Abstract
Introduction
In order to investigate the dynamics of genomic alterations that occur at different developmental stages in vitro, we examined the chromosome content of human preimplantation embryos by molecular-cytogenetic techniques at the single-cell level, up to 13 days post fertilization.
Methods
The embryos were genetically analyzed several times during their development in culture; each embryo was first analyzed by FISH at ‘Day 3’ post fertilization, than during its growth in vitro and the third analysis was performed at development arrest, then the entire blastocyst was analyzed by comparative genomic hybridization (CGH/aCGH).
Results
We found that while on ‘Day 3’ only 31 % of the embryos were detected as normal, on ‘Day 5–6’, 44 % of the embryos were classified as normal and on ‘Day 7’, 57 % were normal. On ‘Days 8–13’, 52 % of the embryos were classified as chromosomally normal. One third of the embryos that were chromosomally abnormal on ‘Day 3’, were found to be normal at development arrest point.
Discussion
These dynamic changes that occur at early developmental stages suggest that testing a single blastomere at ‘Day 3’ post fertilization for PGD might inaccurately reflect the embryo ploidy and increase the risk of false aneuploidy diagnosis. Alternatively, blastocyst stage diagnosis may be more appropriate.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-013-9988-y) contains supplementary material, which is available to authorized users.
Keywords: Preimplantation embryos, Aneuploidy, Mosaicism, Normalization, FISH, CGH, Array CGH
Introduction
Chromosomal abnormalities of preimplantation embryos, mainly until the 8-cell stage, have been the subject of cytogenetic study for a long time. Molecular cytogenetic analysis of interphase nuclei by fluorescent in situ hybridization (FISH) has shown that a large number (35–70 %) of human embryos exhibit chromosomal abnormalities and mosaicism in vitro at these early developmental stages [4, 50]. However, FISH has technical limitations which might influence the interpretations of the findings. The adaptation of comparative genomic hybridization (CGH) to single cells has allowed the study of the full karyotype of blastomeres [48, 49], thus identifying the true level of aneuploidy. In that spirit, SNP array analysis, conducted on day 4 embryos, showed that up to 91 % of preimplantation embryos have mosaicism [47].
These abnormalities may arise from an error during meiosis, resulting in a uniform abnormality present in all cells, or occur during the first three mitotic divisions. These first three mitotic divisions are believed to be without full cell cycle control, leading to chromosomal mosaicism, which defined as the presence of two or more karyotypically distinct cell lines within the same embryo [10, 26, 37].
In spite of the high frequency observed in preimplantation embryos, a low percentage of aneuploidy is found in recognized pregnancies (4 %) and at term birth (0.3 %) [20]. Therefore, it seems that the majority of mosaic embryos disappear, due to either a selection against mosaic embryos (hence, developmental arrest), or to selection against abnormal cells within the embryo (hence, ‘normalization’) [32].
Studies conducted in recent years examined the chromosomal content of cleavage stage embryos in order to decipher the fate of mosaic embryos. Most of the researchers used FISH techniques and found a decrease in the incidence of mosaicism and aneuploidy between day 3 and day 6 preimplantation embryos [5, 30]. However, FISH has technical limitations, and only a limited part of the genome (which is homologues to the probes regions) can be analyzed this way.
Fargouli et al. [15] combined FISH and CGH techniques and found a decrease in the aneuploidy rate in day 5–6 blastocysts. Their conclusion was, that the blastocyst stage does not represent an absolute selective barrier, leading to the possibility that chromosomal changes can occur after this point.
There is limited data on the cytogenetic condition of embryos after day 6; there are only few published papers that studied embryos after day 6, all of which used only FISH; Santos et al. [43] have recently studied day 4, 5 and 8 embryos and Munne’ et al. [38] examined self correction of day 12 embryos. These studies reached different conclusions and proposed arrested development and chromosome self-normalization, respectively, as the mechanism in embryo development.
In order to better understand the genomic variation and development, it is essential to study the genomic content of the embryos for a longer period and at later stages, especially with the use of full karyotype techniques which are available today.
The aim of this study was to follow the embryo development from a molecular cytogenetic point of view, and to test whether the chromosomal status is consistent through its development, and if changes do occur, to examine at what developmental stage they appear. In order to address genomic variation occurring at later developmental stages, we surveyed aneuploidy/polyploidy in the human preimplantation embryo by FISH at the single-cell level and aneuploidy at the blastocyst level by CGH and microarray CGH (aCGH).
Here we report a detailed examination of one hundred human embryos that were genetically analyzed several times during their development in culture. Information obtained from studying preimplantation embryos may change the current approach in clinical assessments of preimplantation diagnosis.
Materials and methods
Donated embryos
Embryos were donated by 22 couples undergoing IVF + PGD (nineteen translocation carriers, three recurrent abortions), at the In-Vitro Fertilization (IVF) unit in Sheba Medical Center. The women ranged in age from 25 to 42 years (mean ± SD of 34 ± 4.1 years). A total of 100 embryos found to be abnormal by FISH-PGD according to signal scoring criteria (see FISH section), were donated. The use of these embryos for this study was approved by the ethics committee for genetic testing in Israel and all couples signed a written informed consent.
Ovarian stimulation, oocyte retrieval and IVF procedures, including assessment of embryo morphology, were performed as previously described [21], without special modifications for this study.
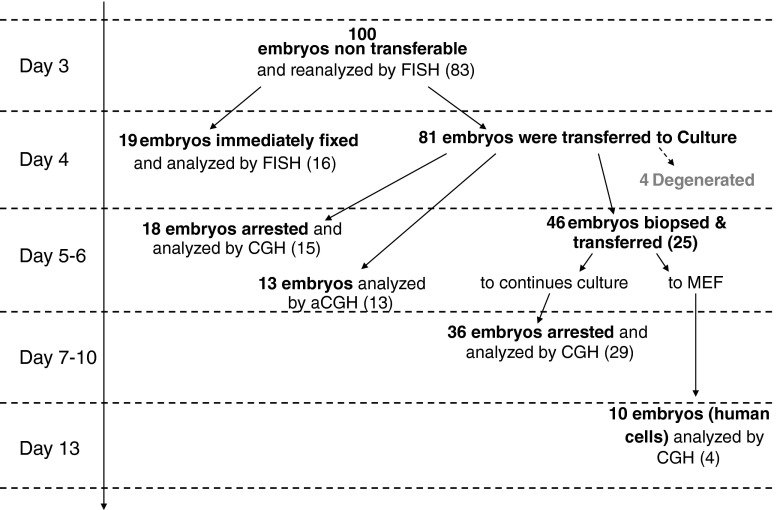
Each embryo was first analyzed by FISH at ‘Day 3’ post fertilization, again through its growth in vitro (not all embryos were sampled at all time points) and last analysis was performed at developmental arrest, at which time the entire blastocyst was analyzed by CGH. Figure 1 summarizes the experimental design.
Fig. 1.
Flow diagram summarizing the experimental design. Each embryo was first analyzed by FISH at ‘Day 3’ post fertilization, again through its growth in vitro (not all embryos were sampled at all time points) and last analysis was performed at developmental arrest. Numbers in brackets represent embryos with complete results
During the course of this study, we studied the aneuploidy rates of 100 human embryos at different stages of early development using FISH and CGH.
The embryos were reanalyzed for aneuploidy using FISH probes on the same cell used for IVF-PGD “‘Day 3’ embryos”.
- The embryos were divided into two groups -
- Dismantled and immediately fixed ‘Day 4’ embryos (19 embryos) – all cells examined by FISH.
- Cultured embryos (81 embryos) - tested by FISH during development and tested by CGH at developmental arrest.
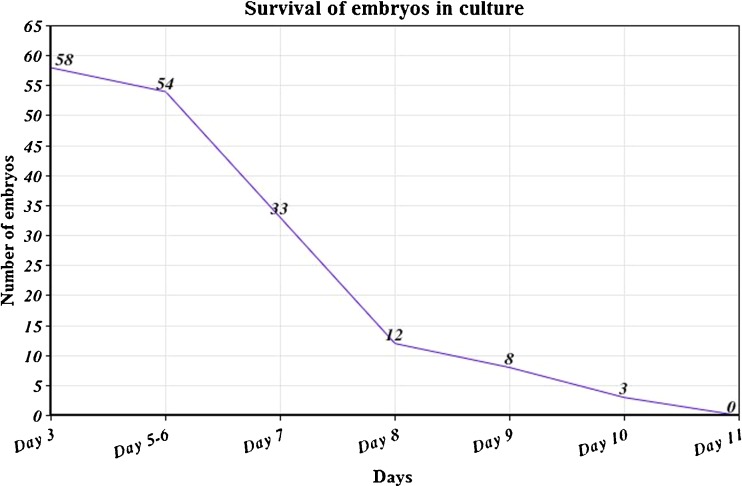
Ten embryos were transferred to MEFs for further growth; thirteen embryos were collected at ‘Day 6’ and examined by aCGH. The rest (n = 58 embryos), were cultured in sub-sequetial medium. Figure 2 summarizes the survival rate of these embryos in culture.
