Abstract
Adolescent female athletes are at increased risk for low bone mineral density (BMD) secondary to exercise-induced hypogonadism. Of particular concern is that the adolescent years are also a critical time for bone accrual, and deficits incurred during this period could lead to suboptimal peak bone mass acquisition and subsequent fracture risk in later life. Although weight bearing exercise is typically associated with an increase in BMD, amenorrheic athletes have lower BMD than eumenorrheic athletes and non-athletic controls as a consequence of low energy availability and subsequent hypogonadism. It is important to recognize that critical interactions exist between net energy availability and the hypothalamo-pituitary-gonadal (H-P-G) axis that are key to the development of a hypogonadal state when energy intake cannot keep pace with expenditure. While the link between energy availability and gonadtotropin pulsatility patterns is well established, the actual metabolic signals that link the two are less clear. Decreased energy availability in athletes is associated with decreases in fat mass, and alterations in adipokines (such as leptin and adiponectin) and fat-regulated hormones (such as ghrelin and peptide YY). These hormones impact the H-P-G axis in animal models, and it is possible that in athletes alterations in fat-related hormones signal the state of energy availability to the hypothalamus and contribute to suppression of gonadotrophin pulsatility, hypothalamic amenorrhea and consequent decreased BMD. A better understanding of pathways linking low energy availability with functional hypothalamic amenorrhea and low BMD is critical for the development of future therapeutic strategies addressing these issues in amenorrheic athletes.
Introduction
The adolescent years are characterized by marked increases in bone mass accrual in association with rising levels of growth hormone (GH), insulin like growth factor-1 (IGF-1) and gonadal steroids. Of importance, more than 90% of peak bone mass is achieved by 18 years of age, and peak bone mass is an important determinant of bone health and fracture risk in later life (1). A lower than expected rate of bone accrual leads to not only low bone mineral density (BMD) Z-scores, but also suboptimal peak bone mass and possibly increased fracture risk in later years.
Conditions associated with decreased rates of bone mass accrual in adolescence include those characterized by decreased secretion of hormones such as GH, IGF-1 and gonadal steroids, and increased secretion of hormones such as cortisol and thyroxine. In addition, alterations in certain adipokines, such as leptin and adiponectin, and peptides that are impacted by changes in BMI and fat mass, such as ghrelin, peptide YY (PYY) and insulin, can impact bone metabolism. Physical exercise is a positive determinant of bone mass accrual in healthy adolescents. However, excessive exercise, particularly in the setting of associated hypogonadism, leads to low BMD in adolescents as well as in adults. Marked alterations in adipokines and fat-regulated hormones have been described in amenorrheic athletes (2, 3), and these alterations and their impact on the hypothalamo-pituitary-gonadal (H-P-G) axis and on bone metabolism are described below.
Bone metabolism in adolescent amenorrheic and eumenorrheic athletes and non-athletic controls
Many studies have reported that adult amenorrheic athletes have lower bone density than normally menstruating athletes (4–7), and in adolescents, endurance activities such as running have been associated with an increased risk of fractures (8). There are limited data, however, regarding bone metabolism in adolescent athletes.
Within adolescent endurance athletes, we have shown that amenorrheic athletes have significantly lower bone density compared with eumenorrheic athletes and also non-athletic controls (9). In a study of 21 amenorrheic adolescent athletes, 18 eumenorrheic athletes, and 18 controls, we demonstrated that amenorrheic athletes had lower bone density Z-scores at the spine and whole body compared with eumenorrheic athletes and non-athletic controls (9). The amenorrheic athletes also had lower hip bone density Z-scores compared with eumenorrheic athletes. In addition, Z-scores for height adjusted measures of bone density, such as spine bone mineral apparent density (BMAD) and whole body bone mineral content for height (WB BMC/Ht) were lower in amenorrheic athletes compared with eumenorrheic athletes and controls. Lower spine bone density in amenorrheic athletes was a consequence of lower BMC for bone area indicative of lighter bones, whereas, lower WB bone density was a consequence of lower bone area for height, indicative of thinner bones. Importantly, levels of bone formation and bone resorption markers were lower in amenorrheic athletes compared with controls, suggestive of a reduced bone turnover state. This is in contrast to normal adolescence, which is a state of increased bone turnover (at least in early adolescence).
