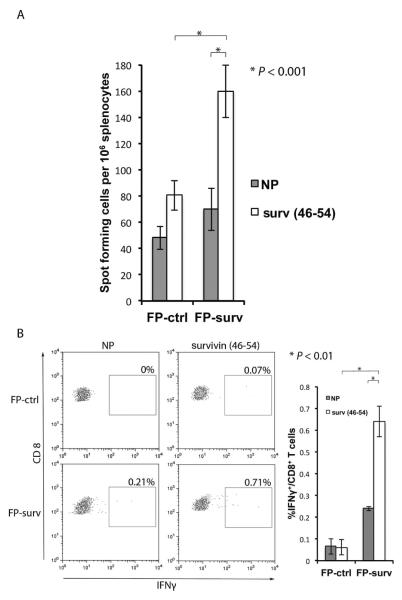
To determine if FP-surv vaccination induces survivin specific CD8+ T cells , we injected 2 groups of BALB/c mice with FP-surv or FP-ctrl and evaluated T cell responses using ELISPOT and intracellular cytokine staining (ICC) to detect IFN-γ6. Cells pooled from spleens and lymph nodes from immunized mice were re-stimulated with survivin46–54 peptide that, in our preliminary experiments, developed higher CD8+ T cell response when compared with other survivin-specific epitopes (Supplementary Figure 4). FP-surv vaccination induced significantly higher levels of IFN- γ -positive CD8+ T cells as detected by ELISPOT and ICC analyses (Fig.4).
Figure 4.
Induction of IFN-γ secreting CD8+ T lymphocytes by survivin vaccination. BALB/c mice were vaccinated with two i.m. injections of FP-surv or FP-ctrl (one week apart). Five days after the last vaccination spleens and inguinal lymph node cells were isolated and enumerated for assays. (A) Secretion of IFN-γ was evaluated by ELISPOT assay. T cells were activated with survivin46–54 peptide in the presence of 5 IU/mL IL-2 for 24 h or incubated with no peptide (NP). The results are presented as spot-forming cells per 106 cells and results represent the mean ± S.E. (N = 5 mice/group). (B) Intracellular IFN-γ in CD8+ T cells was evaluated by flow cytometric ICC analyses of spleen/lymph ± node cells cultured with or without survivin46–54 peptide. Left Panel: Representative data from flow cytometric analysis of survivin-vaccinated and control mice. Lymphocytes were either not stimulated (NP) or stimulated with survivin46–54 peptide. CD8+ T cells were distinguished using a marker gate in the CD3 vs. CD8 dot plot. Right panel: Percentage of IFN-γ expressing CD8+ T cells was evaluated with data are presented as mean ± S.E. (N = 5 mice/group). Statistical significance was determined by Student's t test in both ELISPOT and intracellular IFNγ staining assays.
To further verify the generation of survivin-specific CD8+ T cells by FP-surv, we used a pentamer reagent that labels survivin20–28 epitope-specific CD8+ T cells in spleens and lymph nodes from vaccinated and unvaccinated mice. Flow cytometry analysis revealed that a significantly higher number of survivin-specific CD8+ T cells were detected in FP-surv vaccinated mice compared with control mice (Supplementary Figure 5).
Vaccination with FP-surv improves survival in the i.p. mouse model of MM
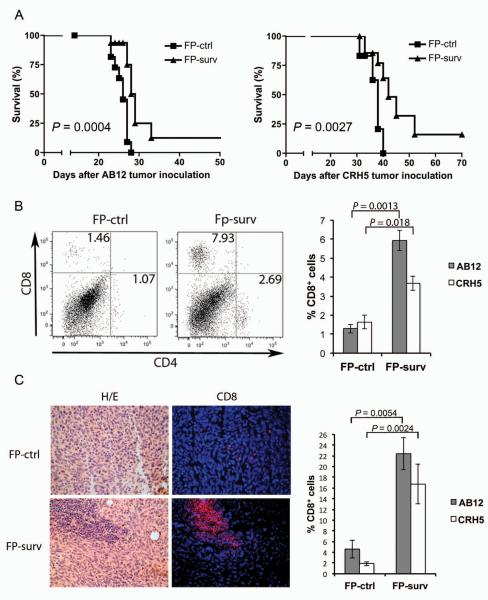
MM usually develops from mesothelial cells lining both thoracic and peritoneal cavities. To develop a clinically relevant MM model testing the therapeutic efficacy of FP-surv, we injected AB12 or CRH5 MM cells into the peritoneum of two groups of BALB/c mice. Seven days later, when tumors started growing and spreading within the peritoneal cavity, mice were primed with FP-surv or FP-ctrl and boosted with FP one week later. Survival analyses showed a significant increase (P=0.0004 and 0.0027 for AB12 and CRH5 injected mice respectively) in median survival of FP-surv vaccinated animals compared to control animals (Fig. 5A). Moreover, 10% of FP-surv vaccinated animals in both MM models displayed complete tumor remission (data not shown).
