Abstract
Propranolol (PRN) has recently been recommended as the first-line medicine for complicated infantile hemangiomas (IHs), because of the significant effect. However, no pharmacokinetic parameters have ever been reported for infants who receive PRN treatment for IH. In this study, we show that plasma PRN concentration is affected by the frequency of administration of PRN. A single daily administration of PRN (1 mg/kg/d) resulted in an early elevation of plasma PRN compared to a twice a day administration of the same dose. In contrast, the twice a day application resulted in a more prolonged expression at a later time-point. Our findings provide pharmacokinetic parameters of PRN action in IH for clinic.
Keywords: Infantile hemangioma, propranolol, pharmacokinetics, RP-HPLC
Introduction
Infantile hemangioma (IH) is the most common benign tumor of childhood (Figures 1 and 2), affecting 10% of newborns, especially females [1]. Approximately 60% of IH commonly occur in the head and neck [2]. IHs are biologically unique, there is two proliferating phase during the first year following birth, followed by a slow spontaneous involutional phase occurring within a period of 5 to 10 years [3]. However, ulceration, blood, deformity even threatens to life when the tumors are great or location on key part. Many patients require treatment to avoid permanent sequelae such as chromatosis telangiectasias and scarring. Propranolol (PRN) has been reported by Léauté-Labrèze et al [4] since 2008, and shows promise effect on IHs (Figure 3), has substituted the corticosteroids for the first line of treatment [5]. However, at present, there is no standard protocol for dose or frequency of PRN administration [6], as the pharmacokinetic parameters of the drug have yet to be established. Clinicians must make prescriptions based on their personal experience. In the present study, PRN was orally administered at two different frequencies and the plasma concentration of PRN was subsequently measured at different time points after the oral administration of PRN.
Figure 1.
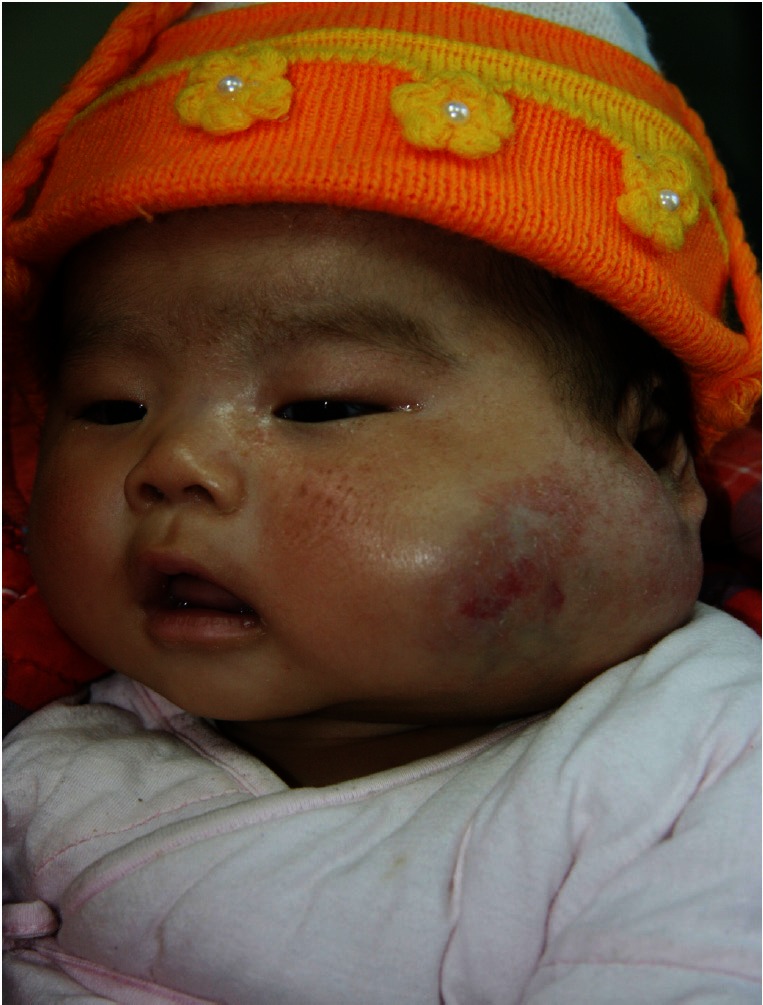
Age 2 month, no treatment.