The two groups of athletes in the study did not differ for activity level, suggesting that factors other than intensity and duration of exercise determine which athletes will develop amenorrhea. The amenorrheic athletes did have lower body mass index (BMI) and fat mass than the eumenorrheic athletes, and the prevalence of disordered eating was much higher in the amenorrheic (62%) compared with the eumenorrheic group (11%). These data suggest that decreased energy availability from decreased caloric intake may contribute to lower BMI and fat mass and subsequent functional hypothalamic amenorrhea in amenorrheic athletes. In a regression model that included the diagnostic category, IGF-1 levels, activity level, bone age, BMI and lean mass, most of these covariates (except activity level) were independent determinants of bone density measures. These data emphasize the impact of the hypogonadal state and overall state of nutrition on bone metabolism in female adolescent athletes.
Similarly, in adult athletes, amenorrhea has been associated with lower bone density (4–7), and lower energy availability (as evidenced by higher scores for disordered eating) has been determined to be an important predictor of lower bone density measures (4). Importantly, not all forms of excessive activity are associated with low bone density even when there is associated amenorrhea. For example, amenorrheic gymnasts do not have lower bone density than non-exercising controls (10, 11). This has been postulated to be a consequence of differences in the nature of exercise. Gymnastics is a non-repetitive impact loading sport, as opposed to running, which is associated with repetitive impact and an endurance sport. The latter is less likely to manifest beneficial effects of mechanical loading on bone, and is more likely to be associated with low bone density, particularly when associated with low energy availability and hypogonadism.
Recent studies in anorexia nervosa, a state of severe negative energy balance, have shown that low bone mass accrual during the adolescent years can be followed by increases in bone mass after recovery of weight and menses (12). However, catch-up is not complete, and this further raises concerns of a suboptimal peak bone mass despite subsequent attainment of a positive state of energy balance in adolescent athletes. Given this incomplete catch-up, therapeutic strategies may be necessary to optimize bone mass accrual, even while recovery from the low energy availability state is being optimized. In order to develop the appropriate therapeutic strategies, it is critical to develop a better understanding of the pathophysiology underlying low bone density in conditions of reduced energy availability, and of factors that contribute to hypogonadism, as well as other determinants of low bone density.
Factors contributing to low bone density in amenorrheic adolescent athletes
The early adolescent years are characterized by increased bone turnover as a consequence of rising levels of GH and IGF-1, both of which are bone anabolic hormones (13, 14) and cause endocortical bone formation. Subsequently, rising levels of estrogen have anti-resorptive effects and reduce endosteal bone resorption. These pubertal changes are responsible for bone modeling and the bone mass accrual that is so characteristic of puberty. Low IGF-1 levels in athletes who are in a state of markedly reduced energy availability and the associated hypogonadism could cause marked decreases in rates of bone accrual, leading to low bone mineral density. Of concern, as many as 23.5% of adolescent athletes are at risk for amenorrhea (15). In our studies, we have noted that low IGF-1 levels and hypogonadism are important determinants of low bone density in athletes (9). Similarly, other studies in adults have indicated that hypogonadism in athletes is associated with decreased bone density and increased fracture risk (4, 16–19).
There are strong data to indicate that patterns of gonadotropin pulsatility change when energy availability falls below a critical threshold of 30 kcal/kg lean body mass/day (20). Recent endeavors have aimed at examining metabolic signals that link low energy availability and impaired gonadotropin secretion, and fat mass and fat-related hormones are possible candidates. In our study, amenorrheic athletes had significantly lower fat mass than eumenorrheic athletes (2, 3). We have also previously shown that increases in fat mass predict recovery of the H-P-G axis in girls with anorexia nervosa, a more severe state of energy deficit (21). Therefore, it is likely that hormones that are secreted by fat or regulated by fat have an impact on the H-P-G axis. Interestingly, many hormones that are secreted or regulated by fat also have an impact on bone metabolism in in vitro and animal studies. Adipokines, such as leptin (22–25) and adiponectin (26–29), and hormones that are altered when body fat changes, such as ghrelin (30, 31) and PYY (32–34), can impact both the H-P-G axis and bone metabolism, and may be important links between net energy availability, reproductive status and bone mineral density.
Adipokines in female athletes
Decreased energy availability can result from increased energy expenditure, decreased energy intake or both (20), and is associated with low fat mass and a suppression of the H-P-G axis. This leads to low levels of gonadal steroids and a decreased rate of bone mass accrual. Associated with low fat mass are low levels of the adipokine, leptin (35–37), and high levels of another adipokine, adiponectin (38, 39); hormones that likely signal information about available energy stores to the hypothalamus.
