Abstract
Certain chemotherapeutic regimens trigger cancer cell death while inducing dendritic cell maturation and subsequent immune responses. However, chemotherapy-induced immunogenic cell death (ICD) has thus far been restricted to select agents. In contrast, several chemotherapeutic drugs modulate antitumor immune responses, despite not inducing classic ICD. In addition, in many cases tumor cells do not die after treatment. Here, using docetaxel, one of the most widely used cancer chemotherapeutic agents, as a model, we examined phenotypic and functional consequences of tumor cells that do not die from immunogenic cell death. Docetaxel treatment of tumor cells did not induce ATP or HMGB1 secretion, or cell death. However, calreticulin exposure was observed in all cell lines examined after chemotherapy treatment. Killing by CEA, MUC-1, or PSA-specific CD8+ CTLs was significantly enhanced after docetaxel treatment. This killing was associated with increases in components of antigen-processing machinery, and mediated largely by calreticulin membrane translocation, as determined by functional knockdown of calreticulin, PERK, or calreticulin-blocking peptide. A docetaxel-resistant cell line was selected (MDR-1+, CD133+) by continuous exposure to docetaxel. These cells, while resistant to direct cytostatic effects of docetaxel, were not resistant to the chemomodulatory effects that resulted in enhancement of CTL killing. Here, we provide an operational definition of “immunogenic modulation,” where exposure of tumor cells to nonlethal/sublethal doses of chemotherapy alters tumor phenotype to render the tumor more sensitive to CTL killing. These observations are distinct and complementary to immunogenic cell death and highlight a mechanism whereby chemotherapy can be used in combination with immunotherapy.
Keywords: Docetaxel, immunogenic cell death, immunogenic modulation, calreticulin, antigen-processing machinery, T-cell response
Introduction
Anticancer therapies such as chemotherapy and radiation aim for direct killing of tumor cells. However, recent studies have demonstrated that these modalities may have immunomodulatory effects, through direct action on tumor cells or on cells of the immune system 1, 2. Docetaxel, a member of the taxane family of drugs, exhibits broad antitumor activity by microtubule stabilization and is currently indicated for the treatment of multiple cancer types. We previously reported that docetaxel modulated CD4+, CD8+, CD19+, natural killer cell, and T-regulatory (Treg) populations in nontumor-bearing mice, and enhanced IFN-γ production by CD8+ T cells 2. We also showed that docetaxel combined with vaccine increased antigen-specific T-cell responses to the antigen expressed by the vaccine and was superior to either agent alone at reducing tumor burden. Here, we examine the ability of this taxane to alter the phenotype of human tumor cells and increase their susceptibility to CD8+ CTL-mediated killing. We also provide an operational definition of “immunogenic modulation,” whereby exposure of tumor cells to nonlethal/sublethal doses of chemotherapy (due to resistance or low-dose delivery) alters tumor phenotype to render the tumor more sensitive to CTL killing.
We hypothesized that immunogenic modulation of tumor-cell phenotype and antigen-processing machinery (APM) by docetaxel could enhance productive interactions between CD8+ CTLs and cancer cells. In this study, we examined the effects of docetaxel on molecules that have been implicated in enhancing T cell-mediated tumor-cell killing through diverse mechanisms, including calreticulin (CRT), Fas, the adhesion/costimulatory molecule ICAM-1, MHC class I, the human tumor antigens carcinoembryonic antigen (CEA) and mucin-1 (MUC-1), and components of the antigen-processing chain 3. In addition, we determined that cells resistant to the direct cytostatic effects of docetaxel were still modified by the drug in a manner that resulted in enhanced lysis by CTLs, compared with untreated cells.
These studies are the first to report a) an evaluation of hallmarks of immunogenic cell death (ICD) and immunogenic modulation following docetaxel treatment of prostate, breast, and colon cancer cell lines, b) the use of docetaxel to functionally enhance antigen-specific CTL-mediated killing, c) the use of docetaxel to functionally enhance antigen-specific CTL-mediated killing of a docetaxel-resistant tumor cell line by modulation of APM, and d) the functional role of CRT in immunogenic modulation. These observations are distinct and complementary to those of immunogenic cell death and highlight a mechanism whereby chemotherapy can be used in combination with active immunotherapy.
Materials and Methods
Tumor cell lines
Cells of human prostate carcinoma (LNCaP), breast carcinoma (MCF-7, MDA-231), and colorectal carcinoma (SW620, LS174T) were obtained from American Type Culture Collection (Manassas, VA) and cultured in media designated by the provider for propagation and maintenance. Cells were incubated at 37°C with 5% CO2.
Chemotherapy
Preparations of docetaxel (Taxotere®, NDC 0075-8003-01; Sanofi Aventis, Bridgewater, NJ) were diluted to 1 mg/mL in phosphate-buffered saline (PBS). The drug was then further diluted in sterile PBS to a working stock of 10 μg/mL. For certain studies, mitoxantrone (1 μM, NDC 61703-343-65; Hospira, Lake Forest, IL) was used as a positive control for ICD. Sterile PBS was added to control samples.
Analysis of immunogenic cell death
Cells treated with chemotherapy were harvested and assessed for viability by 7AAD staining and cell-surface expression of CRT (see below). Supernatant fluids were analyzed for high-mobility group box 1 (HMGB1) protein by ELISA (IBL International, Hamburg, Germany), and for ATP by bioluminesence (Sigma, St. Louis, MO), according to the manufacturer. In all cases, starting (day 0) cell number and media volume was controlled across all 4 cell lines and all 4 assays.
Flow cytometry analysis
Cell surface staining of tumor cells was performed using the primary labeled monoclonal antibodies (mAb) CD95-FITC, CD54-PE, CD66-FITC, CD227-FITC, HLA-ABC-PE (BD Biosciences, San Diego, CA), and calreticulin-PE (R&D Systems, Minneapolis, MN). The appropriate isotype-matched controls were purchased from BD Biosciences. CD133-APC was purchased from Miltenyi Biotech (Auburn, CA). MDR-1 (ABCB1) was purchased from eBioscience (San Diego, CA). Proteins were scored as upregulated if detection levels increased by ≥ 10% or if mean fluorescence intensity (MFI) increased by ≥ 30% following docetaxel treatment. Stained cells were acquired on a FACScan or FacsCalibur flow cytometer using CellQuest software (BD Biosciences). Isotype control staining was < 5% for all samples analyzed. For analysis of APM, LMP2-specific mAb SY-1; TAP-1-specific mAb NOB1; TAP-2-specific mAb NOB2; calnexin-specific mAb TO-5; ERp57-specific mAb TO-2; and tapasin-specific mAb TO-3 were developed and characterized as described 4. Mouse IgG1 mAb isotype control was obtained from BD Biosciences. Cell surface and intracytoplasmic staining of cells was performed as described 5.
In vivo studies
Studies were conducted with 6- to 8-week-old female nude mice (nu/nu) (Charles River, Wilmington, MA). LNCaP tumor cells (8 × 106) were admixed 1:1 with Matrigel (BD Biosciences) and injected s.c. When tumors reached a volume of 1000 mm3, mice were given 0.5 mg of docetaxel or PBS i.p. once every other day over a period of 5 days, equivalent to 75–100 mg/m2. On day 7, tumors were surgically removed and examined by flow cytometry and histochemistry. For flow cytometry, tumors were processed into single-cell suspensions and staining was performed as described above. For immunohistochemistry, fresh tissues were immersed in the buffered zinc fixative Z-fix (Anatech LTD, Battle Creek, MI) and embedded. 5-μm paraffin sections were cut and incubated with serum block, followed by anti-CEA mAb (COL-1) 6, anti-MUC-1 mAb (DF-3) 7, anti-CRT (clone FMC75, Abcam, Cambridge, MA) or isotype-matched control antibodies, followed by ABC peroxidase (Vector Labs, Burlingame, CA) and diaminobenzidine. The sections were rinsed and counterstained with hematoxylin. The entire slides were digitally scanned by an Aperio ScanScope CS scanning system (Aperio Technologies Inc., Vista, CA) and analyzed by the Aperio ImageScope Viewer software. Necrotic regions were excluded from analysis. The positive pixel count v9 algorithm was used to measure CEA+, MUC-1+, or calreticulin+ tumor regions. For CRT membrane staining, the membrane algorithm was used.
CTL lines
The HLA-A2-restricted, CEA-specific, CD8+ cytotoxic T-cell line (designated CEA CTL) recognizes the CEA peptide epitope YLSGANLNL (CAP-1) 8. It was maintained and propagated as previously described 9. The HLA-A2-restricted, prostate-specific antigen (PSA)-specific, CD8+ CTL line (designated PSA CTL) recognizes the PSA peptide epitope VLSNDVCAQV 7. The HLA-A2-restricted, MUC-1-specific, CD8+ CTL line (designated MUC-1 CTL) recognizes the MUC-1 peptide epitope ALWGQDVTSV 7.
