Abstract
Purpose
To identify the role of both genetic (number of CGG repeats in the FMR1 gene) and autoimmune factors (anti-ovarian antibodies) in premature ovarian failure (POF).
Methods
In cross-sectional study, 78 women with POF were divided into 3 groups by the number of CGG repeats (less than 28, 28–36, more than 36) in any of the FMR1 gene alleles. We performed the detection of skewed X-chromosome inactivation, CGG repeats in the FMR1 gene, anti-ovarian antibodies (AOA) and sex hormones tests.
Results
Compared to a higher or lower number of CGG repeats the 28–36 triple CGG counts are strongly associated with the AOA detection (RR = 19.23, 95 % CI = 2.63–100.0). The women with autoimmune-driven POF have significantly higher anti-Mullerian hormone levels in comparison to women with non-autoimmune-driven POF.
Conclusion
The presence of AOA above 10 IU/mL is associated with the normal number of CGG repeats in regard to ovarian reserve and a better preservation of follicular primordial pool in the women with POF.
Keywords: FMR1 (fragile mental retardation) gene of fragile Х-chromosome, CGG nucleotide repeats, Premature ovarian failure (POF), Anti-Mullerian hormone (AMH), Anti-ovarian antibodies
Introduction
Premature ovarian failure (POF) is one of the most mysterious diseases of the reproductive system. It is characterized by secondary hypergonadotropic amenorrhea in women under 40, and affects about 1–2 % of women [20]. Currently, there are no prognostic criteria for POF. The modern markers of ovarian reserve allow the evaluation of preantral and antral follicles counts, but not the individual primordial pool [10]. Hormonal and biochemical tests do not show the monthly follicle loss and thus do not indicate the true biological age of ovaries. The search for new molecular and biological markers, which can help to forecast the rate of ovarian ageing and their premature failure, is on the international medical agenda.
POF is believed to result from various epigenetic and genetic factors. In most cases, premature ovarian senescence is associated with the X chromosome abnormalities. The X chromosome inactivation is the inactivation of one out of the two X chromosomes in female somatic cells [15]. The X chromosome structural abnormalities, such as large deletions and unbalanced translocations, may result in the skewed patterns of the X chromosome inactivation (SXCI) with the abnormal inactive X chromosome in the most cells of the organism. The SXCI can be associated with idiopathic POF [15]. Initially, the damage of the long arm of the X-chromosome, particularly the FMR1 (fragile mental retardation) gene premutation with number of CGG repeats of more than 55, was considered the main cause of POF [2, 6, 22]. Later, lower CGG counts were associated with POF as well [12]. The CGG counts of 26–34 (median 30) indicate the normal ovarian function and its timely switching off [4, 11].
However, POF does not always result from genetic abnormalities. We have found that 45 % of POF patients have the CGG counts of 26–34, that rules out the genetic origin of the disease [16]. The analysis of several POF cases suggests an underlying autoimmune origin of the disease [8]. The clarification of ovarian ageing causes in this group of women was the rationale for the more in-depth clinical and laboratory investigation.
The objective of the study was to identify the role of genetic (CGG repeats in the FMR1 gene) and autoimmune factors (anti-ovarian antibodies) in premature ovarian failure.
Materials and methods
In September, 2009 - December, 2011, we recruited 78 women with POF to participate in the study. The inclusion criteria were: age under 40, desired fertility, the presence of secondary amenorrhea, and two serum FSH levels higher than 40 IU/L, measured with a month interval. All subjects gave written informed consent for participation in the study. The study design was cross-sectional with one-time measurements. The genetic testing was performed in the molecular genetics laboratory of the FMSMU with the genetic analyzer АВI3100. The skewed X-chromosome inactivation was detected by methyl-sensitive quantitative fluorescent polymerase chain reaction (PCR) of CAG repeats in exon 1 of the AR gene [1]. The size of PCR product from each allele was analyzed by Gene Mapper v.3.5 software for the quantification of peak area. Differences in size ratio of the heterozygous two-peak patterns suggested skewed XCI. XCI status was classified as random (XCI < 70 %), or skewed (XCI ≥ 70 %) [17]. The FMR1 gene examination included detection of CGG repeats in 5′- nontranslating region of exon 1 of this gene. Gleicher et al. [11] and other colleagues [4, 9] have defined the number of CGG repeats of 26–34 as normal in regard to ovarian function, however the Russian population was not presented in these studies.
