Abstract
Objective: Left ventricular diastolic dysfunction is receiving more attention in patients with end-stage liver diseases. The importance of diastolic dysfunction observed before orthotopic liver transplantation (OLT) and its adverse effects on hemodynamics and outcomes of OLT patients, have not been fully explored. We carried a retrospective study to investigate the influence of diastolic dysfunction on OLT patients. Methods: Included in this retrospective study were 330 consecutive patients scheduled for cadaveric OLT over a 5-year period. According to preoperative Doppler echocardiogram (ECHO) findings, patients were divided into two groups: DD group (patients with diastolic dysfunction) and control group (patients with normal ECHO). Patient characteristics, operation variables, hemodynamic course, blood products and drug requirements, postoperative courses and outcomes were evaluated. Results: 306 patients met the study entry criteria and 100 had preoperative diastolic dysfunction. Mean artery blood pressure was significantly lower in DD group after graft reperfusion than that in control group (P<0.01). More patients in DD group required epinephrine, and the mean dose of epinephrine was higher in DD group than that in control group (P<0.01). There was no significant difference in postoperative ventilation time, duration of ICU and hospital stay, renal failure and postoperative mortality between the two groups. Conclusion: Diastolic dysfunction is common in liver transplant recipients. Patients with diastolic dysfunction may be associated with substantial hemodynamic alterations after graft reperfusion and need more inotropic support during OLT. Diastolic dysfunction was not associated with significant adverse postoperative outcomes.
Keywords: Anesthesia, liver transplantation, intraoperative complications, postoperative complications, echocardiography
Introduction
Liver disease is associated with heart impairment, including mainly coronary artery disease and the so called cirrhotic cardiomyopathy. Importantly, cirrhotic cardiomyopathy is frequently silent and revealed by exertion or stress including surgery and liver transplantation, and preoperative echographic assessment might be useful [1]. Diastolic dysfunction is characterized by decreased left ventricular compliance and relaxation, and regarded as a feature of cirrhotic cardiomyopathy [2]. It has been indicated as often as by decreased E/A ratio (the ratio of early to late (atrial) phases of ventricular filling) on a Doppler echocardiogram (ECHO) [3]. Rabie et al [4] observed that diastolic dysfunction indicated by reduced E/A ratio is associated with increased mortality after transjugular intrahepatic portosystemic stent shunt (TIPS). Whether diastolic dysfunction also affects outcomes of patients undergoing orthotopic liver transplantation (OLT) was never explored.
OLT carries the risk of perioperative hemodynamic impairment, mainly hypotension associated with hyperkinetic circulation, major bleeding, postreperfusion syndrome (PRS). PRS is characterized by a marked decrease in systemic blood pressure following unclamping of the portal vein and liver reperfusion. At graft reperfusion, whether diastolic dysfunction can herald profound post-reperfusion hemodynamic instability are not understood. We therefore performed a retrospective study to examine whether diastolic dysfunction has altered perioperative hemodynamic changes associated with PRS, and has adverse effects on short-term outcome of patients after OLT.
Methods
Patients
After obtaining institutional ethics committee approval, we performed a retrospective study that included 330 adult patients who had undergone cadaveric OLT at Changzheng Hospital from January 2005 to December 2009. As it was a retrospective observational study, informed consent from patients was not required. All subjects underwent a preoperative Doppler ECHO examination. The exclusion criteria were OLT for alcoholic cirrhosis, hemochromatosis, fulminant hepatic failure, retransplantation, simultaneous liver and kidney transplantation. Patients who had TIPS were also excluded.
Preoperative cardiac assessment
Doppler ECHO including tissue Doppler imaging (TDI) was performed routinely in all recipients 2-3 days before OLT. Mitral inflow velocities including early (E) and late (A) maximal ventricular filling velocities, E/A ratio, and deceleration time (DT) of E were measured. Early (e′) diastolic mitral annular velocity and E/e′ ratio were assessed using TDI. In our center, diastolic dysfunction was reported referring to the recommendations of the European Study Group on Diastolic Heart Failure [5] when the following variables were recorded: (1) Mitral Doppler flow velocity signal: E/A<50 year <1.0 and DT<50 years >220 ms or E/A>50 years <0.5 and DT>50 years >280 ms; (2) TDI: E/e′ ratio >8.
