Abstract
Purpose
The objective of this study was to investigate the predictive value of anti-Mullerian hormone (AMH) on fertilization rate (FR), blastocyst development, embryo quality, the outcome of the pregnancy and the live birth rate (LBR) following in vitro fertilization-embryo transfer (IVF-ET)/intracytoplasmic sperm injection (ICSI).
Method
In this prospective study outcomes were followed in 83 women undergoing cycles of IVF/ICSI within a university hospital. Basal serum AMH, follicle stimulating hormone (FSH), luteinizing hormone (LH) and antral follicle count (AFC) were measured on Day 3. Serum AMH (Gn6 AMH ) level was measured on Day 6 after the administration of gonadotrophin (Gn). AMH was measured in follicle fluid (FF AMH) on the day of ovum pick-up (dOPU). The numbers of retrieved and fertilized oocytes, good quality embryos and blastocysts were counted. Secondary outcome variables included clinical pregnancy rate (CPR) and LBR.
Results
Spearman correlation analysis indicated that the numbers of oocytes, good quality embryos and blastocysts were associated with AMH (P < 0.05) and that LBR was correlated with FF AMH (r = 0.495, P < 0.05). No associations were found between FR and AMH (P > 0.05). Receiver operating characteristic analysis showed that the sensitivity of FF AMH at predicting CPR was 91.2 %; the specificity was 86.5 % and ROCAUC was 0.893 (P < 0.0001).
Conclusion
AMH parameters were correlated with good quality embryos and blastocysts, but only FF AMH showed a significant correlation with LBR and CPR.
Keywords: Anti-Mullerian hormone, Fertilization, Blastocyte, Live birth rate, Pregnancy
Introduction
Antimullerian hormone (AMH), also known as mullerian inhibiting substance (MIS), is a dimeric glycoprotein member of the transforming growth factor-β family. AMH is secreted by granulosa cells within preantral and early antral follicles, <4 mm in diameter. Its secretion decreases as the antral follicles begin to grow, and stops when the follicles are larger 8 mm in diameter, or when atresia occurs [4]. AMH is barely detectable in newborn baby girls and its level peaks after puberty and steadily decreases until menopause when serum concentration becomes undetectable [23].
The role of AMH in the ovary is to participate in the regulation of ovarian function, especially in follicle development and selection. It inhibits the initiation of human primordial follicle growth and prevents multiple selection of a dominant follicle by reducing the sensitivity of follicles to follicle stimulating hormone (FSH) [1]. Several reports suggest that AMH might be a better predictor of ovarian responses to controlled ovarian hyperstimulation (COS) than traditional parameters such as age, FSH, estradiol (E2) and inhibin B (INH-B) [7]. This is because AMH levels remain relatively constant throughout the menstrual cycle, and tend to be unaffected by GnRH-agonist pituitary down-regulation or pregnancy ([15, 17]. A previous study has showed that the performance of AMH as a predictor of poor ovarian response was very similar to that achieved with antral follicle counts (AFC) [3]. However AFC was tested in the early stage of the menstrual cycle and was evaluated using ultrasound [2]. Thus the accuracy and stability of AFC testing is inferior to that achieved with serum AMH.
Previous studies have found associations between AMHs (including serum AMH and follicle fluid AMH), fertilization rate, blastocyst development, embryo quality, pregnancy outcome and live birth rate (LBR). Some studies showed that high serum AMH levels on Day 3 were correlated with high numbers of mature oocytes, resulting in more embryos and ultimately a higher clinical pregnancy rate [10, 6, 16]. Other workers found no associations between basal serum AMH levels and embryo quality [5, 29, 18].
An association has also been found between follicle fluid AMH (FF AMH) levels and the quality of embryos in patients with polycystic ovary syndrome (PCOS) [20]. However, in this study population there was no correlation between FF AMH and the degree of maturation of retrieved oocytes, or the success of fertilization. Other workers, have demonstrated a correlation between FF AMH and live birth rate [15].
The present study was undertaken in light of these findings to investigate whether AMHs are associated with fertilization rate, blastocyst development, embryo quality, clinical pregnancy rate (CPR) and LBR.
Materials and methods
Patients
The study population comprised 83 women who underwent their first cycles of in vitro fertilization (IVF)/ intracytoplasmic sperm injection (ICSI) treatment at the Reproductive Medical Center of First Affiliated Hospital of Wenzhou Medical College, between January 2011 to August 2011. Infertility was due to tubal abnormalities (n = 42), sperm abnormalities (n = 21), tubal abnormalities with sperm abnormalities (n = 13), and unexplained causes (n = 7).
