SUMMARY
Although depletion of CD4+ T cells is a major immunologic manifestation of HIV infection, multiple components of the host defense network are impaired. CD4+ T cells demonstrate impaired proliferative and cytokine responses, despite expression of activation signals. Lung CD8+ T cells are increased in number and cause lymphocytic alveolitis, but do not lyse target cells appropriately. Specific antibody responses and opsonization of microorganisms are impaired. Alveolar macrophages demonstrate intact phagocytosis and killing, enhanced antigen presentation, and increased tumor necrosis factor elaboration. Despite these findings, pulmonary infections in vivo may result from impaired activation signaling from T cells. Further investigation is needed to clarify discrepant results regarding pulmonary neutrophils’ capacities for chemotaxis and phagocytosis. In the future, improved understanding of these impairments in the lung could lead to specific interventions aimed at prevention of lung infection.
Keywords: HIV infections, Lung diseases, Macrophages, alveolar, T-Lymphocytes, B-Lymphocytes, Bronchoalveolar lavage
INTRODUCTION
Since the early era of widespread HIV infection, the variety of pulmonary infections encountered in this population demonstrated that HIV severely impairs lung host defenses 1. While most investigations focus on HIV’s effects on systemic immunity, an increasing body of literature examines pulmonary immune and inflammatory mechanisms during HIV infection 2. As impairments in pulmonary host defense are better understood, strategies to correct these defects may be developed for treatment and prophylaxis of pulmonary infections 3.
The advent of highly active antiretroviral therapy (ART) has decreased the incidence of pulmonary infections in HIV-infected individuals dramatically, but this population remains at risk for infection. This review will focus on immune and inflammatory deficits in HIV-infected individuals who are naïve to HIV treatment; the effects of anti-retroviral therapy on pulmonary host defense are summarized comprehensively in the subsequent review.
Several mechanisms have been postulated to explain susceptibility to pulmonary infections 4. First, HIV can directly infect and kill cells directed against specific pathogens, leaving decreased numbers of cells available to participate in host defense. Second, HIV can impair the metabolic or secretory functions of effector cells. Third, HIV-infected cells may shift their repertoires from elaboration of immunostimulating to immunosuppressive products, such as a shift from Th1 to Th2 cytokine production. Fourth, HIV infection may interfere with the ability of circulating immune cells to migrate into the lungs and to clear pathogens from the alveolar spaces. Finally, co-infection by a second pathogen may contribute to impaired host defense. In all likelihood, all of the mechanisms contribute to some extent to deficits in defense.
Although much useful knowledge has been generated by examination of systemic immunity, caution must be exercised in interpreting studies of systemic host defense and applying them to the pulmonary compartment. Many investigations have extrapolated data obtained from peripheral blood cells to reach conclusions about lung immunity. For example, monocyte-derived macrophages (peripheral blood mononuclear cells that are cultured and develop phenotypic characteristics of macrophages) yield important results, but these data may not be directly applicable to alveolar or other tissue macrophages. It is worth noting that the alveolar milieu has significant influence on cellular function in the lung. Therefore, interpretation of in vitro studies may be somewhat limited. The accessibility of lung cells for study by bronchoalveolar lavage (BAL) provides an opportunity to study the direct effects of HIV on lung host defenses.
Considering the function of lung cells, and not just their numbers, is also of importance. Because of the difficulty in studying functional capabilities of lung cells, current clinical practice often depends upon measuring numbers of cells rather than their function. For example, the most recent US Public Health Service recommendations suggest that providers should discontinue primary and secondary Pneumocystis jirovecii prophylaxis for sustained increases in CD4+ T cell counts of greater than 200 cells/μl for at least 3 months 5. No other tests are available clinically to predict risk of Pneumocystis pneumonia, but it is reasonable to assume that the functional abilities of these CD4+ T cells are at least as important as their numbers. In fact, HIV-infected individuals with weak peripheral blood lymphocyte proliferation to Pneumocystis antigens are at significantly higher risk of infection than individuals whose lymphocytes proliferate vigorously 6. As another example, antibody responses to Pneumocystis major surface glycoprotein recombinant fragment C1 distinguish HIV-infected individuals with and without clinical pneumonia 7. When followed prospectively, lack of immunoglobulin G (IgG) response to the Pneumocystis antigen KEX1 predicts individuals who develop Pneumocystis pneumonia versus other AIDS-defining illnesses 8. Although these tests are not available for clinical use, they emphasize the need for further functional investigation.
HIV INFECTION OF LUNG CELLS
HIV Tropism
HIV strains differ in their tropism for lymphocytes or monocytes/macrophages (Figure 1) 9. The CD4 molecule, present on lymphocytes and monocytes/macrophages, serves as the primary cellular receptor for HIV-1. In the lung, CD4 serves as the primary receptor for HIV on alveolar macrophages 10. Coreceptors are also needed for HIV entry into cells, and these cellular coreceptors define the tropism of HIV strains. Lymphocyte-tropic (T-tropic, X4) strains interact with the chemokine receptor CXCR4 (fusin) to control entry into target cells. Infection can be blocked by the CXC chemokine SDF-1, which is a CXCR4 ligand. Conversely, monocyte-tropic (M-tropic) strains interact with the chemokine receptor CCR5 to control entry into target cells. HIV infection of human alveolar macrophages is preferentially mediated by the CCR5 receptor, although alveolar macrophages also express CXCR4 11. Infection of macrophages can be blocked with the CC chemokines RANTES, MIP-1α and MIP-1β, which are CCR5 ligands.
