ABSTRACT
BACKGROUND
Rapid antigen detection tests (RADT) are commonly used to guide appropriate antibiotic treatment of group A beta-hemolytic streptococcal (GABHS) pharyngitis. In adults, there is controversy about the need for routine backup testing of negative RADT.
OBJECTIVE
Estimate the costs and benefits in adults of routine backup testing by DNA Gen-probe of negative RADT (Acceava).
DESIGN
Observational follow-up study.
PARTICIPANTS
All patients aged 18 years and older visiting a Cleveland Clinic generalist physician in 2009 and 2010 with a visit diagnosis of acute pharyngitis (ICD codes 462, 034.0).
MAIN MEASURES
The patients were identified using the Cleveland Clinic Epic Clarity database. We determined the proportion of false negative RADT, antibiotic prescription patterns and rate of serious suppurative complications within 30 days of the office visit.
KEY RESULTS
Of 25,130 patients with acute pharyngitis, 19 % had no testing and 81 % were tested. Of the 15,555 patients that had a negative RADT and follow-up DNA probe, 6 % had a positive DNA probe. Of the 953 patients who had a negative RADT and a positive DNA strep probe, 48 % received an antibiotic prescription at the time of the visit and 51 % received an antibiotic prescription after an average of 2.3 days. Only one patient with a negative RADT and no follow-up DNA probe developed a peritonsillar abscess. Overall, of the 15,555 DNA probes performed, management was altered in only 3 % of the patients at a total cost of $1,757,715. Fifty-six percent received an antibiotic while only 19.5 % had a confirmed strep throat diagnosis.
CONCLUSIONS
The false negative rate of Acceava RADT for the diagnosis of GABHS pharyngitis was 6 %. We question the benefit of routine DNA probe backup testing in adults because of its substantial cost, an average delay in antibiotic prescribing of over 2 days, and because suppurative complications are very uncommon. We found a high rate of inappropriate antibiotic prescribing.
KEY WORDS: acute pharyngitis, strep throat, testing, rapid antigen detection tests, RADT, DNA probe
INTRODUCTION
Sore throat is a common clinical presentation. According to the National Ambulatory Care Survey, acute pharyngitis is one of the top 20 reported diagnoses, and represents 1.1 % of primary care visits1 or an estimated 12 million visits a year.2 Viruses are the most common cause of acute pharyngitis.3 Group A beta-hemolytic streptococci (GABHS) represent about 10 % of throat infections in adults.4 However, it is important to identify people likely to have GABHS infection,5 because it is the only common form of acute pharyngitis for which antibiotic therapy is indicated.6 When given early in the course of illness, antibiotic therapy can shorten the duration of symptoms, reduce the rate of transmission and prevent suppurative complications (e.g., peritonsillar abscess, cervical lymphadenitis and mastoiditis) and rheumatic fever.7–10 In addition, the cost of GABHS pharyngitis is substantial, estimated to be over 1.2 billion dollars per year in adults in 2008.11
Fever, tonsillar exudate, anterior cervical adenopathy and absence of cough increase the likelihood of GABHS pharyngitis, and the presence or absence of these signs and symptoms are helpful in judging the likelihood of GABHS infection.12,13 However, the signs and symptoms of GABHS pharyngitis and non-streptococcal pharyngitis overlap so much that it is not possible to make an accurate diagnosis based solely on clinical grounds.12 Culture of a throat swab on a sheep-blood agar plate is the gold standard for the confirmation of acute GABHS pharyngitis.14 Rapid antigen detection tests (RADTs), however, are the tests most frequently used for diagnosis because they are easy to do, are reliable and rapidly provide results allowing immediate decision making at the time of the office visit. The initial RADTs used the latex agglutination technique, but newer tests are based on enzyme immunoassay (EIA) techniques, and more recently, on optical immunoassay (OIA) technology. Among the most recent tests is a chemiluminescent single-stranded DNA probe that detects specific rRNA sequences unique to GABHS. This DNA probe test was shown to have a sensitivity ranging between 86 % and 94.8 %, and a specificity above 95 % when compared to the blood agar culture.15,16 While the RADTs for GABHS are very specific, their sensitivity is lower than that of culture,17 so some recommend that all negative RADTs be followed up by a culture. Because of its excellent sensitivity and specificity, DNA probe is often considered equivalent to culture,18 and is commonly used as a confirmatory method instead of throat culture.