Cytotoxicity assays
CTLs specific for CEA, PSA, or MUC-1 were used on day 4 of the restimulation cycle following Ficoll purification. Docetaxel-treated and untreated human tumor cells were cultured with drug for 72 h and subsequently used as targets in a standard cytotoxicity assay using 111In 1, 10. Radiolabeled tumor cells (2 × 103) were incubated with 6 × 104 antigen-specific CTLs (E:T ratio of 30:1) for 18 h at 37°C with 5% CO2. Targets and CTLs were suspended in complete medium supplemented with 10% human AB serum in 96-well U-bottom plates (Costar, Cambridge, MA). After incubation, supernatants were collected and assayed on a Cobra Autogamma (Packard Instruments, Downers Grove, IL). The percentage of specific lysis was determined by the standard equation: % specific lysis = [(experimental-spontaneous)/(maximum-spontaneous)] × 100. For MHC-I-blocking studies, tumor cells were incubated with anti-HLA-A2 mAb (20 μg/mL, AbD Serotec, Raleigh, NC) or isotype control mAb (IgG2b, 20 μg/mL, AbD Serotec) for 1 h at 37 C prior to being used as CTLs. Target tumor cells were then incubated with CTLs, as above. For indicated experiments, CEA-specific CTLs were preincubated for 2 h in the presence of 100 nM concanamycin A to specifically inhibit perforin-dependent lysis, and incubated with target cells; concanamycin A was present during the assay. For indicated experiments, the CTL assay was performed in the presence of CEA peptide or a calreticulin-blocking peptide (0.17 μM, MBL International, Woburn, MA). As a control, a 15 mer peptide, LCMV118-132 (RPQASGVYMGNLTAQ), was used.
RNA isolation, real-time PCR, and siRNA knockdown(s)
For selected cell lines, cells were seeded in T-75 flasks at 0.5–1 × 107 cells/flask. Cells were allowed to adhere for 12 h, then treated with either 25 or 250 ng/mL docetaxel or left untreated. Real-time PCR analysis was performed on cDNA isolated from docetaxel-treated or untreated cells using TaqMan Gene Expression Master Mix (Applied Biosystems, Carlsbad, CA). The primers used to amplify the genes of interest were Hs00189032_m1 (h-calreticulin), Hs00984006_m1 (h-PERK), and human GAPDH endogenous control (Applied Biosystems). Gene expressions were obtained after 40 light cycles using a 7300 Real-Time PCR machine (Applied Biosystems) using the following cycle conditions: 2 min at 50°C and 10 min at 95°C, followed by 40 cycles of 15 sec at 95°C and 1 min at 60°C. Experiments were performed in triplicate. Data were analyzed and genes were normalized against the housekeeping gene GAPDH. For tumor-cell silencing of CRT or PERK, siRNA and negative control siRNA were used according to the manufacturer’s instructions (Silencer siRNA, Life Technologies, Grand Island, NY).
Generation of a docetaxel-resistant tumor cell line
SW620 cells were made resistant to docetaxel as previously described 11. Briefly, cells were passaged in increasing concentrations of docetaxel to generate docetaxel resistance. The drug concentration was increased 3-fold every 2 weeks (2 passages). Cells able to tolerate the highest concentration of drug (3,000 ng/mL) and continue to proliferate were used for subsequent studies.
Statistical analysis
Tests of significance are reported as p values, derived from Student’s t-test using a 2-tailed distribution and calculated at 95% confidence using GraphPad Prism 4.0 for Macintosh. Significant differences in the distribution of flow cytometry analysis data were determined by the Kolmogorov-Smirnov test, using CellQuest software (BD Biosciences).
Results
Tumors treated with docetaxel increase CRT surface expression but fail to undergo immunogenic cell death
Certain chemotherapeutic agents can stimulate immunogenic tumor cell death 12. The cardinal signs of immunogenic cell death (ICD) are a) CRT exposure on the surface of dying cells, b) the release of HMGB1, c) the release of ATP, and most importantly, d) cell death 13. Each of these molecules acts on dendritic cells (DCs) to facilitate the presentation of tumor antigens to the immune system 14. CRT, a chaperone and calcium regulator, when exposed on the surface of a dying cell, serves as a phagocytic signal to DCs 15, 16. When HMGB1, a nonhistone chromatin-binding protein, is released from dying cells, it engages TLR-4 on DCs, leading to maturation 13. The secretion of ATP by dying cells binds purigenic PRX7 receptors on DCs, further supporting T-cell activation 12.
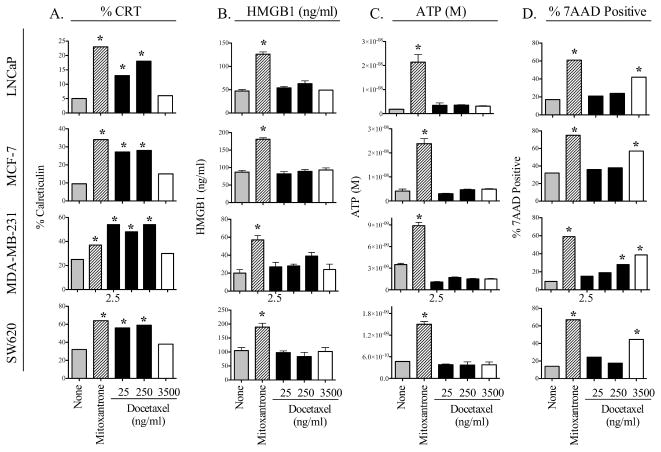
We first examined whether in vitro treatment with therapeutic doses of docetaxel induced ICD in a panel of 4 human carcinoma cell lines (1 prostate, 2 breast, 1 colorectal). Cells were subjected to 0–3500 ng/mL of docetaxel for 72 h. Mitoxantrone was used to induce ICD as a positive control 12. Treatment of LNCaP tumor cells with docetaxel significantly induced translocation of CRT to the cell surface in a dose-dependent manner (Fig. 1A). However, docetaxel treatment did not result in the secretion of HMGB1 (Fig. 1B) or ATP at any concentration (Fig. 1C). Finally, treatment of these tumor cells with docetaxel did not induce cell death at 2.5–250 ng/ml; however, at very high concentrations of docetaxel (3500 ng/ml), cells displayed only significantly decreased viability as determined by 7AAD staining. Similar results were observed with the breast cancer lines MCF-7 and MDA-231, and with the colon cancer cell line SW620 (Fig. 1 A–D). For each cell line, treatment with mitoxantrone unequivocally induced all 4 molecular determinants of ICD. Taken together, these results show that docetaxel treatment, while significantly modulating CRT translocation, fails to induce classic ICD.
Figure 1.
Tumor cells treated with docetaxel show increased surface expression of CRT, but do not undergo ICD. Four human tumor cell lines were treated with 2.5–250 ng/ml (black bars), or 3500 ng/ml docetaxel (open bars). Mitoxantrone (1 μM) was used as a positive control (crosshatched bars). After 72 h of incubation, cells were examined for cardinal signs of ICD. (A) Surface expression of CRT. (B) HMGB1 secretion. (C) ATP secretion. (D) Percentage of dying cells (7AAD+). * = statistical significance relative to untreated cells. This experiment was repeated 2 times with similar results.