The women were ≤38 years of age; with a body mass indexes (BMI) between 18 and 29 kg/m2 and Day 3 serum FSH levels <12 IU/L. The women all had two ovaries together with a history of regular, ovulatory menstrual cycles (every 24 to 35 days). None of the women had received hormonal therapy in the previous 3 months. Women with ovarian cyst (> 3 cm in diameter), PCOS, endometriosis, a history of ovarian surgery or endocrine disorders were excluded from entering the study.
The study was approved by the institutional review board, an informed consent was obtained from all participants.
Hormone measurements
On Day 3 of the menstrual cycle and prior to treatment, blood samples for assay of AMH, FSH, E2 and luteinizing hormone (LH) were collected by venupuncture. The samples were immediately centrifuged to separate the serum and were stored in aliquots at −80 °C.
Ultrasound scanning with a 6.5-MHz transvaginal probe (Logic 180; General Electric, Health care Technology, Wuxy, China) was used to count the number of antral follicles in each ovary that had a mean diameter of 3 to 10 mm. Measurement of serum AMH level was repeated on Day 6 of gonadotropin therapy.
Anti-Mullerian hormone was measured using the Immunotech Enzyme Immune Assay kit (Bechman-Caulter, France) according to the instruction manual. On the day of ovum pick-up (dOPU), under transvaginal ultrasound guidance, fluid from three to five dominant follicles was gently and thoroughly aspirated using a 10 mL syringe. The fluid was maintained at 37 °C until the oocyte was found and isolated. The level of AMH in FF was measured as described above.
Cycle monitoring and IVF/intracytoplasmic sperm injection
All patients received standard ovarian stimulation with recombinant FSH (r-FSH) under pituitary suppression with a GnRH agonist. Briefly, the GnRH agonist (triptorelin 0.5–0.7 mg, Decapeptyl, 3.75 mg Ferring, Kiel, Germany) was administered subcutaneously in the mid-luteal phase of the previous cycle. Stimulation commenced 2 weeks later, when the circulating E2 level was <150 pmol/L, the thickness of endometrium was <5 mm, serum LH was <5 IU/L and a vaginal ultrasonographic scan showed an absence of follicles >10 mm in diameter.
Ovarian stimulation was achieved by administration of 150 IU/day of recombinant FSH (Gonal-F, Merck Serono SA Aubonne Branch, Swiss Confederation). The first response scan was performed on stimulation Day 6 (S). Thereafter, FSH was administered on an individual basis according to the ovarian response, assessed by sequential transvaginal ultrasonography and serum estradiol measurements. The criteria for human chorionic gonadotropin (HCG) administration (Livzon, 5000 to 10,000 IU, Pharmaceutical Group Inc, China,) was the presence of three or more follicles ≥16 mm in diameter with a consistent rise in serum estradiol concentration.
Oocyte aspiration was performed using vaginal ultrasound, 34 to 36 h after hCG injection. Intracytoplasmic sperm injection was performed using standard procedures and the embryos were transferred 2 or 3 days later.
The luteal phase was supported with 40 mg progesterone administered by daily injection (20 mg Prontogest; Zhejiang Xianju Pharmaceuticals, China). A pregnancy test was carried out on Day 14 after embryo transfer. Two weeks later, a transvaginal ultrasound was performed to confirm pregnancy
Study endpoints
Fertilization rate (FR) was calculated as the number of fertilized eggs relative to the number of retrieved oocytes. Good quality embryos were defined as those at the 4 to 6 cell stage on Day 2 or at the 6 to 8 cell stage on Day 3. There was to be less than 20 % difference in the size of blastomeres, and anucleated fragments were to account for less than 20 % of the total embryo volume and to have a localized peripheral distribution within the embryos. The good quality embryos meeting these criteria were either transferred to the recipients or frozen.
Clinical pregnancy was defined by the presence of a gestational sac. Biochemical pregnancy was defined by the presence of β-HCG >50 mIU/mL without ultrasound evidence of a gestational sac.
Clinical pregnancy rate was defined by the ratio of the clinical pregnancy cases to the embryo transfer cases. Live birth rate was calculated by the number of the live babies divided by the number of embryo transfer cases.
Statistical analysis
Statistical analysis was undertaken using Statistical Program for Social Sciences (SPSS) version 17 for Windows (SPSS Inc., Chicago, IL). Values were shown as means and standard deviations (±SD). T-tests were used to compare clinical indexes in pregnant and non-pregnant women.
