Abstract
Purpose
The reduction of the number of embryos transferred while maintaining a satisfactory rate of pregnancy (PR) with in vitro fertilization calls for a refined technique of embryonic selection. This prospective study investigates the significance of early embryonic compaction at day 3 as a marker of the chances of implantation.
Methods
We examined 317 transfers and their outcome involving 509 embryos including 91 compacted embryos.
Results
Early compaction seems linked with the ovarian response to stimulation and embryonic quality. The PR is significantly increased when the embryonic cohort contains at least one compacted embryo (44 % versus 29.5 %, p = 0.01), and when at least one compacted embryo is transferred (44 % versus 31 %, p < 0.05). The analysis of our single embryo transfers shows that the implantation rates are significantly better for compacted embryos (50 % versus 30 %, p < 0.05) (OR 2.98; CI 1.02–5.28).
Conclusion
Thus, early compaction, sometimes observed at day 3, may serve as a useful additional criterion for selecting the embryos transferred.
Keywords: Early compaction, Cleavage stage, Embryonic selection, Transfer at day 3
Introduction
The optimization of in vitro fertilization (IVF) is based on a policy of embryo transfer aiming to reduce the number of multiple pregnancies while maintaining a satisfactory rate of pregnancy. Multiple pregnancies, recognized as a complication of IVF, are associated with increased risks of perinatal mortality and morbidity [3,4]. To avoid these risks, the number of embryos transferred has been progressively reduced over the years. In current practice, only one or two embryos, deemed of optimal quality, are replaced in the maternal uterus. It is therefore of interest to define the relevant criteria and the ideal day for embryo transfer.
Depending on the stage of embryonic development, two types of transfer are generally proposed, either at the cleavage stage on day 2 or day 3, or at the blastocyst stage on day 5. In the first type of transfer, embryonic quality is evaluated according to several criteria such as the appearance of the pronuclei, the time of the first cleavage, the kinetics of embryonic development and the morphological quality of the embryos [11,28,39]. In the second type of transfer, these criteria are supplemented by the data concerning the embryologic changes between day 3 and day 5, and the morphological appearance of the blastocyst [11]. Transfer on day 5 might seem advantageous as it would allow the selection of embryos having undergone compaction and cavitation, reflecting their developmental potential. Indeed, the implantation rates are better for these embryos [14]. However, the risk of developmental arrest, leading to the cancellation of transfer, would need to be considered, particularly if the number of embryos available for extended culture was low. Since conditions of culture are suboptimal [38], there is no evidence that an embryo that had not evolved in vitro between day 3 and day 5 would not have done so in vivo after replacement in the cleavage stage. In addition, a transfer on day 5 presents other disadvantages, such as the epigenetic risks associated with prolonged maintenance of embryos in vitro [8,30]. To avoid such disadvantages while maintaining good implantation rates, it is important to improve the criteria for assessing the developmental potential of the embryos at the cleavage stage.
After fertilization, the embryo usually consists of four blastomeres at 48 h, and an average of eight cells at 72 h. To date, no study has demonstrated the superiority of transfer on day 3 versus day 2 [1,2,20,32,34]. With the current criteria, an extra day at this stage does not provide enough additional information concerning the developmental potential of the embryo [27,36].
Physiologically, the transcription of the embryonic genome, although it begins at day 3, is actually active at day 4 [6,18]. Embryos having passed this step have the greatest potential for development and are able to engage in the process of compaction and cavitation. During the initial phase of compaction, the blastomeres begin to compact tightly, becoming scarcely distinguishable from each other. Cell boundaries then progressively disappear until the embryo is fully compacted. Finally, at the late compaction stage, the cell boundaries reappear, the number of blastomeres increases, and the cavitation starts [36], leading to the formation of the blastocyst at day 5. Only about half the good quality embryos on day 3 appear to be likely to undergo this process on day 4 [29,36]. Some authors suggest that the compaction of embryos on day 4 is a good criterion for the selection of embryos and propose embryo transfer at the morula or compacted stage [10,27,36].
Although the compaction generally occurs at day 4, it may occur as early as day 3. Compaction reflects a commitment of the embryo to the next stage of development, but the significance of its early onset has been little evaluated. Two retrospective studies investigating this phenomenon suggest that the compaction at day 3 could serve as a supplementary criterion for embryonic selection. One of these studies, involving 1047 transferred embryos, found successful implantation rates closely correlated with the compaction level of the embryos [35], and the other study, on 316 transferred embryos, included early compaction as a parameter of the quality score on day 3, reporting a trend toward an improved pregnancy rate [9].
The purpose of our work, which represents the first prospective study on the subject, was to investigate the significance of early embryonic compaction at day 3. We tried to determine whether this phenomenon could serve as a positive marker of embryo quality and be used for embryo selection at the cleavage stage to increase the chances of pregnancy.