According to the IDSA (Infectious Disease Society of America) guidelines for the diagnosis of strep throat in adults, however, a confirmatory culture of a negative RADT is not mandatory. While the guidelines do not recommend against confirmatory throat cultures, they state that the diagnosis of GABHS on the basis of the RADT without confirmation of negative results by culture is an “acceptable alternative”.6 Despite their recommendation, routine confirmation of negative RADTs by culture or DNA probe is still widely performed in many US healthcare organizations, and is a requirement of the Joint Commission unless an exception is granted based on self study of testing results. Because patients are charged an additional $113 for a DNA strep probe test at Cleveland Clinic, we thought it important to determine whether confirmation of negative RADT adds enough clinical value for patients to justify the additional cost of routine testing. We hypothesized that the rate of false negative RADTs would be low, and that patients with a false negative RADT and patients with a negative RADT but no confirmatory test would have good outcomes whether or not they were treated with an antibiotic. As a secondary goal, we were interested in measuring inappropriate use of antibiotics in adults with acute pharyngitis.
METHODS
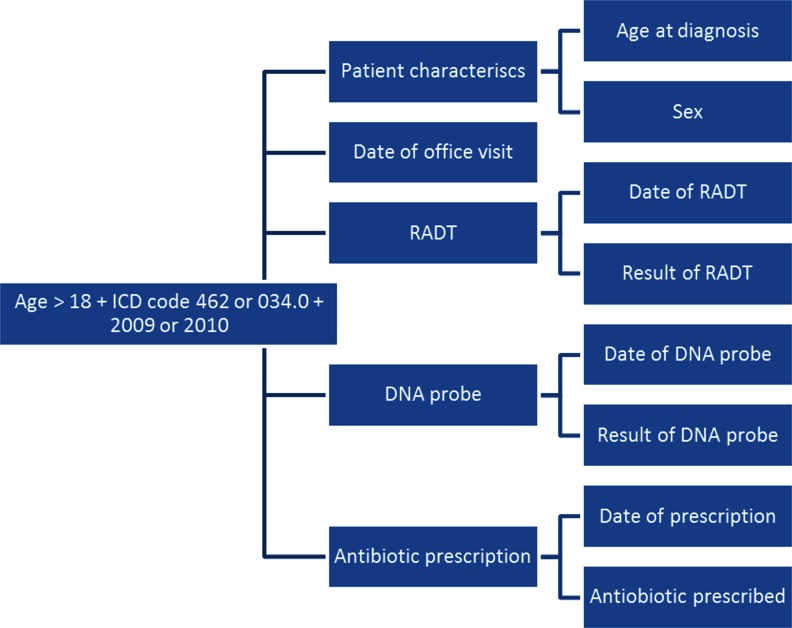
We used the Cleveland Clinic Epic Clarity database to identify a cohort of all patients aged 18 years and older who had an office visit to a Cleveland clinic generalist physician in the years 2009 and 2010 and had a visit diagnosis of acute pharyngitis (ICD codes 462, 034.0). We extracted the following variables: patient age and sex, date of office visit, date of RADT, RADT result, date of DNA probe, DNA probe result, date of antibiotic prescription and name of antibiotic prescribed (Fig. 1). We also extracted pharyngeal abscess (ICD codes 475, 478.22, 478.29) as an additional diagnosis within 30 days of the initial office visit. From this cohort, we identified all patients with a negative RADT and a subsequent positive DNA probe (false negative RADT group), and all patients who had a negative RADT but no confirmatory DNA probe. For these patients, we performed a chart audit to determine if and when these patients were treated with an antibiotic. We calculated the proportion of all patients with a negative RADT and negative DNA probe who were treated with an antibiotic. We also identified all patients with a concomitant diagnosis of pharyngeal abscess within 30 days of the office visit and performed a chart audit in order to determine the microbiological nature of the abscess (streptococcal Vs non Streptococcal), the timing of the diagnosis and the patterns of antibiotic treatment.