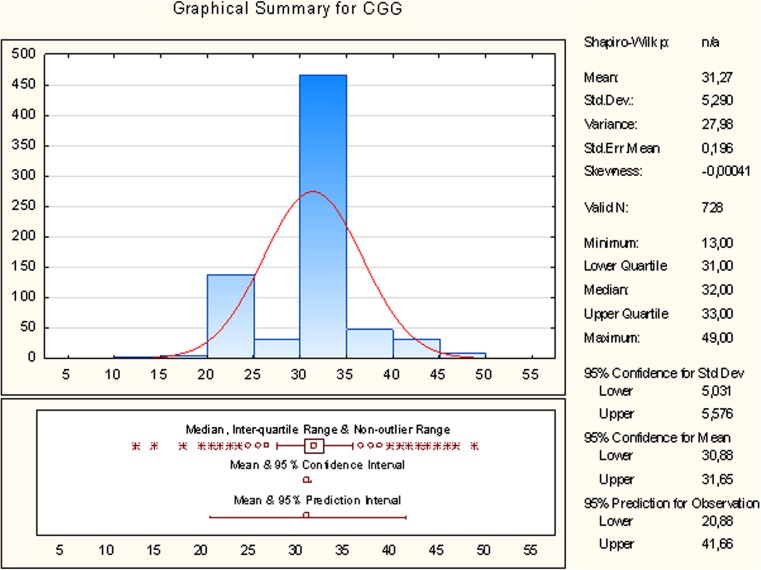
We calculated the reference for CGG repeats by box and whisker plotting using the data of 364 apparently healthy women from the European part of Russia. These women were admitted to the FMSMU for routine examinations. They had regular periods, normal menstrual and obstetrical history. Their mean age was 42.3 ± 0.9 years/range 40–49 years and therefore, they did not have POF. Half of the women had CGG counts of 31–33 (median 32). Addition and subtraction of 1.5-times interquartile range to the upper and lower quartile respectively formed the non-outlier range of 28–36 triple CGG repeats, which was used as the reference value in respect to normal ovarian function (Fig. 1).
Fig. 1.
Distribution of CGG repeats in the FMR1 gene of patients with POF. Median = 32, inter-quartile range composes 31–33 CGG repeats, non-outlier range composes 28–36 CGG repeats
According to the number of CGG repeats in any FMR1 gene allele (allele 1 with lower number, allele 2 with higher number of repeats), the POF patients were divided into 3 groups:
Group А - women with allele 1 < 28 triple CGG repeats, n = 24;
Group В - women with allele 1 ≥ 28 and allele 2 ≤ 36 triple CGG repeats, n = 33;
Group С - women with allele 2 > 36 triple CGG repeats, n = 21.
The investigation of the patients included physical examination, hormone and anti-ovarian antibody tests, and ultrasound imaging. The hormone testing was performed in the hormone laboratory of the RCOGP on blood samples, using the commercial assays: anti-Mullerian hormone (АМH) (“AMH Gen II enzyme-linked immunoabsorbent assay (ELISA)”, REF. A79765, Beckman Coulter, USA), follicle-stimulating hormone (FSH) (“FSH”, REF.L2KFS6, Immulite®2000 Systems, Siemens, USA), testosterone (Т) (“TES Total Testosterone”, REF. L2KTW6, Immulite®2000 Systems, Siemens, USA). Testing of anti-ovarian antibodies (AOA) in blood samples was performed by ELISA in the laboratory of immunology of the RCOGP, using the commercial assays (“Anti-ovary antibody ELISA”, Cat No: BS-40-20, Bioserv Diagnostics GmbH, Germany) [3, 8, 21]. Reference level is 0–10 IU/mL.