Leptin is primarily produced in adipocytes and its circulating levels are highly correlated with body fat content (35). Levels decrease with weight loss and increase as BMI and body fat increases (36, 37, 40). Sufficient fat mass is thought to be necessary for normal reproductive function, although the threshold of fat mass necessary for normal menses may differ from individual to individual. Leptin deficient animals and humans who fail to undergo pubertal development have pubertal onset once leptin is administered (41). This suggests that leptin facilitates GnRH stimulated gonadotropin and gonadal steroid production, and that low leptin levels, as seen in athletes, could lead to hypogonadism and associated bone mineral density deficits. We have shown that leptin levels are significantly lower in adolescent amenorrheic athletes when compared with eumenorrheic athletes and controls (2), and low leptin levels are a positive predictor of levels of gonadal steroids, particularly estradiol, even after controlling for potential confounders. These data are also consistent with a study by Welt et al. in which the authors demonstrated a resumption of ovulatory cycles following recombinant human leptin administration in three out of eight women with hypothalamic amenorrhea despite an overall reduction in body weight (22).
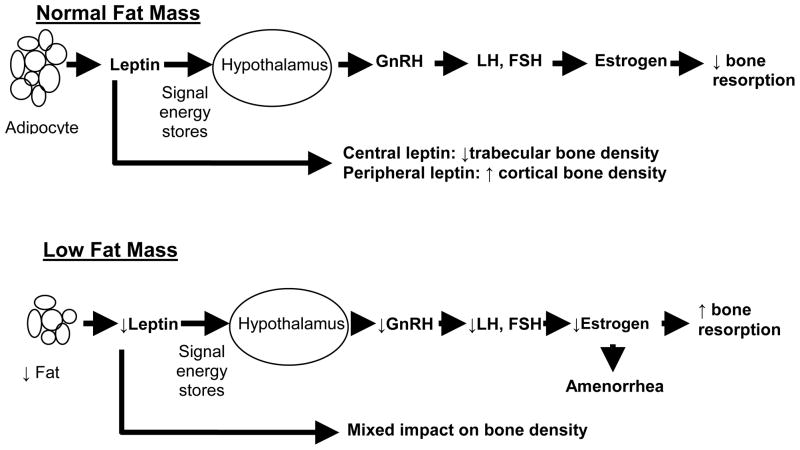
While low leptin levels in amenorrheic athletes can impact bone density through induction of a hypogonadal state, a direct role of leptin on bone cannot be ruled out. Leptin deficient ob/ob mice have a decrease in trabecular bone mass when leptin is administered intracerebroventricularly (23), an effect mediated by beta 2 adrenergic activation. However, there appears to be a dichotomy in the effect of leptin on bone depending on whether it is administered centrally or peripherally. Contrary to central effects, peripherally administered leptin causes an increase in cortical bone mass (24, 25). In addition, central leptin may also positively impact cortical bone by inducing GH secretion. In humans, studies examining associations of leptin and bone have shown variable results. Overall, leptin deficiency has been associated with increased (23), decreased (25), or site variable (24) bone mass. Figure 1 shows a speculated pathway of leptin induced effects on gonadal steroids and bone mineral density.
Figure 1.
Proposed Impact of Leptin on Gonadotropin Secretion and Bone in Conditions of Normal versus Low Fat Mass
Adiponectin is the most abundant product secreted from adipocytes. In contrast to leptin, adiponectin levels are low in obesity (38) and variable in low weight conditions (42, 43). Adiponectin gene expression within adipocytes typically increases with weight loss and is associated with improved insulin sensitivity (39). In vitro studies have demonstrated that adiponectin suppresses gonadotrophin secretion (29) and activates both osteoblasts and osteoclasts (27, 28). A common progenitor cell in bone marrow gives rise to adipocytes and osteoblasts (44, 45). When adiponectin binds to the AdipoR1 receptor, mesenchymal progenitor cells within the marrow undergo osteoblast proliferation and differentiation, while adipogenesis is inhibited (28). However, adiponectin can also increase RANKL and decrease OPG levels causing an increase in osteoclastic activity (27). In adult men and women, as well as in postmenopausal women and adolescents with anorexia nervosa, high adiponectin levels have been associated with low bone density (42, 46, 47), suggesting that bone resorptive effects may trump bone formation effects. We did not find significant differences in adiponectin levels in adolescent amenorrheic athletes compared with eumenorrheic athletes and non-athletic controls (3). However, adiponectin was a significant predictor of levels of testosterone. Contrary to our expectations and in vitro studies (29), the association between adiponectin and testosterone was positive (and not negative). We found no association between adiponectin and bone density measures in our athletes and non-athletic controls.