Materials and methods
Inclusion criteria
This study includes patients who had an embryo transfer on day 3, after IVF, with or without microinjection, between September 2009 and May 2011 at the University Hospital of Angers. The ethics committee of the University Hospital of Angers approved the plan of the study. During this period, transfer on day 2 was reserved for patients having only one or two embryos. Data were collected prospectively for analysis. A total of 317 transfers involving 509 embryos were selected for this study.
Stimulation of ovulation
Long agonist protocols and antagonist protocols were used for the stimulation of ovulation. The long agonist protocol included down-regulation with a GnRH agonist (Triptoreline: Decapeptyl® Ipsen Pharma, Boulogne Billancourt, France) followed by ovarian stimulation with FSH (Menotropine: Menopur® Ferring Pharmaceuticals, Denmark; Follitropine alpha + Lutropine alpha: Pregoveris® Serono, Switzerland; Follitropine alpha: Gonal F® Serono, Switzerland; Follitropine beta: Puregon® Organon, Holland). The antagonist protocol used ovarian stimulation with FSH followed by the administration of a GnRH antagonist (Cetrorelix: Cetrotide® Serono, Switzerland or Ganerelix: Orgalutran® Organon, Holland).
The starting dose of FSH was 150–300 IU daily. The treatments were monitored by pelvic ultrasound examination and blood estradiol assays. Ovulation was induced by injecting human chorionic gonadotrophin (hCG) 5000 UI, when at least three follicles larger than 17 mm were present and the ovarian estradiol level was consistent. Oocytes were retrieved with a transvaginal probe 36 h after the HCG injection.
In vitro fertilization
The cumulus-oocyte complexes (COCs) were washed in multiple dishes with Flushing medium (Origio-France, Limonest, France) and subsequently incubated at 37 °C in Ferticult culture media (Fertipro, Beernem, Belgium).
For cIVF (classical in vitro fertilization), COCs were put in contact with spermatozoa within 2 h after oocyte retrieval. The zygotes were observed the next day after removal of follicular cells by gentle pipetting and immersion in Global culture medium (LifeGlobal, Seattle, WA, USA).
For ICSI (intra-cytoplasmic sperm injection), cumulus cells were discarded using hyaluronidase (80 UI, Fertipro, Beernem, Belgium) and gentle pipetting, just before sperm injection. Only metaphase II oocytes were injected.
The zygotes and the embryos were individually cultured in 30 microlitre drops of culture media (Global® LifeGlobal, Seattle, WA, USA) under oil at 37 °C, in a humidified atmosphere containing 6 % CO2.
Embryonic classification and selection for transfer
Zygotes were observed 18–20 h post-insemination to check for the presence of two pronuclei, and 25–29 h post-insemination to ascertain the existence of early cleavage, a phenomenon known to be associated with higher implantation rates [37]. Embryos were then assessed at day 2 (43–45 h post-insemination) and day 3 (67–69 h post-insemination) according to the BLEFCO (“Biologistes des Laboratoires d’Etudes de la Fécondation et de la Conservation de l’Oeuf”) classification, incorporating the number of blastomeres, uniformity of size (noted 1: homogeneous, or 2: heterogeneous) and the percentage of cytoplasmic fragments of the blastomeres (noted 1: <10 %, 2: 10–30 %, or 3: >30 %). Embryos were considered to be of good quality when homogeneous, with normal kinetics, i.e. four cells at day 2 and 7–9 cells at day 3, and containing less than 10 % of cytoplasmic fragments. All these observations were made by two members of our laboratory.
At day 3 (67–69 h post-insemination) we classified embryos without compaction (Fig. 1) and those with compaction. We considered all the embryos that had entered the compaction process, i.e. embryos at the beginning of compaction when membrane fusion was visible but it was still possible to count the number of cells (Fig. 2), and those at full compaction when it was impossible to distinguish cell boundaries (Fig. 3). The number of embryos selected for transfer was determined according to the embryonic quality, age of the patient, rank of the attempt, and the clinical history. For the first attempt in women under 35 years, we transferred only one embryo if embryo freezing was practicable and two embryos otherwise. For women over 35 years, or for younger women undergoing attempts ranked above one, we transferred two embryos.
Fig. 1.