Tumor cells treated with chemotherapy undergo immunogenic modulation and demonstrate significantly increased sensitivity to antigen-specific cytotoxic T-cell killing
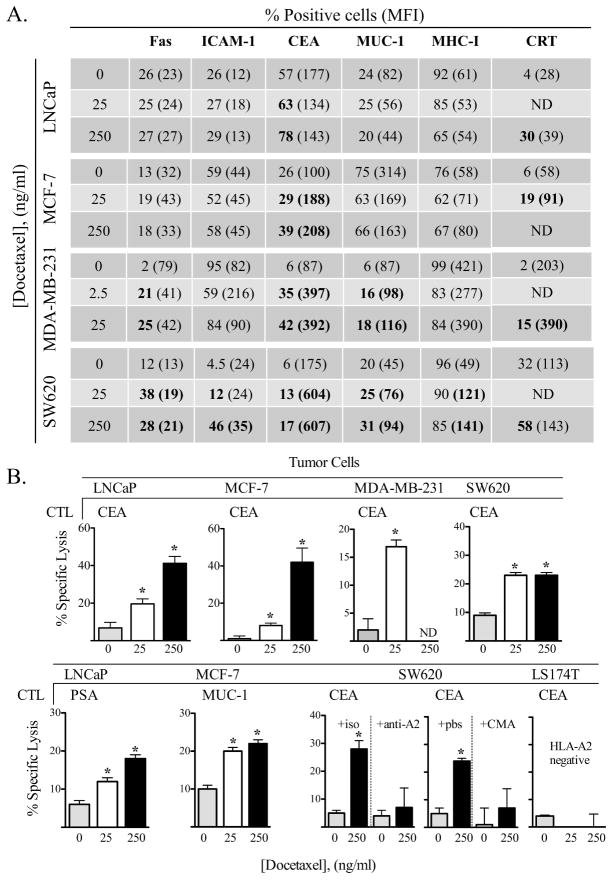
As several cell surface proteins on tumor target cells have previously been demonstrated to be critical for interactions with CD8+ T cells1, we examined the potential role of altered tumor phenotype on CTL sensitivity (immunogenic modulation). Cells subjected to docetaxel were analyzed for surface expression of Fas, ICAM-1, CEA, MUC-1, and MHC-I. CRT was also monitored by flow cytometry. While this chemotherapy treatment was nonlytic, there were notable alterations in expression of the surface proteins analyzed. Marked increased expression of CEA and CRT was the most commonly observed change, with all (4/4) cell lines increasing surface expression of each molecule (Fig. 2A). Upregulation of MUC-1 and Fas (2/4 cell lines) was also observed. In addition, treatment of LNCaP tumor cells with docetaxel significantly induced upregulation of other prostate tumor antigens as determined by RT-PCR: PSA, 1.34 fold increase, PSCA, 1.89 fold increase, PSMA, 1.28 fold increase, and PAP, 1.46 fold-increase (data not shown).
Figure 2.
Tumor cells treated with a chemotherapeutic agent undergo immunogenic modulation and demonstrate significantly increased sensitivity to antigen-specific CTL killing. (A) Human tumor cells were treated in vitro for 72 h with 2.5, 25, or 250 ng/mL of docetaxel, or left untreated. Cells were analyzed after each treatment for surface expression Fas, ICAM-1, CEA, MUC-1, MHC-I, and CRT. Numbers indicate percentage of positive cells. Numbers in parentheses denote MFI. Bold type indicates marked upregulation (≥ 10% increase in percent of cells or 30% increase in MFI not observed in isotype control vs. untreated cells). (B) Human tumor cells treated in vitro for 72 h with 25 (white bars) or 250 (black bars) ng/ml of docetaxel, or left untreated (gray bars), were used as targets in an 18-h CTL lysis assay. CEA-, PSA-, or MUC-1-specific CD8+ T cells were used as effector cells at an E:T ratio of 30:1. For controls, tumor cells were incubated with anti-HLA-A2 mAb or concanamycin A (CMA). CEA+HLA-A2− LS174T cells were used to verify CTL specificity. ND; not determined. * = statistical significance relative to untreated cells. This experiment was repeated 4 times with similar results.
To determine the functional significance of cellular alterations induced by docetaxel, tumor cell lines were treated and coincubated with the CEA-, PSA-, and/or MUC-1-specific CTL. Untreated LNCaP cells were killed with CEA-specific T cells at a level of 8%. Docetaxel treatment of these cells substantially increased CTL killing in a dose-dependent manner (19% and 42%, Fig. 2B, p = < 0.0001 vs. no treatment for both 25 and 250 ng/mL). Moreover, MCF-7, MDA-231, and SW620 tumor cells also demonstrated significantly increased sensitivity to CEA-specific killing after docetaxel treatment (Fig. 2B). In addition, LNCaP tumor cells were killed at significantly greater levels by PSA-specific CTLs, and MCF-7 tumor cells were killed by MUC-1-specific T cells following docetaxel treatment. These data indicate that exposure of cells to docetaxel enhances antigen-specific CTL-mediated killing of tumor cells, and that this effect can be extended to a variety of tumor-associated antigens (TAAs). The CTL killing was MHC-restricted as determined by anti-MHC (HLA-A2) blocking antibody (Fig. 2B), and perforin mediated, as CMA-treated CTLs (which abrogate perforin-based cytotoxic activity) showed significantly reduced killing (p = 0.01) (Fig. 3B). In addition, CEA+, HLA-A2−, LS174T cells were used to verify CTL specificity. *: statistical significance. This experiment was repeated 4 times with similar results. Immunogenic modulation was also observed in cells treated with another taxane; paclitaxel. For LNCaP prostate tumor cells treated with 3.65 μg/mL paclitaxel (equivalent to 175 mg/m2) 17, CEA-specific killing increased 3.2-fold (11% ± 6% killing in untreated or cremophor-treated cells vs. 36% ± 12% in paclitaxel-treated cells, p = 0.032, data not shown).
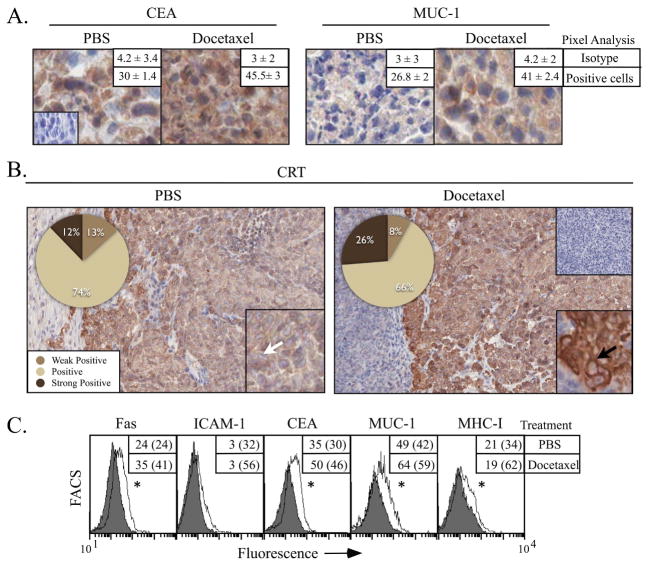
Figure 3.
In vivo treatment with docetaxel modulates tumor phenotype. Nude mice bearing LNCaP xenografts were treated with docetaxel or vehicle (PBS). One week later, tumors were surgically removed, stained, and evaluated by immunohistochemistry for expression of the tumor antigens CEA or MUC-1 (A, 40X, inset: isotype control), or CRT (B, 20X, inset 40X). Numbers indicate percentage of positive cells as determined by pixel analysis (n = 2 mice/treatment group). Arrows indicate CRT membrane staining. (C) Flow cytometry of tumors treated with docetaxel (open histograms) or PBS (shaded histograms). Numbers indicate percentage of positive cells. Numbers in parentheses denote MFI. * = statistical significance relative to untreated cells.
Tumors treated in vivo with docetaxel modulate tumor phenotype
To confirm phenotypic changes induced by docetaxel in vivo, nude mice were implanted with LNCaP tumors and treated with docetaxel or PBS. Prostate tumor cells increased expression of the TAAs CEA and MUC-1 1.5-fold after docetaxel treatment (p = 0.01) (Fig. 3A). CRT is heterogeneously expressed in untreated LNCaP cells. As seen in Figure 3B, 12% of CRT cells stained strongly positive, and cellular localization of CRT was diffuse (inset panel). After treatment with docetaxel, however, there was a sharp and significant increase in expression of CRT, with 26% of cells staining strongly positive (p = 0.03). Moreover, CRT expression in tumors treated with docetaxel was associated with the cell membrane (inset, black arrow). In untreated tumors, CRT membrane staining was 2% at 3+, 9.6% at 2%, and 2.2% at 1+ versus or docetaxel treated tumors, where CRT membrane staining was 3.7% at 3+, 7.6% at 2+, and 4.3% at 1+. We next sought to quantify the expression of Fas, ICAM-1, CEA, MUC-1, and MHC-I by flow cytometry (Fig. 3C). LNCaP tumors responded to docetaxel with a significant increase (1.4-fold) in expression of Fas, CEA, and MUC-1. The increase in CEA and MUC-1 expression corroborated the increase observed by immunohistochemistry (Fig. 3A) and in vitro analysis of tumor cells (Fig. 2A).