Hormones that may be regulated by body fat content
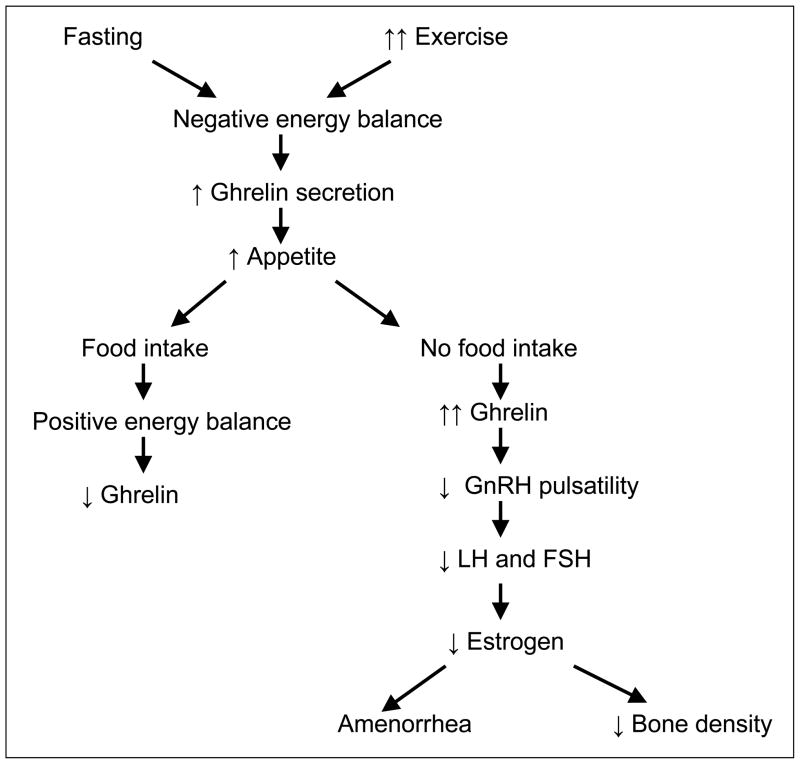
Appetite-regulating hormones such as ghrelin and PYY are predicted inversely by BMI and fat mass. Ghrelin, one of the first orexigenic hormones to be discovered (48, 49), is secreted primarily by the P/D1 cells in the fundus of the stomach. Levels rise during periods of fasting and are low following food intake and in obesity, suggesting that ghrelin may signal the state of energy availability. Ghrelin levels rise in adults following exercise (50, 51) and are also high in anorexia nervosa (52, 53). We evaluated ghrelin levels in amenorrheic adolescent athletes, eumenorrheic athletes, and nonathletic controls, and found that amenorrheic athletes had significantly higher ghrelin levels than eumenorrheic athletes and controls even after controlling for BMI (2). In addition, we found an inverse relationship between levels of ghrelin and gonadal steroids leading us to postulate that ghrelin levels may differentiate between athletes who will or will not develop functional hypothalamic amenorrhea. These data are also consistent with studies in animals and humans that demonstrate a suppression of gonadotropin pulsatility following ghrelin administration (31, 54). Figure 2 demonstrates the effect of food intake on ghrelin levels.
Figure 2.
Effect of Energy Balance and Ghrelin on Gonadotropin Secretion and Bone
Ghrelin binds to and activates the neuropeptide Y (NPY) and agouti-related peptide-producing neurons in the arcuate nucleus of the hypothalamus, and therefore stimulates appetite and food intake (48, 55). It is also a GH secretagogue (56, 57), and GH promotes bone formation. Therefore, ghrelin may have an effect on bone mass accrual by stimulating GH secretion. In addition, osteoblasts express the ghrelin receptor (GHS-R1a), and ghrelin can stimulate osteoblast proliferation and differentiation (30). Therefore, ghrelin may affect bone mass accrual through mechanisms independent of the GH-IGF-1 axis. In a study of adolescent girls with anorexia nervosa and controls, we previously reported that serum ghrelin levels predicted bone mineral density independent of body composition, the GH-IGF-1 axis, and estradiol in healthy adolescent girls but not in girls with anorexia nervosa (58). Thus, ghrelin appears to be involved in pathways that are both detrimental and beneficial to the process of bone mass accrual. While high ghrelin levels may contribute to the hypogonadal state and subsequently to low bone density, ghrelin may stimulate osteoblastic activity through GH dependent or independent mechanisms. The overall impact of these processes is still not clearly understood. In our study of adolescent amenorrheic and eumenorrheic athletes, ghrelin was not an independent predictor of bone density measures, but did emerge as an independent and negative predictor of markers of bone formation and bone resorption, suggesting that high ghrelin levels may contribute to the low bone turnover state in amenorrheic athletes (3).