Fig. 2.
Summary of the survival of embryos in culture (by days)
‘Day 3’ embryos
‘Day 3’ (post fertilization) embryos underwent blastomere biopsy using a micromanipulation system (Narashige, Japan) fitted on an inverted microscope (Diaphot 300, Nikon, Japan). A laser system (ZILOS-tk, Hamilton Thorne) was used for dissection of the zona pellucida prior to biopsy. A single blastomere was removed from each embryo. After the manipulation, embryos were returned to the culture media. The biopsied blastomeres were fixed on a glass slide using 3:1 Acetic acid-Methanol solution (Merck KGaA, Darmstadt, Germany) and were used for the IVF-PGD analysis. After this analysis, the slides were washed and analyzed with the research probes, for ‘Day 3’ analysis (FISH section).
‘Day 4’ embryos
Out of the 100 embryos donated for this study (found to be un-transferable by FISH-PGD), 19 ‘Day 4’ embryos were dismantled and immediately fixed on slides (for fixation of whole embryos on slides see section “Fixation of blastomeres and embryos”). The number of blastomeres per embryo was counted, and all nuclei were analyzed by FISH. The remaining embryos (n = 81), were cultured for further cytogenetic analysis. All embryos were grown in standard embryo culture condition that has been described previously [21].
Embryo culture
On day 5–6, 18 embryos have self-arrested and were collected to PCR tubes for CGH analysis. Four embryos degenerated in this process, could not be collected and were not analyzed. Embryos that were still developing were biopsied on ‘Day 5–6’. One to two cells per embryo were fixed on slides for FISH analysis using the same procedure described for ‘Day 3’ embryo’s biopsy.
After biopsy, these developing embryos were cultured as followed:
36 embryos were returned to the culture until self-arrest (days 7–10), then they were collected and analyzed by CGH.
13 embryos were collected and analyzed by microarray CGH (aCGH) in order to compare between CGH and aCGH.
10 randomly selected embryos were plated onto mouse embryonic fibroblast cells previously mitotically inactivated by mitomycin C on gelatin-coated tissue culture dishes. The embryos were cultures in this system until day 13, and then collected and analyzed by CGH.
Preparation of cells for FISH and CGH
Fixation of blastomeres and embryos
Nuclei were fixed on slides using 3:1 Acetic acid-Methanol solution (Merck KGaA, Darmstadt, Germany). The fixed nuclei were prepared for FISH by dehydration in fresh 70 %, 80 %, and 100 % ethanol (BioLab ltd., Jerusalem, Israel) for 2 min each at −20 °C.
PCR and whole genome amplification
Blastocysts were aspirated into a polymerase chain reaction (PCR) tube for genomic DNA amplification:
For the purpose of CGH analysis: PCR tubes contained 3.5 μL PBSx1 (Repli-g midi kit, QIAGEN GmbH, Hilden, Germany). Isothermal DNA amplification method with 29 DNA polymerase was used (Repli-g midi kit, QIAGEN GmbH, Hilden, Germany) as described in the manufacturers’ manual. The isothermal amplification was performed at 30 °C for 16 h and the reaction was stopped upon incubation at 65 °C for 3 min.
For the purpose of aCGH analysis: PCR tubes contained 2.5 μL PBSx1 (Rubicon Genomics, Inc. Ann Arbor, MI, USA).
Rubicon PicoPlexTM WGA method was used. Thermal cycling library preparation followed by universal-primer PCR, with redundant utilization of template molecules. To determine the success of the amplification, 5 μl of the products were analyzed on a 2 % agarose gel.
Fluorescent in situ hybridization (FISH) and interpretation
FISH analysis was performed following the manufacturer’s instructions and was described previously [13]. FISH analysis took place using three sequential hybridizations. Cycle 1: Specific probes for chromosomes 12, 16, 17 labeled with three different fluorochromes; Cycle 2: Specific probes for chromosomes 18, X, Y labeled with three different fluorochromes; Cycle 3: Specific probes for chromosomes 13, 21 labeled with two different fluorochromes. All probes used during this study were commercial probes, used also for clinical purposes (Abbott Molecular, AbbotPark, IL, USA) and summarized in Table 1. The protocol used was previously described [3].
Table 1.
FISH probes used in this study. All probes came from Abbott (see Fluorescent in-situ hybridization section)
| Chromosome | Locus | Probe name |
|---|---|---|
| 12 | 12p11.1–q11 | CEP 12 (D12Z3) |
| 13 | 13q14 | LSI 13 |
| 16 | 16q11.2 | CEP 16 (D16Z3) |
| 17 | 17p11.1–q11.1 | CEP 17 (D1721) |
| 18 | 18p11.1–q11.1 | CEP 18 (D18Z1) |
| 21 | 21q22.13–22.2 | LSI 21 () |
| X | Xp11.1–q11.1 | CEP X (DXZ1) |
| Y | Yp11.1–q11.1 | CEP Y (DYZ3) |
Signal scoring was performed according to stringent criteria: blastomeres were scored as “normal status” if FISH clearly indicated two separate signals for each probe, while unbalanced blastomere showed deviation from the ‘normal’ signal pattern [36]. Two signals represent two homolog chromosomes when their distance apart was at least two domain diameters [39]. Two signals that are less than two domains apart are considered as one duplicated signal and represent a single homolog chromosome. Chromosomes involved in the translocation were naturally excluded from this embryo’ aneuploidy screening.
We classified blastomeres as normal (nuclei showing the normal amount of signals for the chromosomes investigated), or abnormal.
All nuclei from ‘Day 4’ embryos were immediately fixed and analyzed by FISH. To distinguish between true aneuploidy and FISH artifact, embryos were classified as normal if >90 % of nuclei exhibited normal number of signals. Embryos were classified as aneuploid if >90 % of nuclei showed the same abnormality. Embryos were classified as mosaic, if they had cells with either a normal or an abnormal chromosomal constitution, and in which 10 to 90 % of the cells showed the same chromosomal abnormality. When almost all the cells showed different and complex chromosomal abnormalities they were classified as “Chaotic embryos”.
Comparative Genomic Hybridization (CGH), image analysis and interpretation
The CGH protocol employed for the analysis of the blastocysts has been validated and described in detail previously [27]. As reference DNA, isolated amniotic cells from normal pregnancies were used (tested by g-banding to have 46, XY karyotype). These cells underwent the same amplification as used for the blastocystes. Test and reference DNAs were labeled by nick translation, according to the manufacturer’s instructions (Nick translation kit; Abbott). Co-precipitation of test and reference DNAs, their denaturation, along with that of the slides, and the post-hybridization washes all were conducted as described previously [23]. Digital image analysis was used to facilitate the identification of chromosomal regions with abnormal fluorescence ratios. Images of the hybridized metaphases were evaluated as previously published [23], with a detection resolution ≥5 Mb [28].
Microarray CGH (aCGH)
Amplification products were processed according to the BlueGnome 24sure + protocol (available at www.cytochip.com) and that was previously described [14].
Statistical analysis
Since not all embryos were tested for equal number of probes (due to chromosomes involved in translocations), spearman correlation test was applied to compare samples with different number of variables.
All statistic analyses were done using SPSS, with p-value ≤ 0.05 considered significant.
Results
FISH results
Reanalysis of ‘Day 3’ embryos
A total of 100 blastomeres, representing 100 ‘Day 3’ embryos, were reanalyzed after PGD results were obtained. These embryos were found not suitable for transfer according to their PGD indication. Cells were analyzed in two-three sequential sets of FISH probes, therefore in some cases the fluorescent signal diminished and the cell couldn’t be analyzed. Complete FISH results were obtained for 83 embryos (83 %).
The results demonstrated that 27 embryos were normal (32.5 %), 11 were trisomic (13.2 %), 20 were monosomic (24.1 %), 19 embryos (22.9 %) had aneuploidy in more than one chromosome, 5 embryos (6.0 %) had abnormality in the sex chromosomes and one (1.2 %) was haploid [data presented in Tables 2 and 3].
Table 2.
