Abstract
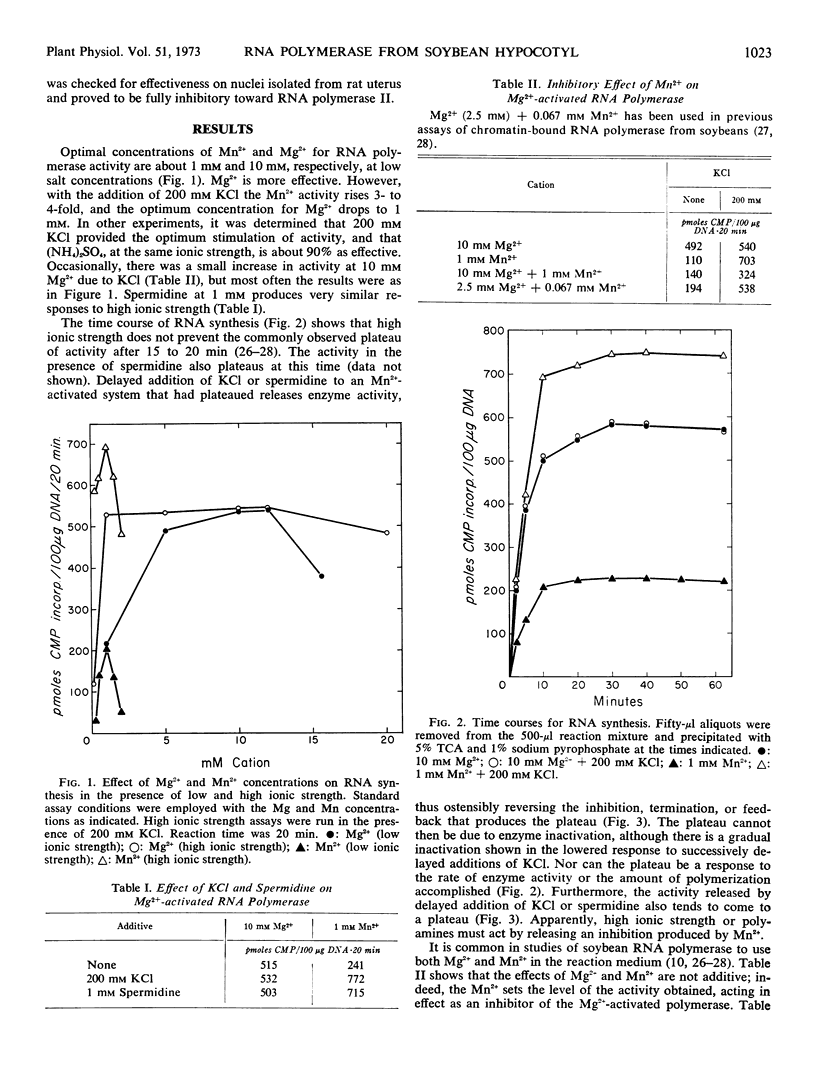
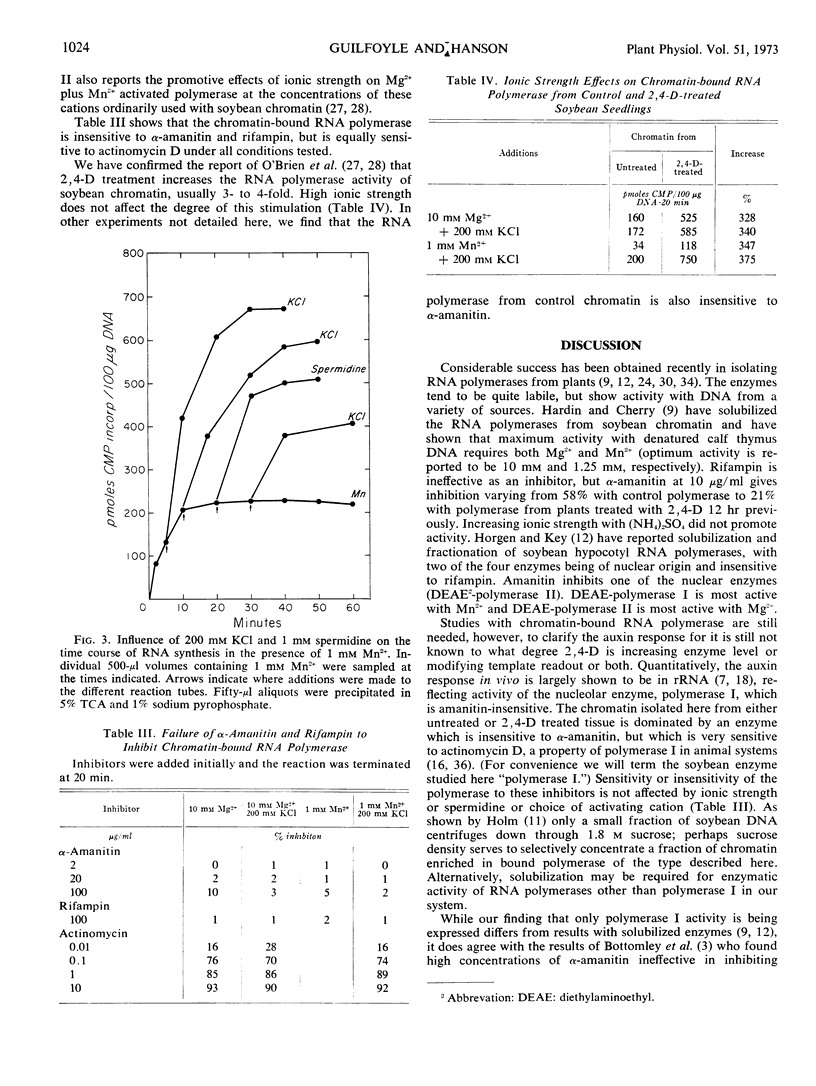
Optimal activity of chromatin-bound RNA polymerase from soybeans is obtained with 1 mm Mn2−, but only when high ionic strength or polyamines are included in the medium. Such inclusion does not increase the Mg2+ activation of the polymerase, but it does lower the concentration needed for optimum activity from 10 mm to 1 mm. Mg2− activation is inhibited by added Mn2+, and the inhibition is relieved by high ionic strength or spermidine. The RNA polymerase with either cation is almost entirely polymerase I at low and high ionic strength as evidenced by insensitivity to α-amanitin. Treatment of soybean seedlings with 2,4-dichlorophenoxyacetic acid does not change these characteristics; although the activity rises 3- to 4-fold.