Spearman analysis was performed to detect the correlations between the different outcomes and the three AMH concentrations. Receiver operating characteristic (ROC) curves were constructed to compare the accuracy of AMH and other parameters in predicting CPR. Sensitivity, specificity, positive and negative predictive values were calculated for each AMH determination and for other parameter cut-off levels.
This was a prospective study with a small sample size, therefore small sample size calculations (PASS11, NCSS, USA) were undertaken to determine the power of the study to detect differences at the 0.05 probability level. Values of P < 0.05 were considered statistically significant.
Results
Patient characteristics
The mean age of the women in the study was 30.2 years (range: 23 to 38 years) and mean basal FSH was 7.79 ± 1.55 IU/L. All women underwent oocyte aspiration and 76 underwent embryo transfer. Forty-seven women achieved clinical pregnancy, including one case of ectopic pregnancy. There were six cases of biochemical pregnancy and 23 women failed to become pregnant. There were 33 full-term live births, four preterm births, and 10 cases of abortion.
Comparison of parameters in pregnancy and non pregnancy cases
The women were divided into a pregnancy group (clinical pregnancy) and non-pregnancy group which included six cases of biochemical pregnancy. As shown in Table 1, FF AMH (power of test >0.999), fertilization rate (power of test: 0.561) and cleavage (power of test: 0.595) were significantly higher in the pregnancy group than in the non pregnancy group.
Table 1.
Comparison of different parameters in the pregnancy and non-pregnancy groups
| Parameter | Mean±SD | P-value | |
|---|---|---|---|
| Pregnancy group (n = 47) | Non-pregnancy group (n = 29) | ||
| Basal AMH, ng/mL | 3.44 ± 1.035 | 3.14 ± 1.29 | 0.273 |
| Gn6 AMH, ng/mL | 2.81 ± 1.25 | 2.42 ± 1.55 | 0.233 |
| FF AMH, ng/mL | 8.28 ± 2.04 | 4.76 ± 2.58 | 0.000* |
| FSH, IU/L | 7.96 ± 1.81 | 7.63 ± 1.30 | 0.368 |
| E2 pmol/L | 156.52 ± 64.31 | 151.86 ± 82.37 | 0783 |
| AFC | 16.19 ± 4.67 | 16.3 ± 3.84 | 0.923 |
| Gn6 E2 pmol/L | 674.56 ± 632.4 | 1002.7 ± 1305.62 | 0.162 |
| E2 Peak value, pmol/L | 9542.08 ± 4294.78 | 7795.35 ± 4835.29 | 0.102 |
| Retrieved oocytes, n | 11.53 ± 4.57 | 9.98 ± 5.15 | 0.171 |
| Mature eggs, n | 10.56 ± 4.07 | 8.78 ± 4.32 | 0.069 |
| Fertilizations, n | 7.81 ± 3.42 | 6,20 ± 3.02 | 0.033 |
| Cleavages, n | 7.67 ± 3.37 | 6.02 ± 2.97 | 0.027 |
| High quality embryos, n | 5.22 ± 3.15 | 4.08 ± 3.08 | 0.113 |
| Frozen embryos, n | 1.86 ± 1.9 | 1.3 ± 1.73 | 0.182 |
Significant P values were denoted in bold; *: defined as P value < 0.05, and the power of test >0.9
Relationships between AMHs and outcomes
Table 2 shows the relationships between AMHs and study outcomes. Numbers of oocytes retrieved, good quality embryos and blastocysts were correlated with baseline serum AMH, Gn6 AMH and FF AMH. AFC was statistically significantly correlated with Gn6 AMH and FF AMH. However, the powers of both tests was <0.9.
Table 2.
Spearman rank correlation results for basal AMH, Gn6 AMH and FF AMH
| Parameter | Basal AMH | Gn6 AMH | FF AMH | |||
|---|---|---|---|---|---|---|
| r | P-value | r | P-value | r | P-value | |
| Age | −0.085 | 0.443 | −0.061 | 0.581 | 0.021 | 0.847 |
| FSH | 0.031 | 0.781 | −0.038 | 0.731 | −0.078 | 0.483 |
| LH | 0.130 | 0.242 | 0.071 | 0.523 | 0.123 | 0.269 |
| AFC | 0.064 | 0.563 | 0.239 | 0.030 | 0.237 | 0.031 |
| Total oocytes retrieved | 0.550 | 0.000* | 0.677 | 0.000* | 0.654 | 0.000* |
| FR | −0.101 | 0.361 | −0.147 | 0.184 | 0.109 | 0.325 |
| Number of good embryos | 0.392 | 0.000* | 0.383 | 0.000* | 0.579 | 0.000* |
| Number of blastocysts | 0.313 | 0.004 | 0.365 | 0.001* | 0.55 | 0.000* |
| LBR | 0.169 | 0.127 | 0.152 | 0.171 | 0.495 | 0.000* |
The spearman analysis was performed to detect the correlations between different outcomes of this study and the concentration of three types of AMHs. P value < 0.05 was considered statistically significant. Significant P values were denoted in bold. *: defined as P value < 0.05, and the power of test > 0.9
LBR was correlated with FF AMH (r = 0.495; P < 0.0001) but not with basal or Gn6 AMH. There were no associations between AMHs, age, FSH, LH, or fertilization rate.