Docetaxel-resistant tumor cells undergo immunogenic modulation and are killed by antigen-specific CTLs at significantly greater levels after treatment with docetaxel
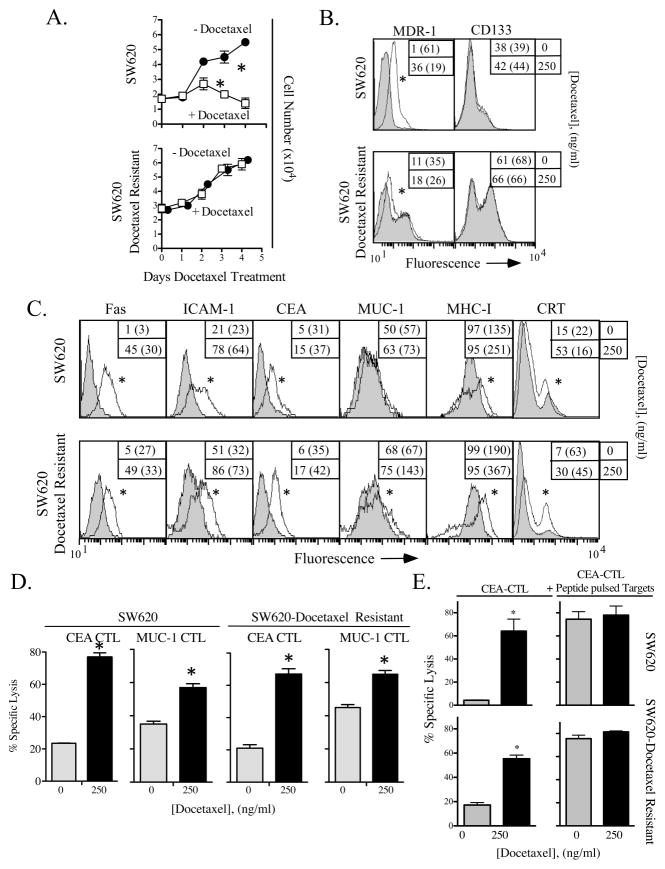
Human carcinomas often become resistant to the cytotoxic effects of chemotherapy 18, 19. We selected the most docetaxel-resistant SW620 cells present in cultures by serially passing the cells through increasing concentrations of docetaxel (up to 3000 ng/mL) to generate a docetaxel-resistant subline. Subsequently, SW620 cells and docetaxel-resistant SW620 cells were treated with docetaxel for up to 96 h. While both cell lines remained viable in the presence of 250 ng/mL of docetaxel, the docetaxel-resistant subline proliferated at levels similar to untreated cells (Fig. 4A). The docetaxel-resistant phenotype of these cells was confirmed by the increased expression of MDR-1, a member of the ATP-binding cassette (ABC) family of transporters (Fig. 4B). SW620 cells have also been reported to contain a resident subpopulation that expresses CD133, which is associated with cancer stem cells that can be selected by chemotherapy 20. Here, we found that 38% of SW620 cells were CD133+ (Fig. 4B), and that this percentage increased to 60% in docetaxel-resistant SW620 cells.
Figure 4.
Docetaxel-resistant tumor cells undergo immunogenic modulation and are killed by antigen-specific CTLs at significantly greater levels after treatment with docetaxel. SW620 cells and SW620 cells passaged in increasing concentrations of docetaxel (designated docetaxel-resistant) were treated with docetaxel for up to 96 h. (A) Tumor cell proliferation was measured after treatment with 0 (closed circles) or 250 (open squares) ng/ml of docetaxel. Viable cells as determined by trypan blue exclusion werereported after 1, 2, 3, and 4 days of continuous exposure to docetaxel. * = statistical significance. (B) Expression of multidrug-resistance pump MDR-1 and stem cell marker CD133 on SW620 and docetaxel-resistant SW620 cells. (C) SW620 and docetaxel-resistant SW620 cells were treated in vitro for 72 h with 250 ng/mL of docetaxel (open histograms) or left untreated (shaded histograms), then analyzed by flow cytometry. Numbers indicate percentage of positive cells. Numbers in parentheses denote MFI. * = statistical significance relative to untreated cells. (D) CEA- and MUC-1-specific CTL lysis. Cells incubated for 72 h with 0 (gray bars) or 250 (black bars) ng/mL of docetaxel were used as targets in an 18-h CTL lysis assay, using either CEA-specific or MUC-1-specific CD8+ T cells as effector cells. (E) Target tumor cells were pulsed with CEA peptide at an E:T ratio of 30:1. * = statistical significance. This experiment was repeated 3 times with similar results.
Although the docetaxel-resistant SW620 cells were resistant to the direct inhibitory effects of docetaxel (Fig. 4A), the phenotype of these cells was modulated after docetaxel treatment to levels similar to those of the parental SW620 tumor cells (Fig. 4C). Both SW620 and docetaxel-resistant SW620 cells showed increased levels of Fas (> 9-fold), ICAM-1 (> 2-fold), and CEA (> 3-fold). These cells also upregulated MUC-1 and MHC-I to similar levels. Furthermore, docetaxel-resistant cells, when exposed to docetaxel, exhibited a 2-fold increase in sensitivity to CEA-specific CTL lysis (Fig. 4D, p = 0.001). Lysis by MUC-1-specific CTLs was also significantly enhanced in docetaxel-resistant SW620 cells treated with docetaxel (Fig. 4D, p = 0.0002). The increased CTL sensitivity was similar to the killing seen with untreated and docetaxel-treated parental SW620 cells (Fig. 4D). These data suggest that cells insensitive to the cytostatic effects of docetaxel can nonetheless be sensitized to CTL lysis by exposure to the drug. To confirm that these cells had functional expression of MHC-I, SW620 cells and docetaxel-resistant SW620 cells were treated with docetaxel and used in a CEA-specific CTL assay with exogenously loaded CEA peptide (Fig. 4E). As before (Fig. 4D), SW620 cells were killed at a significantly greater level after treatment with docetaxel. However, untreated cells were killed at a level similar to docetaxel-treated cells when loaded with CEA peptide. Similarly, killing of docetaxel-resistant SW620 cells was significantly enhanced after docetaxel treatment, and peptide-pulsed tumor cells were killed at this similar level. Thus, despite their resistance to docetaxel, SW620 cells still respond to transient docetaxel treatment with upregulation of APM components thus making them more susceptible to CTL killing.
These data indicate that these cells may have baseline antigen-processing machinery (APM) differences that alter CTL antigen presentation of the peptide required for CTL recognition and killing.
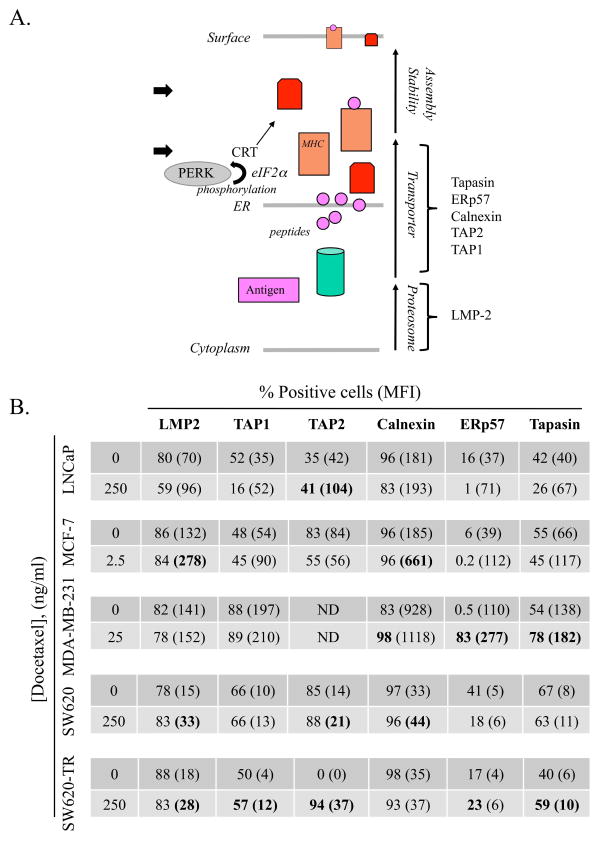
Tumor cells modulate components of the APM chain after treatment with docetaxel
Components of the APM chain cooperate to present appropriate peptide epitopes in the context of MHC-I on the surface of tumor cells. Defects in APM components on tumor cells have a negative impact on T-cell recognition 21–23. To examine the effect of chemotherapy treatment on antigen/MHC-I loading, LNCaP, MCF-7, MDA-231, SW620, and docetaxel-resistant SW620 cells were treated with docetaxel. Cells were then harvested and examined for modulation and translocation of 6 APM components (Fig. 5A). In all 5 tumor cell lines, treatment with docetaxel increased the expression of one or more components of the APM, most commonly TAP2 (3/4 tested lines) and calnexin (3/5 lines) (Fig. 5B). These data indicate that chemotherapy induced upregulation of APM components, which could result in enhanced loading of MHC-I molecules.