Figure 5.
Fowlpox vectors expressing survivin induce CD8+ T cell tumor infiltration which improve survival in i.p. mouse models of MM. (A) BALB/c mice were injected i.p. with AB12 (Left) or CRH5 (Right) MM cells (day 0). After 7 days, mice (n = 10 mice/group) were vaccinated with 1 × 107 PFU of FP-surv and boosted with the same vaccine 7 days later. FP-ctrl was used as control. Mice were followed for signs of distress and then sacrificed when morbidity was evident. Log-rank analysis was used to determine significance. (B) Tumors from survivin-vaccinated and control mice were collected and enzyme-digested and stained with APC-anti-mouse CD8 and FITC-anti-CD4 as described in Materials and Method. Live cells were distinguished from debris using LIVE/DEAD® cell viability dye. Left panel: Representative data from flow cytometric analysis of AB12 tumors in survivin-vaccinated and control mice. Right panel: Percentage of CD8+ T cells in AB12 and CRH5 tumors from survivin-vaccinated and control mice. Results represent mean ± S.E. (N = 5 mice/group) with means of each group compared using a Student's t test. (C) Frozen sections of tumors obtained from survivin-vaccinated and control mice were stained with H&E or used for immunofluorescence. Anti-CD8 in immunofluorescence analysis was detected with Alexa Fluor 594 (red) and nuclei detected with DAPI (blue). Both H&E and fluorescence images represent AB12 tumor tissues (400×). Right panel: Percentage CD8+ T cells were counted on 10 fields with at least 100 cells in the same slide. Values are expressed as the mean ± S.E. percentage of positive cells per total counted and statistical significance was determined by Student's t test.
FP-surv alters the tumor microenvironment by increasing the percentage of CD8+ T cells and immunostimulatory cytokines in i.p. MM
We next evaluated the effects of vaccination in the i.p. MM model for leukocyte cell populations in tumors using multicolor flow cytometer analysis, which allows the simultaneous identification of several different cell types in one sample27. We identified markers for different types of leukocytes including T cells (CD4 and CD8), B cells (B220), granulocytes (Gr-1), natural killer cells (NK1.1), monocytes/macrophages (CD11b) and dendritic cells (CD11c). A higher percent abundance in FP-surv vaccinated mice versus controls was found for CTLs (CD8+) and T helper cells (CD4+), although statistical significance was observed only for CTLs (Fig 5B). Cell types that did not increase in relative abundance included granulocytes, monocytes/macrophages, natural killer and dendritic cells, while B cells decreased in FP-surv vaccinated mice (data not shown). As an additional approach, immunofluorescence analysis was performed and a significant increase of CD8+ T cells was found in AB12 and CRH5 MM tumors from FP-surv vaccinated mice compared to unvaccinated controls (Fig. 5C and Supplementary Figure 6).
To evaluate the effect of FP-surv on the tumor microenvironment, cytokine mRNA levels were measured by real-time PCR in AB12 and CRH5 i.p. tumors (n = 5). As shown in Table 1, FP-surv induced higher mRNA levels of several inflammatory cytokines, with statistically significant increases found for IFN-γ and IL-12 in AB12 tumors, and for IFN-γ in CRH5 tumors. To determine protein levels of cytokines within the tumor microenvironment we also performed cytometric bead array analysis on homogenates of AB12 and CRH5 tumors. As shown in Table 1, values measured in AB12 and CRH5 tumors from FP-surv vaccinated versus control mice, confirmed significant increases in several cytokines. Consistent with the mRNA data, INF-γ protein was increased by vaccination for both tumors.
Table 1.