Figure 2.
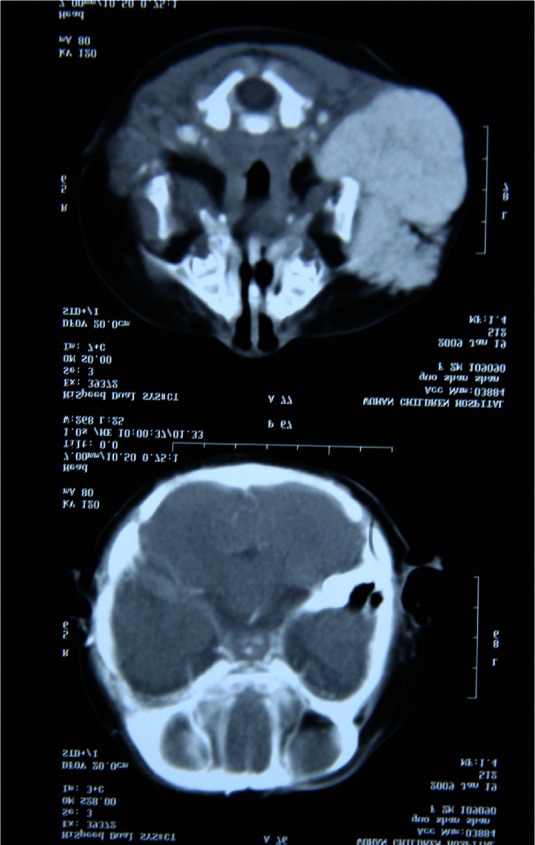
A coronal T2-weighted MRI reveals the lesion.
Figure 3.

Age 12 month, 10 month after starting propranolol.
Materials and methods
We obtained patients’ venous blood under a human-subject protocol approved by the ethics committee at Ninth People’s Hospital, Shanghai Jiao Tong University. All parents of participants approved the study and the plasma sample collections by written informed consent.
Reverse phase high-performance liquid chromatography (RP-HPLC)
12 patients were randomly divided into 2 groups (Table 1), qd (n=6) and bid (n=6). PRN was orally administered to both groups at a dose of 1 mg/kg/d. Group qd received the full dose once a day, while the dose for group bid was halved and given twice a day; the interval between first and second administration was 8 hours. After the first administration, the patients’ venous blood was collected at the selected time points of 2, 6, 10, and 24 hrs, and the plasma was extracted and stored at -20°C. The method of RP-HPLC was carried out according to the description of Salman et al [7]. Briefly, the freed bases of PRN were extracted with 3.5 mL of the extraction solvent (isoamyl-alcohol 1.5 ml: n-heptane 98.5 ml) and were separated using a mixture of a mobile phase consisting of 160 mL water, 180 mL methanol, 70 mL acetonitrile, 2.5 mL acetic acid, and 125 μL triethylamine (v/v). A flow rate of 0.5 mL/min was employed throughout with a 15 μL injec-tion volume. Plasma PRN was measured using a UV Detector (Agilent 1260, USA) at 291 nm. The concentrations of PRN selected for the calibration curve were 15, 30, 60, 120, and 180 ng/ml.
Table 1.
The data of patients who received propranolol treatment at two different dosage
| Group | Quantity | Male | Female | Mean age (month) | Age (month) | Weight (kg) |
|---|---|---|---|---|---|---|
| qd | 6 | 3 | 3 | 5.2 | 2-12 | 7±0.6 |
| bid | 6 | 3 | 3 | 3 | 2-5 | 7.6±0.3 |
qd: once a day; bid: twice a day.
