Abstract
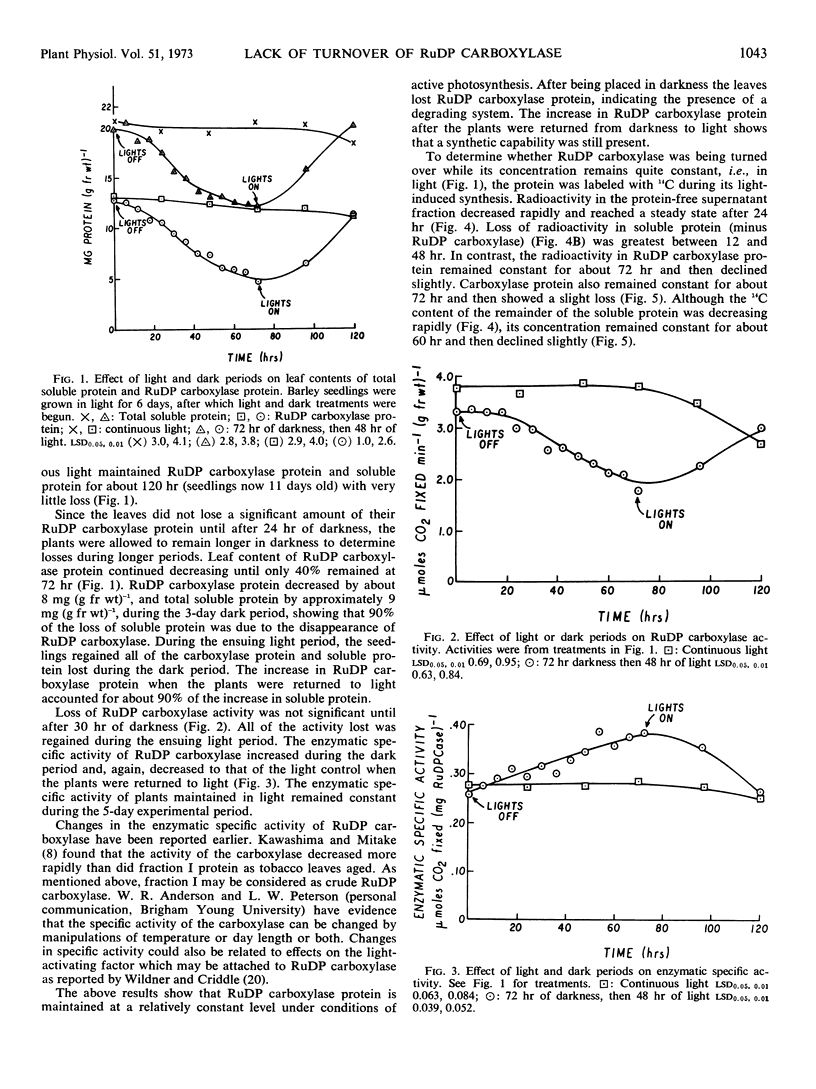
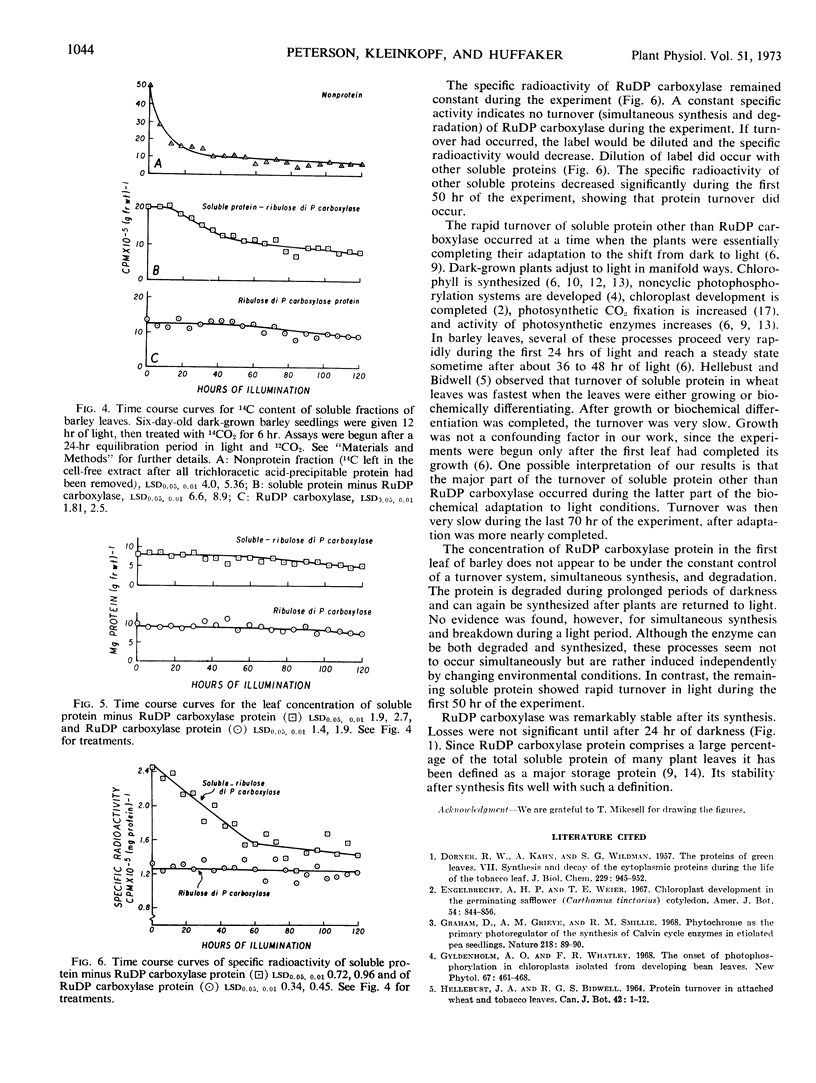
Turnover of ribulose 1,5-diphosphate carboxylase in barley leaves (Hordeum vulgare L.) was followed over time in light and dark. The enzyme was degraded in prolonged darkness and was resynthesized after the plants were returned to light. Labeling with 14C showed that simultaneous synthesis and degradation (turnover) did not occur in light. In contrast, the remaining soluble protein was turned over rapidly in light. Although ribulose 1,5-diP carboxylase can be both degraded and synthesized, these processes seem not to occur simultaneously, but can be induced independently by changing environmental conditions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DORNER R. W., KAHN A., WILDMAN S. G. The proteins of green leaves. VII. Synthesis and decay of the cytoplasmic proteins during the life of the tobacco leaf. J Biol Chem. 1957 Dec;229(2):945–952. [PubMed] [Google Scholar]
- Huffaker R. C., Obendorf R. L., Keller C. J., Kleinkopf G. E. Effects of Light Intensity on Photosynthetic Carboxylative Phase Enzymes and Chlorophyll Synthesis in Greening Leaves of Hordeum vulgare L. Plant Physiol. 1966 Jun;41(6):913–918. doi: 10.1104/pp.41.6.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUPKE D. W. Correlation of a soluble leaf protein with chlorophyll accumulation. J Biol Chem. 1962 Oct;237:3287–3291. [PubMed] [Google Scholar]