Figure 1.
Variables extracted using the Epic Clarity database.
Finally, we calculated the cost of using DNA probes routinely for all negative RADT. At a charge of $113 to the patient per DNA probe, we calculated the cost per false negative RADT, i.e. the number of DNA probes that need to be performed to obtain a single positive result multiplied by the cost of a single DNA probe.
The RADT used at the Cleveland Clinic, Acceava, is based on color immune-chromatographic technology. The manufacturers’ description claims a sensitivity of 97 % and specificity of 95 %.19 These test characteristics have not been validated against blood agar plate culture at Cleveland Clinic, but they were validated by an independent study that compared Acceava and two other RADTs with blood agar culture.20 For backup testing of negative RADTS, Cleveland Clinic uses a DNA probe technique called the Hologic Gen-Probe GASDirect, which uses nucleic acid hybridization for the qualitative detection of group A streptococcal RNA directly from throat swabs. Using throat culture as a gold standard, in a study of 1,005 patients with pharyngitis, Bourbeau found a sensitivity of 90.7 % and a specificity of 98.1 % for the Gen-Probe GASDirect.18 Another study conducted by Chapin et al. on 520 patients found that the DNA probe test was comparable to culture in performance with a sensitivity of 94.8 % and a specificity of 100 %.21 The Gen-probe takes about 1–2 h to result. However, at the Cleveland Clinic, this test is run in batch daily at 11 AM, so the results may not be available to the physician until the following day.
RESULTS
In 2009 and 2010, 25,130 adults seen in Cleveland Clinic primary care practices had a visit diagnosis of acute pharyngitis, of which 69 % were females and 31 % were males. The mean age was 40 years.
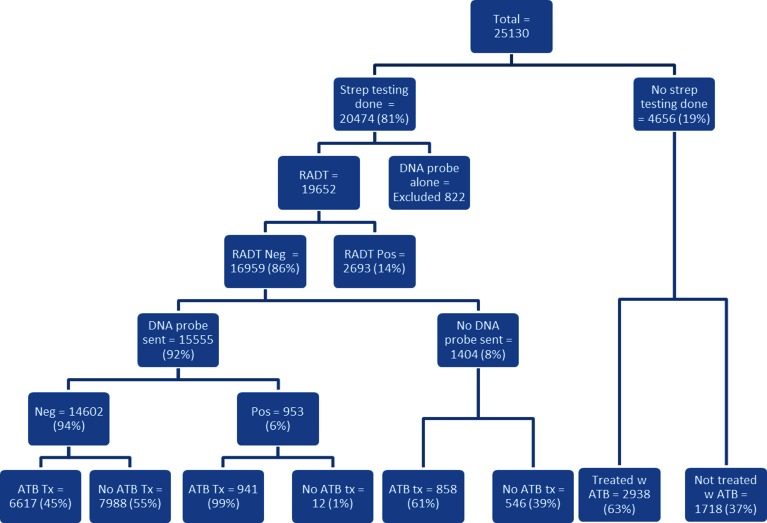
Of these 25,130 patients, 4,656 (19 %) did not have a RADT performed and were treated on the basis of clinical findings. The remaining 20,474 patients (81 %) were tested by RADT alone, DNA probe alone or both. Of the 822 patients who were tested with DNA probe alone, 77 had positive results. Overall, 3,993/20,474 (19.5 %) patients tested positive for GABHS with either RADT or DNA probe test. We excluded from further analysis the 822 patients who had DNA probe testing alone, leaving 19,652 patients who had a RADT test (Fig. 2). Eighty-six percent (16,959) had a negative RADT. Of the 16,959 patients who had negative RADT, 92 % (15,555) had DNA probe backup testing (Fig. 2). Of these 15,555 patients, 94 % (14,602) had a negative DNA probe and 6 % (953) had positive results, a false negative rate of 6 % for the Acceava strep RADT using the DNA probe as the gold standard.