General anesthesia
Anesthesia was induced with intravenous propofol 1.5-2.0 mg/kg, fentanyl 3 μg/kg and succinylcholine 1.5-2.0 mg/kg. After tracheal intubation, anesthesia was maintained with desflurane or sevoflurane, and continuous infusion of fentanyl and atracurium or cisatracurium. The patients were mechanically ventilated with a tidal volume of 8-10 mL/kg, a respiratory frequency of 10-12 breaths per minute, and inhalation of 100% oxygen. After operation, all patients remained intubated and transferred to the intensive care unit (ICU) for mechanical ventilation.
Routine monitoring included electrocardiography, oxygen saturation, end-tidal PCO2, invasive pressure via radial and pulmonary arterial catheters, artery blood gas analysis and urine output. Intravenous fluids (PlasmaLytec-A solution as crystalloid or 20% albumin and 6% hydroxyethyl starch solutions as colloid) were given for volume replacement. Packed red blood cells (RBCs) were administered to keep blood hemoglobin level above 90 g/L. Administration of fresh frozen plasma (FFP) and platelets was performed according to the coagulation profile and platelet count. All patients received 150-250 mL sodium bicarbonate (5%) during the anhepatic period. CaCl2 was used if Ca2+ was lower than 1.0 mmol/L before graft reperfusion.
Before unclamping the portal vein, patients with systolic blood pressure (SAP) <90 mmHg were prophylactically treated with an intravenous bolus of 100 μg phenylephrine, followed by continuous phenylephrine infusion. In patients with a heart rate (HR) lower than 65 bpm, 0.5 mg atropine was prescribed, and in patients with both low SAP and HR (SAP <90 mmHg, HR <65 bpm), 10 μg epinephrine was administered. After unclamping, an intravenous bolus of 200 μg phenylephrine was given when the mean arterial pressure (MAP) was <60 mmHg. In case MAP failed to recover promptly or both MAP and HR were low (HR <60 bpm), 10-30 μg epinephrine was infused intravenously.
Data collection
According to the results of ECHO examination, patients were divided into two groups: DD group (patients with diastolic dysfunction) and control group (patients with normal ECHO) (Figure 1). Data were collected retrospectively from the medical records. Preoperative clinical variables included demographics of the recipients and cold ischemia time of grafts. Intraoperative variables included the surgical type, surgical duration and anhepatic period, blood loss and blood product transfusion, use of inotropic agents during surgery, the incidence of postreperfusion syndrome (PRS) and intraoperative cardiac arrest, and hemodynamic and biological profiles. Postoperative variables included the duration of ventilatory support, ICU and hospital stay, postoperative in-hospital mortality, and postoperative renal complications.
Figure 1.
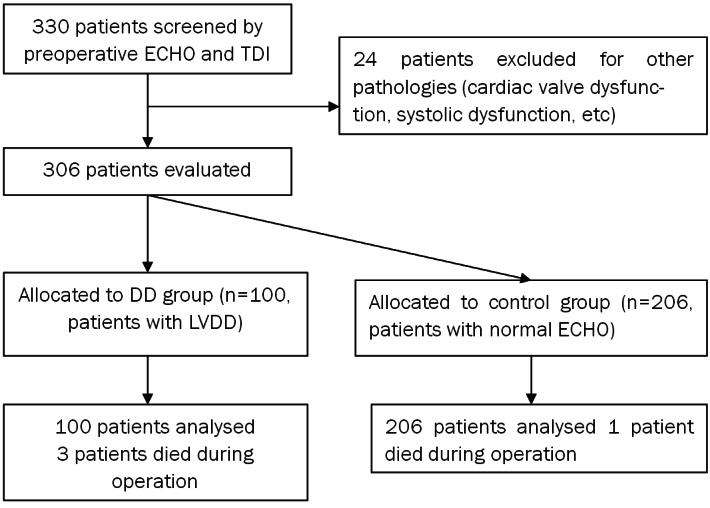
Flow of the patients through the study. ECHO: Echocardiography; TDI: tissue Doppler imaging; LVDD: left ventricular diastolic dysfunctions.
