Abstract
This study underlines the importance of cinnamon, a widely-used food spice and flavoring material, and its metabolite sodium benzoate (NaB), a widely-used food preservative and a FDA-approved drug against urea cycle disorders in humans, in increasing the levels of neurotrophic factors [e.g., brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3)] in the CNS. NaB, but not sodium formate (NaFO), dose-dependently induced the expression of BDNF and NT-3 in primary human neurons and astrocytes. Interestingly, oral administration of ground cinnamon increased the level of NaB in serum and brain and upregulated the levels of these neurotrophic factors in vivo in mouse CNS. Accordingly, oral feeding of NaB, but not NaFO, also increased the level of these neurotrophic factors in vivo in the CNS of mice. NaB induced the activation of protein kinase A (PKA), but not protein kinase C (PKC), and H-89, an inhibitor of PKA, abrogated NaB-induced increase in neurotrophic factors. Furthermore, activation of cAMP response element binding (CREB) protein, but not NF-κB, by NaB, abrogation of NaB-induced expression of neurotrophic factors by siRNA knockdown of CREB and the recruitment of CREB and CREB-binding protein to the BDNF promoter by NaB suggest that NaB exerts its neurotrophic effect through the activation of CREB. Accordingly, cinnamon feeding also increased the activity of PKA and the level of phospho-CREB in vivo in the CNS. These results highlight a novel neutrophic property of cinnamon and its metabolite NaB via PKA – CREB pathway, which may be of benefit for various neurodegenerative disorders.
Introduction
Neurotrophic factors are a family of molecules that stimulate and control neurogenesis and support the survival of existing neurons. Consistently, in neurodegenerative disorders, which are hallmarked by the loss of neurons, neurotrophic factors have been suggested as rescuers of these vulnerable cells. Various neurotrophic molecules including brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3) exhibit protective effects in cell culture as well as animal models of different neurodegenerative disorders (Phillips et al., 1991; Arenas and Persson, 1994; Mamounas et al., 1995; Durany et al., 2000; Howells et al., 2000; Porritt et al., 2005). However, clinical application of those molecules has been limited because of difficulties in delivery. These small proteins do not readily diffuse across the blood-brain barrier (BBB) or ventricular lining and have limited or unstable bioavailability (Rangasamy et al., 2010). Gene delivery and/or protein delivery by stereotactic injection is definitely an option but it has several limitations (Huang and Mucke, 2012). It seems from the therapeutic angle, the best option is to stimulate/induce the production of neurotrophic factors within the CNS of patients with neurodegenerative and neuroinflammatory diseases. Therefore, finding out drugs or small molecules to upregulate neurotrophic factors within the CNS represents an important area of research.
Cinnamon, the brown bark of cinnamon tree, is a commonly used spice and flavoring material for deserts, candies, chocolate, etc. It has a long history as a medicine as well. Medieval physicians used cinnamon in medicines to treat a variety of disorders including arthritis, coughing, hoarseness, sore throats, etc. It was once so highly-prized that several wars were fought over it. In addition to containing manganese, dietary fiber, iron, and calcium, cinnamon contains a major compound, cinnamaldehyde, which is converted into cinnamic acid by oxidation. In the liver, this cinnamic acid is β-oxidized to benzoate (Abd El-Mawla et al., 2001) that exists as sodium salt (NaB) or benzoyl-CoA. It has been reported that minor amount of NaB is also excreted in the urine of human (Bridges et al., 1970; Kubota and Ishizaki, 1991). NaB is of medical importance as it is a component of Ucephan, a FDA-approved drug used in the treatment for hepatic metabolic defects associated with hyperammonemia such as urea cycle disorder in children (Leonard and Morris, 2002; Scaglia et al., 2004). It is also widely used as a preservative in broad range of foods and cosmetic products (Nair, 2001). It is non-toxic and can be administered as a solution in drinking water. It has been reported that 2% solution of NaB in drinking water is safe for lifelong treatment in mice without any noticeable side effects (Toth, 1984). Recently we have delineated that NaB is capable of modulating both innate and adaptive immune responses (Brahmachari and Pahan, 2007; Brahmachari et al., 2009; Brahmachari and Pahan, 2010).
Here we provide the first evidence about the neurotrophic function of cinnamon metabolite NaB. Neurotrophic factors could be produced from both neurons and glial cells. While neurons produce more neurotrophins than glia under physiological conditions, glial cells produce more neurotrophins than neurons under pathophysiological conditions. We show that NaB increases the expression of neurotrophic factors (BDNF and NT-3) in brain cells and in vivo in the CNS of mice. Accordingly, oral administration of cinnamon increased the level of NaB in vivo in the CNS and upregulated CNS expression of neurotrophic factors. Furthermore, we demonstrate that NaB utilizes the PKA – CREB pathway for the upregulation of neurotrophic factors. Our findings raise a possibility that cinnamon and NaB may find further application in neurodegenerative disorders via increased production of neurotrophic factors.
Materials and Methods
Reagents
Sodium benzoate (NaB) and sodium formate (NaFO), were purchased from Sigma Aldrich (St. Louis, MO). Original Ceylon cinnamon (Cinnamonum verum) in ground form was obtained from Indus Organics (San Ramon, CA). Cinnamonum cassia (1000 mg capsules) was purchased from a Chicago area Walgreen. Fetal bovine serum (FBS), Hank's balanced salt solution (HBSS), trypsin, and DMEM/F-12 were from Mediatech (Manassas, VA). Neurobasal media and B27 supplement were purchased from Invitrogen (Grand Island, NY).
Mass spectrometric analysis
A JEOL GCMate II (JEOL USA, Peabody MA) gas chromatograph/ mass spectrometer was used in these experiments. The gas chromatography column was a fused silica capillary column with a nonpolar 5% phenyl 95% dimethylpolysiloxane phase (Agilent HP-5ms), 30 meters long, 0.25 mm internal diameter, 0.25 um film thickness. The carrier gas was Helium (99.9995% Research Grade) run through a STG triple filter (Restek Corp.) at a constant flow rate of 1.1 ml/min. The injector was held at 275 Deg C and was fitted with an Agilent 4mm ID single taper split liner containing deactivated glass wool. The spectrometer was operated in full scan EI mode (+Ve) at 70eV scanning from m/z 10 to m/z 850 using a linear magnet scan. The scan speed was 0.3 sec per scan. The solvent delay was 4.0 min. Data analysis was performed using the TSSPro software (Shrader Analytical & Consulting Laboratoies, Inc., Detroit MI). Total ion current (TIC) chromatographic peaks were used for quantitation. Mass calibration was performed using perflourokerosene.
Isolation of Primary Human Neurons
Human primary neurons were prepared as described by us earlier (Jana et al., 2007; Jana and Pahan, 2010). All of the experimental protocols were reviewed and approved by the Institutional Review Board of the Rush University Medical Center. Briefly, 11–17-week-old fetal brains obtained from the Human Embryology Laboratory (University of Washington, Seattle, WA) were dissociated by trituration and trypsinization (0.25% trypsin in PBS at 37 °C for 15 min). The trypsin was inactivated with 10% heat-inactivated fetal bovine serum (Mediatech). The dissociated cells were filtered through 380- and 140-μm meshes (Sigma Aldrich) and pelleted by centrifugation. The cell pellet was washed once with PBS and once with Neurobasal medium containing 2% B27 and 1% antibiotic-antimycotic mixture (Sigma). In the first step, neurons were enriched by allowing the cells (3 × 106/ml) to adhere to poly-D-lysine-coated plates or coverslips for 5 min. Nonadherent cells were removed, and adherent cells (mostly neurons) were further treated with 10 μM Ara-C to prevent the proliferation of dividing cells. After 10 days of Ara-C treatment, the cells were used for this study. More than 98% of this preparation was positive for microtubule-associated protein-2 (MAP-2), a marker for neurons.
Preparation of Primary Human Astrocytes
Primary human astrocytes were prepared as described by us earlier (Jana et al., 2007; Jana and Pahan, 2010).
Semi-quantitative RT-PCR analysis
To remove any contaminating genomic DNA, total RNA was digested with DNase. Semi-quantitative RT-PCR was carried out as described earlier (Saha et al., 2006; Ghosh and Pahan, 2012; Khasnavis and Pahan, 2012) using a RT-PCR kit from Clonetech (Mountain View, CA). Briefly, 1 μg of total RNA was reverse transcribed using oligo(dT)12-18 as primer and MMLV reverse transcriptase (Clontech). The resulting cDNA was appropriately-diluted, and diluted cDNA was amplified. Amplified products were electrophoresed on a 1.8% agarose gels and visualized by ethidium bromide staining.
| BDNF: | Sense: 5′ -TGAGGACCAGAAAGTTCGGC-3′ |
| Antisense: 5′-CAGTCTTTTTGTCTGCCGCC-3′ | |
| NT-3: | Sense: 5′-TTTCTCGCTTATCTCCGTGGCATCC-3′ |
| Antisense: 5′-GGCAGGGTGCTCTGGTAATTTTCC T-3′ | |
| CREB: | Sense: 5′- ACCAGGAGTGCCAAG GAT TGAAGA-3′ |
| Antisense: 5′- TTTGTACGTCTCCAGAGGCAGCTT-3′ | |
| GAPDH: | Sense: 5′-GGTGAAGGTCGGTGTGAACG3′ |
| Antisense: 5′-TTGGCTCCACCCTTCAAGTG-3′ |
Real-time PCR analysis
It was performed using the ABI-Prism7700 sequence detection system (Applied Biosystems, Foster City, CA) as described earlier (Roy et al., 2007; Ghosh and Pahan, 2012; Khasnavis and Pahan, 2012). The mRNA expressions of respective genes were normalized to the level of GAPDH mRNA. Data were processed by the ABI Sequence Detection System 1.6 software and analyzed by ANOVA.