It is suggested that chromatin as prepared here may be a selected fraction enriched in polymerase I, which is activated by either Mg2+ or Mn2+, and that the Mn2− inhibition of activity is due to a known reaction of Mn2− with DNA which can be relieved by high ionic strength.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. A., Kuntz G. P., Evans H. H., Swift T. J. Preferential interaction of manganous ions with the guanine moiety in nucleosides, dinucleoside monophosphates, and deoxyribonucleic acid. Biochemistry. 1971 Nov 23;10(24):4368–4374. doi: 10.1021/bi00800a003. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbiroli B., Corti A., Caldarera C. M. RNA Polymerase activity in purified nuclei from rat prostate gland in the presence of polyamines. FEBS Lett. 1971 Mar 5;13(3):169–172. doi: 10.1016/0014-5793(71)80227-2. [DOI] [PubMed] [Google Scholar]
- Bottomley W., Spencer D., Wheeler A. M., Whitfeld P. R. The effect of a range of RNA polymerase inhibitors on RNA synthesis in higher plant chloroplasts and nuclei. Arch Biochem Biophys. 1971 Mar;143(1):269–275. doi: 10.1016/0003-9861(71)90209-8. [DOI] [PubMed] [Google Scholar]
- Chambon P., Ramuz M., Mandel P., Doly J. The influence of ionic strength and a polyanion on transcription in vitro. I. Stimulation of the aggregate RNA polymerase from rat liver nuclei. Biochim Biophys Acta. 1968 May 21;157(3):505–519. [PubMed] [Google Scholar]
- Eichhorn G. L., Shin Y. A. Interaction of metal ions with polynucleotides and related compounds. XII. The relative effect of various metal ions on DNA helicity. J Am Chem Soc. 1968 Dec 18;90(26):7323–7328. doi: 10.1021/ja01028a024. [DOI] [PubMed] [Google Scholar]
- Fuchse, Millette R. L., Zillig W., Walter G. Influence of salts on RNA synthesis by DNA-dependent RNA-polymerase from Escherichia coli. Eur J Biochem. 1967 Dec;3(2):183–193. doi: 10.1111/j.1432-1033.1967.tb19514.x. [DOI] [PubMed] [Google Scholar]
- HUANG R. C., BONNER J. Histone, a suppressor of chromosomal RNA synthesis. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1216–1222. doi: 10.1073/pnas.48.7.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin J. W., Cherry J. H. Solubilization and partial characterization of soybean chromatin-bound RNA polymerase. Biochem Biophys Res Commun. 1972 Jul 25;48(2):299–306. doi: 10.1016/s0006-291x(72)80050-0. [DOI] [PubMed] [Google Scholar]
- Holm R. E., O'brien T. J., Key J. L., Cherry J. H. The Influence of Auxin and Ethylene on Chromatin-directed Ribonucleic Acid Synthesis in Soybean Hypocotyl. Plant Physiol. 1970 Jan;45(1):41–45. doi: 10.1104/pp.45.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob S. T., Sajdel E. M., Munro H. N. Different responses of soluble whole nuclear RNA polymerase and soluble nucleolar RNA polymerase to divalent cations and to inhibition by alpha-amanitin. Biochem Biophys Res Commun. 1970 Feb 20;38(4):765–770. doi: 10.1016/0006-291x(70)90647-9. [DOI] [PubMed] [Google Scholar]
- Jacob S. T., Sajdel E. M., Munro H. N. Specific action of alpha-amanitin on mammalian RNA polymerase protein. Nature. 1970 Jan 3;225(5227):60–62. doi: 10.1038/225060b0. [DOI] [PubMed] [Google Scholar]
- Johnson J. D., Jant B. A., Kaufman S., Sokoloff L. Effects of ionic strength on the RNA polymerase activities of isolated nuclei and nucleoli of rat liver. Arch Biochem Biophys. 1971 Feb;142(2):489–500. doi: 10.1016/0003-9861(71)90512-1. [DOI] [PubMed] [Google Scholar]
- KRAKOW J. S. RIBONUCLEIC ACID POLYMERASE OF AZOTOBACTER VINELANDII. III. EFFECT OF POLYAMINES. Biochim Biophys Acta. 1963 Aug 20;72:566–571. [PubMed] [Google Scholar]