PRS is defined as hypotension (mean arterial blood pressure 30% lower than the value immediately at the end of the anhepatic stage, lasting for more than 1 minute within 5 minutes) or asystole after graft reperfusion [6,7]. Renal failure was defined as creatinine clearance under 30 mL/minute according to the Cockroft formula [6].
Statistical analysis
Data were expressed as mean values±SD for data following a normal distribution, or median and interquartile ranges for data with non-normal distributions, number and percentage. The categorical variables were analyzed using the Chi-square test or Fisher’s exact probability test. One-way analysis of variance (ANOVA) or the Mann-Whitney U test was used for other variables. Statistical analysis was made with SPSS version 18.0 (SPSS Inc., Chicago, IL., USA). P<0.05 was considered to be statistically significant.
Results
Of the 330 patients evaluated with ECHO, 100 (30.3%) showed diastolic dysfunction and 206 displayed normal ECHO findings. The remaining 24 patients with other pathologies were excluded from the study.
The baseline characteristics of the patients are shown in Table 1. There was no significant difference in patient characteristics (age, sex, body mass index, MELD score, etiology of liver diseases and cold ischemia time) between DD and control groups.
Table 1.
Recipient Morphometrics
| Total (n=306) | DD group (n=100) | Control group (n=206) | P value | |
|---|---|---|---|---|
| Age (yr) | 47 (41-53) | 48 (45-53) | 47 (41-53) | 0.15 |
| Sex (M/F) | 261/45 | 84/16 | 177/29 | 0.66 |
| BMI (Kg/m2) | 23.3±4.4 | 23.2±4.2 | 23.4±4.5 | 0.68 |
| LT indication | 0.48 | |||
| Cirrhosis | 177 | 53 | 124 | |
| Hepatocellular Carcinoma | 21 | 8 | 13 | |
| Carcinoma+Cirrhosis | 108 | 39 | 69 | |
| MELD score | 10.6±5.0 | 10.0±4.5 | 10.9±5.2 | 0.19 |
| Cold ischemia time (hours) | 9.5±2.8 | 9.7±2.3 | 9.5±3.1 | 0.51 |
Values are expressed as Mean±SD or n or medians (interquartile ranges). Abbreviations: BMI, body mass index; MELD, Model for End-Stage Liver Disease.
Upon preoperative echocardiography, the DD group demonstrated significantly reduced left ventricular end diastolic volume (LVEDV) and E/A ratio, and significantly increased DT and E/e′ (Table 2).
Table 2.
Preoperative transthoracic echocardiography
| DD group (n=100) | Control group (n=206) | P value | |
|---|---|---|---|
| Two-dimensional | |||
| LVIDs (mm) | 31.7±3.7 | 31.6±3.6 | 0.77 |
| LVIDd (mm) | 45.5±5.2 | 44.7±5.1 | 0.25 |
| LA (mm) | 32.5±4.5 | 31.8±4.1 | 0.21 |
| LVESV (mL) | 38.7±7.3 | 40.0±6.9 | 0.15 |
| LVEDV (mL)* | 100.3±13.5 | 103.9±13.8 | 0.03 |
| LVEF (%) | 61.3±4.3 | 61.4±5.0 | 0.83 |
| Doppler | |||
| DT (ms)* | 268.6±27.6 | 200.0±24.5 | <0.01 |
| E/A* | 0.7±0.1 | 1.1±0.2 | <0.01 |
| E/e′* | 10.0±1.4 | 8.3±1.2 | <0.01 |
Values are expressed as Mean±SD Values are expressed as Mean±SD.
P<0.05 compared with DD group.