FISH results of ‘Day 4’ embryos, in comparison to their ‘Day 3’ results. Aneuploidy screening was done with the use of probes for chromosomes 12, 13, 16, 17, 18, 21, X and Y. In brackets noted the percent of abnormal cells in the tested embryo
| Embryo no. | PGD indication | ‘Day 3’ FISH results for aneuploidy screening* one cell analysis | Day 4 FISH analysis-Analysis of the entire embryo | |
|---|---|---|---|---|
| No. cells analyzed | FISH interpretation (%abnormal cells) | |||
| 1 | PGS | 2n | 15 | Mosaic, XX (66 %) |
| 2 | PGS | 2n | 22 | Mosaic, XY (59 %) |
| 3 | PGS | 1x13q14, 1xCEP 18 | 4 | 46,XX (0 %) |
| 4 | PGS | 2n | 9 | Mosaic, XX (55 %) |
| 5 | PGS | 2xCEP X, 1xCEP Y | 21 | Abnormal (91 %) |
| 6 | PGS | 1x21q22 | 5 | 46, XX (0 %) |
| 7 | PGS | 1xCEP 18 | 9 | Mosaic, XX (55 %) |
| 8 | t(13;14) | 1xCEP 16 | 13 | Mosaic, XY (46 %) |
| 9 | t(13;14) | 2n | 11 | 46, XY (9 %) |
| 10 | t(13;14) | 1xCEP 18 | ✘ | NR |
| 11 | t(13;14) | 1xCEP 18 | ✘ | NR |
| 12 | t(13;14) | 3xCEP 17 | 6 | Mosaic, XX (33 %) |
| 13 | t(13;14) | 3xCEP X | 15 | Mosaic, XX (13 %) |
| 14 | t(13;14) | 1xCEP 18 | ✘ | NR |
| 15 | t(15;21) | 2n | 7 | Mosaic, XY (43 %) |
| 16 | t(15;21) | 1xCEP 16, 1xCEP 17 | 20 | Mosaic, XY (15 %) |
| 17 | t(15;21) | 3x13q14 | 13 | Abnormal, XX, 3*13 (100 %) |
| 18 | PGS | 3xCEP X, 3x21q22 | 10 | Mosaic, XX (20 %) |
| 19 | PGS | 2xCEP X,1xCEP Y,1x13q14 | 14 | Chaotic + (X, 18, 13, 21), (81 %) |
*When PGD indication involved one of the research probes, this probe was not tested for aneuploidy. In the results column, only unbalanced chromosomes are mentioned
2n – normal diploid for the tested chromosomes;
CEP – centromeric probe; NR- no results
Table 3.
CGH/aCGH results of ‘development arrest’ embryos, in comparison to their aneuploidy screening. Aneuploidy screening was done based on one cell analysis at ‘Day 3’ and at ‘Day 5/6’ with the use of probes for chromosomes 12, 13, 16, 17, 18, 21, X and Y
| Embryo no. | PGD indication | FISH results for aneuploidy screening* | CGH/aCGH results at development arrest | ||
|---|---|---|---|---|---|
| ‘Day 3’ | ‘Day 5/6’ | Day of self-arrest | Interpretation* | ||
| 20 | t(6;21) | NR | 1xCEP 16 | Day 13 | ✘ |
| 21 | t(6;21) | NR | mosaic | Day 13 | ✘ |
| 22 | t(6;21) | NR | ✘ | Day 6 | 46,XX |
| 23 | t(6;21) | NR | NR | Degenerated | ✘ |
| 24 | t(6;21) | NR | 3xCEP 12 | Day 13 | ✘ |
| 25 | t(6;21) | NR | 2n | Day 13 | ✘ |
| 26 | t(6;21) | 3xCEP 18 | ✘ | Day 6 | 2n |
| 27 | t(6;21) | 1xCEP 18, 3xCEP X | NR | Day 13 | Ish cgh dim(2)(q22qter) |
| 28 | PGS | 3x13q14 | ✘ | Day 6 | Ish cgh dim(X) |
| 29 | PGS | 2xCEP Y | ✘ | Day 6 | Ish cgh dim(22)(p13p11) |
| 30 | t(6;11) | 2n | ✘ | Day 6 | ish cgh enh(7), enh(8), amp(11)(q14q22) |
| 31 | t(6;11) | 3x21q22 | ✘ | Day 6 | 46,XY |
| 32 | t(6;21) | 1xCEP 17 | ✘ | Day 6 | 46,XY |
| 33 | t(6;21) | 2n | 2n | Day 13 | ✘ |
| 34 | t(6;21) | 3xCEP 17 | ✘ | Day 6 | 46,XY |
| 35 | t(6;21) | 2n | ✘ | Day 6 | 46,XY |
| 36 | t(6;11) | 1xCEP 16 | mosaic | Day 9 | 46,XX |
| 37 | t(6;11) | 3x21q22 | 3x21q22 | Day 9 | Ish cgh enh(21) |
| 38 | t(6;11) | 1xCEP 12 | 2n | Day 9 | 46,XY |
| 39 | PGS | 1xCEP 18, 1x21q22 | ✘ | Day 6 | ish cgh amp(1)(p36-p33), dim(6) |
| 40 | PGS | 3x21q22 | ✘ | Day 6 | Ish cgh enh(8)(q248qter) |
| 41 | t(6;21) | NR | ✘ | Day 6 | Ish cgh enh(15), enh(16) |
| 42 | t(6;21) | 1xCEP 12 | ✘ | Day 6 | 46,XY |
| 43 | t(6;21) | 2n | ✘ | Day 6 | Ish cgh enh(1) |
| 44 | t(6;21) | 2n | ✘ | Day 6 | 46,XY |
| 45 | t(6;11) | 2n | 2n | Day 9 | 46,XY |
| 46 | t(6;11) | 2n | 1xCEP 18 | Day 9 | 46,XX |
| 47 | t(13;14) | 1xCEP 12 | 2n | Day 10 | 46,XY |
| 48 | t(13;14) | 2n | 2n | Day 10 | Ish cgh amp(9)(q22q33) |
| 49 | t(7;X) | 3x13q14 | NR | Degenerated | ✘ |
| 50 | t(6;11) | 2n | 2n | Day 9 | 46,XY |
| 51 | t(6;11) | 1xCEP 16, 3x13q14, 1x21q22 | NR | Day 7 | ish cgh amp(2)(q22q32), amp(3)(q12q29), enh(4), enh(13), dim(15), dim(19) |
| 52 | t(10;22) | 2n | NR | Day 7 | 46,XX |
| 53 | t(10;22) | NR | NR | Day 7 | 46,XX |
| 54 | t(13;15) | 2xCEP X, 1xCEP Y | ✘ | Day 6 | ✘ |
| 55 | t(13;15) | 2n | ✘ | Day 6 | ✘ |
| 56 | t(13;15) | 3xCEP 16 | 2n | Day 13 | 46,XX |
| 57 | t(13;14) | 1xCEP 16 | chaos | Day 13 | ish cgh dim(1), dim(16), dim(19), dim(22) |
| 58 | t(11;22) | 1x13q14 | 1x13q14 | Day 13 | 46,XX |
| 59 | t(11;22) | 3x13q14, 1xCEP 17, 1x21q22 | NR | Day 7 | ish cgh enh(1), enh(19), dim(22)(q12qter) |
| 60 | t(11;22) | 3xCEP X | mosaic | Day 13 | Ish cgh enh(X) |
| 61 | t(11;22) | 1xCEP 18 | NR | Day 7 | ✘ |
| 62 | t(11;22) | 2n | ✘ | Day 6 | ✘ |
| 63 | t(13;15) | 1xCEP 12, 4xCEP 17 | NR | Day 7 | ✘ |
| 64 | t(11;22) | 1x21q22 | NR | Day 7 | 46,XY |
| 65 | t(11;22) | 2n | NR | Day 7 | Ish cgh dim(19) |
| 66 | t(13;14) | 1xCEP (16,17) | 3xCEP 16 | Day 8 | ish cgh amp(1)( p36-p33), dim(2)(q22q32), amp(3)(p26p23), enh(16), enh(19), dim(22) |
| 67 | t(13;14) | 3xCEP 16, 1xCEP 17 | chaos | Day 8 | ish cgh enh(1), dim(2), amp(6)(p23p21), enh(11), enh(16), enh(19) |
| 68 | t(13;18) | 1xCEP (12,17) | 2n | Day 7 | Ish cgh amp(9)(q22q33) |
| 69 | t(13;18) | 1XCEP(12,16,17) | 2n | Day 7 | 46,XY |
| 70 | t(2;5) | 1x13q14, 3xCEP 17 | 1xq1413, 1x21q22 | Day 9 | ish cgh amp(8)(q22qter), dim(13), enh(14), enh(17) |
| 71 | t(2;5) | 2n | NR | Degenerated | ✘ |
| 72 | t(2;5) | NR | NR | Degenerated | ✘ |
| 73 | t(2;5) | 2n | NR | Day 9 | ✘ |
| 74 | t(2;12) | 1x21q22 | NR | Day 7 | 46,XY |
| 75 | t(2;12) | 2n | NR | Day 7 | 46,XX |
| 76 | t(2;12) | NR | NR | Day 7 | ✘ |
| 77 | t(2;12) | NR | NR | Day 7 | ✘ |
| 78 | t(2;12) | 1xCEP 18 | NR | Day 7 | ✘ |
| 79 | t(2;12) | 2n | NR | Day 7 | ✘ |
| 80 | t(2;12) | 2n | NR | Day 7 | ✘ |
| 81 | t(13;14) | 2xCEP X, 1xCEP Y, 3x13q14 | 2n | Day 8 | 46,XY |
| 82 | t(13;14) | 3xCEP 17, 1xCEP 18 | 1xCEP 18 | Day 8 | ish cgh amp(2)(p16p14), enh(7), enh(17), dim(18), enh(22) |
| 83 | t(13;14) | 1x21q22 | 1x21q22 | Day 10 | 46,XX |
| 84 | t(5;6) | NR | NR | Day 7 | 46,XX |
| 85 | t(5;6) | NR | NR | Day 6 | ish cgh enh(17), enh(19),enh(22) |
| 86 | t(5;6) | NR | 1x21q22 | Day 7 | ish cgh dim(19), dim(21), |
| 87 | t(10;15) | 2n | NR | Day 7 | 46,XX |
| 88 | t(10;18) | 3x21q22 | NR | Day 6 | 46.XY |
| 89 | t(10;18) | 1xCEP 17 | NR | Day 6 | arr 2x1, arr 9x3, arr 17x1 |
| 90 | t(10;18) | 1xCEP)16, 17) | NR | Day 6 | arr 16 x1, arr 22 x1 |
| 91 | t(6;9) | 1xCEP 16 | NR | Day 6 | arr 19 x1 |
| 92 | t(6;9) | 2n | NR | Day 6 | arr(1-22,X)x2 |
| 93 | t(6;9) | 2n | NR | Day 6 | arr(1-22,X)x2 |
| 94 | t(6;9) | NR | NR | Day 6 | arr22 x1 |
| 95 | t(6;9) | NR | NR | Day 6 | arr(1-22,X)x2 |
| 96 | t(10;22) | 2n | NR | Day 6 | arr(1-22)x2,(XY)x1 |
| 97 | t(10;22) | 3x21q22, 1xCEP 17 | NR | Day 6 | arr 21q11.2q22.3 x3 |
| 98 | t(10;22) | 1x(All) | NR | Day 6 | arr 14 x1 |
| 99 | t(10;22) | NR | NR | Day 6 | arr(1-22)x2,(XY)x1 |
| 100 | t(10;22) | 1xCEP 21 | NR | Day 6 | arr(1-22)x2,(XY)x1 |
*When PGD indication involved one of the research probes, this probe was not tested for aneuploidy. In the results column, only unbalanced chromosomes are mentioned
2n – normal diploid for the tested chromosomes;
CEP – centromeric probe; NR- no results
As presented in Table 2 and Table 3, not all embryos were tested for the same number of probes (due to their specific chromosomes involved in the translocations). Therefore, spearman correlation test was applied and verified that the number of probes analyzed didn’t have an effect on the aneuploidy rate in that embryo (r = 0.2).