Sensitivity of different parameters to predict the pregnancy
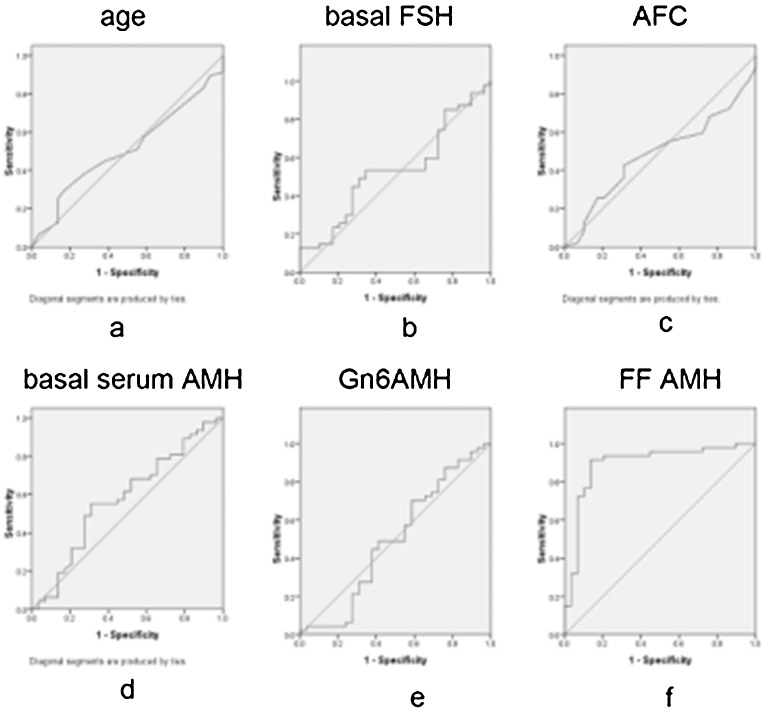
Age, basal FSH, AFC and AMHs were all tested for their ability to predict pregnancy rate in the fresh cycle IVF/ICSI. As shown in Fig. 1, only FF AMH reliably predicted the clinical pregnancy. Using a cut-off value of 1.777 nmol/L, the sensitivity was 91.2 %, the specificity was 86.5 %, and the ROCAUC was 0.893 (P < 0.0001).
Fig. 1.
Comparison of the sensitivity of different parameters to predict the pregnancy rate in the fresh cycle IVF/ICSI. a Age: AUC = 0.51 (95%CI: 0.379–0.641; P = 0.885). b Basal FSH: AUC = 0.532 (95%CI: 0.399–0.665; P = 0.642). c AFC: AUC = 0.482 (95%CI: 0.351–0.613; P = 0.797). d Basal serum AMH: AUC = 0.578 (95%CI: 0.442–0.713; P = 0.257). e Gn6 AMH: AUC = 0.492 (95%CI: 0.349–0.635; P = 0.911). f FF AMH: AUC = 0.893 (95%CI: 0.809–0.977; P = <0.0001)
Discussion
Many factors have the potential to affect the outcome of the pregnancy during IVF/ICSI. Traditional parameters including FSH, E2 and INH-B, are not entirely reliable. However, it has been proposed that estimation of AMH might provide an alternative approach given its high reproducibility and versatility allowing it to be checked at any time during the menstrual cycle [7]. Previous investigations have focused on AMH as a predictor of ovarian response to gonadotrophin stimulation [26, 24]. Consequently, the predictive value of AMH on fertilization rate, blastocyst, embryo quality, pregnancy outcome and LBR remains relatively unexplored, and controversial.