Figure 5.
Tumor cells modulate components of the APM chain after treatment with docetaxel. (A) Schematic of antigen-processing components from cytoplasm to cell surface. Arrows indicate functional checkpoints for CRT and PERK. (B) Human tumor cells were treated in vitro for 72 h with 2.5–250 ng/mL of docetaxel or left untreated, then analyzed for key intracellular components of the APM chain by flow cytometry. Numbers indicate percentage of positive cells. Numbers in parentheses denote MFI. Bold type indicates marked upregulation (≥ 10% increase in percent of cells or 30% increase in MFI not observed in isotype control vs. untreated cells).
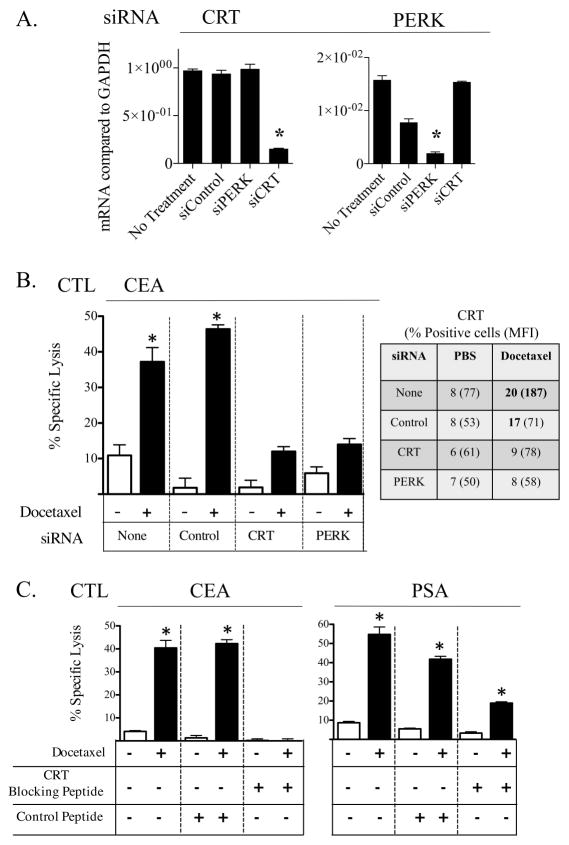
Docetaxel-mediated immunogenic modulation is reduced in the absence of CRT or PERK, and is reduced by CRT blocking peptide
CRT, in addition to being modulated by docetaxel treatment (Figs. 1A, 2B, 3A, and 4C), is a critical component of antigen processing and loading of MHC-I. We thus focused on 3 approaches to determine the functional role of CRT in immunogenic modulation and subsequent sensitivity to CTL-mediated killing: a) knockdown of CRT by siRNA, b) knockdown of the serine/threonine kinase PERK, which normally phosphorylates EIF2α, culminating in the exposure of CRT on the cell surface 24, 25, and c) use of a CRT blocking peptide 26. Knockdown of CRT would normally block APM functions, while silencing of PERK would preserve APM functions but prevent CRT translocation. Peptide blocking would prevent surface CRT/T-cell interactions. LNCaP surface expression of CRT or PERK was reduced by 84% and 88%, respectively (dat not shown), following siRNA silencing (Fig. 6A). Treatment of these cells with docetaxel resulted in a 60% decrease in upregulated CRT (Fig. 6B). As before, LNCaP cells were killed by CEA-specific T cells to a significantly greater level after docetaxel treatment (Fig. 6B). However, the level of increased killing diminished significantly (> 3-fold) in LNCaP cells exhibiting reduced expression of CRT or PERK. The reduction in CTL killing was not observed in cells treated with control siRNA. Finally, LNCaP cells incubated with control blocking peptide were killed to a significantly greater level after docetaxel treatment (Fig. 6C). However, in LNCaP cells coincubated with the CRT blocking peptide, docetaxel-enhanced PSA-specific killing of LNCaP cells was reduced (Fig. 6C). As a control, incubation of the CEA-specific CTL used in these assays with CRT blocking peptide did not decrease T-cell viability (not shown).
Figure 6.
Docetaxel-mediated immunogenic modulation is reduced in the absence of CRT or PERK, and by CRT blocking peptide. (A) Knockdown of CRT or PERK in LNCaP tumor cells. (B) LNCaP tumor cells lacking CRT or PERK were treated with 0 (white bars) or 250 (black bars) ng/mL of docetaxel and used as targets in an 18-h CTL lysis assay using CEA-specific CD8+ T cells. CRT expression was confirmed by flow cytometry. Numbers indicate percentage of positive cells. Numbers in parentheses denote MFI. Bold type indicates marked upregulation (≥ 10% increase in percent of cells or 30% increase in MFI not observed in isotype control vs. untreated cells). (C) LNCaP tumor cells were treated with 0 (white bars) or 250 (black bars) ng/mL of docetaxel and used as targets in an 18-h CTL lysis assay using either CEA-specific or PSA-specific CD8+ T cells, in the presence or absence of CRT blocking peptide (0.17 μM). All assays were done at an E:T ratio of 30:1. * = statistical significance. This experiment was repeated 2 times with similar results.
Discussion
Zitvogel and Kroemer et al. have elegantly shown that certain chemotherapeutic regimens trigger cancer cell death while stimulating endogenous immune responses against the tumor 12–14, 16, 27–29. Chemotherapy-induced ICD has thus far been restricted to only 4 drugs: cyclophosphamide, doxorubicin, oxaliplatin, and mitoxantrone 30. However, many chemotherapeutic agents, including cisplatin, 5-fluorouracil, and vinorelbine, can upregulate multiple surface molecules on tumor cells, rendering them more sensitive to immune-mediated killing 2, 10, 31, without inducing classic ICD32–35. The fact that, in many cases, tumor cells do not die after treatment led us to investigate the fate of tumor cells that do not die directly from chemotherapy exposure or from ICD, using docetaxel as a model chemotherapeutic agent. ICD was initially characterized by Casares et al., who showed that immunizing mice with chemotherapy treated tumor cells protected mice from subsequent challenge 36. For our studies using human tumor cells, the term ‘immunogenic modulation’ is derived from the criterion of continuity (CC) definition of immunogenicity, where an immune cells respond to abrupt modifications of the antigenic patterns with which they are in contact 37. We first examined whether docetaxel could induce ICD in 4 tumor cell lines and found that only CRT translocation was induced after docetaxel treatment (2.5–250 ng/ml, Fig. 1). The doses of docetaxel used here were within the range expected to be administered to patients (100 mg/m2 dose; Cmax of 0.576–3.53 μg/mL, or 668–4095 nM plasma concentration 38. At the highest concentration (Cmax), docetaxel treated cells displayed significantly reduced viability, however CRT translocation, HMGB1 and ATP secretion were not observed. This suggested that an alternative mechanism was active in this type of cell death. Docetaxel has been shown to induce cell death through a non-apoptotic mitotic catastrophe; cells have aberrant mitosis, chromosomal nuclear envelopes, and form large non-viable cells with multiple micronuclei, yet remain intact 39. We previously demonstrated in CEA transgenic mice that docetaxel treatment of MC38-CEA tumors, which did not impact tumor growth, nevertheless induced immune responses to multiple tumor-associated antigens and resulted in synergistic antitumor activity when combined with vaccine 2. It was subsequently determined that MC38-CEA murine tumor cells modulated surface expression of several molecules after in-vitro treatment with 250 ng/ml docetaxel, including Fas (44% positive before treatment, 69% positive after treatment), and MHC-1/H2Db (32% positive before treatment, 72% positive after treatment). In addition, docetaxel treated tumor cells were killed at a much greater level by CEA-specific CTL (30% lysis in untreated cells vs. 81% lysis in docetaxel treated cells), (data not shown). Taken together, these data support the role of docetaxel induced immunogenic modulation in-vivo, and the enhanced subsequent antitumor effects of antigen-specific T-cells induced from a vaccine. This suggested that docetaxel, while not inducing ICD, may nontheless increase the immunogenicity of treated tumor cells. Reports have indicated that induction of ICD depends not on the dose or time of exposure to a given therapeutic, but on the activation of an endoplasmic reticulum (ER) stress module 27. For example, it has been reported that cisplatin does not induce ICD regardless of dose. However, if tumor cells are treated with cisplatin and thapsigargin, an inducer of ER stress, ICD is restored 28. In response to ER stress, the evolutionarily conserved quality control mechanism Unfolded Protein Response (UPR) is triggered with the aim of restoring cellular homeostasis during which several ER chaperones can translocate to the cell surface, including CRT 40. Certain anticancer therapies, whereas not inducing ICD, may modulate immune-relevant pathways in the surviving tumor cells as a direct result of UPR adaptive mechanisms to cellular stress.