Figure 1.
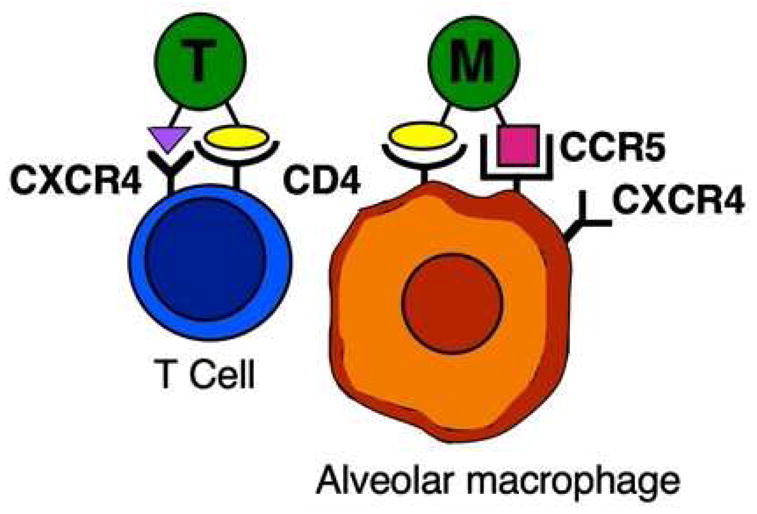
HIV tropism for T cells and alveolar macrophages. T-tropic HIV strains interact with CD4 and CXCR4 (fusin) on T cells for entry. In contrast, M-tropic HIV strains interact with CD4 and CCR5 on alveolar macrophages, although CXCR4 is also present.
As HIV infection progresses, T-tropic virus strains replace M-tropic virus strains, and this change is accompanied by more rapid immunologic decline. Minor chemokine receptors have now been shown to influence progression to AIDS, as well as susceptibility to specific pathogens. For example, variation in CCRL2, which is closely related to CCR5, has been shown to increase progression to AIDS and risk of Pneumocystis pneumonia 12.
HIV Replication
Cytokines and chemokines modulate HIV expression and replication, but conflicts in reported literature probably reflect differences in experimental design. In general, proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), inteleukin-6 (IL-6), and granulocyte monocyte-colony stimulating factor (GM-CSF) induce HIV transcription. Immunosuppressive cytokines (IL-4, IL-10) have dichotomous effects, and chemokines (MIP-1α, RANTES, SDF-1) generally decrease transcription 9.
In the lung, HIV replication occurs in pulmonary lymphocytes and in alveolar macrophages, and infection can be identified in cells sampled by BAL. Alveolar macrophages are likely to be the primary reservoir of HIV in the lung. HIV reverse transcriptase can be detected in alveolar macrophages obtained by lavage from AIDS patients, and alveolar macrophages can be infected with HIV in vitro 13. Interestingly, alveolar macrophages from smokers are more susceptible to HIV infection in vitro than alveolar macrophages from nonsmokers 14. CD8+ T cells in blood 15 and lung 16 can be infected with HIV, and are likely to serve as additional reservoirs.
Reports comparing the HIV burden of alveolar macrophages and peripheral blood monocytes are discrepant. The relative importance of in situ HIV replication in the lung, compared with influx of previously infected cells from bone marrow and blood, is unclear. The percentages of alveolar macrophages expressing HIV antigens have varied considerably among different laboratories 17. Detection of HIV by polymerase chain reaction suggests that HIV infection of alveolar macrophages is common 18, and alveolar macrophages become infected with increasing frequency as HIV infection progresses 19. Direct comparison of alveolar macrophages and peripheral blood monocytes suggests that viral burden is equivalent 20. The frequency of HIV-specific CD4+ and CD8+ T cells has been compared in blood, gut (terminal ileum biopsies), and lung (BAL) in treatment naïve, HIV-infected individuals. Compared with the gut, the lung contained much higher frequencies of HIV-specific CD4+ and CD8+ T cells 21.
Pulmonary infections increase the rate of HIV replication in the lung 22. For example, BAL from lung segments involved by Mycobacterium tuberculosis contains higher viral loads than uninvolved lung segments in the same individual, suggesting increased local replication of HIV 23. One mechanism leading to increased HIV replication is the ability of Mycobacterium tuberculosis infection to increase surface expression of CCR5 by alveolar macrophages 24 (Figure 1). Similarly, Mycobacterium avium infection activates NF-κB in macrophages, leading to increased CCR5 and TNF expression and increased susceptibility to HIV infection 25. Patients with Pneumocystis pneumonia have increased viral loads in BAL compared with asymptomatic, HIV-infected individuals 26. One mechanism of increased HIV replication may be increased production of TNF and IL-6, accompanied by decreased production of IL-10 27.
The effects of anti-retroviral therapy on HIV infection of lung cells, and on replication, are discussed in the following review.