The statistical analysis was performed with Statistica 10 software (USA). The two-sided p < .05 was considered to indicate statistical significance. The continuous data were tested for normality and presented as mean ± standard deviation (M ± SD) with the evaluation of statistical significance between the 2 groups by t-test if normally distributed, or by Wilcoxon sum rank test if abnormally distributed, and between the 3 groups by Kruskal-Wallis test. The categorical data were presented as percentages and assessed by χ2 test. The measure of association was risk ratio (RR) ± 95 % confidence interval (CI).
The study was approved by local IRB.
Results
We examined 78 patients with POF who met the inclusion criteria. The age of the patients was 33.2 ± 5.86 years/range 19–39 years. The main complaint was secondary amenorrhea (range 7 months – 13 years) and infertility (100 % of women). The duration of amenorrhea in the group A was 4.7 ±3.6 years, in the group B - 3.1 ± 2.3 years, in the group C - 4.5 ± 4.6 years (р > 0.05), with start-up at the age of 27.9 ± 7.1, 30.0 ± 7.4 and 30.3 ± 5.8 years respectively (p > 0.05). The patients had the high incidence of mumps (27.3 %, 45.7 % and 38.1 %) and rubella in childhood (27.3 %, 48.6 % and 23.8 %) (p > 0.05). The family history was burdened by high autoimmune morbidity in the relatives (18.2 %, 22.9 % and 9.5 % in the groups A, B and C respectively, (p > 0.05)).
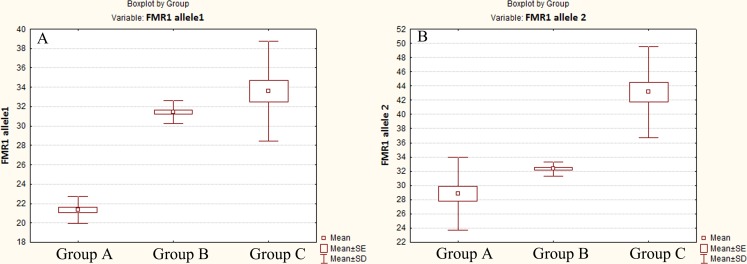
The premutation of the FMR1 gene was identified in 4 of 78 patients (5.1 %), and SXCI - in 19 of 78 patients (24.3 %). In the group А, 9 patients had SXCI (37.5 %); all patients had the triple CGG counts below 28. In the group С, 5 patients had SCXI (23.8 %); all patients had the triple CGG counts above 36. Thus, the premature ovarian senescence in the groups A and C was primarily genetic stipulated (100 % of patients had the CGG counts outside of the reference range in respect to normal ovarian function) or could also result from epigenetic origin (31.1 % of patients in the groups A and C). In the group B, 5 patients had SCXI (15.1 %), and all women had the CGG counts in the range of 28–36. The average number of repeats in allele 1 was 21.58 ± 1.76 (range 18–27) in the group A, 31.60 ± 0.86 (range 28–33) in the group B, and 33.66 ± 5.29 (range 28–45) in the group C (p < 0.00001). The average number of repeats in allele 2 was 29.00 ± 5.11 (range 20–35) in the group A, 32.39 ± 1.08 (range 31–35) in the group B, and 43.52 ± 6.33 (range 37–60) in the group C (p < 0.00001). Figure 2 shows the distribution of the CGG repeats on the FMR1 gene in the POF patients.
Fig. 2.