PYY is another peptide hormone secreted by gut neuroendocrine cells involved in the signaling of energy availability. It is anorexigenic and secreted by the L cells of the distal intestine (59) following food intake (60), and promotes satiety by binding to the Y2 receptor of NPY in the hypothalamus and inhibiting NPY secretion (61). PYY levels are low in obesity (62) and elevated in low energy availability states such as anorexia nervosa (34). We have reported elevated levels of PYY in adolescent athletes with amenorrhea compared with eumenorrheic athletes and nonathletic controls (3). Importantly, animal studies suggest that PYY may suppress gonadotropin secretion, and in our cohort, PYY was an inverse and independent predictor of levels of gonadal steroids. Importantly, the Y2 receptor knockout mouse, which would be PYY resistant, has increased bone mass (32). Consistent with this model, PYY excess has been associated with low bone density in models of low energy availability such as anorexia nervosa (34). In our study of adolescent athletes, PYY was a negative and independent predictor of the bone formation marker PINP and of lumbar bone mineral apparent density Z-scores (3). Thus, high PYY levels in adolescent athletes may contribute to low bone density by suppressing the H-P-G axis and also by reducing bone formation.
Conclusion
Adolescent female athletes are at increased risk for incurring bone mineral density deficits at a critical period of bone mass accrual. Interactions between energy balance, reproductive function and bone mass accrual are interlinked and complex. Hormones produced by adipocytes and gut neuroendocrine cells are important signals of energy balance and play a major role in the control of the H-P-G axis and bone metabolism. Current research to better understand the complex pathways involved in these processes is promising, and with improved understanding of the metabolic and hormonal links between energy availability, reproduction and bone, new therapeutic options should become possible to treat the hypogonadism and low bone density characteristic of amenorrheic athletes.
Acknowledgments
Supported in part by NIH grant R01HD060827
References
- 1.Bachrach L. Acquisition of optimal bone mass in childhood and adolescence. Trends Endocrinol Metab. 2001;12:22–8. doi: 10.1016/s1043-2760(00)00336-2. [DOI] [PubMed] [Google Scholar]
- 2.Christo K, Cord J, Mendes N, et al. Acylated ghrelin and leptin in adolescent athletes with amenorrhea, eumenorrheic athletes and controls: a cross-sectional study. Clin Endocrinol (Oxf) 2008;69:628–33. doi: 10.1111/j.1365-2265.2008.03237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russell M, Stark J, Nayak S, et al. Peptide YY in adolescent athletes with amenorrhea, eumenorrheic athletes and non-athletic controls. Bone. 2009;45:104–9. doi: 10.1016/j.bone.2009.03.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cobb K, Bachrach L, Greendale G, et al. Disordered eating, menstrual irregularity, and bone mineral density in female runners. Med Sci Sports Exerc. 2003;35:711–9. doi: 10.1249/01.MSS.0000064935.68277.E7. [DOI] [PubMed] [Google Scholar]
- 5.Myburgh H, Hutchins J, Fataar A, Hough S, Noakes T. Low bone mineral density is an etiologic factor for stress fratures in athletes. Ann Intern Med. 1990;113:754–759. doi: 10.7326/0003-4819-113-10-754. [DOI] [PubMed] [Google Scholar]
- 6.Myburgh K, Bachrach L, Lewis B, Kent K, Marcus R. Low bone mineral density at axial and appendicular sites in amenorrheic athletes. Med Sci Sports Exerc. 1993;25:1197–202. [PubMed] [Google Scholar]
- 7.Pettersson U, Stalnacke B, Ahlenius G, Henriksson-Larsen K, Lorentzon R. Low bone mass density at multiple skeletal sites, including the appendicular skeleton in amenorrheic runners. Calcif Tissue Int. 1999;64:117–25. doi: 10.1007/s002239900589. [DOI] [PubMed] [Google Scholar]
- 8.Loud KJ, Gordon CM, Micheli LJ, Field AE. Correlates of Stress Fractures Among Preadolescent and Adolescent Girls. Pediatrics. 2005;115:e399–406. doi: 10.1542/peds.2004-1868. [DOI] [PubMed] [Google Scholar]