Analysis of ‘Day 4’ embryos (mosaic screening)
In order to assess the chromosomal constitution, aneuploidy rate and incidence of mosaicism of preimplantation embryos, ‘Day 4’ embryos (n = 19) were dismantled and fixed on slides. Full analysis was obtained for 16 ‘Day 4’ embryos, with a total of 194 blastomeres that were studied (Table 2). An average of 12.1 cells was analyzed for each embryo. Results are summarized in Table 2. Of the 16 embryos successfully analyzed by FISH, three (18.8 %) were composed of normal cells, ten embryos (62.5 %) were classified as mosaic, two embryos were composed only from abnormal cells (12.5 %) and one was chaotic (6.2 %). The number of analyzed cells was variable between the embryos but did not have significant statistical difference. For embryos classified as mosaic, the average percentage of chromosomally abnormal cells (per embryo) was 40 % (range 13–66 %).
Analysis of continuous developing embryos
Forty six embryos have continued to develop in culture beyond ‘Day 5/6’; In order to assess their chromosomal constitution, they underwent biopsy of 1–2 cells at ‘Day 5/6’ and afterwards were returned to a culture medium until self-arrest. FISH was applied with eight probes and full results at this stage were obtained for 54.3 % of the embryos (n = 25). 44 % of them were euploid for all tested chromosomes, 12 % were trisomic and 20 % were monosomic. Thirteen embryos had FISH results for more than one cell, 38.4 % of them showed mosaic aneuploidy.
CGH results
Analysis of ‘Day 5/6’ embryos by chromosomal and microarray CGH
A total of 31 embryos were collected at ‘Day 5/6’ and analyzed, either by CGH or microarray CGH (eighteen embryos reached developmental arrest at ‘Day 5/6’ and were analyzed by CGH; thirteen were collected at ‘Day 6’ and analyzed by aCGH).
Results were obtained for 28 embryos (90.3 %) and are summarized in Table 4. Overall, 53.6 % of the embryos studied at ‘Day 5/6’ were found to be euploid. No statistically significant difference was found between the group of embryos studied by conventional CGH and the group of embryos studied by array (P > 0.05), indicating no differences between these two methods.
Table 4.
Analysis results of ‘Day 5/6’ embryos by CGH and microarray CGH. Embryos were divided to three groups according to their results: (a) normal euploid embryos; (b) embryos carrying single chromosomal aberration; (c) embryos carrying more than one aberration
| Analysis Method | Number of analyzed embryos | Number of embryos with full results | ‘Day 5/6’ results | ||
|---|---|---|---|---|---|
| Normal euploid | Abnormal | ||||
| Single chromosomal aberration | Chromosomal aberration > 1 | ||||
| CGH | 18 | 15 | 53.3 % (8) | 33.3 % (5) | 13.3 % (2) |
| Array CGH | 13 | 13 | 53.8 % (7) | 30.8 % (4) | 15.4 % (2) |
| Total | 31 | 28 | 53.6 % | 32.1 % | 14.3 % |
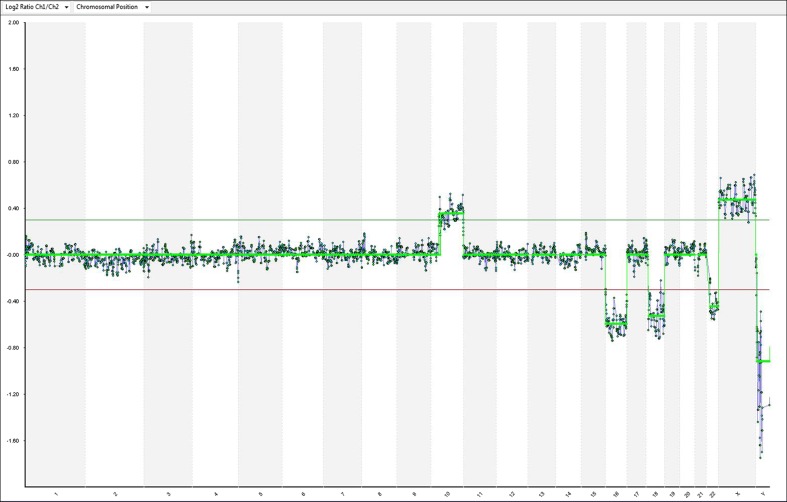
Microarray results of all 13 embryos are presented as ideograms in supplementary data (Supp. 1). Figure 3 is an example for an abnormal array CGH result.
Fig. 3.
Example of array-CGH result from a ‘Day 6’ embryo [sample number 90, Table 3, t (10;18)]. In addition to the unbalanced translocation, the microarray analysis also shows a single copy loss of the entire chromosomes 16 and 22
Analysis of ‘Days 7–13’ embryos
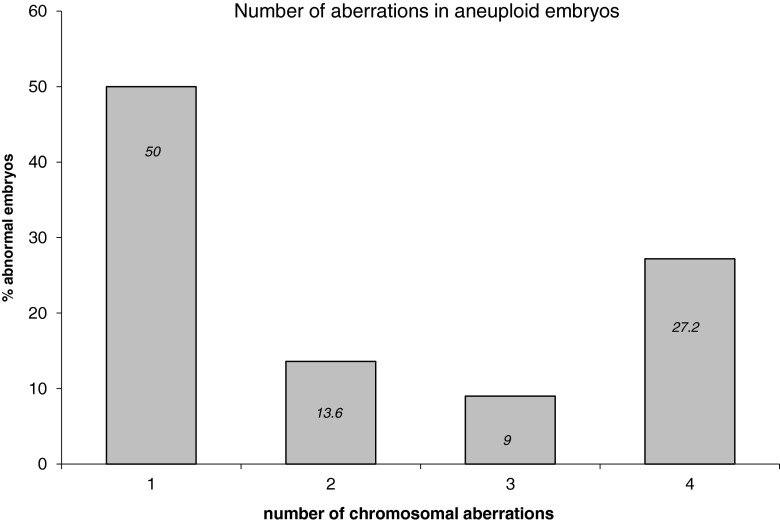
Each embryo that reached developmental arrest in culture was collected and analyzed by chromosomal CGH. Results were obtained for 33 embryos (71.7 %) and are summarized in Table 3. 14 ‘Day 7’ arrested embryos were collected and eight of them (57.1 %) were found to be euploid. 14 embryos that were arrested between ‘Day 8’ and ‘Day 10’ were collected, eight of them (57.1 %) were found to be euploid. Five embryos were collected at ‘Day 13’ (which were grown on MEFs), two (40 %) were normal. All other embryos exhibited stochastic aneuploidies as detailed in Table 3. Changes were observed in almost all chromosomes. Figure 4 exhibits the frequency of each chromosome involvement in the aberrations (aCGH and chromosomal CGH). The median of chromosomes involved in an aneuploid embryo is one (range 1–6), meaning that 50 % of the aneuploid embryos had a single chromosomal aberration (Fig. 5).