Box and whisker plot, defining the distribution of CGG repeats in allele 1 (a) and allele 2 (b) of the FMR1 gene in the patients with POF. Mean of CGG count in the group B differed significantly from the groups A and C in both alleles of the FMR1 gene (p < 0.00001)
The anti-ovarian antibodies above 10 IU/mL were detected in 1 of 24 patients in the group A (4.16 %), in none of 21 patients in the group C, and in 14 of 33 patients in the group B (42.4 %) (р < 0.0001). In 14 women of the group B (42.45 %), the cause of POF was not identified as they did not have SCXI or AOA above 10 IU/mL. Thus, the autoimmune pathology was significantly more often observed in the women with 28–36 CGG counts compared to those with higher or lower CGG counts (RR = 19.23, 95 % CI = 2.63–100.0). The average number of CGG repeats in allele 1 in the women with autoimmune anti-ovarian antibodies was 31.3 ± 2.02 compared to 28.5 ± 6.39 in the women with no autoimmune pathology (p = 0.0405). The average number of CGG repeats in allele 2 was 32.9 ± 1.14 and 34.7 ± 8.05 respectively in these groups of women (р = 0.7854). Hereby, the women with autoimmune-driven POF had significantly higher CGG counts in allele 1 and lower CGG counts in allele 2 of the FMR1 gene.
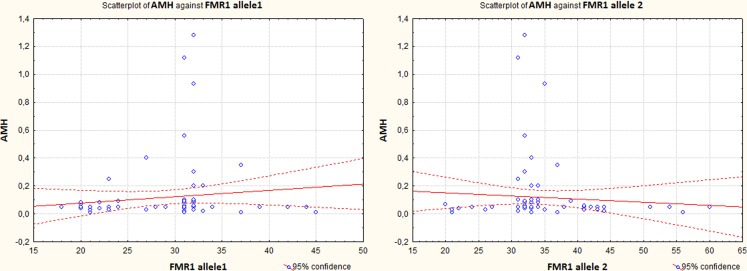
AMH concentration was significantly higher in the group B compared to the other groups of the women (0.07 ± 0.08, 0.19 ± 0.31, 0.05 ± 0.06 ng/mL respectively, (p = 0.0027)) (Fig. 3). In the 15 patients with anti-ovarian antibodies, AMH concentration was higher than in the other patients with POF (0.26 ± 0.36 ng/mL vs. 0.08 ± 0.15 ng/mL respectively, (р = 0.0035)). AMH is an accurate marker of the ovarian reserve. The relatively high concentration of AMH in the women with autoimmune-driven POF can be explained by a better preservation of primordial pool of follicles [14]. However, the FSH concentration, which indicates the ovarian function, did not differ significantly in the groups of the patients (113.8 ± 37.8, 109.4 ± 32.04, 103.02 ± 40.7 IU/L in the groups A, B and C (p = 0.5356); 115.02 ± 29.5 vs. 107.6 ± 37.5 IU/L in the groups with autoimmune and non-autoimmune-driven POF (p = 0.4776)). The testosterone concentration was significantly lower in the patients with autoimmune-driven POF (0.84 ± 0.41 vs. 1.29 ± 0.67 nmole/L, (p = 0.0151)), and in the patients with 28–36 triple CGG repeats compared to the other groups (1.66 ± 0.54 nmole/L in the group A, 0.89 ± 0.52 nmole/L in the group B, 1.17 ± 0.68 nmole/L in the group C, (p = 0.0001)) (Table 1). This may result from the selective autoimmune damage of theca cells as opposed to granulose cells of the ovaries [14].
Fig. 3.
AMH concentrations in women with different CGG count. AMH concentrations were significantly higher in women with normal CGG count (p = 0.0008)
Table 1.