Preparation of Nuclear Extracts and Electrophoretic Mobility Shift Assay
DNA-binding activities of CREB and NF-κB were analyzed by non-radioactive electrophoretic mobility shift assay (EMSA) as described (Corbett et al., 2012; Mondal et al., 2012). After treatment, cells were washed with HBSS, scraped into 1.5 mL tubes and centrifuged in 4°C for 5 min at 500 rpm. The supernatant was aspirated and the pellet was resuspended in a membrane lysis buffer comprised of (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) (HEPES, pH 8.0), MgCl2, KCl, dithiothreitol (DTT) and protease/phosphatase inhibitors (Sigma Aldrich), vortexed and centrifuged in 4°C at 720 × g for 5 minutes. Again, the supernatant was aspirated and the pellet was resuspended in a high salt, nuclear envelope lysis buffer comprised of HEPES (pH 8.0), MgCl2, glycerol, NaCl, ethylenediaminetetraacetic acid (EDTA), DTT and protease/phosphatase inhibitors, rotated vigorously in 4°C for 15 min and centrifuged in 4°C at 13,000 rpm for 15 minutes. The resultant supernatant was complexed with a cocktail of binding buffer (Tris-HCl, KCl, EDTA, DTT, 10x TGE, glycerol and Triton-X 100), custom designed fluorescent CREB-specific or NF-κB-specific probes (Li-Cor Biosciences, Lincoln, NE), and salmon sperm DNA (Invitrogen) for 15 min at room temperature and electrophoresed on custom-cast 6% polyacrylamide-TGE gels in 1X TGE for 2 hrs. Supershift was performed by incubating nuclear extracts with 2 μg ChIP-grade CREB antibody or IgG (EMD Millipore, Billerica, MA) for 30 min prior to addition of the probe. The shift was visualized under the Odyssey® Infrared Imaging System (Li-Cor).
Chromatin immunoprecipitation
Recruitment of CREB to the BDNF promoter was determined using the EZ ChIP kit from EMD Millipore as described by us (Corbett et al., 2012; Ghosh and Pahan, 2012). Briefly, 1 × 106 astrocytes were treated with NaB and after 2 h of treatment, cells were fixed by adding formaldehyde (1% final concentration), and cross-linked adducts were resuspended and sonicated. ChIP was performed on the cell lysate by overnight incubation at 4°C with 2 μg of Abs against CREB and CBP followed by overnight incubation with protein G-agarose (Santa Cruz Biotechnology, Dallas, TX). The beads were washed and incubated with elution buffer. To reverse the cross-linking and purify the DNA, precipitates were incubated in a 65°C incubator overnight and digested with proteinase K. DNA samples were then purified, precipitated, and precipitates were washed with 75% ethanol, air-dried, and resuspended in Tris-EDTA buffer. The following primers were used to amplify fragments flanking the CRE in the mouse exon IV-BDNF promoter: sense: 5′-TCATCACTCACGACCACGTC-3′, antisense: 5′-CCAGCCGGCTACTGCTTTA-3′. PCR products were electrophoresed on 2% agarose gels.
Assay of BDNF by ELISA
Level of BDNF was monitored in supernatants and cell homogenates by high-sensitivity ELISA kit (Promega, Madison, WI) as described before (Saha et al., 2006; Roy et al., 2007).
Immunofluorescence Analysis
It was performed as described earlier (Corbett et al., 2012; Ghosh and Pahan, 2012). Briefly, cover slips containing 100-200 cells/mm2 were fixed with 4% paraformaldehyde followed by treatment with cold ethanol and two rinses in phosphate-buffered saline (PBS). Samples were blocked with 3% bovine serum albumin (BSA) in PBS-Tween-20 (PBST) for 30 min and incubated in PBST containing 1% BSA and following anti-mouse primary antibodies: goat polyclonal BDNF (1:200; Santa Cruz), mouse monolonal β-tubulin (1:500; Millipore), rabbit polyclonal p-CREB (1:600; Cell Signaling), goat polyclonal GFAP (1:300; Santa Cruz), rabbit polyclonal GFAP (1:1000; DAKO), and mouse monoclonal NeuN (1:500; EMD Millipore). After four 15 min washes in filtered PBS, slides were further incubated with Cy2- or Cy5-labeled secondary antibodies (all 1:200; Jackson ImmunoResearch, West Grove, PA) for 1 hr under similar shaking conditions. Following four 15 minute washes with filtered PBS, cells were incubated for 4-5 min with 4′,6-diamidino-2-phenylindole (DAPI, 1:10,000; Sigma). For negative controls, a set of culture slides was incubated under similar conditions void of primary antibodies. The samples were run in an EtOH and Xylene (Thermo Fisher, Waltham, MA) gradient, mounted and observed under a Bio-Rad MRC1024ES confocal laser-scanning microscope
Statistics
Statistical comparisons were made using one-way analysis of variance followed by Student's t test.
Results
Sodium benzoate (NaB) upregulates BDNF and NT-3 in primary human neurons
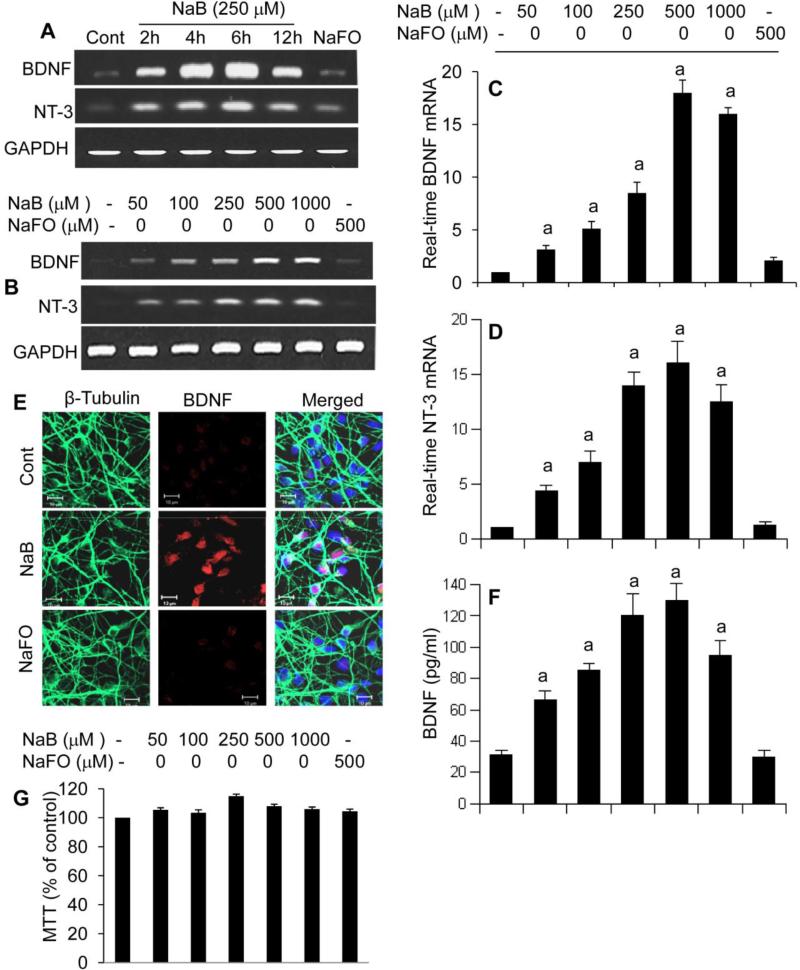
Although there is growing knowledge about the role of neurotrophins in the health and survival of neurons, stem cells as well as oligodendrocytes, little is known about the drugs and associated molecular mechanisms that upregulate neurotrophins. Under physiological conditions, neurons are major producers of neurotrophic factors (Park and Poo, 2013). We examined if NaB could stimulate the expression of neurotrophins in primary human neurons. We found that NaB indeed increased the mRNA expression of both BDNF and NT-3 in a time-dependent (Fig. 1A) and dose-dependent (Fig. 1B-D) manner. This result was specific as sodium formate (NaFO) had no effect on the expression of neurotrophic factors in neurons (Fig. 1A-D). We further checked the protein level of BDNF in neurons. As evident from immunofluorescence in figure 1E and ELISA in figure 1F, NaB, but not NaFO, increased the protein level of BDNF in human neurons. This result was not due to any change in cell viability as MTT assays (Fig. 1G) showed that NaB remained unable to modulate the viability of neurons.
Figure 1. Induction of neurotrophic factors by NaB, but not sodium formate (NaFO), in primary human neurons.