Figure 2.
Population repartition according to testing and antibiotic prescription.
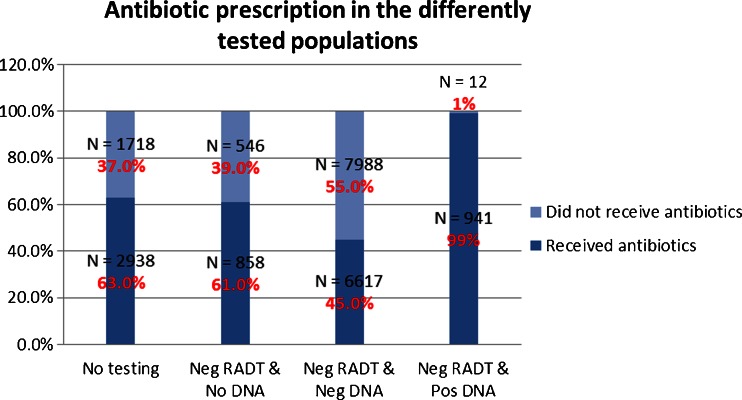
The pattern of antibiotic prescribing for all patients is diagrammed in Figures 2 and 3. Out of 25,130 patients, 14,047 (56 %) were prescribed an antibiotic. Of the 4,656 patients who did not have testing for GABHS, 2,938 (63 %) were prescribed an antibiotic. All patients with positive RADT and no DNA probe confirmation were prescribed an antibiotic. Of the 14,602 patients who had negative RADT and negative DNA probe testing, 6,617 (45 %) were prescribed an antibiotic. Of the 1,404 patients who had a negative RADT with no DNA probe confirmation, 61 % (858) were prescribed an antibiotic.
Figure 3.
Antibiotic prescription according to the testing performed. First bar: Population with no testing performed. Second bar: Population with a negative rapid antigen detection test (RADT) and no DNA probe confirmation. Third bar: Population with a negative RADT and a negative DNA probe confirmation. Fourth bar: Population with a negative RADT and a positive DNA probe confirmation. Light blue: Patients who did not receive antibiotics. Dark blue: Patients who received antibiotics.
Ninety-nine percent of the 953 patients with a negative RADT and a positive DNA probe test were prescribed an antibiotic. Forty-eight percent of these patients were prescribed the antibiotic on the day of the office visit prior to receiving the DNA probe results. The 489 patients not treated on the day of the visit who tested positive via DNA probe (51 %) were prescribed an antibiotic an average of 2.3 days after the office visit. In nearly all cases, the medical record documented that patients receive a phone call from the office to give the test results and a prescription was called or sent to their pharmacy.
Regarding suppurative complications, 45 patients had a diagnosis of pharyngeal abscess within 30 days of the office visit. Thirty-two of these patients presented with the abscess at the index visit. Of the 13 patients diagnosed with a pharyngeal abscess within 30 days after the index visit, 11 had a negative RADT and a negative DNA probe, one had a positive RADT, and only one had a negative RADT with no backup testing. None of the 953 patients with a false negative RADT developed a suppurative complication, based on chart review.
At Cleveland Clinic, patients are charged $113 for a strep DNA probe. In the patients who were tested by both RADT and DNA probe, a total of 15,555 DNA probes at the cost of $1,757,715 were performed, which lead to an antibiotic prescription for 489 patients, 3 % of the 25,130 patients presenting with sore throat. The charges, therefore, per additional patient identified as having strep pharyngitis and treated due to backup testing was $3,595.