Characteristics of the patients with POF and different number of CGG repeats
| Characteristics | Group A | Group B | Group C | p-value |
|---|---|---|---|---|
| n = 24 | n = 33 | n = 21 | ||
| Age (years) | 32.6 ± 6.1 | 33.0 ± 6.0 | 33.9 ± 5.5 | 0.7568 |
| FSH (IU/L) | 113.8 ± 37.8 | 109.4 ± 32.0 | 103.0 ± 40.7 | 0.5356 |
| AMH (ng/mL) | 0.07 ± 0.08 | 0.19 ± 0.31 | 0.05 ±0.06 | 0.0027 |
| Testosterone (nmole/L) | 1.66 ± 0.54 | 0.89 ± 0.52 | 1.17 ± 0.68 | 0.0001 |
| SXCI | 9 (37.5 %) | 5 (15.1 %) | 5 (23.8 %) | 0.1517 |
| CGG repeats in the FMR1 gene | ||||
| Allele 1 | 21.58 ± 1.76 | 31.60 ± 0.86 | 33.66 ± 5.29 | <0.00001 |
| Allele 2 | 29.00 ± 5.11 | 32.39 ± 1.08 | 43.52 ±6.33 | <0.00001 |
| Anti-ovarian antibodies | 1 (4.16 %) | 14 (42.4 %) | 0 (0 %) | <0.0001 |
Continuous data are means ± SD and assessed by Kruskal Wallis test
Categorical data are percentages and assessed by χ2 test
Discussion
Both autoimmune and genetic disorders can cause POF [5, 8, 14]. Autoimmune disorders are usually left undetected and are not considered to be a cause of female infertility.
The genetic mechanism of POF is explained by the translation of peptide of the FMR1 gene and depends upon the number of triple CGG repeats [11, 12]. The FMR1 gene is located on the long arm of the X chromosome in the Хq27.3 locus. The number of copies of trinucleotide repeats increases during gametogenesis. Sullivan et al. [18] showed on mice models that the premature ovarian ageing was commonly caused by excessive transcription of the FMR1 gene product (2005). Gleicher et al. [11] found that both excessive and reduced numbers of CGG repeats in the FMR1 gene contributed to the development of POF. In our study, 42.3 % of women with POF had the number of CGG repeats in the range of 28–36. This range was considered normal in regard to ovarian reserve according to the data of the cohort of women with no signs of POF from the European part of Russia. We assumed that women with the number of CGG triple repeats in the range of 28–36 had a non-genetic cause of primordial pool premature exhaustion.
The autoimmune process involving the ovarian tissue is triggered by various agents: viruses, bacteria, or self-ovarian antigens. The mumps and rubella viruses are well known triggers of autoantibodies production. According to Fenishel et al. [8], up to 30 % of POF cases are of autoimmune origin. In our study, the patients with POF had a high infection index. The incidence of mumps was 38.5 %, of rubella – 35.9 %.
AOA are specific antibodies, which can damage the ovarian tissue [7]. We detected antibodies to the ovarian tissue in the 15 women with POF (19.2 % of cases). The interesting finding was the association between the numbers of CGG repeats in the FMR1 gene and presence of anti-ovarian antibodies. The 28–36 CGG counts were associated with the high rate of anti-ovarian antibodies detection (42.42 % of cases in the group B) as compared with the higher or lower CGG counts (2.2 % in the groups A and C), (RR = 19.23, 95 % CI = 2.63–100.0). We found that the patients with POF can be divided into two groups based on the presence or absence of anti-ovarian autoantibodies: the women with or without autoimmune disorders. The women from the first group had the number of CGG repeats in the range of 28–36. They had the mild form of POF, manifested by a minor decrease of AMH (0.26 ± 0.36 ng/mL), and could preserve the ovarian reserve. The women from the second group had the number of CGG repeats less than 28 or more than 36. They developed a severe form of POF, manifested by an evident decrease of AMH (0.08 ± 0.15 ng/mL).
In the 14 women of the group B (42.45 %) the origin of disease was not clear. They had a normal number of CGG repeats in reference to the ovarian reserve, no SXCI or anti-ovarian antibodies. Gleicher et al. [13] and Tsigkou et al. [19] suggested that the detection of specific, as well as non-specific auto-antibodies and immune cellular response created the condition of an “autoimmune noise”, which was an independent risk factor for the development of the autoimmune form of POF. The further studies of various non-specific auto-antibodies detection are required for better understanding of the possible causes and risk factors of POF in women without the genetic signs of the disease.
The cross-sectional design is a limitation of the study as it does not allow drawing a conclusion about the causality of autoimmune factors and POF. On the other hand, the conduction of longitudinal (cohort) study is not feasible because it takes many years and subjects to detect such a rare outcome as POF. The strength of the study is the presentation of data, including the genetic tests, of quite a big sample of POF patients from the European part of the Russian Federation. Such data are absent in the world scientific publications.
