Abstract
Purpose
To investigate production of progesterone’s precursor, pregnenolone, in the early oocyte donation pregnancy.
Methods
Pregnenolone and progesterone were measured on luteal days 21, 28, 35, 60 and 80. Progesterone was measured via the Immulite system, pregnenolone by liquid chromatography separation with tandem mass spectrometric detection.
Results
Progesterone rose significantly from days 35 today 60. Pregnenolone likewise rose significantly from days 35–60, but at a much higher rate, with an increase of 57 % by day 60, 75 % to day 80. The increase in pregnenolone was statistically more significant than the increase in progesterone (p < .05).
Conclusions
This is the first report describing that progesterone’s precursor, pregnenolone, increases with time in the very early pregnancy. Because no corpus luteum is present in oocyte recipients, the main source of pregnenolone is the early placenta. Measurements of pregnenolone may provide information concerning early trophoblast function and may represent a method of assessing placental competency.
Keywords: Endocrinology of pregnancy, Oocyte donation, Pregnenolone, Progesterone
Introduction
Pregnenolone is the only steroid derived directly from cholesterol, and it is the sole source of every other steroid molecule. Typically, the competency of early luteal/placental function has been indirectly assessed by the measurement of peripheral progesterone levels. However progesterone levels vary during the early pregnancy (first trimester) due to pulsatile secretion by the corpus luteum as well as the expected decline between 7 and 10 weeks of gestation until placenta takes over the progesterone secretion to support the pregnancy [1–3]. Therefore measuring the progesterone levels in early pregnancy is of very limited value and often misleading [3].
Measuring the source of progesterone, pregnenolone, may provide more specific information about the functioning of the fetal-placenta unit and could give further insight into the timing of the luteoplacental shift. Although the placenta and fetal and maternal adrenal glands produce pregnenolone, the placenta is probably the major contributor of pregnenolone production during early pregnancy [3].
We employed the oocyte donation model to study pregnenolone production because such pregnancies exist in the absence of maternal ovarian function, making any changes in peripheral levels indicative of trophoblast output.
In this preliminary study, pregnenolone levels in the early donor egg pregnancy were ascertained to determine if levels changed with time, and if there was a relationship between the levels of pregnenolone and its product, progesterone.
Materials and methods
Oocyte donors underwent standard controlled ovarian hyperstimulation and oocyte retrieval [4]. Oocyte recipients were given estradiol, 6 mg orally per day to thicken the endometrium. On the day after the donor’s ovulation trigger, recipients initiated 50 mg of intramuscular progesterone in oil. Embryo transfer occurred after 5 days of culture. Estrogen and progesterone were continued until menstrual day 60, when the progesterone dose was lowered to 25 mg per day, and all medications were discontinued on day 80. As part of their standard of care, oocyte recipients underwent phlebotomy on luteal days 21, 28, 35, 60 and 80.
Progesterone measurements were performed using the Immulite system (Diagnostic Products Corporation, Los Angeles, USA). The intra assay coefficient of variation (CV) is 7−10 %, 9−12 % for the inter-assay. For pregnenolone analysis, the serum was frozen after progesterone testing, and sent on ice to Endocrine Sciences (Calabasas Hills, CA), where all samples were tested in the same run. Analysis was performed using liquid chromatography separation with tandem mass spectrometric detection (LC-MS/MS) using an MDS-Sciex API5000 triple quadrupole mass spectrometer. The intra-day CV ranges from 2 to 3 %.
Recipients with a normal singleton pregnancy progressing to at least 20 weeks using a fresh transfer from 2009 to 2011 were used. Patients who received any luteal support other than 50 mg of progesterone in oil IM were excluded. Also excluded were women with blood tested in outside labs or those who did not have blood drawn on the prescribed days. We could not include women whose stored serum on any day was of low volume or whose serum was previously used for another study. Thus, cycles of 19 women qualified.