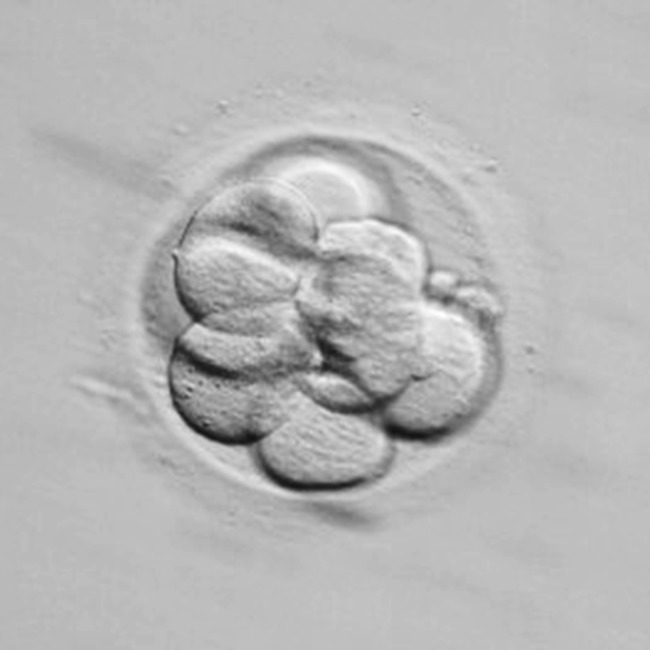
Embryo showing no compaction: individual blastomeres are visible without membrane fusion
Fig. 2.
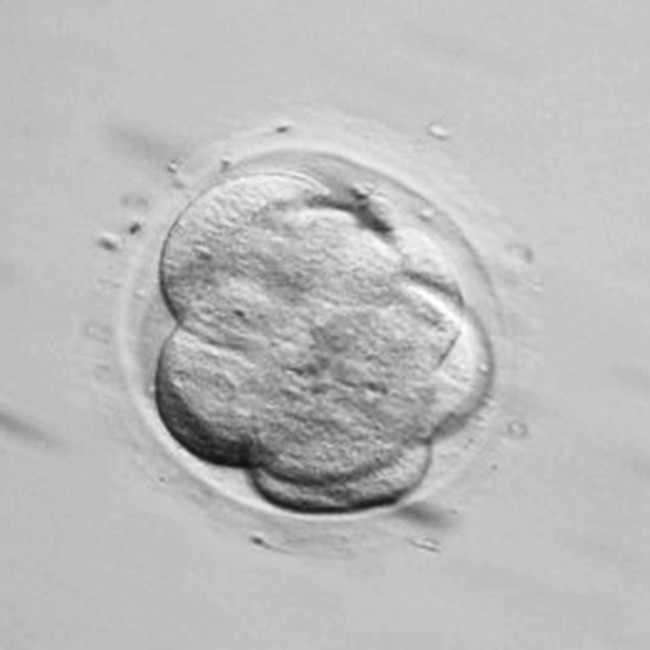
Embryo showing the beginning of compaction: membrane fusion is visible but cells can still be counted
Fig. 3.
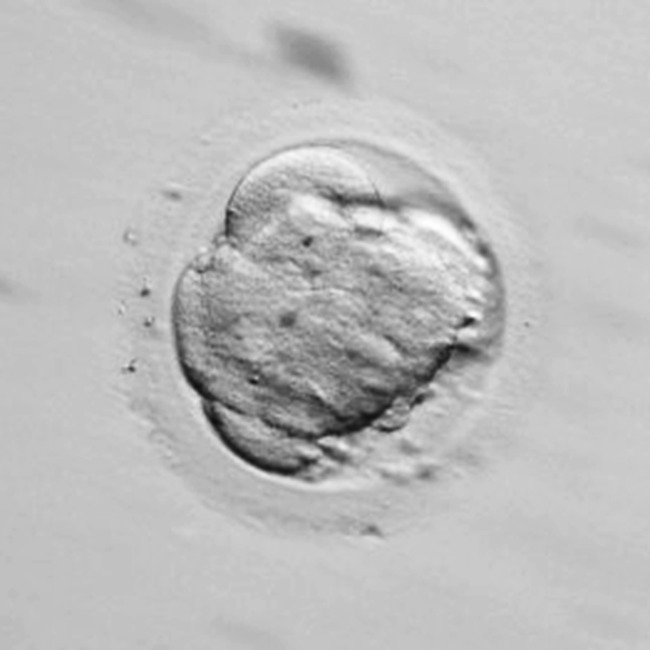
Embryo showing full compaction: cell boundaries can no longer be distinguished
Within the embryonic cohort, we transferred embryos with the best kinetic and morphologic criteria according to the BLEFCO classification. Compacted embryos were prioritized for transfer whether or not they originated from embryos of good quality. Embryonic fragmentation and the number of embryonic cells at day 2 were not taken into account if the embryo was compacted at day 3.
The presence of two pronuclei on day 1 is considered as a good prognostic factor [11]. However, embryos originating from zygotes without pronuclei, or with only one pronucleus, were included in the study since a significant proportion of such embryos has been shown to be diploid and capable of leading to healthy births [12]. The first preference was given to embryos that were compacted at day 3 and in which two pronuclei had been observed at day 1. Nevertheless, embryos that had less than two pronuclei at day 1 were also selected for transfer if they were the only ones that were compacted in the cohort at day 3.
Embryos showing any evident abnormality during their development, such as the existence of three pronuclei or mutinucleation, were systematically discarded because of the high risk of chromosomal aberration [19].