- Kleinkopf G. E., Huffaker R. C., Matheson A. Light-induced de Novo Synthesis of Ribulose 1,5-Diphosphate Carboxylase in Greening Leaves of Barley. Plant Physiol. 1970 Sep;46(3):416–418. doi: 10.1104/pp.46.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Margulies M. M. Effect of Chloramphenicol on Light-Dependent Synthesis of Proteins and Enzymes of Leaves and Chloroplasts of Phaseolus vulgaris. Plant Physiol. 1964 Jul;39(4):579–585. doi: 10.1104/pp.39.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obendorf R. L., Huffaker R. C. Influence of age and illumination on distribution of several calvin cycle enzymes in greening barley leaves. Plant Physiol. 1970 May;45(5):579–582. doi: 10.1104/pp.45.5.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smillie R. M. Photosynthetic & respiratory activities of growing pea leaves. Plant Physiol. 1962 Nov;37(6):716–721. doi: 10.1104/pp.37.6.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TROWN P. W. AN IMPROVED METHOD FOR THE ISOLATION OF CARBOXYDISMUTASE. PROBABLE IDENTITY WITH FRACTION I PROTEIN AND THE PROTEIN MOIETY OF PROTOCHLOROPHYLL HOLOCHROME. Biochemistry. 1965 May;4:908–918. doi: 10.1021/bi00881a018. [DOI] [PubMed] [Google Scholar]
- Tolbert N. E., Gailey F. B. Carbon Dioxide Fixation by Etiolated Plants after Exposure to White Light. Plant Physiol. 1955 Nov;30(6):491–499. doi: 10.1104/pp.30.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VON NOORT, WILDMAN S. G. PROTEINS OF GREEN LEAVES. IX. ENZYMATIC PROPERTIES OF FRACTION-I PROTEIN ISOLATED BY A SPECIFIC ANTIBODY. Biochim Biophys Acta. 1964 Aug 19;90:309–317. [PubMed] [Google Scholar]
- Wildner G. F., Criddle R. S. Ribulose diphosphate carboxylase. I. A factor involved in light activation of the enzyme. Biochem Biophys Res Commun. 1969 Dec 4;37(6):952–960. doi: 10.1016/0006-291x(69)90223-x. [DOI] [PubMed] [Google Scholar]