Some previous reports have demonstrated the value of AMH in predicting oocyte quality, embryo quality, and ICSI outcome. Low AMH levels may be associated with poor oocyte quality resulting in a diminished FR [5, 27]. AMH might, therefore, be a good predictor of fertilization rate following IVF/ICSI. Other workers [30] have indicated that oocytes were more likely to be fertilized when their follicles produced high levels of AMH. This was because follicular fluid AMH levels were more than three times higher in follicles with fertilized oocytes, than in those with no fertilized oocytes.
A study in non-obese patients with non-hyperandrogenemic polycystic ovary syndrome demonstrated a positive correlation between FF AMH level from the first mature follicle, and the numbers of oocytes, 2 pn (pronuclei) and embryos [32]. In the same study there was no correlation between FF AMH and the proportion of oocytes in metaphase II. Other studies have shown that neither baseline serum AMH nor Gn5 AMH, was associated with the quality of oocytes and fertilization rate [26, 29]. It has been proposed that different AMH levels may predict the quality of oocytes ,but not their ability to become fertilized [12].
The results of our study suggested that there was no significant association between basal serum AMH, Gn6 AMH or FF AMH and fertilization rate during IVF/ICSI. Follicular fluid was pooled from three to five mature follicles. Thus, FF AMH concentrations in our study did not reflect AMH levels in individual follicles. This might explain why were unable to find an association between FF AMH and fertilization rate.
Traditionally, good quality embryos are identified on the basis of morphological findings and it has been suggested that AMH may reflect embryo morphology [27]. Studies have shown that when serum AMH concentration on the day of HCG administration were higher than 2.7 ng/mL, good quality of oocytes were always present, which might in turn result in higher implantation and pregnancy rates.
Serum AMH and AFC on Day 3 have been shown to be correlated with the number of good quality embryos and the number of embryos frozen [19]. In other studies serum AMH levels were correlated with the oocyte quality, embryo development and ICSI outcomes [12]. There are also reports showing that embryo morphology scores, non-multiple types, and oocyte quality of oocyte have no direct effect on embryo quality [9].
Our study showed positive correlations between the number of good quality embryos and levels of baseline serum AMH, Gn6 AMH and FF AMH. The most significant correlation was seen with FF AMH. These findings agree with most previous reports [9, 19, 30].
The pregnancy rate associated with blastocysts is generally higher than that seen with Day 2–3 embryos, but there is the risk of the embryo breaking or deteriorating. Thus, due to external or internal environmental factors, the cumulated clinical pregnancy rate with blastocysts is comparable to that seen with D2–3 embryos . Identification of a marker that accurately predicted blastocyst development, would enable the implantation and pregnancy rate associated with Day 2–3 embryos to be improved significantly.
Logistic regression analyses adjusting for age and other variables, have found significant association between serum AMH and Day 5 embryo transfer [11]. In addition, a weak positive correlation has been demonstrated between baseline serum AMH level and subsequent blastocyst development during IVF [28]. Other previous reports stated that further cleavage up to the blastocyst stage was not affected by AMH [5]. Findings from the small number of women in our study suggested that the number of blastocysts was positively correlated with basal serum AMH, Gn6 AMH, and FF AMH (Table 2). These results also indicated for the first time that FF AMH may be a useful marker for predicting blastocyst development. FF AMH was taken on the day of oocyte retrieval, and it might therefore more directly reflect the quality of the oocyte and embryo than baseline serum AMH or Gn6 AMH.
Although AMH is a useful marker for predicting ovarian reserve, its value in predicting pregnancy remains unclear. In monodominant follicle cycles AMH level in the fluid follicle (FF), rather than in the serum, was shown to be positively related to the rate of the ensuing oocyte and embryo implantation. [8]. A study in 276 women showed that concentrations of AMH and inhibin B in serum and in FF were significantly higher in women who became pregnant in the corresponding treatment cycle than in those who did not conceive [31]. Other workers have proposed a threshold basal AMH level of 2.52 ng/mL as a meaningful cutoff for ongoing pregnancy [6].
It has been shown that Day 3 serum AMH level was strongly associated with IVF outcome ,,higher AMH concentrations coincided with a greater number of embryos [10]. However other studies indicated that serum level of AMH did not necessarily predict the outcome of pregnancy [26, 21, 29, 3, 13].
In our study, only FF AMH, fertilization rate and the number of zygotic cleavages distinguished between the pregnancy and non-pregnancy group. Receiver operating characteristic (ROC) curves used to compare the accuracy of different parameters for predicting CPR, showed that only FF AMH had a suitable level of sensitivity (91.2 %) and specificity (86.5 %) (Fig. 1). This finding was in agreement with previously reported results [8].