Assessment of outcome
A luteal phase supplementation was initiated on the day of oocyte retrieval with progesterone. An assay of serum ß-hCG was performed on day 14 after the embryo transfer. A positive result led to an ultrasound examination 6 weeks later in order to objectify the presence of one (or two) gestational sacks with cardiac activity, indicating an ongoing pregnancy.
Statistical analysis
Data were analysed using the χ2 test for categorical variables and the non-parametric Mann-Whitney U-test for continuous variables. For single embryo transfers, a logistic regression was carried out using the Wald method. This analysis gave the odds ratio, and the confidence interval was calculated using the Woolf method. All the calculations were done with Systat software, version 15.0 (SPSS Inc., Chicago, IL, USA). Differences were considered significant at p < 0.05 (*).
Results
We analysed a total of 317 embryo transfers on day 3, comprising 125 single-embryo transfers and 192 two-embryo transfers. Among the 509 embryos transferred, 82 attempts included 91 embryos with a beginning or a full compaction. Approximately one sixth of the embryos transferred were compacted. A quarter of the transfers included at least one compacted embryo with an average of 1.26 compacted embryos transferred. Only 15 % of the embryonic cohorts leading to the transfer of compacted embryo had more than two compacted embryos.
Investigation of the link between early compaction and the parameters of the attempts
We compared the parameters of the attempts between the two groups of patients according to the presence or absence of at least one compacted embryo in their embryonic cohort. There was no significant difference between the occurrence of early compaction and the type of assisted reproductive technology used, the protocol of stimulation, the age of the patient, or the estradiol level on the day of release. However, significantly more FSH units were administered to women having no compacted embryos. More oocytes were retrieved and more embryos were obtained in women having at least one compacted embryo in their cohort (Table 1).
Table 1.
Relationship between early compaction and the characteristics of the IVF attempts. Only the FSH units administered, the number of oocytes retrieved and the embryos obtained differed significantly between attempts with or without compacted embryos
| Attempts | Without any compacted embryo in the cohort | With at least one compacted embryo in the cohort | p | |
|---|---|---|---|---|
| Type of ART | cIVF (n = 131) | 74 % (n = 97) | 26 % (n = 34) | 0.132 χ2test |
| ICSI (n = 186) | 66 % (n = 123) | 34 % (n = 63) | ||
| Protocol | Agonist (n = 181) | 71 % (n = 129) | 29 % (n = 52) | 0.405 χ2test |
| Antagonist (n = 136) | 67 % (n = 91) | 33 % (n = 45) | ||
| Mean age of patients (years) | 31.56 | 31.52 | 0.762 Mann-Whitney U-test | |
| Mean number of FSH units administered | 2029.60 | 1793.81 | 0.008aMann-Whitney U-test | |
| Mean estradiol assay at release | 1828.20 | 1676.77 | 0.375 Mann-Whitney U-test | |
| Mean number of oocytes retrieved | 8.94 | 11.70 | <0.001aMann-Whitney U-test | |
| Mean number of embryos | 5.13 | 7.40 | <0.001aMann-Whitney U-test | |
aSignificant
Investigation of the link between early compaction and the usual prognostic factors
To determine whether the process of early compaction was related to the usual prognostic factors, we examined the link between early embryonic compaction and the existence of two pronuclei, the occurrence of early cleavage, and the embryonic quality on day 2 (Table 2). There was no significant difference between compacted and uncompacted embryos regarding the number of pronuclei and the existence of an early cleavage. However, early compaction was observed more frequently in embryos judged to be of good quality.
Table 2.
Relationship between early compaction and the number of pronuclei at day 1 and the occurrence of early cleavage and embryonic quality at day 2. Early compaction was observed more frequently in embryos judged to be of good quality according to the usual criteria
| Embryos transferred (day 3) | Non-compacted | Compacted | p | |
|---|---|---|---|---|
| Pronuclei (18–20 h post-insemination) | 0 | 9.3 % (n = 39) | 6.6 % (n = 6) | 0.443 χ2test |
| 1 | 0.9 % (n = 4) | 2.2 % (n = 2) | ||
| 2 | 89.8 % (n = 375) | 91.2 % (n = 83) | ||
| Early cleavage (25–29 h post-insemination) | Absent | 69.9 % (n = 292) | 64.8 % (n = 59) | 0.348 χ2test |
| Present | 30.1 % (n = 126) | 35.2 % (n = 32) | ||
| Embryonic quality at day 2 | Good | 68.4 % (n = 286) | 79.1 % (n = 72) | 0.043aχ2test |
| Poor | 31.6 % (n = 132) | 20.9 % (n = 19) | ||
asignificant
Investigation of the link between early compaction and successful pregnancy
Among the 317 embryo transfers realized on day 3, the presence of at least one compacted embryo in the cohort was significantly associated with better pregnancy rates. Indeed, the pregnancy rate (PR) was 44 % (43 pregnancies with 97 transfers) when the cohort contained at least one compacted embryo against 29.5 % (65 pregnancies with 220 transfers) (p = 0.01) (Fig. 4a).