ALTERATIONS IN LUNG LYMPHOCYTES
Cell numbers
Data from the early period of the HIV epidemic indicated that lymphocyte percentages or concentrations 28 are increased in BALs from HIV-infected individuals, compared with uninfected individuals. However, most of these data were obtained during bronchoscopies performed during episodes of clinical pulmonary infections. Examination of BAL lymphocyte subsets from AIDS patients shows decreases in CD4+ T cells and increases in CD8+ T cells. Therefore, CD4 to CD8 ratios in BAL specimens may be even lower than the ratios in peripheral blood 29. During HIV infection, BALs from smokers demonstrate decreased CD4 to CD8 ratios compared with nonsmokers, suggesting that cigarette smoke further suppresses lung defense 30. As in peripheral blood, most T cells in the lung bear αβ T cell receptors on their surfaces, but a minority expresses γδ T cell receptors. Numbers of γδ T cells are reported to be decreased 31 or increased in HIV-infected patients with opportunistic infections 32. Recent work demonstrates that most pulmonary CD4+ T cells are effector memory cells with increased expression of activation markers, at least during episodes of respiratory infection 33.
CD4+ T cells
Decreased numbers of CD4+ T cells in BALs from HIV-infected individuals, undergoing bronchoscopy to diagnose pulmonary infections, predict mortality 34. Low numbers of CD4+ T cells in BAL from asymptomatic, HIV-infected individuals are an independent predictor of mortality 35. In addition to accelerated destruction of CD4+ T cells by HIV infection, underproduction of T cells also occurs. Underproduction can occur from infection-mediated death of progenitor cells and destruction of the hematopoietic stroma 36. Furthermore, proliferative responses are impaired. Peripheral blood T cells from AIDS patients do not proliferate normally in response to mitogens 37. Even in AIDS patients showing serologic evidence of prior infection with cytomegalovirus or herpes simplex virus, lymphocytes fail to proliferate normally in response to these viral antigens 38.
Failure to proliferate may be caused in part by impaired elaboration of IL-2. Peripheral blood T cells from AIDS patients have impaired IL-2 secretion in response to a variety of stimuli 39. In vitro, recombinant IL-2 can restore some mitogenic responses of AIDS patients’ blood lymphocytes40. Clinical trials of IL-2 for HIV infection demonstrate that IL-2 increases CD4+ T cell counts in recipients, without increasing HIV replication, particularly when given intermittently 41. While this therapy initially appeared to be promising, IL-2 may increase risk of bacterial pneumonia when administered frequently 42.
A major defect in T cell host defense during HIV infection is impaired production interferon–γ (IFN–γ) in response to mitogens 37 or antigens 43. The relative ability of peripheral lymphocytes to elaborate IFN–γ correlates with clinical status and CD4+ T cell count 44, and predicts progression to AIDS 45. A clinical example of this impairment occurs in interpretation of IFN–γ release assays for tuberculosis. For example, the performance of commercial assays is negatively influenced in Mycobacterium tuberculosis culture-positive patients with HIV infection and low CD4 counts 46.
Experimental work suggests that progression from asymptomatic HIV infection to AIDS is accompanied by a switch from Th1-like lymphocyte responses to Th2-like lymphocyte responses 47. Prevention of this switch in lymphocyte responses could prevent progression to AIDS. IL-12, a cytokine that favors Th1 development and inhibits Th2 development, has been shown to restore cell-mediated immunity in lymphocytes obtained from HIV-infected individuals 48. Few data exist on the function of lung CD4+ cells, but activation status differs in BAL and blood from HIV-infected and uninfected subjects, and responses to infectious antigens are impaired in BAL CD4+ T cells 49.
CD8+ T cells
CD8+ T cell alveolitis occurs during HIV infection. Some HIV-infected individuals may manifest pulmonary symptoms as a result of CD8+ T cell influx into the lung, clinically diagnosed as lymphoid interstitial pneumonitis 50. The functional capabilities of these cells (and their intended targets) require further investigation.
The alveolitis is a result, in part, of CD8+ T cells directed against HIV antigens, as subpopulations of CD8+ T cells are cytotoxic for macrophages or B cell lines expressing HIV antigens 51. The intensity of CD8+ alveolitis correlates with HIV viral load, and the poor prognosis associated with alveolitis may be a result of the elevated viral burden 52. One mechanism of alveolitis is overproduction of IL-15, a cytokine with IL-2-like effects, by alveolar macrophages. Alveolar macrophages from HIV-infected individuals produce large quantities of IL-15, which enhances antigen presentation by alveolar macrophages and causes proliferation of lung CD8+ T cells 53.
CD8+ T cells obtained from the lungs of HIV-infected individuals do not lyse appropriate targets in vitro 54. For example, CD8+ T cell-mediated cytotoxicity for influenza virus is decreased in HIV-infected individuals 55. Late in the course of HIV infection, numbers of CD8+ T cells decline. Depletion of CD8+ T cells may be associated with the development of disseminated cytomegalovirus and Mycobacterium avium infections 56.