Quantification of cytokines and mRNA levels in Mesothelioma implanted tumors
| AB12 tumors | ||||
|---|---|---|---|---|
| Fold change in mRNA expression | Cytokine level (pg/mL) | |||
|
|
||||
| FP-ctrl | FP-surv | FP-ctrl | FP-surv | |
| IL-6 | 0.023 ± 0.006 | 0.041 ± 0.012 | 5.267 ± 0.606 | 10.76 ± 1.722 |
| IFN-γ | 5.780 ± 1.624 | 20.889 ± 8.201* | 1.516 ± 0.156 | 3.454 ± 0.455* |
| MCP-1 | 9.854 ± 1.275 | 17.398 ± 4.635 | 671.37 ± 130.87 | 1191.01 ± 293.40 |
| TNF-α | 0.830 ± 0.591 | 0.924 ± 0.264 | 1.618 ± 0.118 | 1.902 ± 0.156 |
| IL-10 | 3.271 ± 0.830 | 5.219 ± 2.267 | 5.701 ± 0.059 | 12.054 ± 0.051* |
| IL-12 | 0.124 ± 0.018 | 0.713 ± 0.137* | 11.334 ± 0.449 | 23.004 ± 1.313* |
| CRH5 tumors | ||||
|---|---|---|---|---|
| Fold change in mRNA expression | Cytokine level (pg/mL) | |||
|
|
||||
| FP-ctrl | FP-surv | FP-ctrl | FP-surv | |
| IL-6 | 0.005 ± 0.001 | 0.025 ± 0.017 | 0.652 ± 0.050 | 2.851 ± 0.758 |
| IFN-γ | 0.145 ± 0.051 | 1.025 ± 0.236* | 0.501 ± 0.256 | 3.654 ± 0.757* |
| MCP-1 | 2.664 ± 0.206 | 2.474 ± 0.228 | 113.12 ± 87.176 | 459.64 ± 9.385* |
| TNF-α | 0.192 ± 0.071 | 0.097 ± 0.010 | 1.267 ± 0.202 | 1.806 ± 0.157* |
| IL-10 | 0.031 ± 0.008 | 0.951 ± 0.603 | 5.304 ± 0.081 | 8.023 ± 0.118* |
| IL-12 | 0.489 ± 0.186 | 0.053 ± 0.011 | 9.756 ± 2.374 | 9.634 ± 3.158 |
NOTE: Mesothelioma tumors (n = 5 for each treatment group) from control and survivin-vaccinated mice were harvested, homogenized and cytokine levels were quantified. DNA-free RNA was extracted from each tumor sample, reverse transcribed and subjected to real-time RT-PCR analysis. RNA was normalized using a stable housekeeping RNA (HPRT).
P < 0.05
Survivin vaccination neither induces autoimmunity nor impairs fertility
To be clinically relevant, survivin specific CTLs induced by vaccination should not recognize and eliminate normal cells in which survivin is expressed at physiological levels. Given that survivin expression has been reported in some normal proliferating cells and during embryonic development28, we performed histopathological and fertility analyses in vaccinated mice to assess possible autoimmune reactions. Organs from vaccinated mice were removed after 50 days, embedded in paraffin and stained with H&E. Histopathological analysis showed no infiltration of inflammatory cells, tissue damage or any other sign of autoimmunity. Fertility was evaluated by breeding vaccinated females with unvaccinated males and vice versa. Vaccinated animals showed normal fertility rates and litters indicating that FP-surv vaccine did not negatively impact fertility. In addition, the fertility of the same numbers of progeny males and females was also evaluated with the same protocol and similar to the experiments above no negative effects on fertility were observed (data not shown).
DISCUSSION
In this study we show for the first time that FP vectors expressing survivin can induce an anti-tumor CTL response, which effectively targets tumor cells and limits progression of MM tumors. Expression of survivin has been documented in various types of human cancers, and often high levels of the protein have been associated with tumor progression and resistance to chemotherapy29, 30. Survivin overexpression has been reported in 75 of 112 pleural MM8 and more recently in 29 of 32 peritoneal MM9. Also the present study clearly indicated by immunohistochemistry that survivin was highly abundant in all the examined human MM lesions. This finding was consistent with the overexpression of survivin observed in all cell lines from MM patients included in our study. Survivin expression was consistently absent in normal tissues surrounding MM tumors and in HM as well. Similar results were found in tumor and cells from the animals where MM was induced by asbestos or erionite inoculation.