Primary neurons were treated with 250 μM NaB or NaFO for different time intervals followed by monitoring the mRNA expression of BDNF and NT-3 by semi-quantitative RT-PCR (A). Cells were treated with different concentrations of NaB and NaFO for 6 h followed by monitoring mRNA expression of BDNF and NT-3 by semi-quantitative RT-PCR (B) and real-time PCR (C, BDNF; D, NT-3). After 24 h of treatment, protein level of BDNF was monitored by immunofluorescence analysis (E) and ELISA (F). Cell viability was assayed by MTT (G). Results are mean ± SD of three different experiments. sp < 0.001 vs control.
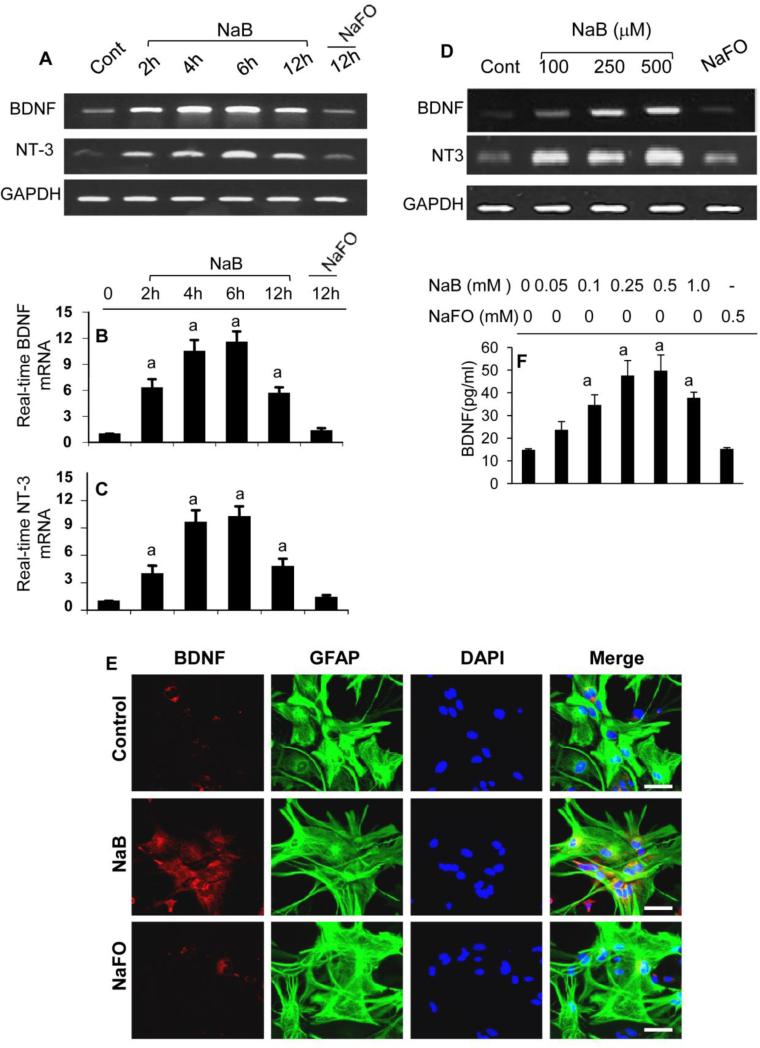
Astrocytes, the major cell type in the CNS, also produce neurotrophic factors (Saha et al., 2006; Park and Poo, 2013). Therefore, next we examined if NaB could stimulate the expression of neurotrophic factors in primary human astrocytes. Consistent to human neurons, NaB strongly increased the mRNA expression of BDNF and NT-3 in human astrocytes in a time-dependent (Fig. 2A-C) and dose-dependent (Fig. 2D) manner. Again, NaFO remained unable to induce the expression of BDNF and NT-3 in astrocytes, suggesting the specificity of this effect. Double-label immunofluorescence showed that NaB, but not NaFO, markedly stimulated the expression of BDNF in astrocytes (Fig. 2E). ELISA results also showed that NaB at a dose of 250 or 500 M increased the level of BDNF protein in human astrocytes by about four fold (Fig. 2F).
Figure 2. Induction of neurotrophic factors by NaB, but not NaFO, in primary human astrocytes.
Primary astrocytes were treated with 250 μM NaB or NaFO for different time intervals followed by monitoring the mRNA expression of BDNF and NT-3 by semi-quantitative RT-PCR (A) and real-time PCR (B, BDNF; C, NT-3). Cells were treated with different concentrations of NaB for 6 h followed by monitoring the mRNA expression of BDNF and NT-3, by semi-quantitative RT-PCR (D). After 24 h of treatment, double-label immunofluorescence analysis was performed for BDNF & GFAP (E). Supernatants were used for BDNF ELISA (F). Results are mean ± SD of three different experiments. sp < 0.001 vs control.
Composition of different types of cinnamon
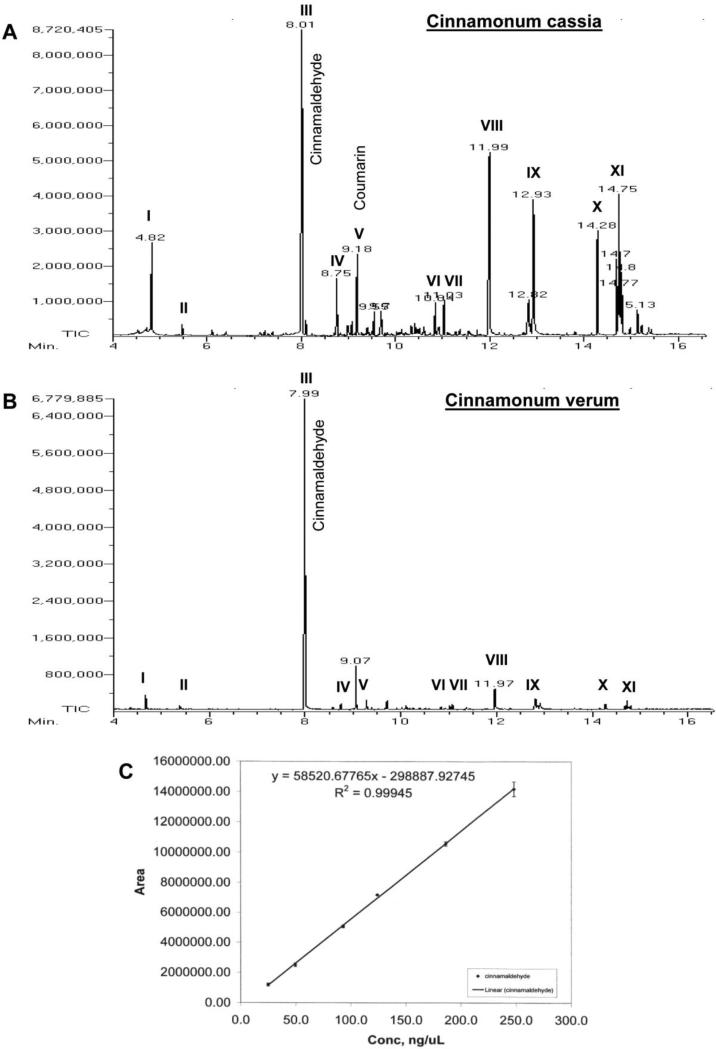
Next, we concentrated on cinnamon itself if cinnamon could upregulate neurotrophic factors in the brain. However, cinnamon contains different types of compounds and there are different types of cinnamon as well. Chinese cinnamon (Cinnamonum cassia) and original Ceylon cinnamon (Cinnamonum verum or Cinnamonum zylencum) are two major types of cinnamon that are available in the US. Therefore, we analyzed the composition of these two types of cinnamon powder by mass spectrometry. Although cinnamaldehyde was present as the major peak in both Cinnamonum cassia (Fig. 3A) and Cinnamonum verum (Fig. 3B), Cinnamonum cassia contained more styrene, benzene, 1,1’-(2-butene-1,4-diyl)bis-, benzene, 1,1’-(1,2-cyclobutanediyl)bis-, palmitic acid, stearic acid, 4-phenylbutyl chloride, and (2,3-diphenylcyclopropyl)methyl phenyl sulfoxide than Cinnamonum verum (Fig. 3A-B). Furthermore, Cinnamonum cassia contained a peak at 9.18 min, which corresponded to 1-benzopyran-2-one (commonly known as coumarin) (Fig. 3A). Interestingly, Cinnamonum verum did not contain any coumarin. Since it has been reported that at higher dose, coumarin could be toxic for the liver and kidney, we performed further studies with only Cinnamonum verum. Before using Cinnamonum verum in mouse, we also quantified the level of cinnamaldehyde in this type of cinnamon using the standard curve (Fig. 3C). We found that the concentration of cinnamaldehyde in acetonitrile extract of Cinnamonum verum was 2.15 mM. Upon calculation, it has been seen that Cinnamonum verum contains 2.84 mg cinnamaldehyde per gm of cinnamon powder. However, it is possible that all cinnamaldehyde of cinnamon powder was not extracted in acetonitrile because only 20% of total mass of cinnamon was solubilized in acetonitrile.
Figure 3. Mass spectrometric analysis of cinnamon extract.