Statistical analysis
Descriptive statistics were compared as mean ± SD. Because progesterone naturally tends to produce much higher levels than pregnenolone, we analyzed progesterone and pregnenolone in separate models. The hormone values were log-transformed to normalize the sample distributions. Then the rise of hormone at each follow-up day against levels at the 21st day (reference day) was evaluated as the percentage change and was individually tested against no change using Student’s two-tailed t test at each follow-up date. One way analysis of variance (ANOVA) with repeated measures in linear mixed model was also applied to analyze the effects of the day-by-day variability on each hormone levels; progesterone and pregnenolone concentrations were analyzed as a continuous dependent and a day indicator nested under the same individual in order to reflect day-variability in a separate model. Two sided p-values < 0.05 were considered to be statistically significant throughout the analysis. R statistical package was used for calculations (www.R-project.org). Statistical power (1-β) of these results was computed using G*Power 3.1
Results
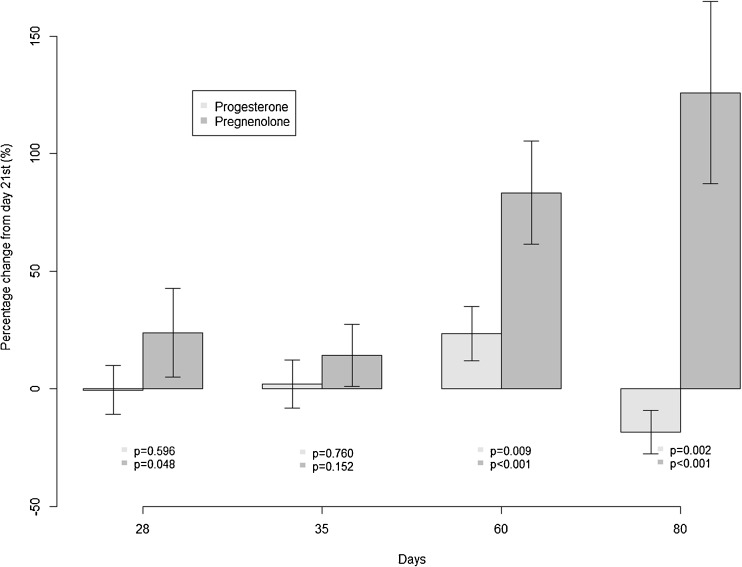
The mean and standard deviation of progesterone and pregnenolone levels from days measured are summarized in Table 1. Levels of both hormones significantly changed over time (p-value < 0.0001). Figure 1 is the graphic representation of the comparison of the changes in pregnenolone levels over time and progesterone levels over time individually. Data were presented as percent changes in each hormone levels at given luteal days compared to the luteal day 21 level (reference group). A marked increase was observed in pregnenolone levels (at 28 days, p-value = 0.048), which occurred earlier than changes in progesterone levels. We achieved 83.6 % power for this finding.
Table 1.
Summary of progesterone and pregnenolone levels early in the DE pregnancy. Data is presented as mean ± SD. Note units
| Days | Pregnenolone (ng/dl) | Progesterone(ng/dl) |
|---|---|---|
| 21 | 62.5 ± 37.8 | 3005.8 ± 852.6 |
| 28 | 75.1 ± 53.8 | 2913.5 ± 820.22 |
| 35 | 69.8 ± 42.8 | 2963.3 ± 618.9 |
| 60 | 109.6 ± 66.0 | 3628.4 ± 1049.3 |
| 80 | 122.4 ± 46.4 | 2363.68 ± 553.05 |
| p-value1 | <0.0001 | <0.0001 |
p-value1 was calculated from one way analysis of variance (ANOVA) with repeated measures in linear mixed model
Fig. 1.
Changes in progesterone and pregnenolone from days 21 to 80
Discussion
This preliminary study demonstrates that pregnenolone levels rise significantly in the early donor egg pregnancy, and that these changes are independent of progesterone levels. Because no corpus luteum is present in oocyte recipients, the main source of this increase is the early placenta. Additional studies following levels in both normal and failing natural pregnancies, including ectopic pregnancies, may be of value.
Pregnenolone is mainly produced by placenta, which converts cholesterol to pregnenolone during the early pregnancy [3, 5]. However maternal adrenal and fetal adrenal gland may also contribute to the pregnenolone production since both these tissues have cytochrome p450 side chain cleavage enzyme that converts cholesterol to pregnenolone [6, 7]. Some authors argue that fetal liver may even contribute to the pregnenolone production by providing the cholesterol as a substrate to the fetal adrenal gland [3].