The development of clinical interventions based on vaccines have been limited so far from the lack of ubiquitous shared antigens in MM. Wilms' tumor-1 (WT-1) antigen is the only candidate antigen that has been targeted by vaccination in MM patients31. The fact that MM cells and tissues from different sources consistently express survivin and MHC-I suggested that a vaccination against survivin may be beneficial for the vast majority of MM patients. In the case of WT-1 vaccination, synthetic peptides were used to generated CD4+ and CD8+ responses32. We decided instead to use recombinant FP vectors. Vaccines based on recombinant vectors induce a strong inflammatory response, directed mainly towards the vector proteins. In turn, this inflammatory response leads to an increased immune response against the protein encoded by the gene of interest inserted into the vector. An advantage of using viral vectors over proteins administered with adjuvants is that the vector-encoded protein is more immunogenic and is expressed within cells for generation of strong CD8+ T cell responses. Pox viral vectors are among the most exploited in vaccine development. Specifically, FP has been shown promising as a vaccine for certain types of infectious diseases and cancers33, 34. FP infection leads to effective antigen presentation by antigen presenting cells, and the generation of antigen-specific CTLs17. Therefore, we generated a FP vector expressing human survivin and evaluated its potential in suppressing MM tumors in mice. The decision to use the human gene in a mouse model may be considered controversial. However, our choice was supported by the fact that the human survivin protein is 84% identical and 91% homologous to the mouse survivin protein35. Furthermore, when human and mouse protein sequences were analyzed by the HLA Peptide Binding Prediction algorithms BIMAS36 and SYFPEITHI 37 identical HLA peptide binding motifs were generated. In this sense, trials in humans may include mouse along with human survivin for more efficacious immunotherapeutic effects. However, due to the differences between human and mouse survivin sequences, the immunogeneicity generated by our vaccine still needs to be carefully evaluated, before the results of this study can be translated to vaccine development for human MM.
In our experiments, FP-surv induced an increased number of tumor-infiltrating CD8+ T cells that correlated with improved animal survival and slower tumor growth in 3 different subcutaneous MM mouse models. Our data suggest a correlation between FP-surv-induced CD8+ T cell responses and survival. However, further experiments utilizing blocking antibody or CD8+ T cell-deficient mice would provide more direct evidence of the role of these effector cells in increasing survival. Moreover, our functional studies were limited to IFN-γ production, and follow-up studies are on-going to determine the involvement of tumor-specific killing mechanisms. The increased number of tumor-infiltrating CD8+ T cells likely included a relevant number of survivin-specific T cells that controlled tumor growth. This hypothesis was supported by the results of ELISPOT and ICC analyses of lymphoid cells from BALB/c FP-surv vaccinated mice that showed a significantly higher IFN-γ production, after survivin peptide in vitro re-stimulation, compared to lymphoid cells from control vaccinated mice. Our findings showed specific T cell responses indeed, which are generated against two different survivin epitopes included in the FP-surv vaccines. Both survivin46–54 and survivin20–28 peptides generated specific T cell responses as determined by intracellular cytokine staining and PENT analyses, respectively. These epitopes have been reported to be immunogenic 38, 39 and our data support this notion. Additional evidence of the generation of survivin specific CTLs by FP-surv came from the staining of splenocytes and lymph node cells from immunized C57BL/6 mice with survivin-specific pentamers. Studies examining the effectiveness of the FP-surv on generating memory T cell responses in currently underway. Also, while the number of NK cells did not change with FP-surv vaccination, it cannot be ruled out that their killing capacity was increased with vaccination and significantly contributed to reduced tumor progression. A further investigation is ongoing to address this relevant issue.
Although survivin vaccination induced complete tumor regression in 33% of mice injected i.p. with EKKH5 MM cells, the majority of vaccinated mice died because of the tumor mass growth. However, in these mice tumor growth was significantly delayed significantly and, in most cases, tumors contained both areas with signs of necrosis exhibiting complete cellular degradation and a small rim or viable tumor cells. This pattern, which was not observed in unvaccinated mice and may be due to the suppression of angiogenesis in the tumor neovasculature. As a matter of fact, proliferating endothelial cells, triggered by tumor cells, overexpress survivin and have been reported as targeted of CTL lysis in survivin vaccinated mice 38. These possible effects need to be confirmed experimentally.