Results
The peak concentration of PRN in plasma of IH patients was observed at approximately 5 minutes when the peak of PRN plasma concentrations were found (Figure 4). For the total dose of PRN was same every day (1 mg/kg/d), the highest peak concentration was observed in 383 ng/mL, lowest in 0 ng/mL. Patients in group qd (n=6) whose plasma concentrations of propranolol on 2 hr, 6 hr, 10 hr, 24 hr after oral administration of propranolol (1 mg/kg/d) were C2hq: 207.8±33.9 ng/mL, C6hq: 150.5±35.6 ng/mL, C10hq: 61.8±17.1 ng/mL, C24hq: 35.8±12.9 ng/mL respectively, and the number in group bid (n=6) were C2hb: 155.2±40.6 ng/mL, C6hb: 92.5±33.8 ng/mL, C10hb: 109.4±18.7 ng/mL, C24hb: 16.2±8.2 ng/mL, respectively. The mean plasma concentrations of PRN on 2 hr, 6 hr and 24 hr following oral administration of PRN were higher in the group given a single daily dose (qd, n=6) compared with the group that was administered the same dose twice a day, (bid, n=6, P<0.05). However, at 10 hrs, the concentration was lower for the qd group compared to the bid group (P<0.05). Therefore, mean plasma concentration of PRN was maintained for a longer time in group bid compared with group qd, which decreased over time (P<0.05). The peak of propranolol concentration was achieved at 2 hr in groups, 207.8±33.9 ng/mL and 155.2±40.6 ng/mL for group’s qd and bid respectively.
Figure 4.
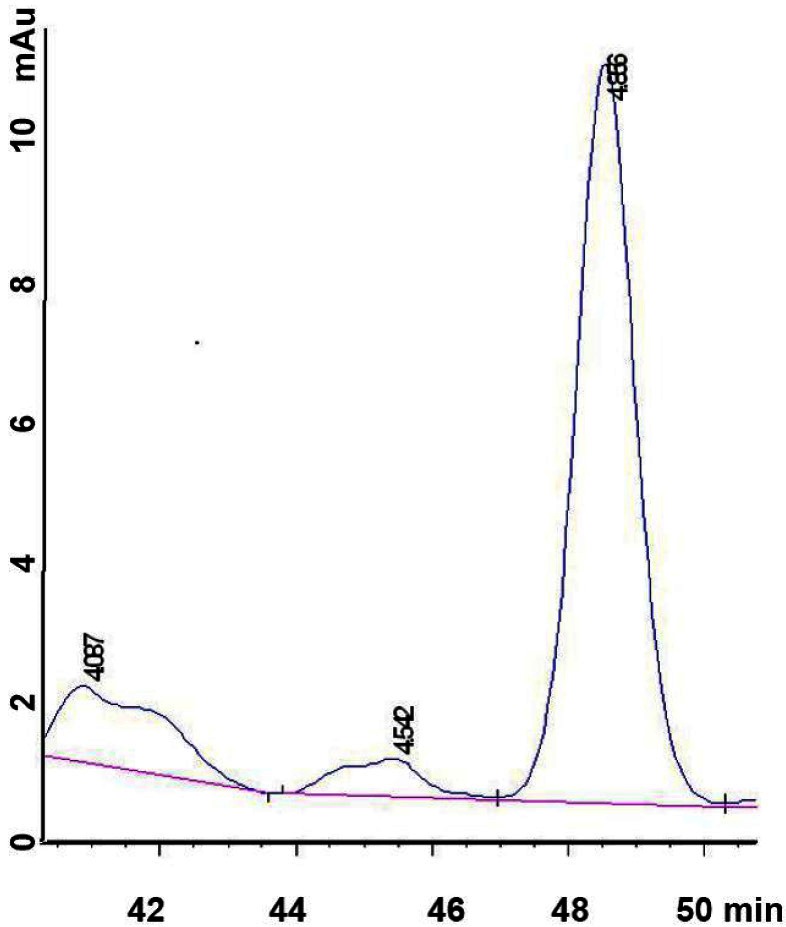
PRN concentration in plasma of IH patients following oral PRN administration. The time of peak concentration; Mean plasma concentrations after a single (group qd, n=6) and twice daily (group bid, n=6) PRN application (*P<0.05).
The elimination half-life (t1/2) were longer than 6 hr in the two groups (P>0.05). The mean plasma concentration of group qd decreased with the time; the plasma concentration was high in the prime 6 hr.