One gm cinnamon powder (A, Cinnamonum cassia; B, Cinnamonum verum) was solubilized in 5 ml acetonitrile by vortexing for 5 min. After centrifugation, the extract was loaded onto a GC mass spectrometer (JEOL GCmate). Different peaks [I, styrene; II, benzaldehyde; III, cinnamaldehyde; IV, cinnamic acid; V, benzopyran-2-one or coumarin; VI, benzene, 1,1’-(2-butene-1,4-diyl)bis-; VII, benzene, 1,1’-(1,2-cyclobutanediyl)bis-, trans-; VIII, palmitic acid; IX, stearic acid; X, 4-phenylbutyl chloride; XI, (2,3-diphenylcyclopropyl)methyl phenyl sulfoxide] were identified in the University of Illinois at Chicago Research Resources Center (UIC RRC) using the NIST Mass Spectral Database Library. C) Since cinnamaldehyde was found to be the major peak, a standard curve for cinnamaldehyde was made to quantify the level of cinnamaldehyde in cinnamon extracts. Results represent three independent experiments.
Oral administration of Cinnamonum verum powder produced NaB in vivo in serum and brain of mice
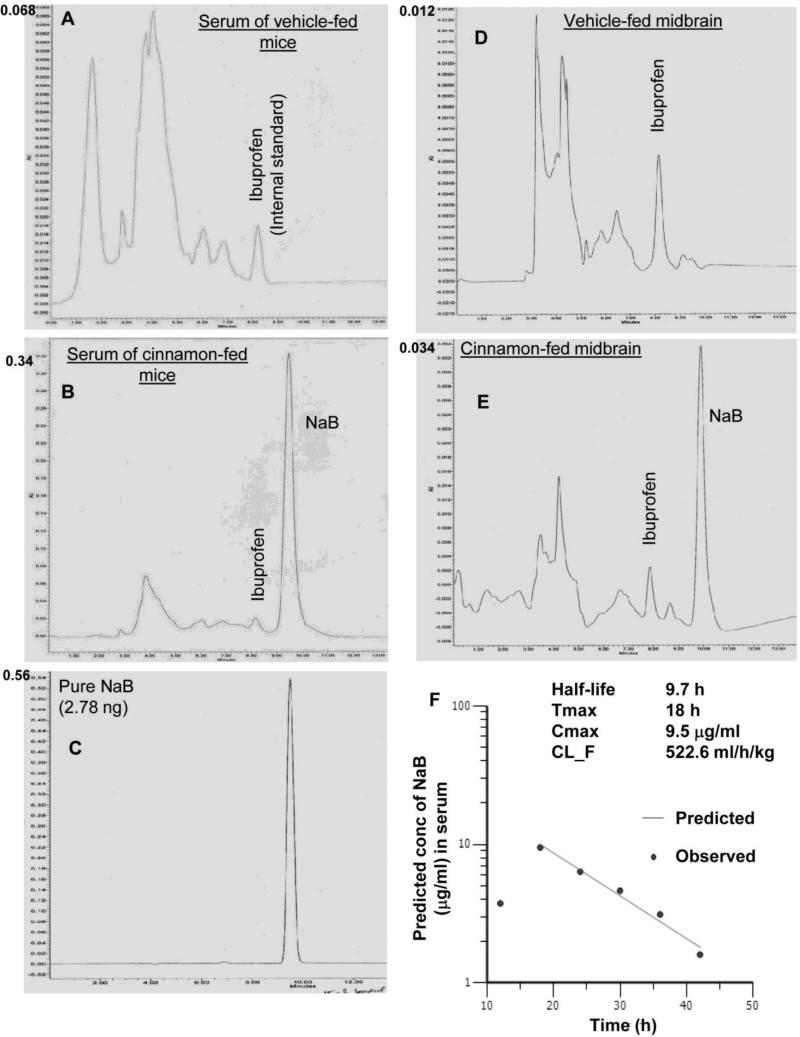
Theoretically cinnamon should be metabolized into NaB. However, there is no quantitative data available on the level of NaB in cinnamon-fed animals. We examined if oral administration of Cinnamonum verum powder to mice increased the level of NaB in serum and brain of C57/BL6 mice. Ibuprofen was used as an internal standard. By HPLC, we could not detect any NaB in serum (Fig. 4A) and midbrain (Fig. 4D) of vehicle-fed mice. The sharp peak in figure 4C represents pure NaB. Oral treatment of mice with ground cinnamon (200 mg/kg body wt/d) via gavage markedly increased the level of NaB in serum (Fig. 4B) and midbrain (Fig. 4E). These results demonstrate that cinnamon is metabolized into NaB and that cinnamon-derived NaB is capable of entering into the CNS.
Figure 4. Oral administration of cinnamon powder (Cinnamonum verum) markedly increases the level of NaB in serum and brain of C57/BL6 mice.
After 10 d of either vehicle (A) or cinnamon (200 mg/kg body wt/d) (B) feeding via gavage, mice were sacrificed followed by isolation of blood serum. Ibuprofen was added to serum as an internal standard. Serum was analyzed for the detection of NaB in Waters 2695 separation module HPLC system with the help of “Em-power pro” software and Phenomenex Luna 5μ C18 100A column (250 × 4.6 mm; 280-nm UV wavelength) using mobile phase A (acetonitrile with 0.01M H3PO4) and mobile phase B (H2O with 0.01M H3PO4) (1:4) at the flow rate of 0.5 ml/min. C) Chromatogram for 2.78 ng pure NaB. Similarly, after cinnamon feeding, midbrain tissue (D, vehicle-fed; E, cinnamon-fed) was collected in chloroform: methanol (2:1) extraction solvent containing 0.05M perchloric acid. Ibuprofen was added as an internal standard. Tissues were homogenized and centrifuged at 20,000g for 10 mins. Organic and aqueous phases were separated carefully and 10 μl aqueous phase was analyzed for the detection of NaB by HPLC. F) For pharmacokinetic analysis in serum, mice were treated with one dose of cinnamon powder (100 mg/kg body wt) via gavage. At different time points, mice (n=3) were sacrificed and the concentration of NaB in serum was monitored by HPLC. These data were analyzed using the Phoenix WinNonlin pharmacokinetics modeling software (Pharsight, Mountain View, CA) to calculate half-life, Tmax, Cmax, and clearance (CL_F).
In order to understand the pharmacokinetics of cinnamon, mice were gavaged cinnamon powder, which was mixed in 0.5% methylcellulose, at a dose of 100 mg/kg body wt/d, and at different time points, the concentrations of NaB in serum was monitored by HPLC. These data were analyzed using the Phoenix WinNonlin pharmacokinetics modeling software (Pharsight, Mountain View, CA) to calculate half-life (9.7 h), Tmax (18 h), Cmax (9.5 μg/ml), and clearance (CL_F) (522.6 ml/h/kg) (Fig. 4F).
Oral administration of Cinnamonum verum powder and NaB increases the expression of neurotrophic factors in the brain of mice
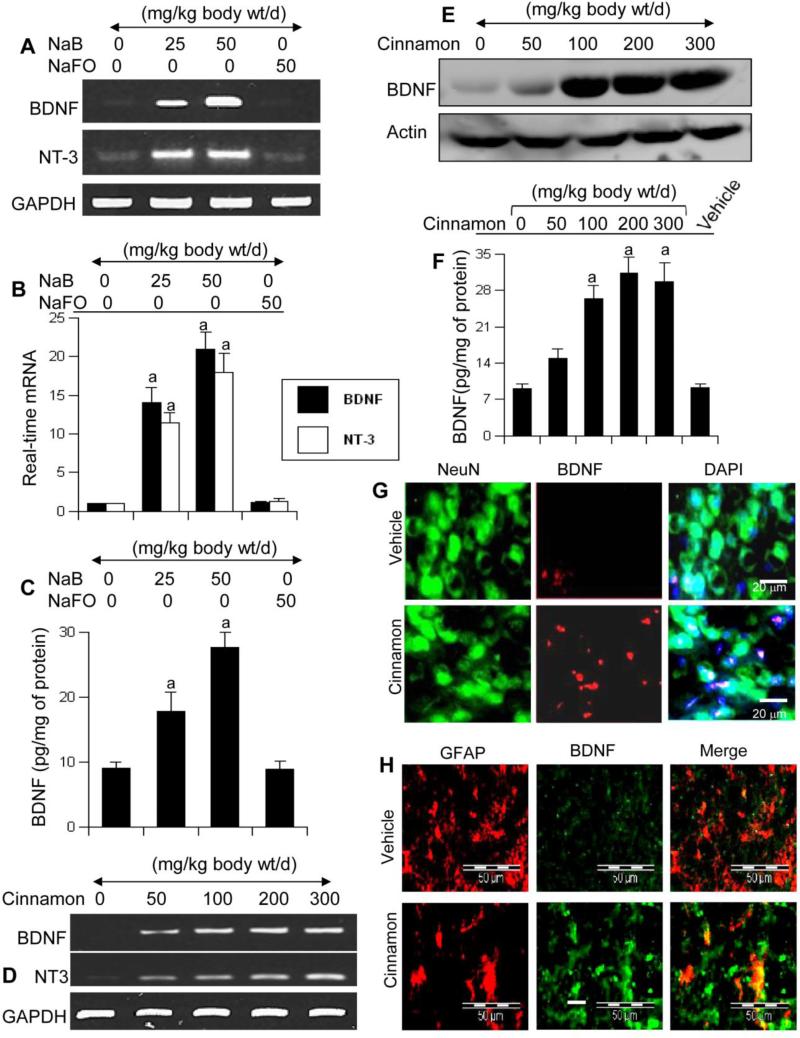
Because NaB increased the expression of neurotrophins in cultured astrocytes and neurons, we examined if NaB was capable of doing so in vivo in the brain. After 10 d of oral administration, NaB markedly increased the mRNA expression of BDNF and NT-3 (Fig. 5A for RT-PCR; Fig. 5B for real-time) in the cortex of male C57/BL6 mice. This result was specific as NaFO feeding did not increase the mRNA expression of these neurotrophic factors (Fig. 5A-B). As evident from ELISA, NaB, but not NaFO, also increased the level of BDNF protein in the cortex (Fig. 5C). Next, we examined if cinnamon itself could also increase these invaluable molecules in vivo in the brain. Interestingly, after 10 d of feeding, Cinnamonum verum powder markedly increased the mRNA expression of BDNF and NT-3 in the cortex (Fig. 5D). Western blot (Fig. 5E) and ELISA (Fig. 5F) results showed that cinnamon feeding markedly increased the level of BDNF protein in vivo in the cortex. These results were also supported by immunofluorescence analysis of cortical sections, which showed that cinnamon treatment increased the level of BDNF in both NeuN-positive neurons (Fig. 5G) and GFAP-positive astrocytes (Fig. 5H).