We first investigated the survivin vaccination in mice by s.c. injection of MM cells, because tumor appearance and dimensions can be easily evaluated over time. More clinically relevant experiments were subsequently performed in mice injected i.p. with AB12 or CRH5 MM cells. When vaccinated with FP-surv these animals displayed better survival rates than controls with 10% of mice exhibiting complete tumor regression. This effect was associated with an increased number of infiltrating CD8+ T cells as detected by flow cytometer analysis and immunofluorescence. In addition, FP-surv vaccination altered the tumor microenvironment resulting in a significant increase in IFN-γ expression in both AB12 and CRH5 tumors. IFN-γ produced at the tumor site is important in stimulating the expression of MHC-I and -II molecules by malignant cells, facilitating the immune response eventually leading to tumor clearance 40. Other immunostimulatory cytokine levels were increased in tumors from survivin vaccinated mice. Although these data did not perfectly match those obtained by RT-PCR and several concerns have been raised on the usefulness of intra-tumor cytokine protein measurements 41, 42, our cytokine profiles suggest that FP-surv is capable of inducing immunologic changes in the tumor microenvironment that may favor for the killing of MM cells.
Importantly, we also performed fertility and histopathological analyses to evaluate autoimmunity. Vaccination of mice against survivin neither affected fertility nor induced tissue damage in healthy organs. Taken together, these results suggest that FP vectors expressing survivin induce specific CTLs and this vaccine strategy effectively suppresses MM tumor growth in vivo without induction of autoimmune response. This vaccine may serve as a basis for the rational design of future treatment and/or prevention strategies for human MM.
Supplementary Material
Novelty and Impact.
Malignant mesothelioma (MM) is a deadly cancer. We show that a Fowlpox-based survivin vaccine (FP-surv) is effective in pre-clinical animal MM models. FP-surv may be used to induce tumor regression in patients with MM at the early stages of disease. In the case of most late-state tumors, tumor burden can be reduced with FP-surv that provides stimulation to the immune system for eliminating any remaining cancer cells after chemotherapy or surgery.
AKNOWLEDGEMENTS
The authors thank Martin W. Kast and Luciano Mutti for the constructive discussions and Fukun Hoffmann for excellent technical assistance.
This research was supported by NIH grants R01AI089999 (P.H.), by NCI grants P01CA114047 (M.C.) and R01CA160715 (H.Y.), by Hawaii Community Foundation grants 12ADVC-51365 (P.B.) and 10ADVC-47420 (G.G.), by the Buttitta Mesothelioma Foundation (M.C.), by the INAIL foundation (A.S.) and by Buzzi Unicem Foundation for the study of Mesothelioma (A.S.). Core facilities supported by P20GM103516, G12RR003061, G12MD007601.
Abbreviations
- MM
Malignant Mesothelioma
- FP-surv
Fowlpox-based survivin vaccine
- CD
Cluster of Differentiation
- PCR
Polymerase Chain Reaction
- ELISPOT
Enzyme-linked immunosorbent spot
- TAAs
tumor-associated antigens
- IAPs
Inhibitory Apoptotic Proteins
- MHC-I
Major Histocompatibility Complex class I
- CTLs
Cytotoxic T Lymphocytes
- HLA
Human Leukocyte Antigen
- FP
Fowlpox virus
- IFN-γ
Interferon-γ
- WHO
World Health Organization
- HM
Human Mesothelial cells
- FBS
Fetal Bovine Serum
- MTT
(3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- IACUC
Institutional Animal Care and Use Committee
- GFP
Green Fluorescent Protein
- eGFP
enhanced GFP
- TPG
Transfer Plasmid Green
- FP-ctrl
control FP
- PFU
plaque-forming unit
- PBS
Phosphate buffered saline
- PE
Phycoerythrin
- IL-2
Interleukin-2
- PENT
PE-labelled H2-Db pentamer
- MCP-1
monocyte chemotactic protein-1
- TNF
Tumor necrosis factor
- H&E
Hematoxylin and eosin
- ANOVA
analysis of variance
- ICC
intracellular cytokine staining
Footnotes
No potential conflicts of interest were disclosed.