DISCUSSION
The prevalence of GABHS infection among patients with pharyngitis seen by primary care physicians varies greatly from study to study, ranging between 4.7 % and 44 %,21–23 but is usually around 10 % in adults.2 Our study showed a prevalence of 19.5 % among patients who had RADT testing, which was done for 81 % of adults in this study, so the prevalence of GABHS among adults with pharyngitis was typical. We cannot know the prevalence of GABHS in the 19 % of patients not tested; a somewhat higher or somewhat lower prevalence is equally plausible in this group of untested patients.
GABHS is a nearly always an uncomplicated, self-limiting disease. Antibiotic therapy aims at reducing the severity of the symptoms, shortening the course of the disease, reducing infectivity and transmission rates and preventing suppurative (e.g., peritonsillar abscess, cervical lymphadenitis and mastoiditis), as well as non-suppurative complications (e.g., rheumatic fever).7–10 It is most beneficial for hastening resolution of symptoms if instituted within the first 2 days of illness.8,9
In our study, the patients that had a negative RADT and a subsequent positive DNA probe test were of particular interest to us, because these were the strep cases ‘missed’ by the RADT. Approximately half of these patients were treated before the DNA probe result was available, and half were treated after it was available. For both groups, the DNA probe added little value. Half of the patients ‘missed’ by the RADTs received an antibiotic an average of 2.3 days after the office visit. Even if we assume that patients presented to the office on the first day of their sickness (which is doubtful), the delay of over 2 days means that these patients were less likely to have benefited from the antibiotic treatment. On the other hand, 48 % of these patients were prescribed antibiotics at the date of the office visit without waiting for DNA probe results, so DNA testing was not used to guide therapy. Moreover, backup testing came at a total cost of $1,757,715, or an equivalent of $3,595 for every patient with GABHS who was initially missed by the RADT. Due to the extremely low prevalence of rheumatic fever in the US and the very low prevalence of suppurative complications, this additional expense does not seem justified.
In regard to suppurative complications, none of the 13 pharyngeal abscesses that occurred in our cohort within 30 days after the index office visit for sore throat could be attributed definitively to streptococcal infection. Of the 1,404 patients who had a negative RADT and no backup DNA test, only one developed a pharyngeal abscess due to an unknown organism (was not cultured), and that patient recovered fully without hospitalization. None of the patients with false negative RADT developed complications. Therefore, backup testing for acute pharyngitis in adults appears to add little value at significant cost.
Prior studies show that antibiotics are prescribed for up to 73 % of adults presenting to primary care clinicians with acute pharyngitis, making this one of the major causes of inappropriate use of antibiotics.24 We were quite surprised to find the high rate of antibiotic prescribing for non-group B strep acute pharyngitis in this study; 56 % percent of patients received an antibiotic, but only 19.5 % had confirmed strep pharyngitis. Of the 1,404 patients who had a negative RADT without DNA probe confirmation, 61 % received an antibiotic prescription. Had they had backup testing, it is likely that only 6 %, 84 patients, would have been positive for strep.
Even more troublesome is antibiotic prescribing for the 6,617 patients who had a negative RADT and subsequent negative DNA probe. It appears that clinicians’ decisions to prescribe an antibiotic for these patients were based solely on clinical grounds, regardless of the RADT results. We did not, however, do chart reviews on these patients to see if they had a household contact with confirmed strep pharyngitis or another co-existing diagnosis for which an antibiotic might be justified, such as acute sinusitis. This seems unlikely because acute sinusitis does not generally cause sore throat. It is possible that some patients were given an antibiotic prescription, but told to not fill the prescription until the culture result was returned. However, we did not have the resources to call 6,617 patients to see if they filled the prescription. This requires another study using pharmacy fulfillment data. Such a high rate of over-prescribing, however, raises concerns about development of antibiotic resistance and antibiotic related complication.