- 9.Christo K, Prabhakaran R, Lamparello B, et al. Bone metabolism in adolescent athletes with amenorrhea, athletes with eumenorrhea, and control subjects. Pediatrics. 2008;121:1127–36. doi: 10.1542/peds.2007-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson TL, Snow-Harter C, Taaffe DR, Gillis D, Shaw J, Marcus R. Gymnasts exhibit higher bone mass than runners despite similar prevalence of amenorrhea and oligomenorrhea. J Bone Miner Res. 1995;10:26–35. doi: 10.1002/jbmr.5650100107. [DOI] [PubMed] [Google Scholar]
- 11.Snow C, Rosen C, Robinson T. Serum IGF-I is higher in gymnasts than runners and predicts bone and lean mass. Med Sci Sports Exerc. 2000;32:1902–7. doi: 10.1097/00005768-200011000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Misra M, Prabhakaran R, Miller KK, et al. Weight gain and restoration of menses as predictors of bone mineral density change in adolescent girls with anorexia nervosa-1. J Clin Endocrinol Metab. 2008;93:1231–7. doi: 10.1210/jc.2007-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohlsson C, Bengtsson BA, Isaksson OG, Andreassen TT, Slootweg MC. Growth hormone and bone. Endocr Rev. 1998;19:55–79. doi: 10.1210/edrv.19.1.0324. [DOI] [PubMed] [Google Scholar]
- 14.Rauch F. Bone accrual in children: adding substance to surfaces. Pediatrics. 2007;119 (Suppl 2):S137–40. doi: 10.1542/peds.2006-2023E. [DOI] [PubMed] [Google Scholar]
- 15.Nichols JF, Rauh MJ, Lawson MJ, Ji M, Barkai HS. Prevalence of the female athlete triad syndrome among high school athletes. Arch Pediatr Adolesc Med. 2006;160:137–42. doi: 10.1001/archpedi.160.2.137. [DOI] [PubMed] [Google Scholar]
- 16.Drinkwater BL, Bruemner B, Chesnut CH., 3rd Menstrual history as a determinant of current bone density in young athletes. JAMA. 1990;263:545–8. [PubMed] [Google Scholar]
- 17.Jones KP, Ravnikar VA, Tulchinsky D, Schiff I. Comparison of bone density in amenorrheic women due to athletics, weight loss, and premature menopause. Obstet Gynecol. 1985;66:5–8. [PubMed] [Google Scholar]
- 18.Rencken ML, Chesnut CH, 3rd, Drinkwater BL. Bone density at multiple skeletal sites in amenorrheic athletes. JAMA. 1996;276:238–40. [PubMed] [Google Scholar]
- 19.Wolman RL, Clark P, McNally E, Harries M, Reeve J. Menstrual state and exercise as determinants of spinal trabecular bone density in female athletes. BMJ. 1990;301:516–8. doi: 10.1136/bmj.301.6751.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loucks AB, Verdun M, Heath EM. Low energy availability, not stress of exercise, alters LH pulsatility in exercising women. J Appl Physiol. 1998;84:37–46. doi: 10.1152/jappl.1998.84.1.37. [DOI] [PubMed] [Google Scholar]
- 21.Misra M, Prabhakaran R, Miller KK, et al. Role of cortisol in menstrual recovery in adolescent girls with anorexia nervosa. Pediatr Res. 2006;59:598–603. doi: 10.1203/01.pdr.0000203097.64918.63. [DOI] [PubMed] [Google Scholar]
- 22.Welt CK, Chan JL, Bullen J, et al. Recombinant Human Leptin in Women with Hypothalamic Amenorrhea. N Engl J Med. 2004;351:987–997. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- 23.Ducy P, Amling M, Takeda S, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 24.Hamrick MW, Pennington C, Newton D, Xie D, Isales C. Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone. 2004;34:376–83. doi: 10.1016/j.bone.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 25.Steppan CM, Crawford DT, Chidsey-Frink KL, Ke H, Swick AG. Leptin is a potent stimulator of bone growth in ob/ob mice. Regul Pept. 2000;92:73–8. doi: 10.1016/s0167-0115(00)00152-x. [DOI] [PubMed] [Google Scholar]
- 26.Lu M, Tang Q, Olefsky JM, Mellon PL, Webster NJ. Adiponectin activates adenosine monophosphate-activated protein kinase and decreases luteinizing hormone secretion in LbetaT2 gonadotropes. Mol Endocrinol. 2008;22:760–71. doi: 10.1210/me.2007-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo XH, Guo LJ, Xie H, et al. Adiponectin stimulates RANKL and inhibits OPG expression in human osteoblasts through the MAPK signaling pathway. J Bone Miner Res. 2006;21:1648–56. doi: 10.1359/jbmr.060707. [DOI] [PubMed] [Google Scholar]