REFERENCES
- 1.Carbone M, Yang H. Molecular pathways: targeting mechanisms of asbestos and erionite carcinogenesis in mesothelioma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:598–604. doi: 10.1158/1078-0432.CCR-11-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertino P, Carbone M, Pass H. Chemotherapy of malignant pleural mesothelioma. Expert opinion on pharmacotherapy. 2009;10:99–107. doi: 10.1517/14656560802631285. [DOI] [PubMed] [Google Scholar]
- 3.Anraku M, Cunningham KS, Yun Z, Tsao MS, Zhang L, Keshavjee S, Johnston MR, de Perrot M. Impact of tumor-infiltrating T cells on survival in patients with malignant pleural mesothelioma. The Journal of thoracic and cardiovascular surgery. 2008;135:823–9. doi: 10.1016/j.jtcvs.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 4.Yamada N, Oizumi S, Kikuchi E, Shinagawa N, Konishi-Sakakibara J, Ishimine A, Aoe K, Gemba K, Kishimoto T, Torigoe T, Nishimura M. CD8+ tumor-infiltrating lymphocytes predict favorable prognosis in malignant pleural mesothelioma after resection. Cancer immunology, immunotherapy : CII. 2010;59:1543–9. doi: 10.1007/s00262-010-0881-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lladser A, Sanhueza C, Kiessling R, Quest AF. Is survivin the potential Achilles' heel of cancer? Advances in cancer research. 2011;111:1–37. doi: 10.1016/B978-0-12-385524-4.00001-5. [DOI] [PubMed] [Google Scholar]
- 6.Nagaraj S, Pisarev V, Kinarsky L, Sherman S, Muro-Cacho C, Altieri DC, Gabrilovich DI. Dendritic cell-based full-length survivin vaccine in treatment of experimental tumors. J Immunother. 2007;30:169–79. doi: 10.1097/01.cji.0000211329.83890.ba. [DOI] [PubMed] [Google Scholar]
- 7.Jin L, Amatya VJ, Takeshima Y, Shrestha L, Kushitani K, Inai K. Evaluation of apoptosis and immunohistochemical expression of the apoptosis-related proteins in mesothelioma. Hiroshima journal of medical sciences. 2010;59:27–33. [PubMed] [Google Scholar]
- 8.Kleinberg L, Lie AK, Florenes VA, Nesland JM, Davidson B. Expression of inhibitor-of-apoptosis protein family members in malignant mesothelioma. Human pathology. 2007;38:986–94. doi: 10.1016/j.humpath.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Zaffaroni N, Costa A, Pennati M, De Marco C, Affini E, Madeo M, Erdas R, Cabras A, Kusamura S, Baratti D, Deraco M, Daidone MG. Survivin is highly expressed and promotes cell survival in malignant peritoneal mesothelioma. Cellular oncology : the official journal of the International Society for Cellular Oncology. 2007;29:453–66. doi: 10.1155/2007/456839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Idenoue S, Hirohashi Y, Torigoe T, Sato Y, Tamura Y, Hariu H, Yamamoto M, Kurotaki T, Tsuruma T, Asanuma H, Kanaseki T, Ikeda H, et al. A potent immunogenic general cancer vaccine that targets survivin, an inhibitor of apoptosis proteins. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11:1474–82. doi: 10.1158/1078-0432.CCR-03-0817. [DOI] [PubMed] [Google Scholar]
- 11.Andersen MH, Svane IM, Becker JC, Straten PT. The universal character of the tumor-associated antigen survivin. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:5991–4. doi: 10.1158/1078-0432.CCR-07-0686. [DOI] [PubMed] [Google Scholar]
- 12.Wobser M, Keikavoussi P, Kunzmann V, Weininger M, Andersen MH, Becker JC. Complete remission of liver metastasis of pancreatic cancer under vaccination with a HLA-A2 restricted peptide derived from the universal tumor antigen survivin. Cancer immunology, immunotherapy : CII. 2006;55:1294–8. doi: 10.1007/s00262-005-0102-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hadrup SR, Gehl J, Sorensen RB, Geertsen PF, Straten PT, Andersen MH. Persistence of survivin specific T cells for seven years in a melanoma patient during complete remission. Cancer biology & therapy. 2006;5:480–2. doi: 10.4161/cbt.5.5.2652. [DOI] [PubMed] [Google Scholar]
- 14.Miyazaki A, Kobayashi J, Torigoe T, Hirohashi Y, Yamamoto T, Yamaguchi A, Asanuma H, Takahashi A, Michifuri Y, Nakamori K, Nagai I, Sato N, et al. Phase I clinical trial of survivin-derived peptide vaccine therapy for patients with advanced or recurrent oral cancer. Cancer science. 2011;102:324–9. doi: 10.1111/j.1349-7006.2010.01789.x. [DOI] [PubMed] [Google Scholar]