Phenotypically, CD8+ T cells from AIDS patients express activation markers 57, and increased percentages of activated cells predict progression of HIV-related disease 58. Peripheral CD8+ T cells in HIV-infected individuals may be poor effectors because they lack required maturation signals 59. Unlike the periphery, local concentrations of IL-2 and IFN–γ may be increased in the lung during HIV infection, due to activation of CD8+ T cells 60. Functionally, however, CD8+ responses may be deficient because of lack of local CD4+ T cell help. CD4+ T cells are needed for priming, maintenance of memory, and functional activation in CD8+ T cells 61.
In theory, modulation of CD8+ T cell populations directed against HIV-infected cells could provide a novel method to augment host defense. Studies have attempted to exploit this finding by infusion of CD8+ T cells into HIV-infected individuals 62. CD8+ T cells, expanded in vitro and infused into the donor, result in increased killing of HIV-infected target cells 63. These infused CD8+ T cells accumulate in the lung, but their eventual benefit as a therapeutic modality is uncertain.
Natural killer (NK) cells
Increased numbers of NK cells in BAL have been observed in HIV-infected individuals, but with progressive HIV disease, they lose functional capabilities. As with CD8+ T cells, NK cells may be impaired during HIV infection because they are dependent on signals from CD4+ T cells for optimal function 64. Biological response modifiers such as recombinant IL-2 restore lytic ability in vitro 65, and IFN–γ may augment NK cell activity in early stages of HIV infection 66. The combination of IL-12 and IL-15 is effective in restoring expression of cytolytic molecules in NK cells from HIV-infected individuals 67.
B cells and immunoglobulins
The polyclonal activation of B cells that occurs systemically during HIV infection has been appreciated since the early era of the epidemic. Compared with uninfected individuals, measurement of immunoglobulins in BAL from AIDS patients with pulmonary symptoms shows increases in total amounts of IgG, IgM, and IgA 29. Local immunoglobulin synthesis may occur in the lung, shown by increased numbers of IgG, IgM, and IgA secreting cells 68. In contrast to results obtained during episodes of clinical infection, BAL from asymptomatic HIV-infected individuals contains decreased concentrations of IgG compared with uninfected controls 69.
While generalized gammopathy occurs, antibody responses to specific antigens are impaired in HIV-infected individuals. B cell abnormalities begin early in HIV infection, with failure to produce antibody in response to mitogen at the time of HIV seroconversion, before T cell function is affected 70. B cells from AIDS patients show impaired proliferation in response to mitogens, and do not initiate normal antibody synthesis in response to newly encountered antigens 71. Altered IgG concentrations in the lung may be a result of impaired ability of alveolar macrophages to induce IgG secretion from B cells, likely as a result of TGF–β secretion 69. Functionally, BAL and serum IgG from HIV-infected individuals demonstrate decreased opsonic activity against Streptococcus pneumoniae than IgG from uninfected controls 72.
Recent studies examining the utility of antibodies in detection of Pneumocystis are presented in the Introduction.
ALTERATIONS IN ALVEOLAR MACROPHAGES
Cell numbers
Macrophage numbers in BALs from AIDS patients are probably normal 28, but percentages are decreased by influx of other cells. As discussed above, HIV infection of alveolar macrophages establishes these cells as reservoirs of infection without depletion of their numbers.
Chemotaxis, phagocytosis, and killing
Elimination of pathogens by alveolar macrophages depends upon an orderly sequence of chemotaxis, phagocytosis, and killing. Peripheral blood monocytes from AIDS patients are reported to be defective in chemotaxis to several chemoattractants 73, but other investigators find unimpaired chemotaxis 74. Alveolar macrophages from asymptomatic, HIV-infected subjects demonstrate enhanced phagocytosis for Staphylococcus aureus 75. In contrast, binding of Pneumocystis to macrophages depends upon a variety of mediators, including mannose receptors 76. Alveolar macrophages from HIV-infected individuals demonstrate decreased binding and phagocytosis of Pneumocystis in vitro, and this defect correlates with mannose receptor downregulation 77. Importantly, phagocytic activity is decreased in alveolar macrophages from HIV-infected individuals who smoke 78.
The magnitude of the respiratory burst of alveolar macrophages from AIDS patients in vitro is not different from uninfected controls, and IFN–γ enhances the response in cells from both groups equivalently 43. Alveolar macrophages and monocyte-derived macrophages do not kill Toxoplasma gondii or Chlamydia psittaci, whether obtained from AIDS patients or from uninfected individuals 43. When exposed in vitro to IFN–γ, however, alveolar macrophages obtained from AIDS patients increase their killing of these organisms in a manner equivalent to uninfected individuals’ cells 43, 79. These data support the theory that suboptimal activation of alveolar macrophages in vivo is a result of impaired signaling from T cells.
Antigen presentation
During HIV infection, blood monocytes do not present antigens to T cells normally 80. Alveolar macrophages are relatively poor antigen-presenting cells, in comparison to blood monocytes, but alveolar macrophages from HIV-infected patients demonstrate enhanced ability to present antigen 81. To explain the pulmonary infectious complications that occur in HIV-infected individuals despite enhanced antigen presentation, the role of dendritic cells must be considered. Dendritic cells may perform the majority of antigen presentation in the lung. HIV infection of dendritic cells is cytopathic for these cells, and the numbers of dendritic cells are decreased in asymptomatic HIV-infected individuals and in AIDS patients 82. Dendritic cells from HIV-infected individuals exhibit defective antigen presentation, and may facilitate HIV infection of T cells 83.