Abbreviations: LVIDs, left ventricle systolic internal dimension; LVIDd, left ventricle diastolic internal dimension; LA, left atrium; LVESV, left ventricular end systolic volume; LVEDV, left ventricular end diastolic volume; LVEF, left ventricle ejection fraction; DT, mitral early deceleration time; E, peak mitral flow velocity in early diastole; A, peak mitral flow velocity in late diastole; e′, early diastolic mitral annular velocity.
Intraoperative data showed that the incidence of PRS and the mean dose of epinephrine in DD group were higher than those in control group (29% vs. 11%, P<0.01; 335±1735 µg vs. 42±557 µg, P<0.01). Compared with the control group, more patients in DD group required epinephrine (33% vs. 15.5%, P<0.01). The type of surgery, duration of the procedure, anhepatic phase, incidence of cardiac arrest after reperfusion, intraoperative blood loss and transfusion requirement for RBCs and FFP were similar between the two groups (Table 3). Hemodynamic and biological profiles are presented in Table 4. No significant difference was observed in these variables at any time between the two groups, except for MAP at 5 minutes after graft reperfusion.
Table 3.
Results of variables during operation
| Total (n=306) | DD group (n=100) | Control group (n=206) | P value | |
|---|---|---|---|---|
| Operative methods | 0.09 | |||
| Piggyback technique | 57 | 24 | 33 | |
| standard OLT | 249 | 76 | 173 | |
| Duration of surgery (hours) | 9.2±1.7 | 9.5±2.0 | 9.1±1.6 | 0.09 |
| Anhepatic duration (minutes) | 57.6±12.3 | 56.6±11.1 | 58.1±12.9 | 0.32 |
| Introperative blood loss (mL) | 2616±2306 | 2713±2551 | 2568±2182 | 0.61 |
| Transfusion of RBC (units) | 8 (3-12) | 7 (3-11) | 8 (3-14) | 0.39 |
| Transfusion of FFP (units) | 10 (4-18) | 9.5 (4-16) | 10 (4-18.25) | 0.52 |
| Mean doses of epinephrine (µg)* | 138±1098 | 335±1735 | 42±557 | <0.01 |
| No. of patients* | 65 | 33 | 32 | <0.01 |
| Mean doses of phenylephrine (µg)* | 318±654 | 450±903 | 254±480 | 0.11 |
| No. of patients | 183 | 63 | 120 | 0.42 |
| PRS* | 52 | 29 | 23 | <0.01 |
| Cardiac arrest after reperfusion | 4 | 3 | 1 | 0.07 |
Values are expressed as Mean±SD or n or medians (interquartile ranges).
P<0.05 compared with DD group.
Abbreviations: OLT, orthotopic liver transplantation; FFP, fresh frozen plasma; RBC, red blood cell.
Table 4.
Hemodynamic and biological profiles during operation
| Hepatic dissection | 1 minute before graft reperfusion | 5 minutes after graft reperfusion | |
|---|---|---|---|
| HR (bpm) | |||
| DD group | 81±11 | 88±10 | 90±20 |
| Control group | 82±12 | 86±11 | 91±13 |
| MAP (mmHg) | |||
| DD group | 76±6 | 76±6 | 65±15* |
| Control group | 75±7 | 75±7 | 70±10* |
| PAWP (mmHg) | |||
| DD group | 12±3 | 11±3 | 17±4 |
| Control group | 12±3 | 12±3 | 16±5 |
| CI (L·min-1m-2) | |||
| DD group | 3.9±0.8 | 3.1±0.9 | 4.1±1.1 |
| Control group | 4.0±0.8 | 3.2±0.9 | 4.3±0.9 |
| PH | |||
| DD group | 7.39±0.07 | 7.31±0.06 | 7.29±0.06 |
| Control group | 7.39±0.06 | 7.32±0.07 | 7.28±0.06 |
| Serum potassium (mmol/L) | |||
| DD group | 3.9±0.6 | 3.8±0.5 | 4.4±0.6 |
| Control group | 4.0±0.5 | 3.9±0.5 | 4.3±0.6 |
| Serum calcium (mmol/L) | |||
| DD group | 1.16±0.13 | 1.10±0.12 | 1.13±0.13 |
| Control group | 1.18±0.15 | 1.13±0.15 | 1.15±0.10 |
| Temperature (°C) | |||
| DD group | 37.0±0.6 | 35.9±0.7 | 35.3±0.7 |
| Control group | 37.0±0.7 | 35.8±0.8 | 35.2±0.8 |
Values are expressed as Mean±SD.