Fig. 4.
Pregnancy rates (PR) and early compaction. a The PR is significantly higher in patients with at least one compacted embryo in their embryonic cohort (CEC+), than in those without any compacted embryos (CEC-). b The PR is significantly higher in transfers involving at least one compacted embryo (CET+) than in those without any compacted embryos (CET-). c For single embryo transfers, the transfer of one compacted embryo (EC+) led to significantly better PR than the transfer of one non-compacted embryo (EC-)
Moreover, there was a significantly greater chance of success when at least one compacted embryo was transferred. The PR was 44 % (36 pregnancies with 82 transfers) for transfers with at least one compacted embryo and 31 % (72 pregnancies with 235 transfers) for transfers concerning only cleaved embryos (p < 0.05) (Fig. 4b).
In the case of single-embryo transfers, the PR was significantly higher when the transfer involved a compacted embryo: 16 pregnancies with 32 transfers using a compacted embryo (PR 50 %), against 28 pregnancies with 93 transfers when a non-compacted embryo was used (PR 30 %; p < 0.05) (Fig. 4c). In this group, PR directly reflects implantation rate. For a compacted embryo, the chances of implanting are approximately three times higher than those of a non- compacted embryo (OR 2.98; CI 1.02–5.28). The logistic regression carried out, taking into account the role of early compaction as well as that of other factors, such as the maternal age, embryonic quality, the type of ART, and the different elements of the treatment used, i.e. agonist or antagonist, gonadotrophin, FSH units, showed that early compaction was significantly linked with the chances of implantation independently of the other factors (p < 0.05).
Discussion
The kinetic of embryo development is commonly used as a criterion of embryonic quality to select the best embryos for transfer. At the cleaved stage, a development that is too fast or too slow has a negative impact on embryonic implantation. Embryos with an unusual number of cells have been shown to have a higher number of chromosomal abnormalities [11].
Slow embryonic cleavage may reflect cytoplasmic incompetence that does not support embryonic divisions and genomic activation [5]. It is a negative factor that has been associated with a low rate of blastulation [33], decreased blastocyst quality [22] and a lesser likelihood of implantation [16]. Similarly, later in embryonic development, embryos not yet compacted at day 4 [10,27,36] and delayed morulas at day 5 [17] have a lower developmental activity.
The rapid cleavage of embryos is also a poor prognostic factor [23] but the literature on the phenomenon of advanced compaction on day 3 is scarce. Two retrospective studies suggest that it may be a rather good prognostic factor for embryonic potential [9,35]. However, these two studies, retrospectively considered the phenomenon of compaction in the embryonic transfers based on classical criteria. In our prospective study the embryos were first classified as compacted or not, and transferred in the maternal uterus, using this consideration as a priority criterion. After transfer, we checked tested the women for pregnancy. We believe that early compaction on day 3 could be a sign of embryonic scalability and we wanted to test its link with embryonic quality and potential of implantation.
We first compared two groups of patients according to whether or not their embryonic cohort had included compacted embryos. The stimulation protocols and the proportion of cIVF and ICSI procedures used were similar in both groups, thus eliminating any bias due to the influence of the method on the frequency of embryonic compaction. In contrast, the response to the treatment differed between the two groups. The number of FSH units required for a satisfactory ovarian response was significantly higher in the group without compacted embryos but in which fewer oocytes had been retrieved. This may indicate a weaker response to stimulation in this group, linked to impairment of the ovarian reserve and the poorer quality of oocytes [7]. Thus, early compaction on day 3 may reflect embryonic potential attributable to oocyte quality.
Moreover, early compaction occurred more frequently in embryos with a good morphology at the cleavage stage. Since embryonic morphology is related to embryonic potential, this is another argument in favour of a link between compaction at day 3 and embryonic scalability. Regardless of our transfer policy, we have considered the existence of two pronuclei and the occurence of an early cleavage on day 1, as the criteria of embryo competence. But we did not find any association between these markers and early compaction, possibly because of the small size of our series.
Beyond the fact that early compaction is a positive marker of embryonic potential, early compaction at day 3 could also help to refine our criteria for embryo selection. In our study, 72 of the 358 embryos deemed to be of good quality compacted precociously whereas 19 of the 151 embryos judged to be of poor quality embryos compacted at day 3. Thus, the criterion of early compaction could serve to select the most scalable among the good embryos and, similarly, to select those that may have good embryonic potential despite pejorative morphological criteria.