TNF elaboration
The data regarding TNF production during HIV infection are discrepant. Some AIDS patients are reported to have elevated serum levels of TNF 84. When peripheral blood monocytes are examined, they are reported to have either high spontaneous release of TNF 85 or to have suboptimal release after appropriate stimulation 86. Paradoxically, some investigators have found that HIV infection of monocytes or monocyte-derived macrophages in vitro does not induce TNF release 87.
Alveolar macrophages from asymptomatic, HIV-seropositive individuals demonstrate increased spontaneous TNF release, which correlates with extent of HIV expression 88. BAL cells from smokers release less TNF than BAL cells from nonsmokers, suggesting that smoking and HIV interact to suppress macrophage function 30. Recent work examining responses of alveolar macrophages to Salmonella typhimurium demonstrates no differences in phagocytosis or killing between cells from HIV-infected individuals and controls, but TNF elaboration is increased significantly in cells from HIV-infected individuals 89. The literature does not reach consensus, however, and there are experimental examples of impaired TNF release as well. Some of this discrepancy may be explained by the stimuli used to provoke TNF elaboration. For example, when stimulated with lipopolysaccharide (LPS), alveolar macrophages show decreased TNF release via a toll-like receptor 4- (TLR4-) dependent mechanism 90. Conversely, it has been demonstrated that TNF release from alveolar macrophages in response to HIV-1 single-stranded RNA is dependent upon TLR8 signaling 91.
TNF may have beneficial and detrimental effects during Pneumocystis pneumonia. Pneumocystis infection stimulates production of TNF by macrophages 92, and alveolar macrophages from HIV-infected individuals with Pneumocystis pneumonia elaborate increased amounts of TNF 93. Expression of TNF by human alveolar macrophages during Pneumocystis pneumonia correlated with decreased arterial oxygenation, suggesting that TNF-induced inflammation is detrimental 94. Part of the beneficial effect of corticosteroid therapy during Pneumocystis pneumonia may be to inhibit TNF elaboration 95.
ALTERATIONS IN LUNG NEUTROPHILS
Cell numbers
Several BAL series have reported increases in the concentrations 96 or in the percentages of neutrophils 28 obtained from AIDS patients, compared with uninfected controls. Although AIDS patients may have increased numbers of neutrophils at the time of BAL, little is known about the host defense capabilities of these cells.
Chemotaxis and phagocytosis
Peripheral neutrophils from some AIDS patients with frequent localized infections, show decreased chemotaxis in vitro 74. Neutrophils from HIV-infected individuals have decreased expression of CD88, the ligand for complement factor 5a, which could contribute to increased susceptibility to bacterial infections 97. The phagocytic capacity of neutrophils during HIV infection is controversial. Individuals with early HIV infection demonstrate enhanced phagocytosis 98. Phagocytosis of opsonized Staphylococcus aureus is decreased in some, but not all, AIDS patients’ peripheral blood neutrophils 99. The defect in phagocytosis can be corrected by in vivo administration of granulocyte colony stimulating factor 100. Neutrophils from individuals with HIV infection express decreased IgG Fc-γ receptor 1 expression compared with uninfected volunteers 101.
Considering Pneumocystis pneumonia, increased numbers of neutrophils in BAL fluid from patients with HIV infection and Pneumocystis pneumonia correlates with impaired oxygenation 102, poor outcome 103, and increases in mechanical ventilation and mortality 104. Neutrophils obtained from non-HIV infected donors are able to ingest and kill Pneumocystis organisms and generate superoxide when challenged by organisms 105. Anti-Pneumocystis IgG and complement are required to opsonize the organism and increase the respiratory burst of neutrophils 106. In vivo, BAL IL-8 concentrations during Pneumocystis pneumonia correlate with clinical severity and mortality 107.
SYNOPSIS.
The broad variety of pulmonary infections encountered in HIV-infected individuals demonstrates that the host defense network is impaired. An improved understanding of these events in the lung can lead to specific interventions aimed at restoration of deficient function. This review summarizes the pulmonary host defense deficits in HIV-infected individuals, focusing on lymphocytes, alveolar macrophages, and neutrophils.
KEY POINTS.
HIV infects lung lymphocytes and alveolar macrophages, and tropic strains infect lung lymphocytes and alveolar macrophages using distinct chemokine coreceptors.
Lung CD4+ T cells are reduced in number, but demonstrate impaired proliferative and cytokine responses, despite expression of activation signals. Lung CD8+ T cells are increased in number and cause lymphocytic alveolitis, but do not lyse target cells appropriately.
Specific antibody responses and opsonization of microorganisms are impaired.
Alveolar macrophages demonstrate intact phagocytosis and killing, enhanced antigen presentation, and increased tumor necrosis factor elaboration. Despite these findings, pulmonary infections in vivo may result from impaired activation signaling from T cells.
Further investigation is needed to clarify discrepant results regarding pulmonary neutrophils’ capacities for chemotaxis and phagocytosis.