Although oocyte quality and embryo quality decreases with advancing age, we were unable to demonstrate a positive correlation between age and pregnancy rate, possibly due to the narrow age range of our patients, with the oldest being only 38 years of age. Both AMH and age have been shown by others to be independent predictors of live birth [14, 25]. The confidence intervals for each age category are wide highlighting the need to assess the value of AMH for predicting LBR. In the present study we also evaluated the association between AMH and LBR and found a positive association with FF AMH but not with the other parameters tested.
Our conclusions were limited by the fact that the study was not conducted in a monodominant follicle cycle. Instead, we pooled follicular fluid from a number of follicles, and consequently individual follicles and oocytes within them could not be tracked. In addition we failed to exclude cases of azoospermia which was a cause of infertility. Even though some reports indicated that pregnancy outcome after ICSI was not affected by the origin or quantity of sperm [22], the possible inclusion of these patients may have had an impact on our results. Further studies with larger numbers of women and more stringent inclusion criteria are required to confirm our findings.
Despite these limitations, the present study suggested AMHs may be correlated with oocytes retrieval, the number of good quality embryos and with blastocyst development. We also described for the first time the role of FF AMH in predicting blastocyst development. FF AMH also significantly correlated with LBR and CPR, indicating that it might be the most suitable parameter, among the three types of AMH, for predicting embryo quality and blastocyst development. It might also be an independent parameter for predicting CPR and LBR following IVF/ICSI.
Acknowledgment
This work was supported by the Department of Education, Zhejiang Province (Y200804987).
Footnotes
Capsule
This prospective study showed that AMH parameters were correlated with good quality embryos and blastocysts, but only FF AMH showed a significant correlation with LBR and CPR.
References
- 1.Arabzadeh S, Hossein G, Rashidi BH, Hosseini MA, Zeraati H. Comparing serum basal and follicular fluid levels of anti-Mullerian hormone as a predictor of in vitro fertilization outcomes in patients with and without polycystic ovary syndrome. Ann Saudi Med. 2010;30(6):442–447. doi: 10.4103/0256-4947.71063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12(6):685–718. doi: 10.1093/humupd/dml034. [DOI] [PubMed] [Google Scholar]
- 3.Broer SL, Mol BW, Hendriks D, Broekmans FJ. The role of antimullerian hormone in prediction of outcome after IVF: comparison with the antral follicle count. Fertil Steril. 2009;91(3):705–14. doi: 10.1016/j.fertnstert.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Durlinger AL, Gruijters MJ, Kramer P, Karels B, Ingraham HA, Nachtigal MW, Uilenbroek JT, Grootegoed JA, Themmen AP. Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology. 2002;143(3):1076–1084. doi: 10.1210/en.143.3.1076. [DOI] [PubMed] [Google Scholar]
- 5.Ebner T, Sommergruber M, Moser M, Shebl O, Schreier-Lechner E, Tews G. Basal level of anti-Mullerian hormone is associated with oocyte quality in stimulated cycles. Hum Reprod. 2006;21(8):2022–6. doi: 10.1093/humrep/del127. [DOI] [PubMed] [Google Scholar]
- 6.Eldar-Geva T, Ben-Chetrit A, Spitz IM, Rabinowitz R, Markowitz E, Mimoni T, Gal M, Zylber-Haran E, Margalioth EJ. Dynamic assays of inhibin B, anti-Mullerian hormone and estradiol following FSH stimulation and ovarian ultrasonography as predictors of IVF outcome. Hum Reprod. 2005;20(11):3178–3183. doi: 10.1093/humrep/dei203. [DOI] [PubMed] [Google Scholar]
- 7.Fanchin R, Taieb J, Lozano DH, Ducot B, Frydman R, Bouyer J. High reproducibility of serum anti-Mullerian hormone measurements suggests a multi-staged follicular secretion and strengthens its role in the assessment of ovarian follicular status. Hum Reprod. 2005;20(4):923–927. doi:10.1093/humrep/deh688. [DOI] [PubMed]