Fig. 4.
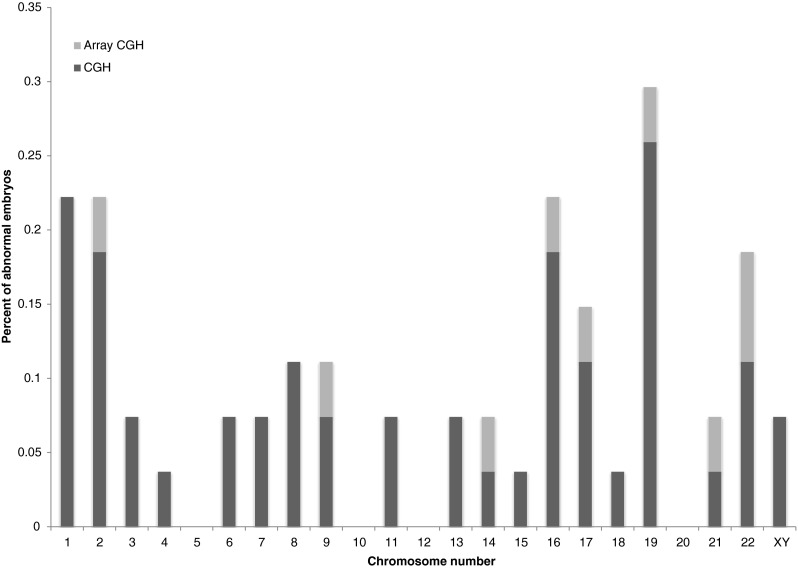
The relative contribution of each chromosome in the aberrant embryos
Fig. 5.
Embryos classified as abnormal at development arrest: percentage of embryos harboring one, two, three and four or more aberrations
Comparison between ‘Day 3’ diagnosis (based on a single cell) and ‘Day 4’ diagnosis (based on findings of the entire embryos)
The goal of this comparison was to determine the prediction rate of ‘Day 3’ PGS analysis (that is based on one cell diagnosis) on the entire embryo status. Since ‘Day 3’ analysis is based on one cell diagnosis, there are only two options for classification – either the embryo is classified as normal or abnormal. At ‘Day 4’ analysis there are three options for classification of the embryo – normal, abnormal and mosaic. Prediction was considered as “true” when either both results were normal or abnormal, or when ‘Day 3’ result was abnormal and ‘Day 4’ result was mosaic (since it also classifies the embryo as abnormal).
Sixteen embryos had both ‘Day 3’ (one cell analysis) and ‘Day 4’ results (average of 12.1 analyzed cells per embryo). Table 5 exhibits the comparison of the results for each embryo; overall, ‘Day 3’ one cell FISH diagnosis predicted correctly (“true”) only 62.5 % of the cases. According to these findings, there is a 20 % chance for biopsy of diploid cell while the embryo is mosaic and a 43 % chance for biopsy of aneuploid cell when the embryo is in fact mosaic.
Table 5.
Prediction rate of ‘Day 3’ PGS analysis (based on one cell diagnosis) on the entire embryo status at ‘Day 4’. Prediction was considered as “true” when either both results were normal or abnormal, or when ‘Day 3’ result was abnormal and ‘Day 4’ result was mosaic (since it also classifies the embryo as abnormal). “True” predictions are marked in the highlighted squares
| ‘Day 3’ diagnosis (Single cell analysis) | ‘Day 4’ diagnosis (Average of 12.1 cells) | ||
|---|---|---|---|
| 2n | Mosaic | Abnormal | |
| 2n | 6.3 % | 25 % | 0 % |
| Abnormal | 12.5 % | 37.5 % | 18.7 % |
Comparison between genetic status of ‘Day 5/6’ embryos analyzed by CGH/aCGH, and their ‘Day 3’ results
24 Embryos had both FISH results at ‘Day 3’ and CGH/aCGH results at ‘Day 5/6’. Comparison between results shows that out of the 24 embryos with both analyses, ten were abnormal at both stages (42 %, some gained other changes that weren’t detected by FISH), five were normal at both analyses (21 %) and seven embryos (29 %) were found to be aneuploid at ‘Day 3’ analysis and euploid at ‘Day 6’ analysis.
Comparison between genetic status of ‘Days 7–13’ embryos, analyzed by CGH, their ‘Day 3’ and ‘Day 5/6’ results
Results at ‘Day 3’ (one cell) and at developmental arrest (blastocyst, days 7–13) were obtained for 29 embryos. 58.6 % showed consistent results (n = 17). 37.9 % (n = 11) were classified as abnormal on ‘Day 3’ and as normal at arrest point.
Out of these 11 embryos: nine (81.8 %) had results at an intermediate stage (‘Day 5/6’ during development). 55 % of them showed the change from the first analysis to the second analysis and the rest showed the change only at the arrest point.
Summary of results
Overall, total of 100 human embryos were tested for aneuploidy during their development in culture. Comparison of aneuploidy levels was conducted at different time points and using different methods. In addition, the mosaic phenomenon was examined.
Genetic status obtained at different days after fertilization have demonstrated that the prediction rate of the embryo status from ‘Day 3’ analysis is only 62.5 %.
Overall, 35.5 % of the embryos that were classified as abnormal at ‘Day 3’ were found to be euploid at later stages (20 out of 69 embryos) and therefore, their ‘Day 3’ diagnosis was misleading.
Almost two thirds of the embryos (62 %) that were found to be trisomic at ‘Day 3’ analysis (in one chromosome) were found to be euploid at developmental arrest compared to 81 % of the embryos with monosomy at ‘Day 3’ analysis.
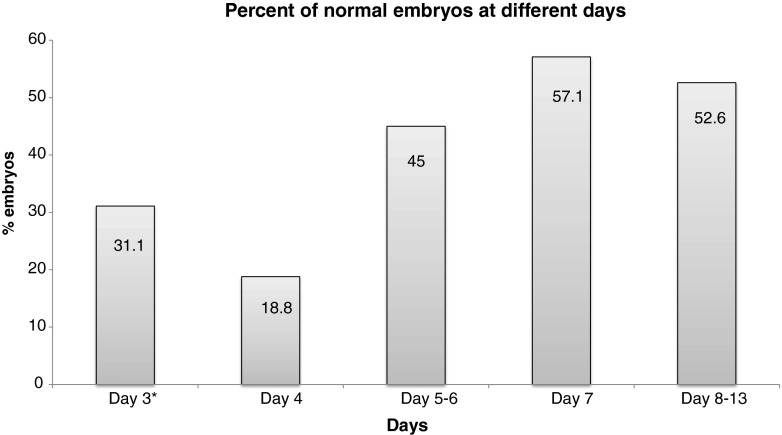
Figure 6 exhibits the percentage of euploid embryos at different days post fertilization. It is notable that ~52 % of all tested embryos at developmental arrest were euploid. According to our findings, 6 days after fertilization the ‘self-correction’ rate is 29 % and 7–13 days after fertilization the ‘self-correction’ rate is 38 %. If analyzed day by day, normalization mostly occurs until 7–8 days post fertilization.
Fig. 6.
The percent of euploid embryos at different days of development. (Asterisks) Based on one cell diagnosis
Discussion
The present study deciphers the genetic status of 100 human IVF embryos throughout their development in culture, up to 13 days post fertilization. We found that while in early developmental stages, a significant number of embryos exhibit aneuploidy (or mosaicism), towards the blastocyst stage and even beyond that stage, the portion of euploid embryos rises. Even though a study based on 100 embryos is not sufficient to reach final conclusions, it certainly gives a direction and creates a strong foundation for additional research in this field.
Cleavage stage embryos have been the subject of many studies over the years, and thus, there is a lot of information about them. However, there is a lack of information regarding the fate of these embryos at post implantation stages. We performed whole genome analysis of embryos through their development in culture, by combining several advanced molecular cytogenetic methods, which provided unique insight into the chromosome dynamics of embryos at different stages of pre-implantation development.
Our results indicate that chromosomal changes are frequent phenomena, documented in early developing stage embryos with the use of FISH, chromosomal CGH and array CGH. These data present evidence for ‘normalization’ of some of the chromosomally abnormal embryos to euploid embryos, occurring after the blastocyst stage.
High levels of mosaicism at ‘Day 4’ embryos
In this study we examined in vitro embryos, as in vivo conceived cleavage embryos are not available for research and embryos lost after the first week(s) of pregnancy are currently unattainable. Therefore, the embryos used in this study are the best available representation of early developing human embryos.
Consistent with previous findings, our FISH results of human ‘Day 3’ IVF embryos demonstrate that only ~30 % of the embryos are euploid for all tested chromosomes. Numbers are variable between different studies (that used FISH strategy of single cell biopsy but with different number of tested chromosomes), reaching up to 80 % of aneuploid ‘Day 3’ embryos [12, 51]. This, however, is based on the assumption that the biopsied cell represented the entire embryo. The growing number of reports about mosaicism at early stage embryos raises doubts regarding this assumption [10, 34].