We next examined whether docetaxel could alter the expression of 6 molecules key to T cell-mediated lysis (Fig. 2A). In this study, CRT and the TAAs MUC-1 and CEA, which are important T-cell targets, were most often upregulated. These phenotypic changes were confirmed in docetaxel-treated nude mice bearing prostate tumor xenografts (Fig. 3). The mechanism for TAA upregulation following docetaxel treatment remains under investigation, focusing on the above-described Unfolded Protein Response. Overexpression of TAAs in tumor tissue could result in increased signal 1, making tumor cells better targets of antigen-specific CTLs. We also report here for the first time the use of docetaxel to functionally enhance antigen-specific CTL-mediated killing in human tumor cell lines. All (4/4) docetaxel-treated tumor cells lines were killed at significantly greater levels by CEA-, MUC-1-, and/or PSA-specific HLA-A2-restricted CD8+ CTLs than their untreated counterparts (Fig. 2). CTL activity was abrogated by CMA, indicating that the killing was perforin/granzyme-based (Fig. 2B).
Drug resistance, estimated to cause treatment failure in > 90% of patients with metastatic disease, is a major consideration in initial treatment and in the adjuvant setting. In this study, we investigated whether SW620 cells (a line established from a patient with stage III colorectal carcinoma41) could be selected for enhanced resistance to docetaxel. Although docetaxel’s ability to inhibit proliferation in these selected cells was abrogated (Fig. 4A), these docetaxel-resistant cells continued to modulate cell surface phenotype in response to docetaxel treatment (Fig. 4C). Moreover, these docetaxel-resistant cells maintained their sensitivity to lysis by antigen-specific CTLs following docetaxel treatment (Fig. 4D). Thus, this study is the first to demonstrate that human tumor cells resistant to the cytostatic effects of docetaxel can still be immunogenically modulated to enhance sensitivity to immune-mediated killing in response to drug exposure. These studies complement those of Shtil et al. 42, who transfected treated murine tumors with the MDR-1 gene to confer drug resistance. In vivo, however, chemotherapy-resistant tumors were killed equally well by T cells induced from vaccination with a whole tumor cell vaccine, showing that the drug-resistant phenotype did not interfere with lysis by pore-forming CTLs. Our findings are similar, in that cells resistant to docetaxel (Fig. 4) remained sensitive to CTL killing via the perforin pathway (Fig. 2). Furthermore, SW620 tumor cells, when pulsed with exogenous CEA peptide, were capable of being killed by a CEA-specific CTL to levels observed with docetaxel-treated tumor cells (Fig. 5E), indicating that the MHC complex was structurally intact. We hypothesized that indolent defects in antigen processing, loading, and/or presentation could be influenced by exposure to docetaxel. Components of the APM chain cooperate to present appropriate peptide epitopes in the context of MHC-I on the surface of tumor cells. APM components include (from cytoplasm to surface, Fig. 5A) proteosome subunits LMP-2, peptide transporters TAP-1, TAP-2, chaperones calnexin and CRT, peptide loaders Erp57 and tapasin, and HLA3. APM defects have been described in several tumors of different histologies, as well as in established cell lines, 43, 44 and have been reported to have a negative impact on T-cell recognition 22, 23. Exposure of tumor cells to docetaxel resulted in substantial upregulation of one or more APM components, which would positively impact the loading of MHC. Modulation of APM components by docetaxel was also observed in tumor cells resistant to docetaxel (SW620-TR, Fig. 5B). These data extend the findings of 2 other investigators: Setiadi et al., who reported that histone deacetylase inhibitors also modulated APM23, including TAP-1, TAP-2, LMP-2, and tapasin; and Lopez-Albaitero 22, who described several levels of APM defects that interfered with CTL recognition and killing in squamous cell carcinoma of the head and neck. Treating APM components in these cells with IFN-γ restored CTL sensitivity.
CRT is a major Ca2+ binding protein, normally located in the lumen of the ER, that binds to misfolded proteins and acts as a molecular chaperone. Emerging data suggest that CRT expressed on the surface of cells undergoing ICD also acts as a phagocytic signal 15, 16. CRT is closely associated with MHC-I and is an important peptide-loading complex. If little or no CRT is present, MHC-I molecules are still able to appear on the cell surface, but the majority are loaded with suboptimal peptides 45. Most importantly, Fu et al. have reported that CRT maintains the low threshold of peptide required for efficient antigen presentation. Given that CRT translocation was observed in docetaxel-treated cells (Figs. 1, 2A, 3B, and 4C), and that CRT enriches endogenous peptides in the ER, which is critical for efficient antigen presentation 45, we next investigated the role of CRT in immunogenic modulation. CTL killing of docetaxel-treated tumor cells was largely inhibited following CRT knockdown (Fig. 6B). The translocation of CRT from the cytoplasm to the cell surface has been shown to be mainly governed by PERK-mediated phosphorylation of the translation initiation factor eIF2α24. Knockdown of PERK in our model significantly interfered with the increased level of CTL killing observed after docetaxel treatment (Fig. 6B). These data are consistent with the findings of Garg et al., who reported that the absence of PERK following shRNA knockdown in CT26 murine tumor cells compromised the ability of mitoxantrone-treated cells to secrete ATP and translocate CRT to the cell surface 46. Finally, because CTL killing was abrogated in the presence of a CRT blocking peptide (Fig. 6C), CTL killing of docetaxel-treated tumor cells was determined to be dependent on CRT’s interaction with CTLs. Low-density lipoprotein receptor-related protein 1 (LRP-1/CD91) has been identified as a potential candidate receptor for CRT by binding interaction analysis 46. In addition, Li et al. have shown that activation of lymphocytes promotes cell surface expression of thrombospondin-1, which binds to LPR-1/CD91 and CRT47. However, the CEA-specific T cells used in these studies did not express LPR-1/CD91 (data not shown). Additional CRT receptors on T cells and/or DCs need to be identified 16; candidates include scavenger receptor A and scavenger receptor class F member 2 15.
CRT exposure has been shown to be an important factor for the up-take of tumor cells by DCs or the phagocytosis of apoptotic cells. However, it is unclear whether docetaxel treatment may also affect these processes. To examine this, Human PBMC derived immature dendritic cells (GM-CSF/IL-4) were co-incubated with LNCaP tumor cells or LNCaP tumor cells treated for 48h with 250ng/ml docetaxel and subsequently examined by flow cytometry for DC maturation markers as an indirect indication of tumor cell phagocytosis. As a positive control, DC were matured with CD40L. DC incubated with LNCaP cells, or LNCaP cells treated with docetaxel failed to undergo maturation as measured by increased expression of CD80, CD83, CD54, CD58, HLA-ABC and HLA-DR/DP/DQ (data not shown). These data suggest that docetaxel treated tumor cells may interact primarily with CD8+ T -cells for direct tumor cell killing and that the DC in the environment may play a greater role in the uptake of cellular debris resulting in the induction of T-cell responses to multiple tumor antigens (antigen cascade/epitope spreading).
We propose here that immunogenic modulation of tumor cell phenotype (Figs. 1, 2A, 3, and 4C) and APM (Fig. 5B) by sublethal chemotherapy enhances productive interactions between CTLs and cancer cells, resulting in enhanced tumor cell killing (Figs. 2B, 4D, 6B, and 6C). The data presented here suggest that optimal tumor therapies are those that can capitalize on the changes induced by conventional therapies to achieve synergy with immunotherapies. In patients, it is possible that treatment of tumors with chemotherapy could induce a continuum of responses ranging from necrosis, ‘classic’ immunogenic cell death, non-classical immunogenic cell death, to immunogenic modulation. Indeed, in clinical trials docetaxel has shown evidence of clinical benefit when combined with a) a poxviral vaccine targeting PSA 48 and b) sipuleucel-T (Provenge®), an autologous DC-based vaccine 49. These trials suggest that docetaxel treatment has effects that go beyond directly inhibiting tumor growth 50. Our finding of enhanced CTL lysis following docetaxel treatment suggests that drug-induced cellular changes could be of clinical benefit in the treatment of some types of tumors for which docetaxel is not currently the standard of care, when combined with immunotherapies. Furthermore, the enhanced CTL lysis observed in a docetaxel-resistant cell line suggests that combining immunotherapy and chemotherapy may lead to improved outcomes in patients who have previously failed chemotherapy alone.