Clinicians face a dilemma in using backup strep testing. On the one hand, the longer a clinician waits before prescribing an antibiotic, the less likely the patient is to benefit from it. But on the other hand, if a clinician prescribes an antibiotic without waiting for the DNA probe results, this leads to significant over-prescribing. Because the false negative rate for RADT was only 6 %, clinicians should trust a negative RADT in nearly all cases, and treat patients symptomatically with pain medication until the confirmatory test result is available. Also, it is quite possible that some of the 6 % of patients “missed” by the RADT are actually strep carriers who have viral pharyngitis. Alternatively, since the RADT false negative rate is quite low, it is reasonable to not use backup testing routinely. Backup testing could be reserved for patients with a higher clinical probability of streptococcal infection and those who appear seriously ill.
CONCLUSION
Routine backup DNA probe testing led to a change in the management of only 3 % of adults treated at Cleveland Clinic in 2010–2011, at the cost of $1,757,715 or an equivalent of $3,595 for each patient missed by the RADT. We found little value for patients despite this high cost, and there were no reported suppurative complications that could be attributed to GABHS in the patients who did not receive an antibiotic prescription. Furthermore, acute pharyngitis was over-treated with antibiotics in this cohort of patients, despite adherence to an accepted diagnostic algorithm.
Acknowledgements
Contributors
We are grateful to Serge Harb, MD, Kelly Nottingham, Virginia Molina and Sarah Schramm for their assistance in obtaining and organizing the clinical data used in this study.
Funders
This publication was made possible by the Case Western Reserve University/Cleveland Clinic CTSA Grant Number UL1 RR024989 from the National Center for Research Resources (NCRR) and the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR, NCATS or NIH.
Prior presentations
American College of Physicians, oral presentation, April 20th 2012.
Conflict of Interest
The authors declare that they do not have a conflict of interest
REFERENCES
- 1.Cherry DK, Woodwell DA. National Ambulatory Medical Care Survey: 2000 summary. Adv Data. 2002;328:1–32. [PubMed] [Google Scholar]
- 2.Schappert SM, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2006. Natl Health Stat Report. 2008;8:1–29. [PubMed] [Google Scholar]
- 3.Bisno AL. Acute pharyngitis: etiology and diagnosis. Pediatrics. 1996;97:949–954. [PubMed] [Google Scholar]
- 4.Snow V, Mottur-Pilson C, Cooper RJ, et al. Principles of appropriate antibiotic use for acute pharyngitis in adults. Ann Intern Med. 2001;134:506. doi: 10.7326/0003-4819-134-6-200103200-00018. [DOI] [PubMed] [Google Scholar]
- 5.Linder JA. Evaluation and management of adult pharyngitis. Compr Ther. 2008;34(3–4):196–203. [PubMed] [Google Scholar]
- 6.Bisno AL, Gerber MA, Gwaltney JM, Jr, Kaplan EL, Schwartz RH. Diagnosis and management of group A streptococcal pharyngitis: a practice guideline. Infectious Diseases Society of America. Clin Infect Dis. 1997;25(3):574–583. doi: 10.1086/513768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brook I, Dohar JE. Management of group A beta-hemolytic streptococcal pharyngotonsillitis in children. J Fam Pract. 2006;55(12):S1–S11. [PubMed] [Google Scholar]
- 8.Randolph MF, Gerber MA, DeMeo KK, Wright L. Effect of antibiotic therapy on the clinical course of streptococcal pharyngitis. J Pediatr. 1985;106:870–875. doi: 10.1016/S0022-3476(85)80228-6. [DOI] [PubMed] [Google Scholar]