Both >36 or <28 CGG repeats in the FMR1 gene suggests the molecular-genetic form of POF, and the 28–36 triple CGG repeats may indicate the autoimmune origin of POF. This hypothesis is confirmed by the double increase of SXCI in the case of higher or lower CGG counts (31.1 % in the groups А and С vs. 15.1 % in the group В). The autoimmune-driven form of POF is milder compared to the genetic form of POF and is characterized by preservation of follicular primordial pool. Both genetic and immunological tests may help in the preclinical examination of women with POF and allow their family planning, including fertility preservation methods.
Acknowledgements
Funding for this study was provided by Ministry of Health of the Russian Federation (grant # 2010-06 reg. #01201066000).
The authors thank Tatyana Y. Ivanets, M.D., Ph.D., the Chief of Scientific-Diagnostic Laboratory of the RCOGP for the assistance in the implementation of the study.
Declaration
The authors report no financial or commercial conflicts of interest
Footnotes
Capsule
Detection of anti-ovarian antibodies is associated with the normal number of CGG repeats in regard to ovarian reserve and a better preservation of follicular primordial pool in the women with premature ovarian failure.
References
- 1.Allen RC, Zoghbi HY, Moseley AB, Rosenblatt HM, Belmont JW. Methylation of Hpa II and Hha I sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X-chromosome inactivation. Am J Hum Genet. 1992;51:1229–39. [PMC free article] [PubMed] [Google Scholar]
- 2.Allen EG, Sullivan AK, Marcus M, Small C, Dominguez C, Epstein MP, et al. Examination of reproductive aging milestones among women who carry the FMR1 premutation. Hum Reprod. 2007;22(8):2142–52. doi: 10.1093/humrep/dem148. [DOI] [PubMed] [Google Scholar]
- 3.Ashrafi M, Fallahian M, Eshrati M, Yazdi RS. The Presence of Anti Thyroid and Anti Ovarian Auto-Antibodies in Familial Premature Ovarian Failure. Int J Fertil Steril. 2008;1(4):171–4. [Google Scholar]
- 4.Chen LS, Tassone F, Sahota P, Hagerman PJ. The CGG repeat element within the 5′ untranslated region of the FMR1 message provides both positive and negative effects on in vivo translation of a downstream reporter. Hum Mol Genet. 2003;12(23):3067–74. doi: 10.1093/hmg/ddg331. [DOI] [PubMed] [Google Scholar]
- 5.Conway GS, Katlas G, Patel A, Davies MS, Jacobs HS. Characterization of idiopathic premature ovarian failure. Fertil Steril. 1996;65(2):337–41. doi: 10.1016/s0015-0282(16)58095-9. [DOI] [PubMed] [Google Scholar]
- 6.Conway GS, Payne NN, Webb J, Murray A, Jacobs PA. Fragile X premutation screening in women with premature ovarian failure. Hum Reprod. 1998;13(5):1184–7. doi: 10.1093/humrep/13.5.1184. [DOI] [PubMed] [Google Scholar]
- 7.Coulam CB, Ryan RJ. Premature menopause.I. Etiology. Am J Obstet Gynecol. 1979;133(6):639–43. doi: 10.1016/0002-9378(79)90011-5. [DOI] [PubMed] [Google Scholar]
- 8.Fenishel P, Sosset C, Barbarino-Monnier P, Gobert B, Hieronimus S, Bene MC, Harter M. Prevalence, specificity and significance of antiovarian antibodies during spontaneous premature ovarian failure. Hum Reproad. 1997;12(12):2623–8. doi: 10.1093/humrep/12.12.2623. [DOI] [PubMed] [Google Scholar]