- 15.Honma I, Kitamura H, Torigoe T, Takahashi A, Tanaka T, Sato E, Hirohashi Y, Masumori N, Tsukamoto T, Sato N. Phase I clinical study of antiapoptosis protein survivin-derived peptide vaccination for patients with advanced or recurrent urothelial cancer. Cancer immunology, immunotherapy : CII. 2009;58:1801–7. doi: 10.1007/s00262-009-0691-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weli SC, Tryland M. Avipoxviruses: infection biology and their use as vaccine vectors. Virology journal. 2011;8:49. doi: 10.1186/1743-422X-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diener KR, Lousberg EL, Beukema EL, Yu A, Howley PM, Brown MP, Hayball JD. Recombinant fowlpox virus elicits transient cytotoxic T cell responses due to suboptimal innate recognition and recruitment of T cell help. Vaccine. 2008;26:3566–73. doi: 10.1016/j.vaccine.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 18.Beukema EL, Brown MP, Hayball JD. The potential role of fowlpox virus in rational vaccine design. Expert review of vaccines. 2006;5:565–77. doi: 10.1586/14760584.5.4.565. [DOI] [PubMed] [Google Scholar]
- 19.Davis MR, Manning LS, Whitaker D, Garlepp MJ, Robinson BW. Establishment of a murine model of malignant mesothelioma. International journal of cancer. Journal international du cancer. 1992;52:881–6. doi: 10.1002/ijc.2910520609. [DOI] [PubMed] [Google Scholar]
- 20.Pass HI, Stevens EJ, Oie H, Tsokos MG, Abati AD, Fetsch PA, Mew DJ, Pogrebniak HW, Matthews WJ. Characteristics of nine newly derived mesothelioma cell lines. The Annals of thoracic surgery. 1995;59:835–44. doi: 10.1016/0003-4975(95)00045-m. [DOI] [PubMed] [Google Scholar]
- 21.Bocchetta M, Di Resta I, Powers A, Fresco R, Tosolini A, Testa JR, Pass HI, Rizzo P, Carbone M. Human mesothelial cells are unusually susceptible to simian virus 40-mediated transformation and asbestos cocarcinogenicity. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:10214–9. doi: 10.1073/pnas.170207097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kroczynska B, Cutrone R, Bocchetta M, Yang H, Elmishad AG, Vacek P, Ramos-Nino M, Mossman BT, Pass HI, Carbone M. Crocidolite asbestos and SV40 are cocarcinogens in human mesothelial cells and in causing mesothelioma in hamsters. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:14128–33. doi: 10.1073/pnas.0604544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Lullo G, Soprana E, Panigada M, Palini A, Agresti A, Comunian C, Milani A, Capua I, Erfle V, Siccardi AG. The combination of marker gene swapping and fluorescence-activated cell sorting improves the efficiency of recombinant modified vaccinia virus Ankara vaccine production for human use. Journal of virological methods. 2010;163:195–204. doi: 10.1016/j.jviromet.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Soprana E, Panigada M, Knauf M, Radaelli A, Vigevani L, Palini A, Villa C, Malnati M, Cassina G, Kurth R, Norley S, Siccardi AG. Joint production of prime/boost pairs of Fowlpox Virus and Modified Vaccinia Ankara recombinants carrying the same transgene. Journal of virological methods. 2011;174:22–8. doi: 10.1016/j.jviromet.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Bertino P, Porta C, Barbone D, Germano S, Busacca S, Pinato S, Tassi G, Favoni R, Gaudino G, Mutti L. Preliminary data suggestive of a novel translational approach to mesothelioma treatment: imatinib mesylate with gemcitabine or pemetrexed. Thorax. 2007;62:690–5. doi: 10.1136/thx.2006.069872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bertino P, Piccardi F, Porta C, Favoni R, Cilli M, Mutti L, Gaudino G. Imatinib mesylate enhances therapeutic effects of gemcitabine in human malignant mesothelioma xenografts. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:541–8. doi: 10.1158/1078-0432.CCR-07-1388. [DOI] [PubMed] [Google Scholar]