Figure 5. Oral administration of NaB and cinnamon increases the level of neurotrophic factors in vivo in the CNS of mice.
Six to eight week old male C57/BL6 mice (n=5 in each group) received different amounts of NaB via gavage and after 10 d of feeding, the mRNA expression of BDNF and NT-3 was monitored in the cortex by semi-quantitative RT-PCR (A) and real-time PCR (B). The protein level of BDNF in the cortex was quantified by ELISA (C). Similarly, mice (n=5 in each group) received different amounts of cinnamon powder (Cinnamonum verum) via gavage and after 10 d of feeding, the mRNA expression of BDNF and NT-3 was monitored in the cortex by semi-quantitative RT-PCR (D). Control mice received only vehicle (0.5% methylcellulose). The protein level of BDNF in the cortex was monitored by Western blot (E) and ELISA (F). Data are means ± SEM of five mice per group. sp < 0.001 vs control. Cortical sections were also double-labeled for NeuN & BDNF (G) and GFAP & BDNF (H). Results represent analysis of two sections of each of five mice per group.
NaB requires CREB for the upregulation of neurotrophic factors
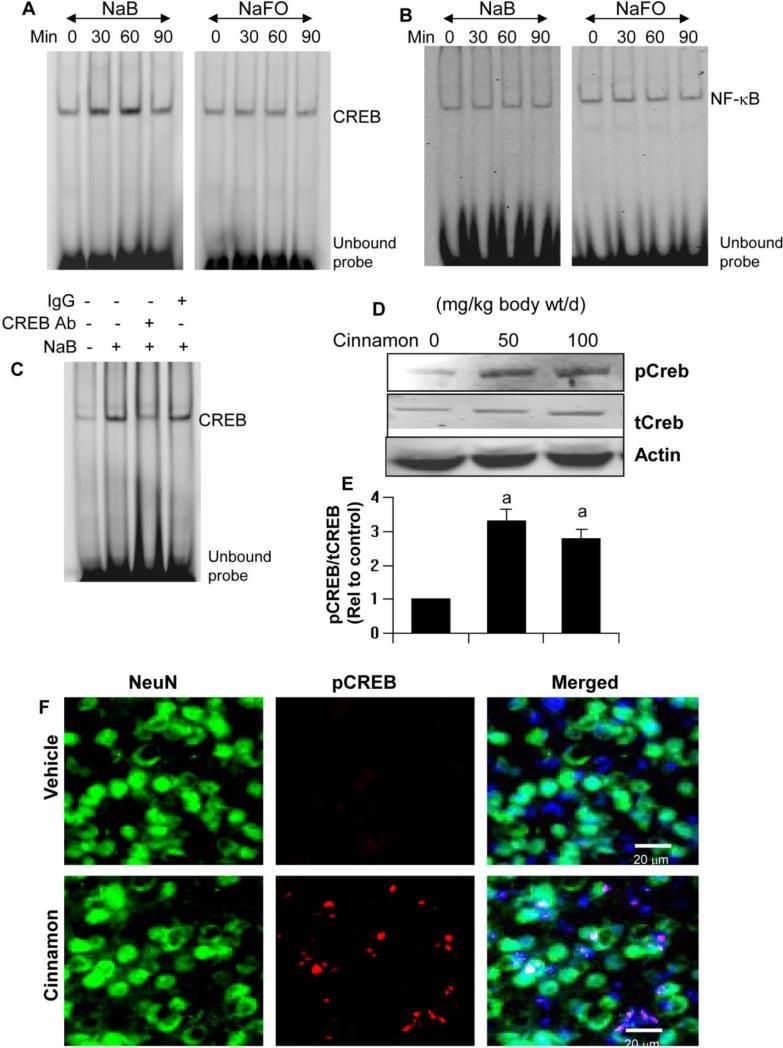
Next, we investigated mechanisms by which NaB upregulated neurotrophic factors in brain cells. Because neurotrophins have long been thought to be under the transcriptional control of CREB (West et al., 2001; Tardito et al., 2006), we analyzed the effect of NaB on the activation of CREB. As analyzed by EMSA, NaB, but not NaFO, induced the DNA-binding activity of CREB in primary human astrocytes in a time-dependent manner with maximum activation visible at 60 min of stimulation (Fig. 6A). Super-shift assay confirmed the presence of CREB in NaB-induced DNA:protein complex (Fig. 6C). Activation of CREB by NaB was specific as neither NaB nor NaFO had any effect on the activation of NF-κB (Fig. 6B). Next, we examined the effect of cinnamon on the activation of CREB in vivo in the brain. In this case, we examined the activation of CREB by monitoring levels of phosphorylated CREB (p-CREB). Similar to the effect of NaB in cultured astrocytes, oral administration of cinnamon markedly increased the phosphorylation of CREB in vivo in the cortex of mice as depicted by Western blot (Fig. 6D-E). This finding was also confirmed by double-label immunofluorescence that shows increase in phosphor-CREB in NeuN-positive neurons in the cortex of cinnamon-, but not vehicle-, fed mice (Fig. 6F).
Figure 6. Activation of CREB by NaB in primary human astrocytes and by cinnamon in vivo in the cortex of mice.
Cells were incubated with 250 μM NaB or NaFO for different time periods followed by monitoring the activation of CREB (A) and NF-κB (B) by EMSA. C) Supershift assay was performed using antibodies against CREB. IgG was used as control. Mice (n=5 in each group) received different amounts of cinnamon powder (Cinnamonum verum) via gavage and after 10 d of feeding, levels of phospho-CREB and total CREB were monitored in the cortex by Western blot (D). Bands were scanned and presented as relative to control (E). Data are means ± SEM of five mice per group. sp < 0.001 vs control. Control mice received only vehicle (0.5% methylcellulose). Cortical sections were also double-labeled for NeuN & phospho-CREB (F). Results represent analysis of two sections of each of five mice per group.
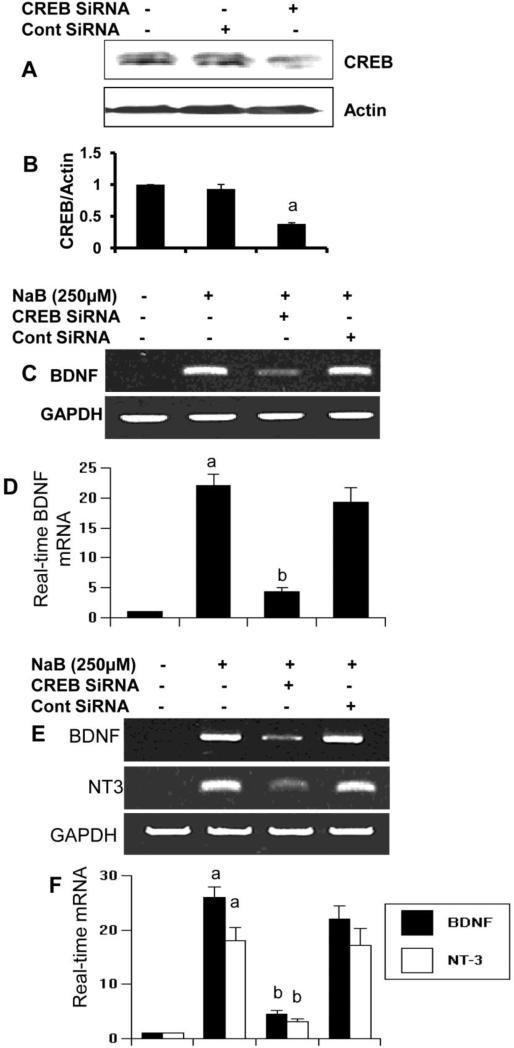
Next, we investigated if NaB required CREB for the upregulation of neurotrophic factors in brain cells. At first, we examined if siRNA knockdown of CREB was capable of suppressing the expression of CREB protein in astrocytes. As evident from figure 7A-B, CREB siRNA, but not control siRNA, decreased the expression of CREB protein in control astrocytes. Accordingly, CREB siRNA, but not control siRNA, abrogated NaB-mediated upregulation of BDNF mRNA in astrocytes (Fig. 7C-D). Similarly, CREB siRNA, but not control siRNA, also decreased NaB-induced upregulation of BDNF and NT-3 mRNAs in neurons (Fig. 7E-F). These results suggest that NaB induces the activation of CREB, but not NF-κB, and that CREB is required for increased expression of neurotrophic factors in brain cells.