Indeed, when tested for mosaicism at ‘Day 4’, a great portion of the embryos in our study was mosaic (62.5 %), with 13–66 % aneuploid cells within the embryo. Other studies that tested ‘Day 4’ embryos that used techniques such as FISH or array based methods, reported similar levels of mosaicism [42, 47]. A meta-analysis of studies on the chromosomal constitution of human pre-implantation embryos, conducted recently by Van Echten-Arends et al. [46], examined 815 embryos and found that 73 % of them were mosaic.
The mosaic phenomenon is considered a major contribution in the process of embryos development. Several mechanisms may be involved in aneuploidy mosaicism. The most investigated one is “anaphase lag” [8] but chromosome duplication or postzygotic nondisjunction may also lead to chromosome loss or gain in the daughter cells [11].
The high prevalence of mosaic embryos can be interpreted in several ways. One option is that chromosomal mosaicism is a casual biological phenomenon in developing embryos with no or limited relevance to embryo survival [22]. Another possible explanation is that chromosome self-normalization occurs during development in chromosomally abnormal embryos, perhaps by cell arrest, apoptotic pathway or trisomic rescue. Supporting the latter is the fact that examination of human embryos genome at later developmental stages, as we conducted here, revealed higher rate of chromosomally normal embryos.
Our work compared ‘Day 3’ and ‘Day 4’ FISH results of the same embryo. The results emphasize the problematic issue of pre-implantation genetic screening (PGS), as the prediction rate of aneuploidy from a single cell to the entire embryo was only 62.5 %. We found that FISH diagnosis based on single ‘Day 3’ blastomere was not predictive enough of the subsequent chromosomal status of the developing embryo. According to our finding, and base on other recent studies [18, 52], it seems inevitable that PGS at ‘Day 3’ with the use of FISH is problematic as there are major obstacles such as chromosomal mosaicism and incomplete analysis. Therefore, other analysis methods (polar body and/or trophectoderm analysis using 24 chromosome microarrays) should be highly considered.
‘Normalization’ of the embryo occurs mainly up to 7–8 days post fertilization
In this study we examined the chromosomal content of embryos through their development in culture. Each embryo was first analyzed by FISH at ‘Day 3’ post fertilization, once again through its growth in vitro and a last analysis was performed at developmental arrest, when the entire blastocyst was analyzed by CGH techniques.
While at ‘Day 3’ post fertilization the rate of euploid embryos was ~30 %, we found that at developmental arrest more than half of the embryos (~52 %) were euploid.
This is the first report that follows the embryo’s status up to 13 days post fertilization and combines FISH at different stages of development and CGH at arrest point. Most of the published reports to date focus on the cytogenetic condition up to blastocyst stage, around 6 days after fertilization. Barbash-Hazan et al. [5] investigated the incidence of embryos’ self-correction by comparing the chromosomal status of ‘Day 3’ and ‘Day 5’ embryos using FISH technique. They found that normalization takes place in correlation to the developmental status and reported that almost 40 % of the embryos that developed to the blastocyst stage underwent self-correction
Munné et al. [38] reported the first evidence for normalization of chromosomally abnormal embryos during the process of hESC derivation on feeder cells. They observed an increase in the frequency of normal cells from day 6 to day 12 in culture. They suggested that once the embryo is mosaic with disomic cells, those cells might develop differently than the abnormal ones, and differences in cleavage performance between disomic and aneuploidy cells might result in enrichment in disomic cells. Santos et al. [43] also found, using FISH, a decrease in mosaicism over time, from 82 % on Day 4 to 42 % on Day 8.
When analyzed by days post fertilization, our results demonstrated that 35.5 % of the embryos underwent some kind of ‘normalization’ or ‘self-correction’- embryos that had been classified as abnormal at ‘Day 3’ were found to be euploid at later stages. According to our findings, most of the ‘normalization’ takes place until ‘Day 7’ post fertilization. It seems that the mechanism for dynamic changes is by a selection against abnormal cells within the embryo rather than selection against abnormal embryos.
With the use of CGH, small mosaics might be missed, and so, embryos classified as normal could in fact carry small mosaicism (<15 %). However, embryos carrying such small mosaicism will probably become normal anyway [30].
One of the explanations for the rise and fall of mosaicism rate during embryonic development can be the fact that there are some reported data indicating that the first three mitotic cell divisions lack some important cell cycle control elements. In the early developing embryo, cell cycle control is performed by maternal transcripts [7, 31]. However, some of the checkpoints of the cell cycle control system are inactive in the first postzygotic cell divisions [1, 2]. Only after the beginning of embryonic genome expression, does cell cycle control become gradually present from the 8-cell stage onwards during the morula stage [7, 31, 45]. Mechanism of programmed cell death (apoptosis) becomes active during the morula stage [19, 24, 25].
It can be assumed that at early developmental stages, the embryo has some sort of “genomic flexibility” that might allow the evolution processes to continue shaping the human genome.
Los et al. [32] suggested a theoretical model of the development of cytogenetically normal, abnormal and mosaic embryos. In this model, the number of mosaic embryos is at its peak at the 8-cell stage of embryonic development and decreases after the embryo’s genome becomes fully activated (mainly due to mitotic arrest mechanism of the abnormal cells that becomes activated with the activation of cell cycle controls).
As we found here, aneuploid embryos (or mosaic embryos containing normal blastomeres) may result in chromosomally normal fetuses. These dynamic changes, as reported in several studies [30, 44], should be monitored closely by several molecular techniques.
The concept of arising mosaicism during the cleavage stage and the disappearance of mosaic embryo in later developmental stages may have an effect on pre-implantation genetic diagnosis. Mosaicism and ‘normalization’ makes the correlation between the genetic status determined in ‘Day 3’ to be of low compatibility with the chromosomal status of the embryo at later stages (or at developmental arrest). This raises serious questions about the whole concept of PGS as a prenatal method of screening, regardless of the diagnosis method, as it is based on 1–2 cells diagnosis and at an early developmental stage,
The benefits of the use of comprehensive cytogenetic screening in embryo research
It is possible that the incidence of chromosome abnormality at conception is high and natural selection probably occurs before and after implantation. In order to investigate this issue, we studied the chromosome content of human embryos at different days post fertilization. When evaluating the chromosomal content of embryos, one should consider the limitation of FISH technique. Alongside the fact that FISH has technical elements that might influence the interpretations of the results, FISH can assess only a few chromosomes in inter-phase nuclei, hence, the information obtained may not always be representative of the real chromosomal status of the cells. Using whole genome analysis gives more comprehensive data regarding the genomic stability of the embryos and overcomes some technical limitations described above.
Therefore, our study design included the use of several techniques that can complement one another. The adaptation of comparative genomic hybridization (CGH) to single cells allowed us to study the full karyotype of embryos as an experimental design. In recent years an even more detailed characterization of blastocyst cytogenetics analysis has become available, with the introduction of methods such as CGH and SNP microarrays [15, 40].
Comparison between several amplification techniques (MDA and PCR based method) followed by CGH and aCGH allowed us to verify that the use of MDA didn’t generate multiple artifacts during metaphase FISH. Results obtained by using these two methods were very similar (with no statistically significant differences); therefore we analyzed all embryos that were collected on the same day as one group, regardless of the method in which they were examined.
Chromosomal imbalances in embryos
In this study we found that chromosome gains and losses appear to be stochastic, with no significantly recurrent aneuploidy of a specific chromosome. Chromosome 1 and 2 did seem to be aneuploid more frequently but it might have been the result of their size rather than anything else. Chromosome 19 also exhibits high aneuploidy rate but there are reports about technical factors regarding this chromosome which might affect the reliability of the results, mainly due to failed hybridization in its GC rich areas [35, 48]. A significant difference was found in our research between monosomy and trisomy rate. The cause for that difference might be technical elements (FISH interpretations) but it may also suggest that chromosome loss is more common in aneuploid embryo [9].
Taken together, genome-wide analyses using microarray CGH (or SNP array) is currently the optimal method which provides an improved standardized genetic screening of human embryos for all chromosomes.
Another interesting finding is that most of the aneuploid embryos had only one aberration. Our results are consistent with Fragouli’s study, that reported of 60 % of the embryos with one aberration and 25 % with two aberrant chromosomes [16]. Interestingly, we also found a significant portion of aneuploid embryos (27 %) harboring four or more aberrations (chaotic embryos), supporting the hypothesis that the first event of chromosome instability may lead to sequential events and to chaos in the embryo chromosome status. [29].
Summary
High levels of chromosome abnormalities were observed in embryos at early development stages. The chromosome instability observed in vitro most probably also occurs in vivo, as only 30 % of human conceptions result in a live birth [33] and more than 50 % of spontaneous abortions have chromosomal imbalances [6, 17, 41]. This “genomic flexibility” might allow the evolution processes to continue shaping the human genome. Moreover, a significant portion of abnormal embryos at day 3 were found to be chromosomally normal at day 7.