Acknowledgments
Supported by: NHLBI U01 HL98961
Footnotes
Conflict of interest: The author has nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beck JM. The immunocompromised host: HIV infection. Proc Am Thorac Soc. 2005;2:423–7. doi: 10.1513/pats.200507-077JS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agostini C, Zambello R, Trentin L, et al. HIV and pulmonary immune responses. Immunol Today. 1996;17:359–64. doi: 10.1016/0167-5699(96)30022-4. [DOI] [PubMed] [Google Scholar]
- 3.Beck JM, Rosen MJ, Peavy HH. Pulmonary complications of HIV infection: report of the fourth NHLBI workshop. Am J Respir Crit Care Med. 2001;164:2120–6. doi: 10.1164/ajrccm.164.11.2102047. [DOI] [PubMed] [Google Scholar]
- 4.White NC, Agostini C, Israël-Biet D, et al. The growth and the control of human immunodeficiency virus in the lung: implications for highly active antiretroviral therapy. Eur J Clin Invest. 1999;29:964–72. doi: 10.1046/j.1365-2362.1999.00550.x. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents. MMWR. 2009;58:1–207. [Google Scholar]
- 6.Atzori C, Clerici M, Trabattoni D, et al. Assessment of immune reconstitution to Pneumocystis carinii in HIV-1 patients under different highly active antiretroviral therapy regimens. J Antimicrob Chemother. 2003;52:276–81. doi: 10.1093/jac/dkg314. [DOI] [PubMed] [Google Scholar]
- 7.Djawe K, Huang L, Daly KR, et al. Serum antibody levels to the Pneumocystis jirovecii major surface glycoprotein in the diagnosis of P. jirovecii pneumonia in HIV+ patients. PLoS ONE. 2010;5:e14259. doi: 10.1371/journal.pone.0014259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gingo MR, Lucht L, Daly KR, et al. Serologic responses to Pneumocystis proteins in HIV patients with and without Pneumocystis jirovecii pneumonia. JAIDS. 2011;57:190–6. doi: 10.1097/QAI.0b013e3182167516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benfield TL, Lundgren JD, Masur H. HIV-1, cytokines, and the lung. In: Nelson S, Martin TR, editors. Cytokines in pulmonary disease: infection and inflammation. New York: Marcel Dekker; 2000. pp. 331–64. [Google Scholar]
- 10.Guay LA, Sierra-Madero JG, Finegan CK, et al. Mediation of entry of human immunodeficiency virus-1 into alveolar macrophages by CD4 without facilitation by surfactant-associated protein-A. Am J Respir Cell Mol Biol. 1997;16:421–8. doi: 10.1165/ajrcmb.16.4.9115753. [DOI] [PubMed] [Google Scholar]
- 11.Park IW, Koziel H, Hatch W, et al. CD4 receptor-dependent entry of human immunodeficiency virus type-1 env-pseudotypes into CCR5-, CCR3-, and CXCR4-expressing human alveolar macrophages is preferentially mediated by the CCR5 coreceptor. Am J Respir Cell Mol Biol. 1999;20:864–71. doi: 10.1165/ajrcmb.20.5.3547. [DOI] [PubMed] [Google Scholar]
- 12.An P, Li R, Wang JM, et al. Role of exonic variation in chemokine receptor genes on AIDS: CCRL2 F167Y association with Pneumocystis pneumonia. PLoS Genetics. 2011;7:e1002328. doi: 10.1371/journal.pgen.1002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salahuddin SZ, Rose RM, Groopman JE, et al. Human T lymphotropic virus type III infection of human alveolar macrophages. Blood. 1986;68:281–4. [PubMed] [Google Scholar]
- 14.Abbud RA, Finegan CK, Guay LA, et al. Enhanced production of human immunodeficiency virus type 1 by in vitro-infected alveolar macrophages from otherwise healthy cigarette smokers. J Infect Dis. 1995;172:859–63. doi: 10.1093/infdis/172.3.859. [DOI] [PubMed] [Google Scholar]
- 15.De Maria A, Pantaleo G, Schnittman SM, et al. Infection of CD8+ lymphocytes: requirement for interaction with infected CD4+ cells and induction of infectious virus from chronically infected CD8+ cells. J Immunol. 1991;146:2220–6. [PubMed] [Google Scholar]
- 16.Semenzato G, Agostini C, Ometto L, et al. CD8+ T lymphocytes in the lung of acquired immunodeficiency syndrome patients harbor human immunodeficiency virus type 1. Blood. 1995;85:2308–14. [PubMed] [Google Scholar]
- 17.Agostini C, Trentin L, Zambello R, et al. HIV-1 and the lung: infectivity, pathogenic mechanisms and cellular immune responses taking place in the lower respiratory tract. Am Rev Respir Dis. 1993;147:1038–49. doi: 10.1164/ajrccm/147.4.1038. [DOI] [PubMed] [Google Scholar]
- 18.Rose RM, Krivine A, Pinkston P, et al. Frequent identification of HIV-1 DNA in bronchoalveolar lavage cells obtained from individuals with the acquired immunodeficiency syndrome. Am Rev Respir Dis. 1991;143:850–4. doi: 10.1164/ajrccm/143.4_Pt_1.850. [DOI] [PubMed] [Google Scholar]