P<0.05 compared with DD group at the same time.
Abbreviations: HR, heart rate; MAP, mean arterial blood pressure; PAWP, pulmonary artery wedge pressure; CI, cardiac index.
There was no significant difference in postoperative ventilation time, ICU and hospital stay, renal failure and postoperative mortality between the two groups (Table 5).
Table 5.
Results of variables after operation
| Total (n=306) | DD group (n=97) | Control group (n=205) | P value | |
|---|---|---|---|---|
| Postoperative ventilation time (hours) | 4.0 (2.0-6.0) | 4.0 (2.3-6.0) | 3.5 (2.0-6.0) | 0.78 |
| ICU Stay (days) | 7.7±5.5 | 7.3±6.1 | 7.8±5.3 | 0.47 |
| Hospital Stay (days) | 37.7±17.0 | 35.8±17.3 | 38.6±16.8 | 0.20 |
| Renal failure | 31 | 14 | 17 | 0.10 |
| Hospital deaths after operation | 43 | 13 | 30 | 0.77 |
Values are expressed as Mean±SD or n or medians (interquartile ranges). Abbreviations: ICU, intensive care unit.
Discussion
Our study showed that diastolic dysfunction is prevalent in end-stage liver disease (ESLD) patients and is associated with high incidence of PRS, while did not alter the patients’ outcome.
Various heart impairments have been reported in patients with liver disease. Coronary artery disease (CAD) is frequent. In a large study, CAD has been detected in 13.3% in ESLD patients [8]. Diastolic dysfunction is also a frequent occurrence in these patients, and regarded as a feature of cirrhotic cardiomyopathy. Diastolic dysfunction refers to abnormalities in ventricular relaxation and filling with prolonged or incomplete return to presystolic length and force [9]. The pathophysiological background of diastolic dysfunction in cirrhosis is increased stiffness of the myocardial wall, most probably because of a combination of mild myocardial hypertrophy, fibrosis, and subendothelial oedema [1,2]. It is generally clinically silent and that it is revealed by physiologic stress such as liver transplantation, or discovered on echocardiographic assessment. Doppler ECHO is an accepted reliable practical method for diagnosing diastolic dysfunction [10]. TDI can add to the diagnosis by its assessment of myocardial wall motion dynamics during diastole [11]. Using Doppler ECHO, ventricular diastolic compliance and corresponding diastolic function can be assessed by measuring the velocity of blood flow from the left atrium to the left ventricle during early diastole (the E wave) and late diastole (the A wave) and calculating the E/A ratio [2]. Several studies have demonstrated decreased E/A ratios in cirrhotics. Finucci et al [12] evaluated diastolic dysfunction in 42 cirrhotic patients and 16 healthy controls. Compared to controls, cirrhotic patients exhibited higher late diastolic flow velocities and decreased E/A ratios. Rabie et al [4] observed that the incidence of diastolic dysfunction was 40.6% in their 101 cirrhotic patients, as indicated by reduced E/A ratio. Patients with TIPS with an E/A ratio <1 seem to have a poorer survival rate than patients without signs of diastolic dysfunction [13]. In the present study, the incidence of diastolic dysfunction was 30.3% in our 330 patients, but we did not find association between diastolic dysfunction and patients outcomes after OLT.