Here, we show that treatment with a chemotherapeutic agent that does not induce ICD can nonetheless generate the molecular and immunologic hallmarks of immunogenic modulation, expanding the set of chemotherapies that can be effectively used in combination with immunotherapy.
Novelty and Impact of Work.
This study is the first to show that tumor treatment with chemotherapy that does not induce immunogenic cell death nonetheless leads to immunogenic modulation, in that exposure of tumor cells to chemotherapy alters tumor phenotype to render them more sensitive to immune-mediated killing, mediated largely by calreticulin translocation. This immunogenic modulation was also observed in a chemotherapy-resistant cell line, suggesting that chemotherapy and immunotherapy could improve outcomes in patients who have previously failed chemotherapy alone.
Acknowledgments
Grant support: This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
The authors thank Dr. Jeffrey Schlom for his helpful suggestions, Andressa Smith and Momodou Jammeh for ICD analysis, and Marion Taylor for excellent technical assistance. We also wish to thank Bonnie L. Casey and Debra Weingarten for editorial assistance in the preparation of this manuscript.
Abbreviations used
- APM
antigen-processing machinery
- CRT
calreticulin
- DC
dendritic cell
- ER
endoplasmic reticulum
- HMGB
high-mobility group box
- ICD
immunogenic cell death
References
- 1.Garnett CT, Palena C, Chakraborty M, Tsang KY, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004;64:7985–94. doi: 10.1158/0008-5472.CAN-04-1525. [DOI] [PubMed] [Google Scholar]
- 2.Garnett CT, Schlom J, Hodge JW. Combination of docetaxel and recombinant vaccine enhances T-cell responses and antitumor activity: effects of docetaxel on immune enhancement. Clin Cancer Res. 2008;14:3536–44. doi: 10.1158/1078-0432.CCR-07-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seliger B, Maeurer MJ, Ferrone S. Antigen-processing machinery breakdown and tumor growth. Immunol Today. 2000;21:455–64. doi: 10.1016/s0167-5699(00)01692-3. [DOI] [PubMed] [Google Scholar]
- 4.Bandoh N, Ogino T, Cho HS, Hur SY, Shen J, Wang X, Kato S, Miyokawa N, Harabuchi Y, Ferrone S. Development and characterization of human constitutive proteasome and immunoproteasome subunit-specific monoclonal antibodies. Tissue Antigens. 2005;66:185–94. doi: 10.1111/j.1399-0039.2005.00462.x. [DOI] [PubMed] [Google Scholar]
- 5.Ogino T, Wang X, Ferrone S. Modified flow cytometry and cell-ELISA methodology to detect HLA class I antigen processing machinery components in cytoplasm and endoplasmic reticulum. J Immunol Methods. 2003;278:33–44. doi: 10.1016/s0022-1759(03)00224-2. [DOI] [PubMed] [Google Scholar]
- 6.Muraro R, Wunderlich D, Thor A, Lundy J, Noguchi P, Cunningham R, Schlom J. Definition by monoclonal antibodies of a repertoire of epitopes on carcinoembryonic antigen differentially expressed in human colon carcinomas versus normal adult tissues. Cancer Res. 1985;45:5769–80. [PubMed] [Google Scholar]
- 7.Tsang KY, Palena C, Gulley J, Arlen P, Schlom J. A human cytotoxic T-lymphocyte epitope and its agonist epitope from the nonvariable number of tandem repeat sequence of MUC-1. Clin Cancer Res. 2004;10:2139–49. doi: 10.1158/1078-0432.ccr-1011-03. [DOI] [PubMed] [Google Scholar]
- 8.Tsang KY, Zaremba S, Nieroda CA, Zhu MZ, Hamilton JM, Schlom J. Generation of human cytotoxic T cells specific for human carcinoembryonic antigen epitopes from patients immunized with recombinant vaccinia-CEA vaccine. J Natl Cancer Inst. 1995;87:982–90. doi: 10.1093/jnci/87.13.982. [DOI] [PubMed] [Google Scholar]
- 9.Tsang KY, Zhu M, Nieroda CA, Correale P, Zaremba S, Hamilton JM, Cole D, Lam C, Schlom J. Phenotypic stability of a cytotoxic T-cell line directed against an immunodominant epitope of human carcinoembryonic antigen. Clin Cancer Res. 1997;3:2439–49. [PubMed] [Google Scholar]
- 10.Gelbard A, Garnett CT, Abrams SI, Patel V, Gutkind JS, Palena C, Tsang KY, Schlom J, Hodge JW. Combination chemotherapy and radiation of human squamous cell carcinoma of the head and neck augments CTL-mediated lysis. Clin Cancer Res. 2006;12:1897–905. doi: 10.1158/1078-0432.CCR-05-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown I, Shalli K, McDonald SL, Moir SE, Hutcheon AW, Heys SD, Schofield AC. Reduced expression of p27 is a novel mechanism of docetaxel resistance in breast cancer cells. Breast Cancer Res. 2004;6:R601–7. doi: 10.1186/bcr918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martins I, Tesniere A, Kepp O, Michaud M, Schlemmer F, Senovilla L, Seror C, Metivier D, Perfettini JL, Zitvogel L, Kroemer G. Chemotherapy induces ATP release from tumor cells. Cell Cycle. 2009;8:3723–8. doi: 10.4161/cc.8.22.10026. [DOI] [PubMed] [Google Scholar]
- 13.Kepp O, Galluzzi L, Martins I, Schlemmer F, Adjemian S, Michaud M, Sukkurwala AQ, Menger L, Zitvogel L, Kroemer G. Molecular determinants of immunogenic cell death elicited by anticancer chemotherapy. Cancer Metastasis Rev. 2011;30:61–9. doi: 10.1007/s10555-011-9273-4. [DOI] [PubMed] [Google Scholar]
- 14.Locher C, Conforti R, Aymeric L, Ma Y, Yamazaki T, Rusakiewicz S, Tesniere A, Ghiringhelli F, Apetoh L, Morel Y, Girard JP, Kroemer G, et al. Desirable cell death during anticancer chemotherapy. Ann N Y Acad Sci. 2010;1209:99–108. doi: 10.1111/j.1749-6632.2010.05763.x. [DOI] [PubMed] [Google Scholar]
- 15.Martins I, Kepp O, Galluzzi L, Senovilla L, Schlemmer F, Adjemian S, Menger L, Michaud M, Zitvogel L, Kroemer G. Surface-exposed calreticulin in the interaction between dying cells and phagocytes. Ann N Y Acad Sci. 2011;1209:77–82. doi: 10.1111/j.1749-6632.2010.05740.x. [DOI] [PubMed] [Google Scholar]
- 16.Zitvogel L, Kepp O, Senovilla L, Menger L, Chaput N, Kroemer G. Immunogenic tumor cell death for optimal anticancer therapy: the calreticulin exposure pathway. Clin Cancer Res. 2010;16:3100–4. doi: 10.1158/1078-0432.CCR-09-2891. [DOI] [PubMed] [Google Scholar]
- 17.Panis C, Lemos LG, Victorino VJ, Herrera AC, Campos FC, Colado Simao AN, Pinge-Filho P, Cecchini AL, Cecchini R. Immunological effects of taxol and adryamicin in breast cancer patients. Cancer immunology, immunotherapy: CII. 2012;61:481–8. doi: 10.1007/s00262-011-1117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fojo AT, Menefee M. Microtubule targeting agents: basic mechanisms of multidrug resistance (MDR) Semin Oncol. 2005;32:S3–8. doi: 10.1053/j.seminoncol.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Komarova NL, Wodarz D. Drug resistance in cancer: principles of emergence and prevention. Proc Natl Acad Sci U S A. 2005;102:9714–9. doi: 10.1073/pnas.0501870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawamoto H, Yuasa T, Kubota Y, Seita M, Sasamoto H, Shahid JM, Hayashi T, Nakahara H, Hassan R, Iwamuro M, Kondo E, Nakaji S, et al. Characteristics of CD133(+) human colon cancer SW620 cells. Cell Transplant. 2010;19:857–64. doi: 10.3727/096368910X508988. [DOI] [PubMed] [Google Scholar]
- 21.Leibowitz MS, Andrade Filho PA, Ferrone S, Ferris RL. Deficiency of activated STAT1 in head and neck cancer cells mediates TAP1-dependent escape from cytotoxic T lymphocytes. Cancer Immunol Immunother. 2011;60:525–35. doi: 10.1007/s00262-010-0961-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez-Albaitero A, Nayak JV, Ogino T, Machandia A, Gooding W, DeLeo AB, Ferrone S, Ferris RL. Role of antigen-processing machinery in the in vitro resistance of squamous cell carcinoma of the head and neck cells to recognition by CTL. J Immunol. 2006;176:3402–9. doi: 10.4049/jimmunol.176.6.3402. [DOI] [PubMed] [Google Scholar]