We found that FISH diagnosis based on single ‘Day 3’ blastomere was not predictive enough of the subsequent chromosomal normality potential. This raises fundamental questions about the use of PGS with FISH as a single method to determine the chromosomal integrity of the embryo. Furthermore, our findings of ‘normalization’ occurring later on in development undermine the effectiveness of ‘Day 3’ post fertilization analysis as a diagnostic tool. Our results suggest that testing a single blastomere at ‘Day 3’ post fertilization for pre-implantation genetic diagnosis might inaccurately reflect the embryo ploidity, increase the risk of false aneuploidy diagnosis and reduce the number of transferable embryos. Alternatively, blastocyst stage diagnosis may be more appropriate.
Electronic supplementary material
(PPT 416 kb)
Acknowledgments
Conflict of interest
The authors declare no conflict of interest.
Footnotes
This work was performed in partial fulfillment of the requirements for a PhD degree of Michal Dekel, Sackler Faculty of medicine, Tel Aviv University, Ramat Aviv, Israel.
Capsule
We studied genomic alterations occurring between different developmental stages of 100 human pre-implantation embryos, and found that proximally one third of the embryos that are classified as chromosomally abnormal on ‘Day 3’ post fertilization are normal at development arrest point.
References
- 1.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. The cell cycle and programmed cell death. In Molecular Biology of the Cell. 4. New York: Garland Science; 2002a. pp. 984–1026. [Google Scholar]
- 2.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. The mechanisms of cell division. In Molecular Biology of the Cell. 4. New York: Garland Science; 2002b. pp. 1027–64. [Google Scholar]
- 3.Aviram-Goldring A, Daniely M, Dorf H, Chaki R, Goldman B, Barkai G. Use of interphase fluorescence in situ hybridization in third trimester fetuses with anomalies and growth retardation. Am J Med Genet. 1999;87:203–6. doi: 10.1002/(SICI)1096-8628(19991126)87:3<203::AID-AJMG2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 4.Baart EB, Martini E, van den Berg I, Macklon NS, Galjaard RJ, Fauser BC, Van Opstal D. Preimplantation genetic screening reveals a high incidence of aneuploidy and mosaicism in embryos from young women undergoing IVF. Hum Reprod. 2006;21(1):223–33. doi: 10.1093/humrep/dei291. [DOI] [PubMed] [Google Scholar]
- 5.Barbash-Hazan S, Frumkin T, Malcov M, Yaron Y, Cohen T, Azem F, Amit A, Ben-Yosef D. Preimplantation aneuploid embryos undergo self-correction in correlation with their developmental potential. Fertil Steril. 2009;92(3):890–6. doi: 10.1016/j.fertnstert.2008.07.1761. [DOI] [PubMed] [Google Scholar]
- 6.Benkhalifa M, Kasakyan S, Clement P, Baldi M, Tachdjian G, Demirol A, Gurgan T, Fiorentino F, Mohammed M, Qumsiyeh MB. Array comparative genomic hybridization profiling of firsttrimester spontaneous abortions that fail to grow in vitro. Prenat Diagn. 2005;25:894–900. doi: 10.1002/pd.1230. [DOI] [PubMed] [Google Scholar]
- 7.Braude P, Bolton V, Moore S. Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature. 1988;332(6163):459–61. doi: 10.1038/332459a0. [DOI] [PubMed] [Google Scholar]
- 8.Coonen E, Derhaag JG, Dumoulin JC, van Wissen LC, Bras M, Janssen M, Evers JL, Geraedts JP. Anaphase lagging mainly explains chromosomal mosaicism in human preimplantation embryos. Hum Reprod. 2004;19(2):316–24. doi: 10.1093/humrep/deh077. [DOI] [PubMed] [Google Scholar]
- 9.Cooper ML, Darilek S, Wun WS, Angus SC, Mensing DE, Pursley AN, Dunn RC, Grunert GM, Cheung SW. A retrospective study of preimplantation embryos diagnosed with monosomy by fluorescence in situ hybridization (FISH) Cytogenet Genome Res. 2006;114(3–4):359–66. doi: 10.1159/000094226. [DOI] [PubMed] [Google Scholar]
- 10.Delhanty JD, Harper JC, Ao A, Handyside AH, Winston RM. Multicolour FISH detects frequent chromosomal mosaicism and chaotic division in normal preimplantation embryos from fertile patients. Hum Genet. 1997;99:755–60. doi: 10.1007/s004390050443. [DOI] [PubMed] [Google Scholar]
- 11.Delhanty JD. Mechanisms of aneuploidy induction in human oogenesis and early embryogenesis. Cytogenet Genome Res. 2005;111(3–4):237–44. doi: 10.1159/000086894. [DOI] [PubMed] [Google Scholar]
- 12.Donoso P, Staessen C, Fauser BC, Devroey P. Current value of preimplantation genetic aneuploidy screening in IVF. Hum Reprod Update. 2007;13(1):15–25. doi: 10.1093/humupd/dml043. [DOI] [PubMed] [Google Scholar]
- 13.Feldman B, Aviram-Goldring A, Evans MI. Interphase FISH for prenatal diagnosis of common a neuploidies. Methods Mol Biol. 2002;204:219–41. doi: 10.1385/1-59259-300-3:219. [DOI] [PubMed] [Google Scholar]
- 14.Fiorentino F, Spizzichino L, Bono S, Biricik A, Kokkali G, Rienzi L, Ubaldi FM, Iammarrone E, Gordon A, Pantos K. PGD for reciprocal and Robertsonian translocations using array comparative genomic hybridization. Hum Reprod. 2011;26(7):1925–35. doi: 10.1093/humrep/der082. [DOI] [PubMed] [Google Scholar]
- 15.Fragouli E, Lenzi M, Ross R, Katz-Jaffe M, Schoolcraft WB, Wells D. Comprehensive molecular cytogenetic analysis of the human blastocyst stage. Hum Reprod. 2008;23(11):2596–608. doi: 10.1093/humrep/den287. [DOI] [PubMed] [Google Scholar]
- 16.Fragouli E, Wells D. Aneuploidy in the human blastocyst. Cytogenet Genome Res. 2011;133(2–4):149–59. doi: 10.1159/000323500. [DOI] [PubMed] [Google Scholar]
- 17.Fritz B, Hallermann C, Olert J, Fuchs B, Bruns M, Aslan M, Schmidt S, Coerdt W, Müntefering H, Rehder H. Cytogenetic analyses of culture failures by comparative genomic hybridisation (CGH)—re-evaluation of chromosome aberration rates in early spontaneous abortions. Eur J Hum Genet. 2001;9:539–47. doi: 10.1038/sj.ejhg.5200669. [DOI] [PubMed] [Google Scholar]
- 18.Geraedts JP. Does additional hybridization also improve preimplantation genetic screening results? Expert Rev Mol Diagn. 2010;10(8):981–5. doi: 10.1586/erm.10.94. [DOI] [PubMed] [Google Scholar]
- 19.Hardy K, Spanos S, Becker D, Iannelli P, Winston RML, Stark J. From cell death to embryo arrest: Mathematical models of human preimplantation embryo development. Proc Natl Acad Sci USA. 2001;98:1655–60. doi: 10.1073/pnas.98.4.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassold T, Abruzzo M, Adkins K, Griffin D, Merrill M, Millie E, Saker D, Shen J, Zaragoza M. Human aneuploidy: incidence, origin, and etiology. Environ Mol Mutagen. 1996;28(3):167–75. doi: 10.1002/(SICI)1098-2280(1996)28:3<167::AID-EM2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 21.Heijnen EM, Eijkemans MJ, De Klerk C, Polinder S, Beckers NG, Klinkert ER, Broekmans FJ, Passchier J, Te Velde ER, Macklon NS, Fauser BC. A mild treatment strategy for in-vitro fertilisation: a randomised non-inferiority trial. Lancet. 2007;369:743–9. doi: 10.1016/S0140-6736(07)60360-2. [DOI] [PubMed] [Google Scholar]
- 22.Iourov IY, Vorsanova SG, Yurov YB. Chromosomal mosaicism goes global. Mol Cytogenet. 2008;1:26. doi: 10.1186/1755-8166-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Israeli O, Gotlieb WH, Friedman E, Goldman B, Ben-Baruch G, Aviram-Goldring A, Rienstein S. Familial vs sporadic ovarian tumors:characteristic genomic alterations analyzed by CGH. Gynecol Oncol. 2003;90:629–36. doi: 10.1016/S0090-8258(03)00375-5. [DOI] [PubMed] [Google Scholar]
- 24.Jurisicova A, Antenos M, Varmuza S, Tilly JL, Casper RF. Expression of apoptosis related genes during human preimplantation embryo development: potential roles for the Harakiri gene product and Caspase 3 in blastomere fragmentation. Mol Hum Reprod. 2003;9:133–41. doi: 10.1093/molehr/gag016. [DOI] [PubMed] [Google Scholar]