Hemodynamic changes likely to occur during liver transplantation are massive bleeding less frequently at the present time. Hypotension associated with graft reperfusion is common, this phenomena is referred to as PRS. The reported incidence of PRS during OLT is 8-30% [6,7,14,15]. PRS is characterized by a decrease in systemic mean arterial blood pressure, decreased systemic vascular resistance and cardiac output, and an increase in pulmonary capillary wedge pressure and central venous pressure [14]. But the pathophysiology of PRS is not fully understood. Many proposed mechanisms are attributed to abrupt influx of cold, acidic and hyperkalemic blood, to air or thrombotic embolization, or to the release of cardio depressive substances and proinflammatory cytokines from the grafted liver [16-18]. In a recent study, Paugam-Burtz et al. for the first time showed that PRS was only influenced by the extended duration of cold ischemia and absence of a portocaval shunt within the limits of their institution practices [6]. The two risk factors are not hemodynamic factors. In contrast with them, we showed that diastolic dysfunction may influence the occurrence of PRS. The difference may result from various reasons. Preoperative ECHO results were not collected and only patients with cirrhosis were included in their study. They used piggyback technique for all transplants, while we only used it in bout 20% of transplants. Some of the strengths of the present study are that it has larger sample and intact preoperative ECHO data, and it excluded systemic diseases that affect the heart and the liver, such as chronic excessive consumption of alcohol, and hemochromatosis.
The present study has some weak points. First, this is a retrospective study. Second, this is a single centre study. Third, diastolic dysfunction is normally classified in four categories based on ECHO values: normal; impaired relaxation; pseudonormal filling and restrictive filling. While the diagnosis of diastolic dysfunction in this study is limited to patients with impaired relaxation, the data analysis does not allow to determine the effect of diastolic dysfunction severity on outcome. Fourth, the hemodynamic and outcome data collection is incomplete due to the retrospective study design. All the limitations definitely warrant future study.
During the occurrence of PRS, most of hemodynamic data were kept within physiological ranges, and following PRS both groups recovered because we have got efficient pharmacologic support (epinephrine and phenylephrine). It may show that once more cirrhotic cardiopathy was decompensated by stress and did not persist after stress. In conclusion, preoperative diastolic dysfunction is prevalent in nearly 33% of patients undergoing OLT. Patients with preoperative diastolic dysfunction have a higher incidence of perioperative hemodynamic disturbances requiring treatment with vasopressors and inotrope. However, if appropriately managed, those patients have outcome results comparable to those with normal preoperative diastolic dysfunction. Based on those results, preoperative diastolic dysfunction does not represent a significant risk factor for major morbidity or mortality in patients undergoing OLT.
Conflict of interest
None.
Funding
This study was supported by the key project of biomedical research of Science and Technology Commission of Shanghai Municipality (NO.10411951300).
References
- 1.Moller S, Henriksen JH. Cirrhotic cardiomyopathy. J Hepatol. 2010;53:179–190. doi: 10.1016/j.jhep.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 2.Lee RF, Glenn TK, Lee SS. Cardiac dysfunction in cirrhosis. Best Pract Res Clin Gastroenterol. 2007;21:125–140. doi: 10.1016/j.bpg.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Moller S, Henriksen JH. Cardiovascular complications of cirrhosis. Gut. 2008;57:268–278. doi: 10.1136/gut.2006.112177. [DOI] [PubMed] [Google Scholar]