We then confirmed the hypothesis that early compaction on day 3 is a positive marker since significantly more pregnancies occurred with attempts involving at least one compacted embryo (p < 0.01). Similarly, the transfer of at least one compacted embryo led to pregnancy more frequently (p < 0.05). This is in accordance with a report indicating an increase of the pregnancy rate with the number of compacted embryos replaced [9]. However, when two embryos were transferred and only one of which was compacted, it was not possible to determine which was actually implanted. We therefore focused on 125 single-embryo transfers and found that the transfer of a compacted embryo led more frequently to pregnancy (p < 0.05), with an implantation rate approximately triple that of non-compacted embryos. Moreover early compaction is significantly linked with the chances of implantation independently of the other factors considered to condition the chances of implantation, such as the maternal age and embryonic quality (p < 0.05).
Recent progress in genomic, metabolomic and proteomic research may be expected to provide non-invasive markers of embryonic quality [31]. But to date, embryonic assessment has been based only on morphological and kinetic criteria. The importance of these criteria has been highlighted by many studies using time-lapse imaging of the developing embryo [21,24–26]. They notably defined new kinetics parameters associated with embryonic quality and some authors have reported an increase in the pregnancy rate of about 20 % [25]. Moreover these studies principally focused on the first divisions of the embryo until the 6-cell stage. To our knowledge, none of these investigated the onset of embryonic compaction in relation with the chance of implantation. Moreover such time-lapse incubators are time-consuming and expensive, and cannot be afforded by all IVF centers. Our observations suggest that compaction could serve as an easily applicable criterion to assess embryonic quality and viability. This would promote the embryo transfer at the cleavage stage with the advantage of reducing the time during which embryos are maintained in vitro. Indeed, suboptimal conditions of embryonic culture are likely to have a negative impact on embryonic development [8,30]. With a mouse model, in vitro culture was found to be linked to abnormalities of the imprinted genes [13], suggesting that assisted reproductive technologies might give rise to diseases due to imprinted genes [15]. However, it has been postulated that the developmental timing of human embryos produced in vitro may vary according to the conditions of culture [11]. The occurrence of early compaction could thus be the expression of embryonic potential depending on particular conditions of growth. This hypothesis needs to be tested and the phenomenon of early embryonic compaction event should be evaluated by each laboratory taking into account the specificity of its own conditions of culture.
Our results show that early embryonic compaction is rather uncommon since only a quarter of the attempts allowed the transfer of at least one compacted embryo, and since more than two compacted embryos were obtained in only 15 % of the attempts. It would be interesting to study larger cohorts to confirm our results and determine whether this process is linked with patient characteristics or to specific conditions for a given attempt.
The precise role played by early compaction in embryonic development still awaits elucidation. Nevertheless, early embryonic compaction at day 3 may serve as an additional criterion of selection, thereby contributing to successful pregnancy.
Acknowledgments
We thank Ms. C. Douillard, M. Guezenec, S. Lehais and C. Moyon, technicians at the In Vitro Fertilization Laboratory of the University Hospital of Angers, for their participation in the classification of embryos. We thank P. Saulnier for his help in the statistical analysis. We are grateful to Kanaya Malkani for his critical reading and comments on the manuscript.
Footnotes
Contribution for each author
SLC and PMP were the principal investigators and take primary responsibility for the paper. PMP and VFH contributed to the conception of design and coordinated the research. CaM, SL and PhD recruited the patients. SLC and PMP contributed to the collection and assembly of data. SLC and PMP contributed to the data analysis and interpretation. SLC and PMP contributed to manuscript writing. PMP, VFH, CaM, SL, PR, and PhD contributed to the drafting of the article, revised it and approved the final version.
Capsule
Early compaction at day 3 may serve as an additional criterion for embryo selection to improve pregnancy rates in in vitro fertilization.