- Kedinger C., Gniazdowski M., Mandel J. L., Jr, Gissinger F., Chambon P. Alpha-amanitin: a specific inhibitor of one of two DNA-pendent RNA polymerase activities from calf thymus. Biochem Biophys Res Commun. 1970 Jan 6;38(1):165–171. doi: 10.1016/0006-291x(70)91099-5. [DOI] [PubMed] [Google Scholar]
- Lindell T. J., Weinberg F., Morris P. W., Roeder R. G., Rutter W. J. Specific inhibition of nuclear RNA polymerase II by alpha-amanitin. Science. 1970 Oct 23;170(3956):447–449. doi: 10.1126/science.170.3956.447. [DOI] [PubMed] [Google Scholar]
- Luck G., Zimmer C. Conformational aspects and reactivity of DNA. Effects of manganese and magnesium ions on interaction with DNA. Eur J Biochem. 1972 Sep 25;29(3):528–536. doi: 10.1111/j.1432-1033.1972.tb02018.x. [DOI] [PubMed] [Google Scholar]
- Mondal H., Mandal R. K., Biswas B. B. RNA polymerase from eukaryotic cells. Isolation and purification of enzymes and factors from chromatin of coconut nuclei. Eur J Biochem. 1972 Feb;25(3):463–470. doi: 10.1111/j.1432-1033.1972.tb01716.x. [DOI] [PubMed] [Google Scholar]
- Novello F., Stirpe F. Simultaneous assay of RNA polymerase I and II in nuclei isolated from resting and growing rat liver with the use of alpha-amanitin. FEBS Lett. 1970 May 11;8(1):57–60. doi: 10.1016/0014-5793(70)80225-3. [DOI] [PubMed] [Google Scholar]
- O'Brien T. J., Jarvis B. C., Cherry J. H., Hanson J. B. Enhancement by 2,4-dichlorophenoxyacetic acid of chromatin RNA polymerase in soybean hypocotyl tissue. Biochim Biophys Acta. 1968 Nov 20;169(1):35–43. doi: 10.1016/0005-2787(68)90006-3. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Korner A. The effect of trypsin digestion and ionic strength on RNA polymerase of rat liver. Arch Biochem Biophys. 1967 Feb;118(2):362–366. doi: 10.1016/0003-9861(67)90361-x. [DOI] [PubMed] [Google Scholar]
- Polya G. M., Jagendorf A. T. Wheat leaf RNA polymerases. I. Partial purification and characterization of nuclear, chloroplast and soluble DNA-dependent enzymes. Arch Biochem Biophys. 1971 Oct;146(2):635–648. doi: 10.1016/0003-9861(71)90172-x. [DOI] [PubMed] [Google Scholar]
- Richardson J. P. Reinitiation of RNA chain synthesis in vitro. Nature. 1970 Mar 21;225(5238):1109–1112. doi: 10.1038/2251109a0. [DOI] [PubMed] [Google Scholar]
- Stevens L. The biochemical role of naturally occurring polyamines in nucleic acid synthesis. Biol Rev Camb Philos Soc. 1970 Feb;45(1):1–27. doi: 10.1111/j.1469-185x.1970.tb01073.x. [DOI] [PubMed] [Google Scholar]
- Stirpe F., Fiume L. Studies on the pathogenesis of liver necrosis by alpha-amanitin. Effect of alpha-amanitin on ribonucleic acid synthesis and on ribonucleic acid polymerase in mouse liver nuclei. Biochem J. 1967 Nov;105(2):779–782. doi: 10.1042/bj1050779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain G. C., Mullinix K. P., Bogorad L. RNA polymerases of maize: nuclear RNA polymerases. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2647–2651. doi: 10.1073/pnas.68.11.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIDNELL C. C., TATA J. R. EVIDENCE FOR TWO DNA-DEPENDENT RNA POLYMERASE ACTIVITIES IN ISOLATED RAT-LIVER NUCLEI. Biochim Biophys Acta. 1964 Jul 22;87:531–533. doi: 10.1016/0926-6550(64)90133-1. [DOI] [PubMed] [Google Scholar]
- Widnell C. C., Tata J. R. Studies on the stimulation by ammonium sulphate of the DNA-dependent RNA polymerase of isolated rat-liver nuclei. Biochim Biophys Acta. 1966 Sep;123(3):478–492. doi: 10.1016/0005-2787(66)90216-4. [DOI] [PubMed] [Google Scholar]