Figure 7. SiRNA knockdown of CREB abrogates the ability of NaB to induce neurotrophic factors in primary human astroglia and neurons.
Primary astrocytes were transfected with CREB-siRNA and control siRNA. After 48 h of transfection, the specificity of siRNA knockdown was monitored by Western blot for CREB and actin (A). Bands were scanned and presented as relative to control (B). After 48 h of siRNA transfection, either astrocytes (C & D) or neurons (E & F) were treated with NaB and after 6 h of treatment, the expression of BDNF was monitored by semi-quantitative RT-PCR (C & E) and real-time PCR (D & F). Results are mean ± S.D. of three different experiments. ap < 0.001 vs control; bp < 0.001 vs NaB.
NaB induced the activation of CREB and the upregulation of BDNF via protein kinase A (PKA)
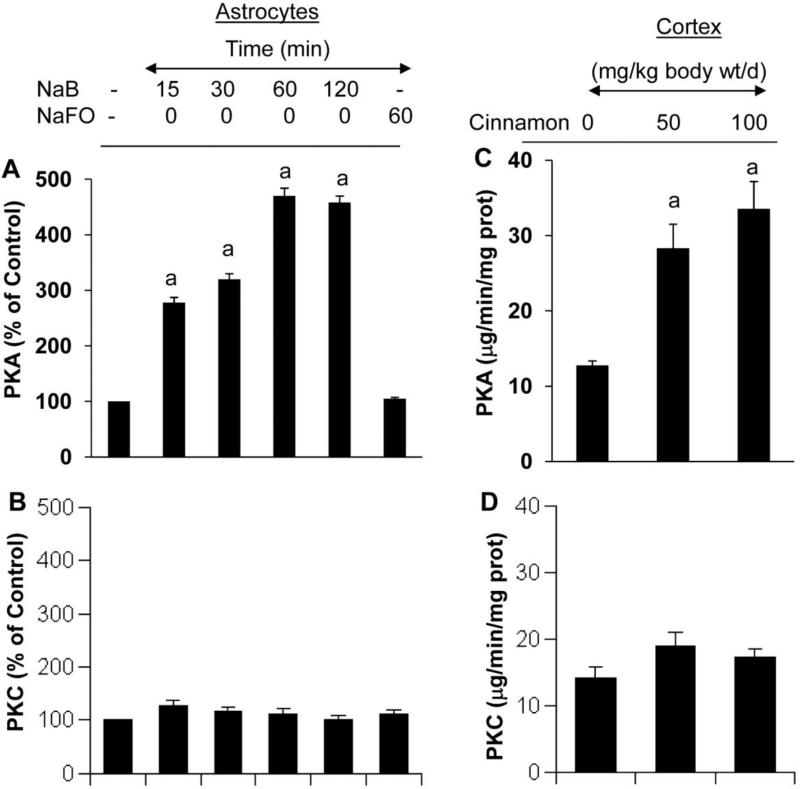
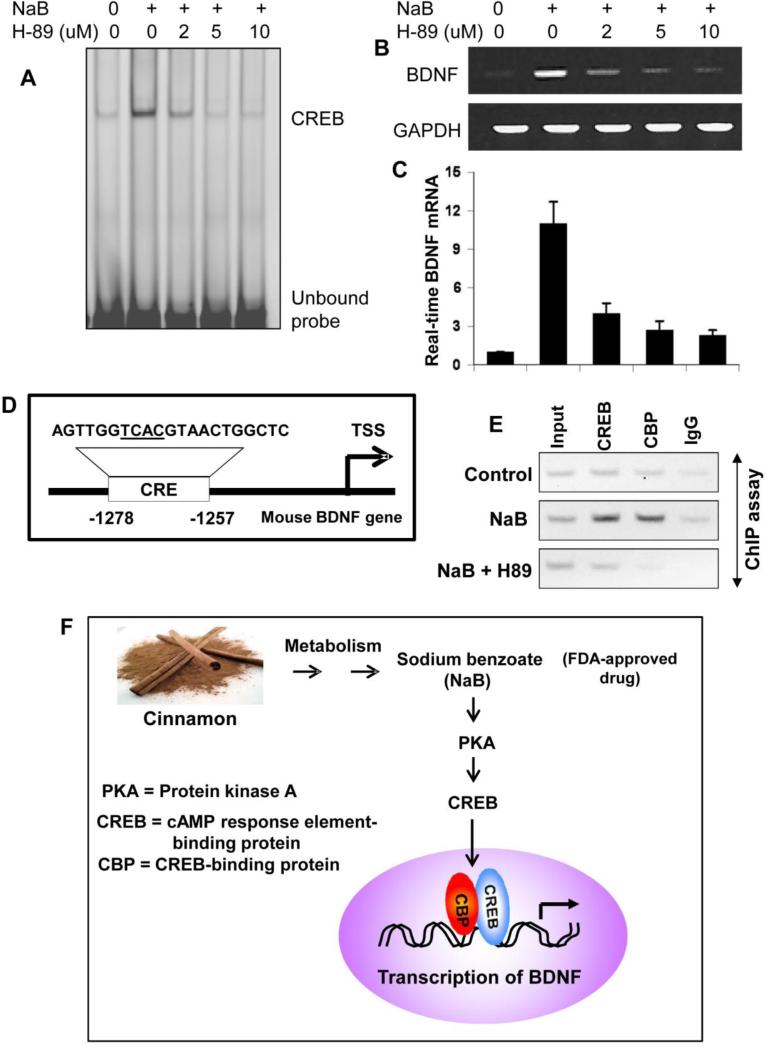
CREB activity can be regulated by a number of signaling pathways. For example, stimuli that activate PKA and PKC result in CREB phosphorylation (Kandel, 2012). This prompted us to determine the upstream regulator of the NaB-mediated CREB activation. At first, we monitored if NaB alone was capable of activating PKA in human astrocytes. As evident from figure 8A, NaB alone induced the activation of PKA in astrocytes in a time-dependent manner and this activation was evident from as early as 15 min of stimulation. However, NaFO remained unable to activate PKA. Furthermore, NaB had no effect on the activation protein kinase C (PKC) (Fig. 8B), indicating the specificity of its effect. Similarly, oral administration of cinnamon also caused the activation of PKA (Fig. 8C), but not PKC (Fig. 8D), in vivo in the cortex of mice. Next, we examined if NaB required PKA for the activation of CREB in astrocytes. Suppression of NaB-mediated activation of CREB by H-89 (Fig. 9A) suggests that NaB induces the activation of CREB via PKA. Accordingly, suppression of NaB-induced expression of BDNF mRNA (Fig. 9B-C) in astrocytes by H-89, a specific inhibitor of PKA, suggests that NaB upregulates BDNF in astrocytes via PKA.
Figure 8. Activation of protein kinase A (PKA) by NaB in primary human astrocytes and by cinnamon in vivo in the cortex.
Primary astrocytes were incubated with 250 μM NaB or NaFO for different time periods followed by monitoring the activation of PKA (A) and PKC (B) as described under “Materials and Methods”. Results are mean ± S.D. of three different experiments. ap < 0.001 vs control. Mice (n=5 in each group) received different amounts of cinnamon powder (Cinnamonum verum) via gavage and after 10 d of feeding, activities of PKA and PKC were monitored in cortical homogenates. Data are means ± SEM of five mice per group. sp < 0.001 vs control.
Figure 9. Inhibition of PKA abrogates the ability of NaB to induce the activation of CREB and the transcription of BDNF in astrocytes.
Cells pretreated with different concentrations of H-89 for 30 min were stimulated with 250 μM NaB followed by monitoring the activation of CREB by EMSA (A) and the mRNA expression of BDNF by semi-quantitative RT-PCR (B) and real-time PCR (C). Results are mean ± S.D. of three different experiments. ap < 0.001 vs control; bp < 0.001 vs NaB. D) Presence of one consensus cAMP response element (CRE) in the exon IV-BDNF promoter. E) Cells pretreated with 2 μM H-89 for 30 min were stimulated with 250 μM NaB for 2 h under serum-free condition followed by monitoring the recruitment of CREB and CREB-binding protein (CBP) to the indicated position of the BDNF promoter by ChIP analysis. Normal IgG was used as control. Results represent three independent experiments. F) Schematic representation of the induction of BDNF transcription by cinnamon.