- 4.Rabie RN, Cazzaniga M, Salerno F, Wong F. The use of E/A ratio as a predictor of outcome in cirrhotic patients treated with transjugular intrahepatic portosystemic shunt. Am J Gastroenterol. 2009;104:2458–2466. doi: 10.1038/ajg.2009.321. [DOI] [PubMed] [Google Scholar]
- 5.European Study Group on Diastolic Heart Failure. How to diagnose diastolic heart failure. Eur Heart J. 1998;19:990–1003. doi: 10.1053/euhj.1998.1057. [DOI] [PubMed] [Google Scholar]
- 6.Paugam-Burtz C, Kavafyan J, Merckx P, Dahmani S, Sommacale D, Ramsay M, Belghiti J, Mantz J. Postreperfusion syndrome during liver transplantation for cirrhosis: outcome and predictors. Liver Transpl. 2009;15:522–529. doi: 10.1002/lt.21730. [DOI] [PubMed] [Google Scholar]
- 7.Garutti Martinez I, Olmedilla L, Perez-Pena JM, Zaballos M, Sanz J, Vigil MD, Navia J. Response to clamping of the inferior vena cava as a factor for predicting postreperfusion syndrome during liver transplantation. Anesth Analg. 1997;84:254–259. doi: 10.1097/00000539-199702000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Carey WD, Dumot JA, Pimentel RR, Barnes DS, Hobbs RE, Henderson JM, Vogt DP, Mayes JT, Westveer MK, Easley KA. The prevalence of coronary artery disease in liver transplant candidates over age 50. Transplantation. 1995;59:859–864. [PubMed] [Google Scholar]
- 9.Hamlin SK, Villars PS, Kanusky JT, Shaw AD. Role of diastole in left ventricular function, II: diagnosis and treatment. Am J Crit Care. 2004;13:453–466. quiz 467-458. [PubMed] [Google Scholar]
- 10.Villars PS, Hamlin SK, Shaw AD, Kanusky JT. Role of diastole in left ventricular function, I: Biochemical and biomechanical events. Am J Crit Care. 2004;13:394–403. quiz 404-395. [PubMed] [Google Scholar]
- 11.Mogelvang R, Sogaard P, Pedersen SA, Olsen NT, Marott JL, Schnohr P, Goetze JP, Jensen JS. Cardiac dysfunction assessed by echocardiographic tissue Doppler imaging is an independent predictor of mortality in the general population. Circulation. 2009;119:2679–2685. doi: 10.1161/CIRCULATIONAHA.108.793471. [DOI] [PubMed] [Google Scholar]
- 12.Finucci G, Desideri A, Sacerdoti D, Bolognesi M, Merkel C, Angeli P, Gatta A. Left ventricular diastolic function in liver cirrhosis. Scand J Gastroenterol. 1996;31:279–284. doi: 10.3109/00365529609004879. [DOI] [PubMed] [Google Scholar]
- 13.Cazzaniga M, Salerno F, Pagnozzi G, Dionigi E, Visentin S, Cirello I, Meregaglia D, Nicolini A. Diastolic dysfunction is associated with poor survival in patients with cirrhosis with transjugular intrahepatic portosystemic shunt. Gut. 2007;56:869–875. doi: 10.1136/gut.2006.102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nanashima A, Pillay P, Crawford M, Nakasuji M, Verran DJ, Painter D. Analysis of postrevascularization syndrome after orthotopic liver transplantation: the experience of an Australian liver transplantation center. J Hepatobiliary Pancreat Surg. 2001;8:557–563. doi: 10.1007/s005340100025. [DOI] [PubMed] [Google Scholar]
- 15.Aggarwal S, Kang Y, Freeman JA, Fortunato FL Jr, Pinsky MR. Postreperfusion syndrome: hypotension after reperfusion of the transplanted liver. J Crit Care. 1993;8:154–160. doi: 10.1016/0883-9441(93)90021-c. [DOI] [PubMed] [Google Scholar]
- 16.Chui AK, Shi L, Tanaka K, Rao AR, Wang LS, Bookallil M, Mayr M, Chiu E, Verran DJ, Mears D, Sheil AG. Postreperfusion syndrome in orthotopic liver transplantation. Transplant Proc. 2000 Nov;32:2116–7. doi: 10.1016/s0041-1345(00)01595-5. [DOI] [PubMed] [Google Scholar]
- 17.Gologorsky E, De Wolf AM, Scott V, Aggarwal S, Dishart M, Kang Y. Intracardiac thrombus formation and pulmonary thromboembolism immediately after graft reperfusion in 7 patients undergoing liver transplantation. Liver Transpl. 2001;7:783–789. doi: 10.1053/jlts.2001.26928. [DOI] [PubMed] [Google Scholar]
- 18.Ayanoglu HO, Ulukaya S, Tokat Y. Causes of postreperfusion syndrome in living or cadaveric donor liver transplantations. Transplant Proc. 2003;35:1442–1444. doi: 10.1016/s0041-1345(03)00483-4. [DOI] [PubMed] [Google Scholar]