References
- 1.Aboulghar MM, Aboulghar MA, Mansour RT, Serour GI, Amin YM, Abou-Setta AM. Pregnancy rate is not improved by delaying embryo transfer from days 2 to 3. Eur J Obstet Gynecol Reprod Biol. 2003;107:176–9. doi: 10.1016/S0301-2115(02)00400-1. [DOI] [PubMed] [Google Scholar]
- 2.Bahceci M, Ulug U, Ciray HN, Akman MA, Erden HF. Efficiency of changing the embryo transfer time from day 3 to day 2 among women with poor ovarian response: a prospective randomized trial. Fertil Steril. 2006;86:81–5. doi: 10.1016/j.fertnstert.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 3.Blondel B, Kaminski M. Trends in the occurrence, determinants, and consequences of multiple births. Semin Perinatol. 2002;26:239–49. doi: 10.1053/sper.2002.34775. [DOI] [PubMed] [Google Scholar]
- 4.Blondel B, Kogan MD, Alexander GR, Dattani N, Kramer MS, Macfarlane A, et al. The impact of the increasing number of multiple births on the rates of preterm birth and low birthweight: an international study. Am J Public Health. 2002;92:1323–30. doi: 10.2105/AJPH.92.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolton VN, Braude PR. Development of the human preimplantation embryo in vitro. Curr Top Dev Biol. 1987;23:93–114. doi: 10.1016/S0070-2153(08)60621-3. [DOI] [PubMed] [Google Scholar]
- 6.Braude P, Bolton V, Moore S. Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature. 1988;332:459–61. doi: 10.1038/332459a0. [DOI] [PubMed] [Google Scholar]
- 7.Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. 2009;30:465–93. doi: 10.1210/er.2009-0006. [DOI] [PubMed] [Google Scholar]
- 8.Chason RJ, Csokmay J, Segars JH, DeCherney AH, Armant DR. Environmental and epigenetic effects upon preimplantation embryo metabolism and development. Trends Endocrinol Metab. 2011;22:412–20. doi: 10.1016/j.tem.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desai NN, Goldstein J, Rowland DY, Goldfarb JM. Morphological evaluation of human embryos and derivation of an embryo quality scoring system specific for day 3 embryos: a preliminary study. Hum Reprod. 2000;15:2190–6. doi: 10.1093/humrep/15.10.2190. [DOI] [PubMed] [Google Scholar]
- 10.Ebner T, Moser M, Shebl O, Sommergruber M, Gaiswinkler U, Tews G. Morphological analysis at compacting stage is a valuable prognostic tool for ICSI patients. Reprod Biomed Online. 2009;18:61–6. doi: 10.1016/S1472-6483(10)60425-7. [DOI] [PubMed] [Google Scholar]
- 11.Embryology A. S. i. R. M. a. E. S. I. G. o. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26:1270–83. doi: 10.1093/humrep/der037. [DOI] [PubMed] [Google Scholar]
- 12.Feenan K, Herbert M. Can ‘abnormally’ fertilized zygotes give rise to viable embryos? Hum Fertil (Camb) 2006;9:157–69. doi: 10.1080/14647270600636269. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez-Gonzalez R, Moreira P, Bilbao A, Jimenez A, Perez-Crespo M, Ramirez MA, et al. Long-term effect of in vitro culture of mouse embryos with serum on mRNA expression of imprinting genes, development, and behavior. Proc Natl Acad Sci U S A. 2004;101:5880–5. doi: 10.1073/pnas.0308560101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glujovsky D, Blake D, Farquhar C, Bardach A. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev. 2012;7:CD002118. doi: 10.1002/14651858.CD002118.pub4. [DOI] [PubMed] [Google Scholar]
- 15.Gosden R, Trasler J, Lucifero D, Faddy M. Rare congenital disorders, imprinted genes, and assisted reproductive technology. Lancet. 2003;361:1975–7. doi: 10.1016/S0140-6736(03)13592-1. [DOI] [PubMed] [Google Scholar]
- 16.Huisman GJ, Alberda AT, Leerentveld RA, Verhoeff A, Zeilmaker GH. A comparison of in vitro fertilization results after embryo transfer after 2, 3, and 4 days of embryo culture. Fertil Steril. 1994;61:970–1. doi: 10.1016/s0015-0282(16)56715-6. [DOI] [PubMed] [Google Scholar]
- 17.Ivec M, Kovacic B, Vlaisavljevic V. Prediction of human blastocyst development from morulas with delayed and/or incomplete compaction. Fertil Steril. 2011;96:1473–8. doi: 10.1016/j.fertnstert.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Kanka J. Gene expression and chromatin structure in the pre-implantation embryo. Theriogenology. 2003;59:3–19. doi: 10.1016/S0093-691X(02)01267-0. [DOI] [PubMed] [Google Scholar]
- 19.Kligman I, Benadiva C, Alikani M, Munne S. The presence of multinucleated blastomeres in human embryos is correlated with chromosomal abnormalities. Hum Reprod. 1996;11:1492–8. doi: 10.1093/oxfordjournals.humrep.a019424. [DOI] [PubMed] [Google Scholar]
- 20.Laverge H, De Sutter P, Van der Elst J, Dhont M. A prospective, randomized study comparing day 2 and day 3 embryo transfer in human IVF. Hum Reprod. 2001;16:476–80. doi: 10.1093/humrep/16.3.476. [DOI] [PubMed] [Google Scholar]