Pregnenolone is converted to progesterone via 3-beta hydroxysteroid dehydrogenase [8]. The actions of this complex system result in a reaction that flows in one direction only: i.e. the reaction is irreversible [3]. Therefore, increases in pregnenolone levels are not due to conversion of administered progesterone.
Now that an early increase in pregnenolone production has been observed, a number of possible research areas can be explored. Measurements of pregnenolone may provide additional information concerning early placental function and may represent an improved method of assessing placental competency. While at this time theoretic, pregnenolone levels could identify pathology related to disorders of implantation or even metabolic and genetic disorders related to the fetus. A precedent for using hormones to assess fetal aneuploidy has been established, as in the cases of human placental growth hormone [9] and even progesterone [10].
It remains unclear why an increase in placental pregnenolone production does not correlate with an increase in progesterone levels. Differential rates of hormone production, metabolism, protein binding, or excretion [8, 11, 12] could be involved. Reaction rates can be dependent on electron donator cofactors [8], phosphorylation reactions [13] and can be inhibited by the concentrations of precursors or products [12]. It has also been hypothesized that pregnenolone can saturate 3-beta hydroxysteroid dehydrogenase enzyme [11].
The fetus may be a contributor of maternal pregnenolone, but not until later in pregnancy. While fetal adrenal cells have been shown to produce dehydroepiandrostenedione sulfate at 8–10 weeks and cortisol at 7–10 weeks gestation, the increases in pregnenolone measured in this study began much earlier [14]. In our study, which includes early pregnancies, one may argue that the source of pregnenolone may be the fetal and/or maternal adrenal gland. However it has been shown that although the fetal and maternal adrenal gland may contribute to the pregnenolone synthesis during the second and third trimester of pregnancy, the major pregnenolone production comes from the placenta during the early pregnancy [15, 16]. The observation that maternal urinary estradiol was very low in human pregnancies with an anencephalic fetus provided the initial insights into the role of the fetal adrenal in steroid production. In fetuses with anencephaly, the fetal adrenal glands are also smaller than that of normal fetuses and the maternal pregnenolone and progesterone concentrations are within normal range suggesting that the main source of pregnenolone and progesterone is placenta and the degree of fetal adrenal gland’s contribution to pregnenolone synthesis in early pregnancy is minimal [16]. It is well known that a functioning fetal circulation is unimportant for the regulation of progesterone levels in the maternal unit [15]. In fact, fetal death, ligation of the umbilical cord or anencephaly, which all are associated with a decrease in estrogen production, have no significant effect on progesterone levels in the maternal compartment. In addition a study by Carr et al. showed that at 6 and 9 weeks of gestation, the fetal adrenal gland weighs 2.6 ± 0.7 mg and 11.5 ± 2.2 mg respectively whereas it weighs 375 ± 3 mg at 16.5 weeks of gestation. Fetal adrenal gland at 16.5 weeks of gestation is 145 fold heavier than the 6-week-old fetal or embryonic adrenal gland. These findings also indirectly suggested that during the early gestation, the fetal adrenal glands are too small to be able to synthesize the majority of pregnenolone and the main source of pregnenolone is the placenta [17]. Based on all these above-mentioned reasons, it is fair to suggest that the main source of the pregnenolone in our study is the early placenta and not the fetal adrenal gland or any other potential source.
In summary, we have demonstrated that pregnenolone increases early in pregnancy and the source of this increase is mainly the trophoblast. These results provide novel information, and ignite interest in the further study of trophoblast pregnenolone production throughout gestation.
Acknowledgement
The authors would like to acknowledge our compliance officer Lucy Lu for her work in obtaining institutional approval of this research.
Ms. Lu has given written permission for this acknowledgment.
Footnotes
Capsule
Measurable increases in progesterone’s precursor, pregnenolone, occur in the early oocyte donation pregnancy, indicating trophoblast as the source.
References
- 1.Schneider MA, Davies MC, Honour JW. The timing of placental competence in pregnancy after oocyte donation. Fertil Steril. 1993;59:1059–64. doi: 10.1016/s0015-0282(16)55928-7. [DOI] [PubMed] [Google Scholar]
- 2.Mishell DR, Jr, Thorneycroft IH, Nagata Y, Murata T, Nakamura RM. Serum gonadotropin and steroid patterns in early human gestation. Am J Obstet Gynecol. 1973;117:631–42. doi: 10.1016/0002-9378(73)90205-6. [DOI] [PubMed] [Google Scholar]
- 3.Fritz M, Speroff L. Clinical gynecologic endocrinology and infertility. Textbook 2011;8th edition: 270–5.