- 25.Jurisicova A, Varmuza S, Casper RF. Programmed cell death and human embryo fragmentation. Mol Hum Reprod. 1996;2:93–8. doi: 10.1093/molehr/2.2.93. [DOI] [PubMed] [Google Scholar]
- 26.Katz-Jaffe MG, Trounson AO, Cram DS. Mitotic errors in chromosome 21 of human preimplantation embryos are associated with non-viability. Mol Hum Reprod. 2004;10:143–7. doi: 10.1093/molehr/gah017. [DOI] [PubMed] [Google Scholar]
- 27.Keskintepe L, Sher G, Keskintepe M. Reproductive oocyte/embryo genetic analysis: comparison between fluorescence in-situ hybridization and comparative genomic hybridization. Reprod Biomed Online. 2007;15(3):303–9. doi: 10.1016/S1472-6483(10)60343-4. [DOI] [PubMed] [Google Scholar]
- 28.Kirchhoff M, Gerdes T, Maahr J. Deletions below 10 megabasepairs are detected in comparative genomic hybridization by standard reference intervals. Genes Chromosomes. Cancer. 1999;25:410–3. doi: 10.1002/(sici)1098-2264(199908)25:4<410::aid-gcc17>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 29.Lejeune J. Autosomal disorders. Pediatrics. 1963;32:326–37. [PubMed] [Google Scholar]
- 30.Li M, DeUgarte CM, Surrey M, Danzer H, DeCherney A, Hill DL. Fluorescence in situ hybridization reanalysis of day-6 human blastocysts diagnosed with aneuploidy on day 3. Fertil Steril. 2005;84(5):1395–400. doi: 10.1016/j.fertnstert.2005.04.068. [DOI] [PubMed] [Google Scholar]
- 31.Lighten AD, Hardy K, Winston RML, Moore GE. IGF2 is parentally imprinted in human preimplantation embryos. Nature Genet. 1997;15:122–3. doi: 10.1038/ng0297-122. [DOI] [PubMed] [Google Scholar]
- 32.Los FJ, Van Opstal D, van den Berg C. The development of cytogenetically normal, abnormal and mosaic embryos: a theoretical model. Hum Reprod Update. 2004;10(1):79–94. doi: 10.1093/humupd/dmh005. [DOI] [PubMed] [Google Scholar]
- 33.Macklon NS, Geraedts JP, Fauser BC. Conception to ongoing pregnancy: the ‘black box’ of early pregnancy loss. Hum Reprod Update. 2002;8:333–43. doi: 10.1093/humupd/8.4.333. [DOI] [PubMed] [Google Scholar]
- 34.Márquez C, Sandalinas M, Bahçe M, Alikani M, Munné S. Chromosome abnormalities in 1255 cleavage-stage human embryos. Reprod Biomed Online. 2000;1(1):17–26. doi: 10.1016/S1472-6483(10)61988-8. [DOI] [PubMed] [Google Scholar]
- 35.Moore DH, Pallavicini M, Cher ML, Gray JW. A t-statistic for objective interpretation of comparative genomic hybridization (CGH) profiles. Cytometry. 1997;28(3):183–90. doi: 10.1002/(SICI)1097-0320(19970701)28:3<183::AID-CYTO1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 36.Munné S, Márquez C, Magli C, Morton P, Morrison L. Scoring criteria for preimplantation genetic diagnosis of numerical abnormalities for chromosomes X, Y, 13, 16, 18 and 21. Mol Hum Reprod. 1998;4:863–70. doi: 10.1093/molehr/4.9.863. [DOI] [PubMed] [Google Scholar]
- 37.Munné S, Sandalinas M, Escudero T, Marquez C, Cohen J. Chromosome mosaicism in cleavage-stage human embryos: evidence of a maternal age effect. Reprod Biomed Online. 2002;4:223–32. doi: 10.1016/S1472-6483(10)61810-X. [DOI] [PubMed] [Google Scholar]
- 38.Munné S, Velilla E, Colls P, Garcia Bermudez M, Vemuri MC, Steuerwald N, Garrisi J, Cohen J. Self-correction of chromosomally abnormal embryos in culture and implications for stem cell production. Fertil Steril. 2005;84(5):1328–34. doi: 10.1016/j.fertnstert.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 39.Munne S, Weier HU. Simultaneous enumeration of chromosomes 13, 18, 21, X, and Y in interphase cells for preimplantation genetic diagnosis of aneuploidy. Cytogenet Cell Genet. 1996;75:263–70. doi: 10.1159/000134497. [DOI] [PubMed] [Google Scholar]
- 40.Northrop LE, Treff NR, Levy B, Scott RT., Jr SNP microarray-based 24 chromosome aneuploidy screening demonstrates that cleavage-stage FISH poorly predicts aneuploidy in embryos that develop to morphologically normal blastocysts. Mol Hum Reprod. 2010;16(8):590–600. doi: 10.1093/molehr/gaq037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pflueger S. Cytogenetics of spontaneous abortion. The principles of clinical cytogenetics. New Jersey: Humana Press; 1999. pp. 317–43. [Google Scholar]
- 42.Rius M, Daina G, Obradors A, Ramos L, Velilla E, Fernández S, Martínez-Passarell O, Benet J, Navarro J. Comprehensive embryo analysis of advanced maternal age-related aneuploidies and mosaicism by short comparative genomic hybridization. Fertil Steril. 2011;95(1):413–6. doi: 10.1016/j.fertnstert.2010.07.1051. [DOI] [PubMed] [Google Scholar]
- 43.Santos MA, Teklenburg G, Macklon NS, Van Opstal D, Schuring-Blom GH, Krijtenburg PJ, de Vreeden-Elbertse J, Fauser BC, Baart EB. The fate of the mosaic embryo: chromosomal constitution and development of Day 4, 5 and 8 human embryos. Hum Reprod. 2010;25(8):1916–26. doi: 10.1093/humrep/deq139. [DOI] [PubMed] [Google Scholar]
- 44.Staessen C, Platteau P, Van Assche E, Michiels A, Tournaye H, Camus M, Devroey P, Liebaers I, Van Steirteghem A. Comparison of blastocyst transfer with or without preimplantation genetic diagnosis for aneuploidy screening in couples with advanced maternal age: a prospective randomized controlled trial. Hum Reprod. 2004;19(12):2849–58. doi: 10.1093/humrep/deh536. [DOI] [PubMed] [Google Scholar]
- 45.Tesarõik J, Kopecõny V, Plachot M, Mandelbaum J. Activation of nucleolar and extranucleolar RNA–synthesis and changes in the ribosomal content of human embryos developing in vitro. J Reprod Fertil. 1986;78:463–70. doi: 10.1530/jrf.0.0780463. [DOI] [PubMed] [Google Scholar]
- 46.Van Echten-Arends J, Mastenbroek S, Sikkema-Raddatz B, Korevaar JC, Heineman MJ, van der Veen F, Repping S. Chromosomal mosaicism in human preimplantation embryos: a systematic review. Hum Reprod Update. 2011;17(5):620–7. doi: 10.1093/humupd/dmr014. [DOI] [PubMed] [Google Scholar]
- 47.Vanneste E, Voet T, Le Caignec C, Ampe M, Konings P, Melotte C, Debrock S, Amyere M, Vikkula M, Schuit F, Fryns JP, Verbeke G, D’Hooghe T, Moreau Y, Vermeesch JR. Chromosome instability is common in human cleavage-stage embryos. Nat Med. 2009;15(5):577–83. doi: 10.1038/nm.1924. [DOI] [PubMed] [Google Scholar]
- 48.Voullaire L, Slater H, Williamson R, Wilton L. Chromosome analysis of blastomeres from human embryos by using comparative genomic hybridization. Hum Genet. 2000;106(2):210–7. doi: 10.1007/s004390051030. [DOI] [PubMed] [Google Scholar]
- 49.Wells D, Delhanty JD. Comprehensive chromosomal analysis of human preimplantation embryos using whole genome amplification and single cell comparative genomic hybridization. Mol Hum Reprod. 2000;6(11):1055–62. doi: 10.1093/molehr/6.11.1055. [DOI] [PubMed] [Google Scholar]
- 50.Wilton L. Preimplantation genetic diagnosis for aneuploidy screening in early human embryos: a review. Prenat Diagn. 2002;22(6):512–8. doi: 10.1002/pd.388. [DOI] [PubMed] [Google Scholar]
- 51.Ye YH, Xu CM, Jin F, Qian YL. Identification of embryonic chromosomal abnormality using FISH-based preimplantation genetic diagnosis. J Zhejiang Univ Sci. 2004;5(10):1249–54. doi: 10.1631/jzus.2004.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zamora S, Clavero A, Gonzalvo MC, de Dios Luna Del Castillo J, Roldán-Nofuentes JA, Mozas J, Castilla JA. PGS-FISH in reproductive medicine and perspective directions for improvement: a systematic review. J Assist Reprod Genet. 2011;28(8):747–57. doi: 10.1007/s10815-011-9578-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PPT 416 kb)