- 28.Luo XH, Guo LJ, Yuan LQ, et al. Adiponectin stimulates human osteoblasts proliferation and differentiation via the MAPK signaling pathway. Exp Cell Res. 2005;309:99–109. doi: 10.1016/j.yexcr.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Pacheco F, Martinez-Fuentes AJ, Tovar S, et al. Regulation of pituitary cell function by adiponectin. Endocrinology. 2007;148:401–10. doi: 10.1210/en.2006-1019. [DOI] [PubMed] [Google Scholar]
- 30.Fukushima N, Hanada R, Teranishi H, et al. Ghrelin directly regulates bone formation. J Bone Miner Res. 2005;20:790–8. doi: 10.1359/JBMR.041237. [DOI] [PubMed] [Google Scholar]
- 31.Kluge M, Schussler P, Uhr M, Yassouridis A, Steiger A. Ghrelin suppresses secretion of luteinizing hormone in humans. J Clin Endocrinol Metab. 2007;92:3202–5. doi: 10.1210/jc.2007-0593. [DOI] [PubMed] [Google Scholar]
- 32.Baldock PA, Sainsbury A, Couzens M, et al. Hypothalamic Y2 receptors regulate bone formation. J Clin Invest. 2002;109:915–921. doi: 10.1172/JCI14588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernandez-Fernandez R, Aguilar E, Tena-Sempere M, Pinilla L. Effects of polypeptide YY(3-36) upon luteinizing hormone-releasing hormone and gonadotropin secretion in prepubertal rats: in vivo and in vitro studies. Endocrinology. 2005;146:1403–10. doi: 10.1210/en.2004-0858. [DOI] [PubMed] [Google Scholar]
- 34.Misra M, Miller KK, Tsai P, et al. Elevated peptide YY levels in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 2006;91:1027–33. doi: 10.1210/jc.2005-1878. [DOI] [PubMed] [Google Scholar]
- 35.Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–5. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 36.Kennedy A, Gettys TW, Watson P, et al. The metabolic significance of leptin in humans: gender-based differences in relationship to adiposity, insulin sensitivity, and energy expenditure. J Clin Endocrinol Metab. 1997;82:1293–300. doi: 10.1210/jcem.82.4.3859. [DOI] [PubMed] [Google Scholar]
- 37.Saad MF, Riad-Gabriel MG, Khan A, et al. Diurnal and ultradian rhythmicity of plasma leptin: effects of gender and adiposity. J Clin Endocrinol Metab. 1998;83:453–9. doi: 10.1210/jcem.83.2.4532. [DOI] [PubMed] [Google Scholar]
- 38.Cambuli VM, Musiu MC, Incani M, et al. Assessment of adiponectin and leptin as biomarkers of positive metabolic outcomes after lifestyle intervention in overweight and obese children. J Clin Endocrinol Metab. 2008;93:3051–7. doi: 10.1210/jc.2008-0476. [DOI] [PubMed] [Google Scholar]
- 39.Yang WS, Lee WJ, Funahashi T, et al. Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. J Clin Endocrinol Metab. 2001;86:3815–9. doi: 10.1210/jcem.86.8.7741. [DOI] [PubMed] [Google Scholar]
- 40.Misra M, Miller KK, Kuo K, et al. Secretory dynamics of leptin in adolescent girls with anorexia nervosa and healthy adolescents. Am J Physiol Endocrinol Metab. 2005;289:E373–81. doi: 10.1152/ajpendo.00041.2005. [DOI] [PubMed] [Google Scholar]
- 41.Farooqi IS. Leptin and the onset of puberty: insights from rodent and human genetics. Semin Reprod Med. 2002;20:139–44. doi: 10.1055/s-2002-32505. [DOI] [PubMed] [Google Scholar]
- 42.Misra M, Miller KK, Cord J, et al. Relationships between serum adipokines, insulin levels, and bone density in girls with anorexia nervosa. J Clin Endocrinol Metab. 2007;92:2046–52. doi: 10.1210/jc.2006-2855. [DOI] [PubMed] [Google Scholar]
- 43.Pannacciulli N, Bunt JC, Ortega E, et al. Lower total fasting plasma adiponectin concentrations are associated with higher metabolic rates. J Clin Endocrinol Metab. 2006;91:1600–3. doi: 10.1210/jc.2005-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gimble JM, Zvonic S, Floyd ZE, Kassem M, Nuttall ME. Playing with bone and fat. J Cell Biochem. 2006;98:251–66. doi: 10.1002/jcb.20777. [DOI] [PubMed] [Google Scholar]