- 4.Mullin CM, Fino ME, Talebian S, Keegan D, Grifo JA, Licciardi F. Comparison of pregnancy outcomes in anonymous shared versus exclusive donor oocyte in vitro fertilization cycles. Fertil Steril. 2010;93:574–8. doi: 10.1016/j.fertnstert.2009.07.1669. [DOI] [PubMed] [Google Scholar]
- 5.Tuckey RC. Progesterone synthesis by the human placenta. Placenta. 2005;26:273–81. doi: 10.1016/j.placenta.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 6.Carr BR, Parker CR, Jr, Milewich L, Porter JC, MacDonald PC, Simpson ER. The role of low density, high density, and very low density lipoproteins in steroidogenesis by the human fetal adrenal gland. Endocrinology. 1980;106:1854–60. doi: 10.1210/endo-106-6-1854. [DOI] [PubMed] [Google Scholar]
- 7.Klepac R. Fetal rat adrenal gland steroidogenesis in vitro in prolonged pregnancy. Biol Neonate. 1981;39:165–70. doi: 10.1159/000241422. [DOI] [PubMed] [Google Scholar]
- 8.Kominami S, Owaki A, Iwanaga T, Tagashira-Ikushiro H, Yamazaki T. The rate-determining step in P450 C21-catalyzing reactions in a membrane-reconstituted system. J Biol Chem. 2001;276:10753–8. doi: 10.1074/jbc.M006043200. [DOI] [PubMed] [Google Scholar]
- 9.Sifakis S, Akolekar R, Syngelaki A, De Cruz J, Nicolaides KH. Maternal serum human placental growth hormone at 11 to 13 weeks in trisomy 21 and trisomy 18 pregnancies. Prenat Diagn. 2010;30:212–5. doi: 10.1002/pd.2555. [DOI] [PubMed] [Google Scholar]
- 10.Christiansen M, Sorensen TL, Larsen SO, Norgaard-Pedersen B. First-trimester maternal serum progesterone in aneuploid pregnancies. Prenat Diagn. 2008;28:319–22. doi: 10.1002/pd.1843. [DOI] [PubMed] [Google Scholar]
- 11.Hill M, Parizek A, Jirasek JE, Jirkovska M, Velikova M, Duskova M, et al. Is maternal progesterone actually independent of the fetal steroids? Physiol Res. 2010;59:211–24. doi: 10.33549/physiolres.931807. [DOI] [PubMed] [Google Scholar]
- 12.Yamazaki T, Ohno T, Sakaki T, Akiyoshi-Shibata M, Yabusaki Y, Imai T, et al. Kinetic analysis of successive reactions catalyzed by bovine cytochrome p450(17alpha, lyase) Biochemistry. 1998;37:2800–6. doi: 10.1021/bi9715066. [DOI] [PubMed] [Google Scholar]
- 13.Lohr JB, Kuhn-Velten WN. Protein phosphorylation changes ligand-binding efficiency of cytochrome P450c17 (CYP17) and accelerates its proteolytic degradation: putative relevance for hormonal regulation of CYP17 activity. Biochem Biophys Res Commun. 1997;231:403–8. doi: 10.1006/bbrc.1997.6113. [DOI] [PubMed] [Google Scholar]
- 14.Ishimoto H, Jaffe RB. Development and function of the human fetal adrenal cortex: a key component in the feto-placental unit. Endocr Rev. 2011;32:317–55. doi: 10.1210/er.2010-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kenneth LB. Endocrinology of the female. Principles and practice of endocrinology and metabolism. 1990:888–90.
- 16.Fritz F, Kropper, A. Metabolism of cortisol. Endocrinology of pregnancy 1983;3rd edition.
- 17.Carr BR, Casey ML. Growth of the adrenal gland of the normal human fetus during early gestation. Early Hum Dev. 1982;6:121–4. doi: 10.1016/0378-3782(82)90098-6. [DOI] [PubMed] [Google Scholar]