- 19.Sierra-Madero JG, Toossi Z, Hom DL, et al. Relationship between load of virus in alveolar macrophages from human immunodeficiency virus type 1-infected persons, production of cytokines, and clinical status. J Infect Dis. 1994;169:18–27. doi: 10.1093/infdis/169.1.18. [DOI] [PubMed] [Google Scholar]
- 20.Lewin SR, Kirihara J, Sonza S, et al. HIV-1 DNA and mRNA concentrations are similar in peripheral blood monocytes and alveolar macrophages in HIV-1-infected individuals. AIDS. 1998;12:719–27. doi: 10.1097/00002030-199807000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Brenchley JM, Knox KS, Asher AI, et al. High frequencies of polyfunctional HIV-specific T cells are associated with preservation of mucosal CD4 T cells in bronchoalveolar lavage. Mucosal Immunol. 2008;1:49–58. doi: 10.1038/mi.2007.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orenstein JM, Fox C, Wahl SM. Macrophages as a source of HIV during opportunistic infections. Science. 1997;276:1857–61. doi: 10.1126/science.276.5320.1857. [DOI] [PubMed] [Google Scholar]
- 23.Nakata K, Rom WN, Honda Y, et al. Mycobacterium tuberculosis enhances human immunodeficiency virus-1 replication in the lung. Am J Respir Crit Care Med. 1997;155:996–1003. doi: 10.1164/ajrccm.155.3.9117038. [DOI] [PubMed] [Google Scholar]
- 24.Fraziano M, Cappelli G, Santucci M, et al. Expression of CCR5 is increased in human monocyte-derived macrophages and alveolar macrophages in the course of in vivo and in vitro Mycobacterium tuberculosis infection. AIDS Res Hum Retrovir. 1999;15:869–74. doi: 10.1089/088922299310575. [DOI] [PubMed] [Google Scholar]
- 25.Wahl SM, Greenwell-Wild T, Hale-Donze H, et al. Permissive factors for HIV-1 infection of macrophages. J Leuk Biol. 2000;68:303–10. [PubMed] [Google Scholar]
- 26.Koziel H, Kim S, Reardon C, et al. Enhanced in vivo human immunodeficiency virus-1 replication in the lungs of human immunodeficiency virus-infected persons with Pneumocystis carinii pneumonia. Am J Respir Crit Care Med. 1999;160:2048–55. doi: 10.1164/ajrccm.160.6.9902099. [DOI] [PubMed] [Google Scholar]
- 27.Israël-Biet D, Esvant H, Laval AM, et al. Impairment of beta chemokine and cytokine production in patients with HIV related Pneumocystis jiroveci pneumonia. Thorax. 2004;59:247–51. doi: 10.1136/thx.2003.013763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White DA, Gellene RA, Gupta S, et al. Pulmonary cell populations in the immunosuppressed patient: bronchoalveolar lavage findings during episodes of pneumonitis. Chest. 1985;88:352–9. doi: 10.1378/chest.88.3.352. [DOI] [PubMed] [Google Scholar]
- 29.Young KR, Jr, Rankin JA, Naegel GP, et al. Bronchoalveolar lavage cells and proteins in patients with the acquired immunodeficiency syndrome: an immunologic analysis. Ann Intern Med. 1985;103:522–33. doi: 10.7326/0003-4819-103-4-522. [DOI] [PubMed] [Google Scholar]
- 30.Wewers MD, Diaz PT, Wewers ME, et al. Cigarette smoking in HIV infection induces a suppressive inflammatory environment in the lung. Am J Respir Crit Care Med. 1998;158:1543–9. doi: 10.1164/ajrccm.158.5.9802035. [DOI] [PubMed] [Google Scholar]
- 31.Hermier F, Comby E, Delaunay A, et al. Decreased blood TcRγδ+ lymphocytes in AIDS and p24-antigenemic HIV-1-infected patients. Clin Immunol Immunopathol. 1993;69:248–50. doi: 10.1006/clin.1993.1176. [DOI] [PubMed] [Google Scholar]
- 32.Kägi MK, Fierz W, Grob PJ, et al. High proportion of gamma-delta T cell receptor positive T cells in bronchoalveolar lavage and peripheral blood of HIV-infected patients with Pneumocystis carinii pneumonias. Respiration. 1993;60:170–7. doi: 10.1159/000196194. [DOI] [PubMed] [Google Scholar]
- 33.Rubbo PA, Tuaillon E, Balloré K, et al. The potential impact of CD4+ T cell activation and enhanced Th1/Th2 cytokine ratio on HIV-1 secretion in the lungs of individuals with advanced AIDS and active pulmonary infection. Clin Immunol. 2011;139:142–54. doi: 10.1016/j.clim.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Agostini C, Adami F, Poulter LW, et al. Role of bronchoalveolar lavage in predicting survival of patients with human immunodeficiency virus infection. Am J Respir Crit Care Med. 1997;156:1501–7. doi: 10.1164/ajrccm.156.5.9611109. [DOI] [PubMed] [Google Scholar]
- 35.Day RB, Wang Y, Knox KS, et al. Alveolar macrophages from HIV-infected subjects are resistant to Mycobacterium tuberculosis in vitro. Am J Respir Cell Mol Biol. 2004;30:403–10. doi: 10.1165/rcmb.2003-0059OC. [DOI] [PubMed] [Google Scholar]