- 21.Lemmen JG, Agerholm I, Ziebe S. Kinetic markers of human embryo quality using time-lapse recordings of IVF/ICSI-fertilized oocytes. Reprod Biomed Online. 2008;17:385–91. doi: 10.1016/S1472-6483(10)60222-2. [DOI] [PubMed] [Google Scholar]
- 22.Luna M, Copperman AB, Duke M, Ezcurra D, Sandler B, Barritt J. Human blastocyst morphological quality is significantly improved in embryos classified as fast on day 3 (> or = 10 cells), bringing into question current embryological dogma. Fertil Steril. 2008;89:358–63. doi: 10.1016/j.fertnstert.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 23.Magli MC, Gianaroli L, Ferraretti AP, Lappi M, Ruberti A, Farfalli V. Embryo morphology and development are dependent on the chromosomal complement. Fertil Steril. 2007;87:534–41. doi: 10.1016/j.fertnstert.2006.07.1512. [DOI] [PubMed] [Google Scholar]
- 24.Meseguer M, Herrero J, Tejera A, Hilligsoe KM, Ramsing NB, Remohi J. The use of morphokinetics as a predictor of embryo implantation. Hum Reprod. 2011;26:2658–71. doi: 10.1093/humrep/der256. [DOI] [PubMed] [Google Scholar]
- 25.Meseguer M, Rubio I, Cruz M, Basile N, Marcos J, Requena A. Embryo incubation and selection in a time-lapse monitoring system improves pregnancy outcome compared with a standard incubator: a retrospective cohort study. Fertil Steril. 2012;98:1481–9. doi: 10.1016/j.fertnstert.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 26.Mio Y, Maeda K. Time-lapse cinematography of dynamic changes occurring during in vitro development of human embryos. Am J Obstet Gynecol. 2008;199:660. doi: 10.1016/j.ajog.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 27.Pantos K, Makrakis E, Chronopoulou M, Biba M, Perdikaris A, Dafereras A. Day 4 versus day 3 embryo transfer: a prospective study of clinical outcomes. Fertil Steril. 2008;89:573–7. doi: 10.1016/j.fertnstert.2007.03.056. [DOI] [PubMed] [Google Scholar]
- 28.Puissant F, Van Rysselberge M, Barlow P, Deweze J, Leroy F. Embryo scoring as a prognostic tool in IVF treatment. Hum Reprod. 1987;2:705–8. doi: 10.1093/oxfordjournals.humrep.a136618. [DOI] [PubMed] [Google Scholar]
- 29.Rijnders PM, Jansen CA. The predictive value of day 3 embryo morphology regarding blastocyst formation, pregnancy and implantation rate after day 5 transfer following in-vitro fertilization or intracytoplasmic sperm injection. Hum Reprod. 1998;13:2869–73. doi: 10.1093/humrep/13.10.2869. [DOI] [PubMed] [Google Scholar]
- 30.Schultz RM. From egg to embryo: a peripatetic journey. Reproduction. 2005;130:825–8. doi: 10.1530/rep.1.00902. [DOI] [PubMed] [Google Scholar]
- 31.Seli E, Robert C, Sirard MA. OMICS in assisted reproduction: possibilities and pitfalls. Mol Hum Reprod. 2010;16:513–30. doi: 10.1093/molehr/gaq041. [DOI] [PubMed] [Google Scholar]
- 32.Shahine LK, Milki AA, Westphal LM, Baker VL, Behr B, Lathi RB. Day 2 versus day 3 embryo transfer in poor responders: a prospective randomized trial. Fertil Steril. 2011;95:330–2. doi: 10.1016/j.fertnstert.2010.06.093. [DOI] [PubMed] [Google Scholar]
- 33.Shapiro BS, Harris DC, Richter KS. Predictive value of 72-hour blastomere cell number on blastocyst development and success of subsequent transfer based on the degree of blastocyst development. Fertil Steril. 2000;73:582–6. doi: 10.1016/S0015-0282(99)00586-5. [DOI] [PubMed] [Google Scholar]
- 34.Shen S, Rosen MP, Dobson AT, Fujimoto VY, McCulloch CE, Cedars MI. Day 2 transfer improves pregnancy outcome in in vitro fertilization cycles with few available embryos. Fertil Steril. 2006;86:44–50. doi: 10.1016/j.fertnstert.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 35.Skiadas CC, Jackson KV, Racowsky C. Early compaction on day 3 may be associated with increased implantation potential. Fertil Steril. 2006;86:1386–91. doi: 10.1016/j.fertnstert.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 36.Tao J, Tamis R, Fink K, Williams B, Nelson-White T, Craig R. The neglected morula/compact stage embryo transfer. Hum Reprod. 2002;17:1513–8. doi: 10.1093/humrep/17.6.1513. [DOI] [PubMed] [Google Scholar]
- 37.Tsai YC, Chung MT, Sung YH, Tsai TF, Tsai YT, Lin LY. Clinical value of early cleavage embryo. Int J Gynaecol Obstet. 2002;76:293–7. doi: 10.1016/S0020-7292(01)00591-4. [DOI] [PubMed] [Google Scholar]
- 38.Vajta G, Rienzi L, Cobo A, Yovich J. Embryo culture: can we perform better than nature? Reprod Biomed Online. 2010;20:453–69. doi: 10.1016/j.rbmo.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 39.Ziebe S, Petersen K, Lindenberg S, Andersen AG, Gabrielsen A, Andersen AN. Embryo morphology or cleavage stage: how to select the best embryos for transfer after in-vitro fertilization. Hum Reprod. 1997;12:1545–9. doi: 10.1093/humrep/12.7.1545. [DOI] [PubMed] [Google Scholar]