- 9.Fu YH, Kuhl DP, Pizzuti A, Pieretti M, Sutcliffe JS, Richards S, et al. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991;67(6):1047–58. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- 10.Gleicher N, Weghofer A, Barad D. Defining ovarian reserve to better understand ovarian aging. Reprod Biol Endocrinol. 2011;9:23. doi: 10.1186/1477-7827-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gleicher N, Weghofer A, Barad DH. Ovarian reserve determinations suggest new function of FMR1 (fragile X gene) in regulating ovarian ageing. Reprod Biomed Online. 2010;20(6):768–75. doi: 10.1016/j.rbmo.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 12.Gleicher N, Weghofer A, Oktay K, Barad D. Relevance of triple CGG repeats in the FMR1 gene to ovarian reserve. Reprod Biomed Online. 2009;19(3):385–390. doi: 10.1016/S1472-6483(10)60173-3. [DOI] [PubMed] [Google Scholar]
- 13.Gleicher N, Weghofer A, Oktay K, Barad DH. Is the immunological noise of abnormal autoimmunity an independent risk factor for premature ovarian aging? Menopause. 2009;16(4):760–4. doi: 10.1097/gme.0b013e318193c48b. [DOI] [PubMed] [Google Scholar]
- 14.La Marka A, Marzotti S, Bronzetti A, Stabile G, Artenisio AC, Bini V, et al. Primary ovarian insufficiency due to steroidogenic cell autoimmunity is associated with a preserved pool of functioning follicles. J Clin Endocrinol Metab. 2009;94(10):3816–23. doi: 10.1210/jc.2009-0817. [DOI] [PubMed] [Google Scholar]
- 15.Sato K, Uehara S, Hashiyada M, Nabeshima H, Sugawara J, Terada Y, et al. Genetic significance of skewed X-chromosome inactivation in premature ovarian failure. Am J Med Genet. 2004;130A(3):240–4. doi: 10.1002/ajmg.a.30256. [DOI] [PubMed] [Google Scholar]
- 16.Shamilova NN, Marchenko LA, Shamilova NN, Marchenko LA. The FMR1 gene: new possibilities of assessing the ovarian reserve. Akusherstvo & Gynecologiya. 2011;2:58–64. [Google Scholar]
- 17.Sugawa F, Wada Y, Maruyama T, Uchida H, Ishizuka B, Ogata T. Premature ovarian failure and androgen receptor gene CAG repeat lengths weighted by X chromosome inactivation patterns. Fertil Steril. 2009;91(2):649–52. doi: 10.1016/j.fertnstert.2007.11.085. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan AK, Marcus M, Epstein MP, Allen EG, Anido AE, Paquin JJ, et al. Association of FMR1 repeat size with ovarian dysfunction. Hum Reprod. 2005;20(2):402–12. doi: 10.1093/humrep/deh635. [DOI] [PubMed] [Google Scholar]
- 19.Tsigkou A, Marzotti S, Borges L, Brozzetti A, Reis F, Candeloro P, et al. High serum inhibin concentration discriminates autoimmune oophoritis from other forms of primary ovarian insufficiency. J Clin Endocrinol Metab. 2008;93(4):1263–9. doi: 10.1210/jc.2007-1675. [DOI] [PubMed] [Google Scholar]
- 20.Welt CK. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol (Oxf) 2008;68(4):499–509. doi: 10.1111/j.1365-2265.2007.03073.x. [DOI] [PubMed] [Google Scholar]
- 21.Wheatcroft NJ, Salt C, Milford-Ward A, Cooke ID, Weetman AP. Identification of ovarian antibodies by immunofluorescence, enzyme-linked immunosorbent assay or immunoblotting in premature ovarian failure. Hum Reprod. 1997;12(12):2617–22. doi: 10.1093/humrep/12.12.2617. [DOI] [PubMed] [Google Scholar]
- 22.Wittenberger MD, Hagerman RJ, Sherman SL, McConkie-Rosell A, Welt CK, Rebar RW, et al. The FMR1 premutation and reproduction. Fertil Steril. 2007;87(3):456–65. doi: 10.1016/j.fertnstert.2006.09.004. [DOI] [PubMed] [Google Scholar]