NaB induces the recruitment of CREB to the BDNF promoter in astrocytes
To further confirm the role of CREB in NaB-induced transcription of BDNF, we monitored the recruitment of CREB to the exon IV-BDNF promoter. Mouse exon IV-BDNF promoter harbors one CRE between 1257 and 1278 base pairs upstream of the transcriptional start site (Fig. 9D). At first, we used ChIP analysis to study if NaB induced the recruitment of CREB to this CRE. After immunoprecipitation of NaB-treated astroglial chromatin fragments by Abs against CREB, we were able to amplify 165 bp fragment flanking the CRE and this amplification was missing in control astrocytes (Fig. 9E), suggesting that NaB induces the recruitment of CREB to the BDNF promoter. Because CREB-binding protein (CBP), a histone acetyl transferase, plays an important role in CREB-mediated many transcriptional activities, we investigated if CBP was also involved in NaB-induced transcription of BDNF. As evident from Figure 9E, NaB treatment also induced the recruitment of CBP to the BDNF promoter. In contrast, no amplification product was observed in any of the immunoprecipitates obtained with control IgG (last lanes of Fig. 9E), suggesting the specificity of these interactions. These results suggest that NaB alone is capable of increasing the recruitment of both CREB and CBP to the mouse exon IV-BDNF promoter. Therefore, next we examined if NaB stimulated this recruitment via PKA. Consistent to the inhibition of BDNF mRNA expression (Fig. 9B-C), H-89 inhibited the recruitment of both CREB and CBP to the BDNF promoter in NaB-treated astrocytes (Fig. 9E). These results demonstrate that NaB increases the recruitment of CREB and CBP to the BDNF promoter in astrocytes via PKA.
Discussion
Neurotrophic factors are secreted small proteins that are capable of signaling neurons to survive, differentiate, or grow. Furthermore, neurotrophic factors have also been suggested as rescuers of vulnerable neurons in many neurodegenerative diseases including Alzheimer's disease, Parkinson's disease, HIV-associated dementia, in which the levels of some neurotrophic factors are significantly reduced in the brain. For example, it has been shown that the levels of BDNF and NT-3 are significantly down-regulated in patients with Alzheimer's disease (AD) (Phillips et al., 1991; Durany et al., 2000). Accordingly, these neurotrophic factors exhibit protective effects in cell culture as well as animal models of different neurodegenerative disorders (Arenas and Persson, 1994; Howells et al., 2000; Porritt et al., 2005). Therefore, increasing the levels of these neurotrophic factors and/or maintaining their physiological levels in the CNS of patients with neurodegenerative disorders is/are an important area of research. However, such mechanisms are poorly understood.
Cinnamon is a commonly used flavoring material and its metabolite sodium benzoate (NaB) is a widely used food preservative and a FDA-approved drug for the treatment of urea cycle disorders in children. Earlier we have demonstrated that NaB modifies T cells at multiple steps and protects experimental allergic encephalomyelitis, an animal model of MS (Brahmachari and Pahan, 2007). Recently, it has been shown that orally administered cinnamon extract protects memory in a mouse model of AD (Frydman-Marom et al., 2011). Several lines of evidence presented in this study clearly support the conclusion that cinnamon and its metabolite NaB are capable of upregulating neurotrophic factors. Our conclusion is based on the following observations. First, NaB dose-dependently induced the expression of BDNF and NT-3 in primary human astrocytes and neurons. This upregulation was specific as sodium formate (NaFO), a compound structurally similar to NaB but without having the benzene moiety, had no effect. Second, after oral feeding, NaB entered into the brain and upregulated BDNF and NT-3 in vivo in the brain. Third, oral administration of ground cinnamon increased the level of NaB in blood and brain of mice and upregulated BDNF and NT-3 in vivo in the brain. Because the loss of these neurotrophic factors has been implicated in the pathogenesis of various neurodegenerative diseases, our results provide a potentially important mechanism whereby cinnamon and its metabolite NaB may ameliorate or delay neurodegeneration.
The signaling events required for the transcription of neurotrophic factors are becoming clear. Upon analysis of the exon IV-BDNF promoter using the Genomatix Software Suite, we have found consensus binding sites for CREB, and NF-κB, C/EBPβ, and AP-1 in BDNF promoter. Although these transcription factors (CREB, NF-κB, C/EBPβ, and AP-1) may play a role in the expression of various neurotrophic factors, activation of CREB seems essential for the transcription of most of the neurotrophic factors (Saha et al., 2006; Roy et al., 2007; Kandel, 2012). Therefore, for a drug to exhibit neurotrophic effect, it is almost mandatory to stimulate the activation of CREB. Activation of CREB in cultured glial cells by NaB and in vivo in the CNS by cinnamon feeding and abrogation of the ability of NaB to induce neurotrophic factors by siRNA knockdown of CREB suggest that NaB increases the levels of neurotrophic factors via CREB. Although the activation of NF-κB has also been shown to be involved in the expression of neurotrophic factors, NaB alone does not induce the activation of NF-κB. Furthermore, earlier we have found that in activated glial cells, NaB inhibits the activation of NF-κB (Brahmachari et al., 2009). Therefore, NaB does not upregulate the expression of trophic factors via NF-κB. However, it was unknown by which mechanisms NaB induced the activation of CREB in brain cells. The cAMP-protein kinase A (PKA) pathway, one of the most common and versatile signal pathways in eukaryotic cells, is involved in the regulation of multiple cellular functions including cell growth, differentiation, survival, and synaptic plasticity (Taylor et al., 2008; Kandel, 2012). PKA is a holoenzyme composed of two distinct subunits: regulatory (R) and catalytic (C). These regulatory and catalytic subunits form a tetrameric holoenzyme (R2C2) (Taylor et al., 1995; Taylor et al., 2008). In the absence of cAMP, PKA exists as a stable inactive tetramer (Taylor et al., 1995; Taylor et al., 2008). After an increase in intracellular cAMP, the regulatory PKA subunits bind to cAMP in a cooperative manner, resulting in the conformational change in the regulatory subunits and disassociation of the holoenzyme into an R2(cAMP)4 dimer and two monomers of catalytically active kinase (Taylor et al., 1995; Taylor et al., 2008). Here we present evidence that NaB induced the activation of CREB via PKA. While both protein kinase C (PKC) and protein kinase A (PKA) are known to phosphorylate and activate CREB, NaB specifically induced the activation of PKA. Suppression of PKA by H-89 inhibited NaB-induced activation of CREB and expression of neurotrophic factors. However, at present, we do not know mechanisms by which NaB induces PKA. In general, PKA signaling pathway is transduced from G proteins (Taylor et al., 1995; Taylor et al., 2008). Of the various Gα isoforms, Gsα stimulates adenylyl cyclase, whereas Giα mediates the inhibition of adenylyl cyclase. The activation of adenylyl cyclase catalyzes the conversion of adenosine triphosphate to cAMP, which then serves as a second messenger and activates PKA. Because NaB induces the activation of PKA within minutes, it may not be surprising if NaB uses any of the G proteins to activate PKA.
PKA has several key functions in the cell, including of glycogen, sugar and lipid metabolism. Therefore, our results suggest that cinnamon and NaB may also participate in glucose and lipid metabolism. In fact, in a 2003 clinical trial, cinnamon has been shown to reduce blood sugar in patients with type II diabetes (Khan et al., 2003). The same study has also reported a decrease of cholesterol by 12 to 26 percent by oral administration of cinnamon in same patients. In a recent study, we have also demonstrated that NaB antagonizes the mevalonate pathway and reduces blood cholesterol at a level that is comparable to cholesterol-lowering drug pravastatin (Brahmachari et al., 2009).
There are several advantages of NaB and cinnamon over other proposed anti-neurodegenerative therapies. First, both NaB and cinnamon are fairly nontoxic. Cinnamon has been widely used as flavoring material and spice throughout the world for centuries. Cinnamon is metabolized to NaB. NaB is excreted through the urine, if in excess. NaB is an FDA-approved drug against Urea cycle disorders in children. Second, cinnamon and NaB can be taken orally, the least painful route. Recently, we have demonstrated that NaB treatment of mice with relapsing-remitting EAE, an animal model of MS, via drinking water suppressed the disease process of EAE (Brahmachari and Pahan, 2007). Oral administration of cinnamon extract exhibits neuroprotective effect in a mouse model of AD.(Frydman-Marom et al., 2011) Third, cinnamon and NaB are very economical compared to other existing anti-neurodegenerative therapies. Fourth, after oral administration, NaB rapidly diffuses through the BBB. Similarly, after oral administration of cinnamon, we also detected NaB in the brain. Fifth, glycine toxicity is a problem in different neurological diseases because for movement disorders, glycine is one of the factors for inhibiting motor neurons (Iwasaki et al., 1992). When impaired, glycinergic inhibition leads to spastic and hypertonic disorders such as featured in PD, multiple sclerosis (MS) and spinal cord trauma. NaB is known to combine with glycine to produce hippurate, a compound that is readily excreted in the urine (Mitch and Brusilow, 1982). Because PD and MS patients exhibit significant elevation in plasma level of glycine (Iwasaki et al., 1992; Barkhatova et al., 1998), NaB and cinnamon may have added benefits for MS and PD.
In summary, we have demonstrated that cinnamon (an easily-available and widely-used natural compound) and NaB (a metabolite of cinnamon, commonly-used food additive and a FDA-approved drug for urea cycle disorders) upregulate neurotrophic factors via PKA-mediated activation of CREB. These results highlight undiscovered properties of cinnamon and NaB and indicate that these compounds may be used for therapeutic intervention in neurodegenerative disorders as primary or adjunct therapy.
Acknowledgements
This study was supported by grants from Alzheimer's Association (IIRG-12-241179) and NIH (AT6681). The authors would like to thank Dr. Yang Yuan for assistance with the pharmacokinetics analysis.
Footnotes
Conflict of interest
Authors do not have any conflict of interest.