- 45.Pei L, Tontonoz P. Fat’s loss is bone’s gain. J Clin Invest. 2004;113:805–6. doi: 10.1172/JCI21311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jurimae J, Rembel K, Jurimae T, Rehand M. Adiponectin is associated with bone mineral density in perimenopausal women. Horm Metab Res. 2005;37:297–302. doi: 10.1055/s-2005-861483. [DOI] [PubMed] [Google Scholar]
- 47.Lenchik L, Register TC, Hsu FC, et al. Adiponectin as a novel determinant of bone mineral density and visceral fat. Bone. 2003;33:646–51. doi: 10.1016/s8756-3282(03)00237-0. [DOI] [PubMed] [Google Scholar]
- 48.Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–8. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 49.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–13. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 50.De Souza MJ, Leidy HJ, O’Donnell E, Lasley B, Williams NI. Fasting ghrelin levels in physically active women: relationship with menstrual disturbances and metabolic hormones. J Clin Endocrinol Metab. 2004;89:3536–42. doi: 10.1210/jc.2003-032007. [DOI] [PubMed] [Google Scholar]
- 51.Jurimae J, Jurimae T, Purge P. Plasma ghrelin is altered after maximal exercise in elite male rowers. Exp Biol Med (Maywood) 2007;232:904–9. [PubMed] [Google Scholar]
- 52.Hotta M, Ohwada R, Katakami H, Shibasaki T, Hizuka N, Takano K. Plasma levels of intact and degraded ghrelin and their responses to glucose infusion in anorexia nervosa. J Clin Endocrinol Metab. 2004;89:5707–12. doi: 10.1210/jc.2004-0353. [DOI] [PubMed] [Google Scholar]
- 53.Misra M, Miller KK, Kuo K, et al. Secretory dynamics of ghrelin in adolescent girls with anorexia nervosa and healthy adolescents. Am J Physiol Endocrinol Metab. 2005;289:E347–56. doi: 10.1152/ajpendo.00615.2004. [DOI] [PubMed] [Google Scholar]
- 54.Vulliemoz NR, Xiao E, Xia-Zhang L, Germond M, Rivier J, Ferin M. Decrease in Luteinizing Hormone Pulse Frequency during a Five-Hour Peripheral Ghrelin Infusion in the Ovariectomized Rhesus Monkey. J Clin Endocrinol Metab. 2004;89:5718–5723. doi: 10.1210/jc.2004-1244. [DOI] [PubMed] [Google Scholar]
- 55.Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and Agouti-related protein mRNA levels and body weight in rats. Diabetes. 2001;50:2438–43. doi: 10.2337/diabetes.50.11.2438. [DOI] [PubMed] [Google Scholar]
- 56.Arvat E, Maccario M, Di Vito L, et al. Endocrine activities of ghrelin, a natural growth hormone secretagogue (GHS), in humans: comparison and interactions with hexarelin, a nonnatural peptidyl GHS, and GH-releasing hormone. J Clin Endocrinol Metab. 2001;86:1169–74. doi: 10.1210/jcem.86.3.7314. [DOI] [PubMed] [Google Scholar]
- 57.Takaya K, Ariyasu H, Kanamoto N, et al. Ghrelin strongly stimulates growth hormone release in humans. J Clin Endocrinol Metab. 2000;85:4908–11. doi: 10.1210/jcem.85.12.7167. [DOI] [PubMed] [Google Scholar]
- 58.Misra M, Miller KK, Stewart V, et al. Ghrelin and bone metabolism in adolescent girls with anorexia nervosa and healthy adolescents. J Clin Endocrinol Metab. 2005;90:5082–7. doi: 10.1210/jc.2005-0512. [DOI] [PubMed] [Google Scholar]
- 59.Huda MS, Wilding JP, Pinkney JH. Gut peptides and the regulation of appetite. Obes Rev. 2006;7:163–82. doi: 10.1111/j.1467-789X.2006.00245.x. [DOI] [PubMed] [Google Scholar]
- 60.Ballantyne GH. Peptide YY(1-36) and peptide YY(3-36): Part II. Changes after gastrointestinal surgery and bariatric surgery. Obes Surg. 2006;16:795–803. doi: 10.1381/096089206777346619. [DOI] [PubMed] [Google Scholar]
- 61.Keire DA, Mannon P, Kobayashi M, Walsh JH, Solomon TE, Reeve JR., Jr Primary structures of PYY, [Pro(34)]PYY, and PYY-(3-36) confer different conformations and receptor selectivity. Am J Physiol Gastrointest Liver Physiol. 2000;279:G126–31. doi: 10.1152/ajpgi.2000.279.1.G126. [DOI] [PubMed] [Google Scholar]
- 62.Batterham RL, Cohen MA, Ellis SM, et al. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 2003;349:941–8. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]