- 23.Setiadi AF, Omilusik K, David MD, Seipp RP, Hartikainen J, Gopaul R, Choi KB, Jefferies WA. Epigenetic enhancement of antigen processing and presentation promotes immune recognition of tumors. Cancer Res. 2008;68:9601–7. doi: 10.1158/0008-5472.CAN-07-5270. [DOI] [PubMed] [Google Scholar]
- 24.Panaretakis T, Kepp O, Brockmeier U, Tesniere A, Bjorklund AC, Chapman DC, Durchschlag M, Joza N, Pierron G, van Endert P, Yuan J, Zitvogel L, et al. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. Embo J. 2009;28:578–90. doi: 10.1038/emboj.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panaretakis T, Joza N, Modjtahedi N, Tesniere A, Vitale I, Durchschlag M, Fimia GM, Kepp O, Piacentini M, Froehlich KU, van Endert P, Zitvogel L, et al. The co-translocation of ERp57 and calreticulin determines the immunogenicity of cell death. Cell death and differentiation. 2008;15:1499–509. doi: 10.1038/cdd.2008.67. [DOI] [PubMed] [Google Scholar]
- 26.Chao MP, Jaiswal S, Weissman-Tsukamoto R, Alizadeh AA, Gentles AJ, Volkmer J, Weiskopf K, Willingham SB, Raveh T, Park CY, Majeti R, Weissman IL. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci Transl Med. 2010;2:63ra94. doi: 10.1126/scitranslmed.3001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hannani D, Sistigu A, Kepp O, Galluzzi L, Kroemer G, Zitvogel L. Prerequisites for the antitumor vaccine-like effect of chemotherapy and radiotherapy. Cancer J. 2011;17:351–8. doi: 10.1097/PPO.0b013e3182325d4d. [DOI] [PubMed] [Google Scholar]
- 28.Martins I, Kepp O, Schlemmer F, Adjemian S, Tailler M, Shen S, Michaud M, Menger L, Gdoura A, Tajeddine N, Tesniere A, Zitvogel L, et al. Restoration of the immunogenicity of cisplatin-induced cancer cell death by endoplasmic reticulum stress. Oncogene. 2011;30:1147–58. doi: 10.1038/onc.2010.500. [DOI] [PubMed] [Google Scholar]
- 29.Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 2011;8:151–60. doi: 10.1038/nrclinonc.2010.223. [DOI] [PubMed] [Google Scholar]
- 30.Vacchelli E, Galluzzi L, Fridman WH, Galon J, Sautes-Fridman C, Tartour E, Kroemer G. Trial watch: Chemotherapy with immunogenic cell death inducers. Oncoimmunology. 2012;1:179–88. doi: 10.4161/onci.1.2.19026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gameiro SR, Caballero JA, Higgins JP, Apelian D, Hodge JW. Exploitation of differential homeostatic proliferation of T-cell subsets following chemotherapy to enhance the efficacy of vaccine-mediated antitumor responses. Cancer immunology, immunotherapy: CII. 2011;60:1227–42. doi: 10.1007/s00262-011-1020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan OT, Yang LX. The immunological effects of taxanes. Cancer Immunol Immunother. 2000;49:181–5. doi: 10.1007/s002620000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eckert K, Fuhrmann-Selter T, Maurer HR. Docetaxel enhances the expression of E-cadherin and carcinoembryonic antigen (CEA) on human colon cancer cell lines in vitro. Anticancer Res. 1997;17:7–12. [PubMed] [Google Scholar]
- 34.Matsuzaki I, Suzuki H, Kitamura M, Minamiya Y, Kawai H, Ogawa J. Cisplatin induces fas expression in esophageal cancer cell lines and enhanced cytotoxicity in combination with LAK cells. Oncology. 2000;59:336–43. doi: 10.1159/000012192. [DOI] [PubMed] [Google Scholar]
- 35.Prete SP, Aquino A, Masci G, Orlando L, Giuliani A, De Santis S, De Vecchis L, De Filippi R, Greiner JW, Bonmassar E, Graziani G. Drug-induced changes of carcinoembryonic antigen expression in human cancer cells: effect of 5-fluorouracil. J Pharmacol Exp Ther. 1996;279:1574–81. [PubMed] [Google Scholar]
- 36.Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, Schmitt E, Hamai A, Hervas-Stubbs S, Obeid M, Coutant F, Metivier D, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202:1691–701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pradeu T, Carosella ED. On the definition of a criterion of immunogenicity. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17858–61. doi: 10.1073/pnas.0608683103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minami H, Kawada K, Sasaki Y, Tahara M, Igarashi T, Itoh K, Fujii H, Saeki T, Ozawa K, Sato H. Population pharmacokinetics of docetaxel in patients with hepatic dysfunction treated in an oncology practice. Cancer Sci. 2009;100:144–9. doi: 10.1111/j.1349-7006.2009.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morse DL, Gray H, Payne CM, Gillies RJ. Docetaxel induces cell death through mitotic catastrophe in human breast cancer cells. Mol Cancer Ther. 2005;4:1495–504. doi: 10.1158/1535-7163.MCT-05-0130. [DOI] [PubMed] [Google Scholar]
- 40.Luo B, Lee AS. The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies. Oncogene. 2012 doi: 10.1038/onc.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huerta S, Heinzerling JH, Anguiano-Hernandez YM, Huerta-Yepez S, Lin J, Chen D, Bonavida B, Livingston EH. Modification of gene products involved in resistance to apoptosis in metastatic colon cancer cells: roles of Fas, Apaf-1, NFkappaB, IAPs, Smac/DIABLO, and AIF. J Surg Res. 2007;142:184–94. doi: 10.1016/j.jss.2006.12.551. [DOI] [PubMed] [Google Scholar]
- 42.Shtil AA, Turner JG, Durfee J, Dalton WS, Yu H. Cytokine-based tumor cell vaccine is equally effective against parental and isogenic multidrug-resistant myeloma cells: the role of cytotoxic T lymphocytes. Blood. 1999;93:1831–7. [PubMed] [Google Scholar]
- 43.Racanelli V, Leone P, Frassanito MA, Brunetti C, Perosa F, Ferrone S, Dammacco F. Alterations in the antigen processing-presenting machinery of transformed plasma cells are associated with reduced recognition by CD8+ T cells and characterize the progression of MGUS to multiple myeloma. Blood. 2011;115:1185–93. doi: 10.1182/blood-2009-06-228676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seliger B, Stoehr R, Handke D, Mueller A, Ferrone S, Wullich B, Tannapfel A, Hofstaedter F, Hartmann A. Association of HLA class I antigen abnormalities with disease progression and early recurrence in prostate cancer. Cancer Immunol Immunother. 2010;59:529–40. doi: 10.1007/s00262-009-0769-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fu H, Liu C, Flutter B, Tao H, Gao B. Calreticulin maintains the low threshold of peptide required for efficient antigen presentation. Mol Immunol. 2009;46:3198–206. doi: 10.1016/j.molimm.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 46.Garg AD, Krysko DV, Verfaillie T, Kaczmarek A, Ferreira GB, Marysael T, Rubio N, Firczuk M, Mathieu C, Roebroek AJ, Annaert W, Golab J, et al. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. Embo J. 2012;31:1062–79. doi: 10.1038/emboj.2011.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li SS, Liu Z, Uzunel M, Sundqvist KG. Endogenous thrombospondin-1 is a cell-surface ligand for regulation of integrin-dependent T-lymphocyte adhesion. Blood. 2006;108:3112–20. doi: 10.1182/blood-2006-04-016832. [DOI] [PubMed] [Google Scholar]
- 48.Arlen PM, Gulley JL, Parker C, Skarupa L, Pazdur M, Panicali D, Beetham P, Tsang KY, Grosenbach DW, Feldman J, Steinberg SM, Jones E, et al. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin Cancer Res. 2006;12:1260–9. doi: 10.1158/1078-0432.CCR-05-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petrylak D. Defining the optimal role of immunotherapy and chemotherapy: advanced prostate cancer patients who recieve sipuleucel-T (PROVENGE) followed by docetaxel derive greatest survivial benefit. 14th Annual Meeting of the Chemotherapy Foundation Symposium; 2006. [Google Scholar]
- 50.Schlom J, Gulley JL, Arlen PM. Paradigm shifts in cancer vaccine therapy. Exp Biol Med (Maywood) 2008;233:522–34. doi: 10.3181/0708-MR-226. [DOI] [PMC free article] [PubMed] [Google Scholar]