Discussion
There is currently very little research about plasma concentrations and pharmacokinetics of PRN for treatment of IH. Clinical questions such as frequency of PRN administration (i.e., once a day or twice a day) and the exact dose required to achieve an effective plasma concentration remain un-answered. In this study, examination of the plasma concentrations of PRN in IH patients after oral administration of PRN demonstrated that the plasma concentration fluctuating from 0 ng/mL to 383 ng/mL (~1.39 μM); although the total dose of PRN was same every day (1 mg/kg/d).
Comparison of the two different administration regimes, (a single daily dose, group ‘qd’ and the same dose halved and administered twice a day, group ‘bid’), showed that there were benefits and drawbacks in both groups, respectively, plasma concentrations are higher in group qd at first 6 hours. After the elimination half-life, the mean concentration of PRN was significantly decreased in this group. In contrast, higher plasma concentrations can be maintained in group bid for more than 10 hr. Therefore, in terms of time-effectiveness of plasma PRN, a twice a day administration would be preferable. It should also be noted that the β-adrenergic antagonist elimination half-life of pharmacodynamics is longer than the time of pharmacokinetics [8], when the PRN cannot be detected in the blood, it may still be present in the head, heart and lungs [9]. This implies that group bid would have a more prolonged action on the cardiovascular system than group qd. It is important for the patients with cardiovascular systemic disease to administer PRN once a day. The twice a day application may be better than the single daily administration.
Acknowledgements
We are very grateful to Professor James Hupp from East Carolina University School of Dental Medicine for his careful revisions and valuable suggestions for this manuscript. Written informed patient consent was obtained for publication.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Amir J, Metzker A, Krikler R, Reisner SH. Strawberry hemangioma in preterm infants. Pediatr Dermatol. 1986;3:331–332. doi: 10.1111/j.1525-1470.1986.tb00535.x. [DOI] [PubMed] [Google Scholar]
- 2.Finn MC, Glowacki J, Mulliken JB. Congenital vascular lesions: clinical application of a new classification. J Pediatr Surg. 1983;18:894–900. doi: 10.1016/s0022-3468(83)80043-8. [DOI] [PubMed] [Google Scholar]
- 3.Haggstrom AN, Drolet BA, Baselga E, Chamlin SL, Garzon MC, Horii KA, Lucky AW, Mancini AJ, Metry DW, Newell B, Nopper AJ, Frieden IJ. Prospective study of infantile hemangiomas: clinical characteristics predicting complications and treatment. Pediatrics. 2006;118:882–887. doi: 10.1542/peds.2006-0413. [DOI] [PubMed] [Google Scholar]
- 4.Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taïeb A. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649–2651. doi: 10.1056/NEJMc0708819. [DOI] [PubMed] [Google Scholar]
- 5.Holmes WJ, Mishra A, Gorst C, Liew SH. Propranolol as first-line treatment for rapidly proliferating Infantile Haemangiomas. JPRAS. 2011;64:445–451. doi: 10.1016/j.bjps.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Haider KM, Plager DA, Neely DE, Eikenberry J, Haggstrom A. Outpatient treatment of periocular infantile hemangiomas with oral propranolol. J AAPOS. 2010;14:251–256. doi: 10.1016/j.jaapos.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Salman SA, Sulaiman SA, Ismail Z, Gan SH. Quantitative determination of propranolol by ultraviolet HPLC in human plasma. Toxicol Mech Methods. 2010;20:137–142. doi: 10.3109/15376511003602112. [DOI] [PubMed] [Google Scholar]
- 8.Lin H, Gounder MK, Bertino JR, Kong AN, DiPaola RS, Stein MN. A validated HPLC assay for the determination of R-(-)-gossypol in human plasma and its application in clinical pharmacokinetic studies. J Pharm Biomed Anal. 2012;66:371–375. doi: 10.1016/j.jpba.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reiter MJ. Cardiovascular drug class specificity: beta-blockers. Prog Cardiovasc Dis. 2004;47:11–33. doi: 10.1016/j.pcad.2004.04.004. [DOI] [PubMed] [Google Scholar]