References
- Abd El-Mawla AM, Schmidt W, Beerhues L. Cinnamic acid is a precursor of benzoic acids in cell cultures of Hypericum androsaemum L. but not in cell cultures of Centaurium erythraea RAFN. Planta. 2001;212:288–293. doi: 10.1007/s004250000394. [DOI] [PubMed] [Google Scholar]
- Arenas E, Persson H. Neurotrophin-3 prevents the death of adult central noradrenergic neurons in vivo. Nature. 1994;367:368–371. doi: 10.1038/367368a0. [DOI] [PubMed] [Google Scholar]
- Barkhatova VP, Zavalishin IA, Askarova L, Shavratskii V, Demina EG. Changes in neurotransmitters in multiple sclerosis. Neurosci Behav Physiol. 1998;28:341–344. doi: 10.1007/BF02464784. [DOI] [PubMed] [Google Scholar]
- Brahmachari S, Pahan K. Sodium benzoate, a food additive and a metabolite of cinnamon, modifies T cells at multiple steps and inhibits adoptive transfer of experimental allergic encephalomyelitis. J Immunol. 2007;179:275–283. doi: 10.4049/jimmunol.179.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmachari S, Pahan K. Myelin basic protein priming reduces the expression of Foxp3 in T cells via nitric oxide. J Immunol. 2010;184:1799–1809. doi: 10.4049/jimmunol.0804394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmachari S, Jana A, Pahan K. Sodium benzoate, a metabolite of cinnamon and a food additive, reduces microglial and astroglial inflammatory responses. J Immunol. 2009;183:5917–5927. doi: 10.4049/jimmunol.0803336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges JW, French MR, Smith RL, Williams RT. The fate of benzoic acid in various species. Biochem J. 1970;118:47–51. doi: 10.1042/bj1180047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett GT, Roy A, Pahan K. Gemfibrozil, a lipid-lowering drug, upregulates IL-1 receptor antagonist in mouse cortical neurons: implications for neuronal self-defense. J Immunol. 2012;189:1002–1013. doi: 10.4049/jimmunol.1102624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durany N, Michel T, Kurt J, Cruz-Sanchez FF, Cervos-Navarro J, Riederer P. Brain-derived neurotrophic factor and neurotrophin-3 levels in Alzheimer's disease brains. Int J Dev Neurosci. 2000;18:807–813. [PubMed] [Google Scholar]
- Frydman-Marom A, Levin A, Farfara D, Benromano T, Scherzer-Attali R, Peled S, Vassar R, Segal D, Gazit E, Frenkel D, Ovadia M. Orally administrated cinnamon extract reduces beta-amyloid oligomerization and corrects cognitive impairment in Alzheimer's disease animal models. PLoS One. 2011;6:e16564. doi: 10.1371/journal.pone.0016564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Pahan K. Gemfibrozil, a lipid-lowering drug, induces suppressor of cytokine signaling 3 in glial cells: implications for neurodegenerative disorders. J Biol Chem. 2012;287:27189–27203. doi: 10.1074/jbc.M112.346932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howells DW, Porritt MJ, Wong JY, Batchelor PE, Kalnins R, Hughes AJ, Donnan GA. Reduced BDNF mRNA expression in the Parkinson's disease substantia nigra. Exp Neurol. 2000;166:127–135. doi: 10.1006/exnr.2000.7483. [DOI] [PubMed] [Google Scholar]
- Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148:1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki Y, Ikeda K, Shiojima T, Kinoshita M. Increased plasma concentrations of aspartate, glutamate and glycine in Parkinson's disease. Neurosci Lett. 1992;145:175–177. doi: 10.1016/0304-3940(92)90015-y. [DOI] [PubMed] [Google Scholar]
- Jana A, Pahan K. Fibrillar amyloid-beta-activated human astroglia kill primary human neurons via neutral sphingomyelinase: implications for Alzheimer's disease. J Neurosci. 2010;30:12676–12689. doi: 10.1523/JNEUROSCI.1243-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana M, Jana A, Pal U, Pahan K. A simplified method for isolating highly purified neurons, oligodendrocytes, astrocytes, and microglia from the same human fetal brain tissue. Neurochem Res. 2007;32:2015–2022. doi: 10.1007/s11064-007-9340-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol Brain. 2012;5:14. doi: 10.1186/1756-6606-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A, Safdar M, Ali Khan MM, Khattak KN, Anderson RA. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care. 2003;26:3215–3218. doi: 10.2337/diacare.26.12.3215. [DOI] [PubMed] [Google Scholar]
- Khasnavis S, Pahan K. Sodium benzoate, a metabolite of cinnamon and a food additive, upregulates neuroprotective Parkinson disease protein DJ-1 in astrocytes and neurons. J Neuroimmune Pharmacol. 2012;7:424–435. doi: 10.1007/s11481-011-9286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota K, Ishizaki T. Dose-dependent pharmacokinetics of benzoic acid following oral administration of sodium benzoate to humans. Eur J Clin Pharmacol. 1991;41:363–368. doi: 10.1007/BF00314969. [DOI] [PubMed] [Google Scholar]
- Leonard JV, Morris AA. Urea cycle disorders. Semin Neonatol. 2002;7:27–35. doi: 10.1053/siny.2001.0085. [DOI] [PubMed] [Google Scholar]
- Mamounas LA, Blue ME, Siuciak JA, Altar CA. Brain-derived neurotrophic factor promotes the survival and sprouting of serotonergic axons in rat brain. J Neurosci. 1995;15:7929–7939. doi: 10.1523/JNEUROSCI.15-12-07929.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitch WE, Brusilow S. Benzoate-induced changes in glycine and urea metabolism in patients with chronic renal failure. J Pharmacol Exp Ther. 1982;222:572–575. [PubMed] [Google Scholar]
- Mondal S, Roy A, Jana A, Ghosh S, Kordower JH, Pahan K. Testing NF-kappaB-based therapy in hemiparkinsonian monkeys. J Neuroimmune Pharmacol. 2012;7:544–556. doi: 10.1007/s11481-012-9377-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair B. Final report on the safety assessment of Benzyl Alcohol, Benzoic Acid, and Sodium Benzoate. Int J Toxicol. 2001;20(Suppl 3):23–50. doi: 10.1080/10915810152630729. [DOI] [PubMed] [Google Scholar]
- Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14:7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- Phillips HS, Hains JM, Armanini M, Laramee GR, Johnson SA, Winslow JW. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer's disease. Neuron. 1991;7:695–702. doi: 10.1016/0896-6273(91)90273-3. [DOI] [PubMed] [Google Scholar]
- Porritt MJ, Batchelor PE, Howells DW. Inhibiting BDNF expression by antisense oligonucleotide infusion causes loss of nigral dopaminergic neurons. Exp Neurol. 2005;192:226–234. doi: 10.1016/j.expneurol.2004.11.030. [DOI] [PubMed] [Google Scholar]
- Rangasamy SB, Soderstrom K, Bakay RA, Kordower JH. Neurotrophic factor therapy for Parkinson's disease. Prog Brain Res. 2010;184:237–264. doi: 10.1016/S0079-6123(10)84013-0. [DOI] [PubMed] [Google Scholar]
- Roy A, Liu X, Pahan K. Myelin basic protein-primed T cells induce neurotrophins in glial cells via alphavbeta3 [corrected] integrin. J Biol Chem. 2007;282:32222–32232. doi: 10.1074/jbc.M702899200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha RN, Liu X, Pahan K. Up-regulation of BDNF in astrocytes by TNF-alpha: a case for the neuroprotective role of cytokine. J Neuroimmune Pharmacol. 2006;1:212–222. doi: 10.1007/s11481-006-9020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaglia F, Carter S, O'Brien WE, Lee B. Effect of alternative pathway therapy on branched chain amino acid metabolism in urea cycle disorder patients. Mol Genet Metab. 2004;81(Suppl 1):S79–85. doi: 10.1016/j.ymgme.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Tardito D, Perez J, Tiraboschi E, Musazzi L, Racagni G, Popoli M. Signaling pathways regulating gene expression, neuroplasticity, and neurotrophic mechanisms in the action of antidepressants: a critical overview. Pharmacol Rev. 2006;58:115–134. doi: 10.1124/pr.58.1.7. [DOI] [PubMed] [Google Scholar]
- Taylor SS, Radzio-Andzelm E, Hunter T. How do protein kinases discriminate between serine/threonine and tyrosine? Structural insights from the insulin receptor protein-tyrosine kinase. FASEB J. 1995;9:1255–1266. doi: 10.1096/fasebj.9.13.7557015. [DOI] [PubMed] [Google Scholar]
- Taylor SS, Kim C, Cheng CY, Brown SH, Wu J, Kannan N. Signaling through cAMP and cAMP-dependent protein kinase: diverse strategies for drug design. Biochim Biophys Acta. 2008;1784:16–26. doi: 10.1016/j.bbapap.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth B. Lack of tumorigenicity of sodium benzoate in mice. Fundam Appl Toxicol. 1984;4:494–496. doi: 10.1016/0272-0590(84)90208-2. [DOI] [PubMed] [Google Scholar]
- West AE, Chen WG, Dalva MB, Dolmetsch RE, Kornhauser JM, Shaywitz AJ, Takasu MA, Tao X, Greenberg ME. Calcium regulation of neuronal gene expression. Proc Natl Acad Sci U S A. 2001;98:11024–11031. doi: 10.1073/pnas.191352298. [DOI] [PMC free article] [PubMed] [Google Scholar]