- 9.Krober MS, Bass JW, Michels GN. Streptococcal pharyngitis: placebo-controlled double-blind evaluation of clinical response to penicillin therapy. JAMA. 1985;253:1271–1274. doi: 10.1001/jama.1985.03350330069024. [DOI] [PubMed] [Google Scholar]
- 10.Nelson JD. The effect of penicillin therapy on the symptoms and signs of streptococcal pharyngitis. Pediatr Infect Dis J. 1984;3:10–13. doi: 10.1097/00006454-198401000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Salkind AR, Wright JM. Economic burden of adult pharyngitis: the payer’s perspective. ISPOR. 2008;11:621–627. doi: 10.1111/j.1524-4733.2007.00286.x. [DOI] [PubMed] [Google Scholar]
- 12.Wannamaker LW. Perplexity and precision in the diagnosis of streptococcal pharyngitis. Am J Dis Child. 1972;124(3):352–358. doi: 10.1001/archpedi.1972.02110150050009. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan EL, Top FH, Jr, Dudding BA, Wannamaker LW. Diagnosis of streptococcal pharyngitis: differentiation of active infection from the carrier state in the symptomatic child. J Infect Dis. 1971;123(5):490–501. doi: 10.1093/infdis/123.5.490. [DOI] [PubMed] [Google Scholar]
- 14.Breese BB, Disney FA. The accuracy of diagnosis of beta-streptococcal infections on clinical grounds. J Pediatr. 1954;44:670–673. doi: 10.1016/S0022-3476(54)80008-4. [DOI] [PubMed] [Google Scholar]
- 15.Pokorski SJ, Vetter EA, Wollan PC, Cockerill FR., 3rd Comparison of Gen-Probe Group A streptococcus Direct Test with culture for diagnosing streptococcal pharyngitis. J Clin Microbiol. 1994;32:1440–1443. doi: 10.1128/jcm.32.6.1440-1443.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heelan JS, Wilbur S, Depetris G, Letourneau C. Rapid antigen testing for group A Streptococcus by DNA probe. Diagn Microbiol Infect Dis. 1996;24:65–69. doi: 10.1016/0732-8893(95)00275-8. [DOI] [PubMed] [Google Scholar]
- 17.Gerber MA. Comparison of throat cultures and rapid strep tests for diagnosis of streptococcal pharyngitis. Pediatr Infect Dis J. 1989;8:820–824. doi: 10.1097/00006454-198911000-00032. [DOI] [PubMed] [Google Scholar]
- 18.Chapin KC, Blake P, Wilson CD. Performance characteristics and utilization of rapid antigen test, DNA probe, and culture for detection of group a streptococci in an acute care clinic. J Clin Microbiol. 2002;40(11):4207–4210. doi: 10.1128/JCM.40.11.4207-4210.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogo T, Schwartz RH, Ascher DP. Comparison of the Inverness Medical Acceava Strep A test with the Genzyme OSOM and Quidel QuickVue Strep A tests. Clin Pediatr. 2011;50:294–296. doi: 10.1177/0009922810385675. [DOI] [PubMed] [Google Scholar]
- 20.Bourbeau PP, Heiter BJ. Use of swabs without transport media for the Gen-Probe Group A Strep Direct Test. J Clin Microbiol. 2004;42:3207–3211. doi: 10.1128/JCM.42.7.3207-3211.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peter GS, Bisno AL. Group A streptococcal pharyngitis in adults: diagnosis and management. In: Pechera JC, Kaplan EL, eds. Streptococcal pharyngitis issues in infectious diseases. Basel. Karger. 2004;3:22.
- 22.Poses RM, Cebul RD, Collins M, Fager SS. The accuracy of experienced physicians’ probability estimates for patients with sore throats. Implications for decision making. JAMA. 1985;254:925. doi: 10.1001/jama.1985.03360070063024. [DOI] [PubMed] [Google Scholar]
- 23.Woods WA, Carter CT, Stack M, et al. Group A streptococcal pharyngitis in adults 30 to 65 years of age. South Med J. 1999;92:491. doi: 10.1097/00007611-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Linder JA, Stafford RS. Antibiotic treatment of adults with sore throat by community primary care physicians: a national survey, 1989–1999. JAMA. 2001;286:1181. doi: 10.1001/jama.286.10.1181. [DOI] [PubMed] [Google Scholar]