- 27.Hoffmann PR, Gurary A, Hoffmann FW, Jourdan-Le Saux C, Teeters K, Hashimoto AC, Tam EK, Berry MJ. A new approach for analyzing cellular infiltration during allergic airway inflammation. Journal of immunological methods. 2007;328:21–33. doi: 10.1016/j.jim.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Connor DS, Schechner JS, Adida C, Mesri M, Rothermel AL, Li F, Nath AK, Pober JS, Altieri DC. Control of apoptosis during angiogenesis by survivin expression in endothelial cells. The American journal of pathology. 2000;156:393–8. doi: 10.1016/S0002-9440(10)64742-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altieri DC. Survivin and apoptosis control. Advances in cancer research. 2003;88:31–52. doi: 10.1016/s0065-230x(03)88303-3. [DOI] [PubMed] [Google Scholar]
- 30.Zaffaroni N, Pennati M, Daidone MG. Survivin as a target for new anticancer interventions. Journal of cellular and molecular medicine. 2005;9:360–72. doi: 10.1111/j.1582-4934.2005.tb00361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bagia M, Nowak AK. Novel targeted therapies and vaccination strategies for mesothelioma. Current treatment options in oncology. 2011;12:149–62. doi: 10.1007/s11864-011-0149-1. [DOI] [PubMed] [Google Scholar]
- 32.Krug LM, Dao T, Brown AB, Maslak P, Travis W, Bekele S, Korontsvit T, Zakhaleva V, Wolchok J, Yuan J, Li H, Tyson L, et al. WT1 peptide vaccinations induce CD4 and CD8 T cell immune responses in patients with mesothelioma and non-small cell lung cancer. Cancer immunology, immunotherapy : CII. 2010;59:1467–79. doi: 10.1007/s00262-010-0871-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vergati M, Intrivici C, Huen NY, Schlom J, Tsang KY. Strategies for cancer vaccine development. Journal of biomedicine & biotechnology. 2010;2010 doi: 10.1155/2010/596432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skinner MA, Laidlaw SM, Eldaghayes I, Kaiser P, Cottingham MG. Fowlpox virus as a recombinant vaccine vector for use in mammals and poultry. Expert review of vaccines. 2005;4:63–76. doi: 10.1586/14760584.4.1.63. [DOI] [PubMed] [Google Scholar]
- 35.Li F, Altieri DC. The cancer antiapoptosis mouse survivin gene: characterization of locus and transcriptional requirements of basal and cell cycle-dependent expression. Cancer research. 1999;59:3143–51. [PubMed] [Google Scholar]
- 36.Parker KC, Bednarek MA, Coligan JE. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J Immunol. 1994;152:163–75. [PubMed] [Google Scholar]
- 37.Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanovic S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics. 1999;50:213–9. doi: 10.1007/s002510050595. [DOI] [PubMed] [Google Scholar]
- 38.Lladser A, Ljungberg K, Tufvesson H, Tazzari M, Roos AK, Quest AF, Kiessling R. Intradermal DNA electroporation induces survivin-specific CTLs, suppresses angiogenesis and confers protection against mouse melanoma. Cancer immunology, immunotherapy : CII. 2010;59:81–92. doi: 10.1007/s00262-009-0725-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang H, Wang Y, Liu C, Zhang L, Xia Q, Zhang Y, Wu J, Jiang C, Chen Y, Wu Y, Zha X, Yu X, et al. DNA and adenovirus tumor vaccine expressing truncated survivin generates specific immune responses and anti-tumor effects in a murine melanoma model. Cancer immunology, immunotherapy : CII. 2012;61:1857–67. doi: 10.1007/s00262-012-1296-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmitz-Winnenthal FH, Escobedo LV, Beckhove P, Schirrmacher V, Bucur M, Ziouta Y, Volk C, Schmied B, Koch M, Antolovic D, Weitz J, Buchler MW, et al. Specific immune recognition of pancreatic carcinoma by patient-derived CD4 and CD8 T cells and its improvement by interferon-gamma. International journal of oncology. 2006;28:1419–28. doi: 10.3892/ijo.28.6.1419. [DOI] [PubMed] [Google Scholar]
- 41.Kim S, Buchlis G, Fridlender ZG, Sun J, Kapoor V, Cheng G, Haas A, Cheung HK, Zhang X, Corbley M, Kaiser LR, Ling L, et al. Systemic blockade of transforming growth factor-beta signaling augments the efficacy of immunogene therapy. Cancer research. 2008;68:10247–56. doi: 10.1158/0008-5472.CAN-08-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia-Hernandez Mde L, Gray A, Hubby B, Klinger OJ, Kast WM. Prostate stem cell antigen vaccination induces a long-term protective immune response against prostate cancer in the absence of autoimmunity. Cancer research. 2008;68:861–9. doi: 10.1158/0008-5472.CAN-07-0445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.