- 8.Fanchin R, Mendez Lozano DH, Frydman N, Gougeon A, di Clemente N, Frydman R, Taieb J. Anti-Mullerian hormone concentrations in the follicular fluid of the preovulatory follicle are predictive of the implantation potential of the ensuing embryo obtained by in vitro fertilization. J Clin Endocrinol Metab. 2007;92(5):1796–1802. doi: 10.1210/jc.2006-1053. [DOI] [PubMed] [Google Scholar]
- 9.Gnoth C, Schuring AN, Friol K, Tigges J, Mallmann P, Godehardt E. Relevance of anti-Mullerian hormone measurement in a routine IVF program. Hum Reprod. 2008;23(6):1359–65. doi: 10.1093/humrep/den108. [DOI] [PubMed] [Google Scholar]
- 10.Hazout A, Bouchard P, Seifer DB, Aussage P, Junca AM, Cohen-Bacrie P. Serum antimullerian hormone/mullerian-inhibiting substance appears to be a more discriminatory marker of assisted reproductive technology outcome than follicle-stimulating hormone, inhibin B, or estradiol. Fertil Steril. 2004;82(5):1323–1329. doi: 10.1016/j.fertnstert.2004.03.061. [DOI] [PubMed] [Google Scholar]
- 11.Honnma H, Baba T, Sasaki M, Hashiba Y, Oguri H, Fukunaga T, Endo T, Asada Y. Serum anti-Mullerian hormone levels affect the rate of ongoing pregnancy after in vitro fertilization. Reprod Sci. 2013;20(1):51–9. doi: 10.1177/1933719112450329. [DOI] [PubMed] [Google Scholar]
- 12.Irez T, Ocal P, Guralp O, Cetin M, Aydogan B, Sahmay S. Different serum anti-Mullerian hormone concentrations are associated with oocyte quality, embryo development parameters and IVF-ICSI outcomes. Arch Gynecol Obstet. 2011;284(5):1295–1301. doi: 10.1007/s00404-011-1979-6. [DOI] [PubMed] [Google Scholar]
- 13.Jayaprakasan K, Campbell B, Hopkisson J, Johnson I, Raine-Fenning N. A prospective, comparative analysis of anti-Mullerian hormone, inhibin-B, and three-dimensional ultrasound determinants of ovarian reserve in the prediction of poor response to controlled ovarian stimulation. Fertil Steril. 2010;93(3):855–864. doi: 10.1016/j.fertnstert.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 14.Khader A, Lloyd SM, McConnachie A, Fleming R, Grisendi V, La Marca A, Nelson SM. External validation of anti-Mullerian hormone based prediction of live birth in assisted conception. J Ovarian Res. 2013;6(1):3. doi: 10.1186/1757-2215-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.La Marca A, Nelson SM, Sighinolfi G, Manno M, Baraldi E, Roli L, Xella S, Marsella T, Tagliasacchi D, D’Amico R, Volpe A. Anti-Mullerian hormone-based prediction model for a live birth in assisted reproduction. Reprod Biomed Online. 2011;22(4):341–9. doi: 10.1016/j.rbmo.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Lekamge DN, Barry M, Kolo M, Lane M, Gilchrist RB, Tremellen KP. Anti-Mullerian hormone as a predictor of IVF outcome. Reprod Biomed Online. 2007;14(5):602–610. doi: 10.1016/S1472-6483(10)61053-X. [DOI] [PubMed] [Google Scholar]
- 17.Liberty G, Ben-Chetrit A, Margalioth EJ, Hyman JH, Galoyan N, Eldar-Geva T. Does estrogen directly modulate anti-mullerian hormone secretion in women? Fertil Steril. 2010;94(6):2253–2256. doi: 10.1016/j.fertnstert.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 18.Lie Fong S, Baart EB, Martini E, Schipper I, Visser JA, Themmen AP, de Jong FH, Fauser BJ, Laven JS. Anti-Mullerian hormone: a marker for oocyte quantity, oocyte quality and embryo quality? Reprod Biomed Online. 2008;16(5):664–670. doi: 10.1016/S1472-6483(10)60480-4. [DOI] [PubMed] [Google Scholar]
- 19.Majumder K, Gelbaya TA, Laing I, Nardo LG. The use of anti-Mullerian hormone and antral follicle count to predict the potential of oocytes and embryos. Eur J Obstet Gynecol Reprod Biol. 2010;150(2):166–70. doi: 10.1016/j.ejogrb.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 20.Mashiach R, Amit A, Hasson J, Amzalzg S, Almog B, Ben-Yosef D, Lessing JB, Limor R, Azem F. Follicular fluid levels of anti-Mullerian hormone as a predictor of oocyte maturation, fertilization rate, and embryonic development in patients with polycystic ovary syndrome. Fertil Steril. 2010;93(7):2299–2302. doi: 10.1016/j.fertnstert.2009.01.125. [DOI] [PubMed] [Google Scholar]
- 21.McIlveen M, Skull JD, Ledger WL. Evaluation of the utility of multiple endocrine and ultrasound measures of ovarian reserve in the prediction of cycle cancellation in a high-risk IVF population. Hum Reprod. 2007;22(3):778–785. doi: 10.1093/humrep/del435. [DOI] [PubMed] [Google Scholar]
- 22.Moghadam KK, Nett R, Robins JC, Thomas MA, Awadalla SG, Scheiber MD, Williams DB. The motility of epididymal or testicular spermatozoa does not directly affect IVF/ICSI pregnancy outcomes. J Androl. 2005;26(5):619–23. doi: 10.2164/jandrol.05018. [DOI] [PubMed] [Google Scholar]
- 23.Mulders AG, Laven JS, Eijkemans MJ, de Jong FH, Themmen AP, Fauser BC. Changes in anti-Mullerian hormone serum concentrations over time suggest delayed ovarian ageing in normogonadotrophic anovulatory infertility. Hum Reprod. 2004;19(9):2036–42. doi: 10.1093/humrep/deh373. [DOI] [PubMed] [Google Scholar]
- 24.Nardo LG, Gelbaya TA, Wilkinson H, Roberts SA, Yates A, Pemberton P, Laing I. Circulating basal anti-Mullerian hormone levels as predictor of ovarian response in women undergoing ovarian stimulation for in vitro fertilization. Fertil Steril. 2009;92(5):1586–1593. doi: 10.1016/j.fertnstert.2008.08.127. [DOI] [PubMed] [Google Scholar]
- 25.Nelson SM, Yates RW, Fleming R. Serum anti-Mullerian hormone and FSH: prediction of live birth and extremes of response in stimulated cycles–implications for individualization of therapy. Hum Reprod. 2007;22(9):2414–21. doi: 10.1093/humrep/dem204. [DOI] [PubMed] [Google Scholar]
- 26.Penarrubia J, Fabregues F, Manau D, Creus M, Casals G, Casamitjana R, Carmona F, Vanrell JA, Balasch J. Basal and stimulation day 5 anti-Mullerian hormone serum concentrations as predictors of ovarian response and pregnancy in assisted reproductive technology cycles stimulated with gonadotropin-releasing hormone agonist--gonadotropin treatmen. Hum Reprod. 2005;20(4):915–922. doi: 10.1093/humrep/deh718. [DOI] [PubMed] [Google Scholar]
- 27.Silberstein T, MacLaughlin DT, Shai I, Trimarchi JR, Lambert-Messerlian G, Seifer DB, Keefe DL, Blazar AS. Mullerian inhibiting substance levels at the time of HCG administration in IVF cycles predict both ovarian reserve and embryo morphology. Hum Reprod. 2006;21(1):159–63. doi: 10.1093/humrep/dei270. [DOI] [PubMed] [Google Scholar]
- 28.Sills ES, Collins GS, Brady AC, Walsh DJ, Marron KD, Peck AC, Walsh AP, Salem RD. Bivariate analysis of basal serum anti-Mullerian hormone measurements and human blastocyst development after IVF. Reprod Biol Endocrinol. 2011;9:153. doi: 10.1186/1477-7827-9-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smeenk JM, Sweep FC, Zielhuis GA, Kremer JA, Thomas CM, Braat DD. Antimullerian hormone predicts ovarian responsiveness, but not embryo quality or pregnancy, after in vitro fertilization or intracyoplasmic sperm injection. Fertil Steril. 2007;87(1):223–226. doi: 10.1016/j.fertnstert.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi C, Fujito A, Kazuka M, Sugiyama R, Ito H, Isaka K. Anti-Mullerian hormone substance from follicular fluid is positively associated with success in oocyte fertilization during in vitro fertilization. Fertil Steril. 2008;89(3):586–91. doi: 10.1016/j.fertnstert.2007.03.080. [DOI] [PubMed] [Google Scholar]
- 31.Wunder DM, Guibourdenche J, Birkhauser MH, Bersinger NA. Anti-Mullerian hormone and inhibin B as predictors of pregnancy after treatment by in vitro fertilization/intracytoplasmic sperm injection. Fertil Steril. 2008;90(6):2203–2210. doi: 10.1016/j.fertnstert.2007.10.078. [DOI] [PubMed] [Google Scholar]
- 32.Yilmaz N, Uygur D, Dogan M, Ozgu E, Salman B, Mollamahmutoglu L. The effect of follicular antimullerian hormone levels of non-obese, non-hyperandrogenemic polycystic ovary syndrome patients on assisted reproduction outcome. Gynecol Endocrinol. 2012;28(3):162–165. doi: 10.3109/09513590.2011.593667. [DOI] [PubMed] [Google Scholar]