- 36.McCune JM. The dynamics of CD4+ T-cell depletion in HIV disease. Nature. 2001;410:974–9. doi: 10.1038/35073648. [DOI] [PubMed] [Google Scholar]
- 37.Rudy T, Opelz G, Gerlack R, et al. Correlation of in vitro immune defects with impaired gamma interferon response in human-immunodeficiency-virus-infected individuals. Vox Sang. 1988;54:92–5. doi: 10.1111/j.1423-0410.1988.tb01623.x. [DOI] [PubMed] [Google Scholar]
- 38.Krowka J, Stites D, Mills J, et al. Effects of interleukin 2 and large envelope glycoprotein (gp120) of human immunodeficiency virus (HIV) on lymphocyte proliferative responses to cytomegalovirus. Clin Exp Immunol. 1988;72:179–85. [PMC free article] [PubMed] [Google Scholar]
- 39.Alcocer-Varela J, Alarcon-Segovia D, Abud-Mendoza C. Immunoregulatory circuits in the acquired immune deficiency syndrome and related complex. Production of and response to interleukins 1 and 2, NK function and its enhancement by interleukin-2 and kinetics of the autologous mixed lymphocyte reaction. Clin Exp Immunol. 1985;60:31–8. [PMC free article] [PubMed] [Google Scholar]
- 40.Sheridan JF, Aurelian L, Donnenberg AD, et al. Cell-mediated immunity to cytomegalovirus (CMV) and herpes simplex virus (HSV) antigens in the acquired immune deficiency syndrome: interleukin-1 and interleukin-2 modify in vitro responses. J Clin Immunol. 1984;4:304–11. doi: 10.1007/BF00915298. [DOI] [PubMed] [Google Scholar]
- 41.Sereti I, Lane HC. Immunopathogenesis of human immunodeficiency virus: implications for immune-based therapies. Clin Infect Dis. 2001;32:1738–55. doi: 10.1086/320758. [DOI] [PubMed] [Google Scholar]
- 42.INSIGHT-ESPRIT Study Group. Predictors of bacterial pneumonia in evaluation of subcutaneous interleukin-2 in a randomized international trial (ESPRIT) HIV Med. 2010;12:219–27. doi: 10.1111/j.1468-1293.2010.00875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murray HW, Gellene RA, Libby DM, et al. Activation of tissue macrophages from AIDS patients: in vitro response of AIDS alveolar macrophages to lymphokines and interferon-γ. J Immunol. 1985;135:2374–7. [PubMed] [Google Scholar]
- 44.Murray HW, Scavuzzo DA, Kelly CD, et al. T4+ cell production of interferon gamma and the clinical spectrum of patients at risk for and with acquired immunodeficiency syndrome. Arch Intern Med. 1988;148:1613–6. [PubMed] [Google Scholar]
- 45.Murray HW, Hillman JK, Rubin BY, et al. Patients at risk for AIDS-related opportunistic infections: clinical manifestations and impaired gamma interferon production. N Engl J Med. 1985;313:1504–10. doi: 10.1056/NEJM198512123132403. [DOI] [PubMed] [Google Scholar]
- 46.Aabye MG, Ravn P, PrayGod G, et al. The impact of HIV infection and CD4 cell count on the performance of an interferon gamma release assay in patients with pulmonary tuberculosis. PLoS ONE. 2009;4:e4220. doi: 10.1371/journal.pone.0004220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clerici M, Shearer GM. A TH1ØTH2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993;14:107–11. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- 48.Clerici M, Lucey DR, Berzofsky JA, et al. Restoration of HIV-specific cell-mediated immune responses by interleukin-12 in vitro. Science. 1993;262:1721–4. doi: 10.1126/science.7903123. [DOI] [PubMed] [Google Scholar]
- 49.Jambo KC, Sepako E, Fullerton DG, et al. Bronchoalveolar CD4+ T cell responses to respiratory antigens are impaired in HIV-infected adults. Thorax. 2011;66:375–82. doi: 10.1136/thx.2010.153825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Autran B, Sadat-Sowti B, Hadida F, et al. HIV-specific cytotoxic T lymphocytes against alveolar macrophages: specificities and downregulation. Res Virol. 1991;142:113–8. doi: 10.1016/0923-2516(91)90046-6. [DOI] [PubMed] [Google Scholar]
- 51.Autran B, Plata F, Guillon JM, et al. HIV-specific cytotoxic T lymphocytes directed against alveolar macrophages in HIV-infected patients. Res Virol. 1990;141:131–6. doi: 10.1016/0923-2516(90)90014-a. [DOI] [PubMed] [Google Scholar]
- 52.Twigg HL, Soliman DM, Day RB, et al. Lymphocytic alveolitis, bronchoalveolar lavage viral load, and outcome in human immunodeficiency virus infection. Am J Respir Crit Care Med. 1999;159:1439–44. doi: 10.1164/ajrccm.159.5.9808031. [DOI] [PubMed] [Google Scholar]
- 53.Agostini C, Zambello R, Facco M, et al. CD8 T-cell infiltration in extravascular tissues of patients with human immunodeficiency virus infection: interleukin-15 upmodulates costimulatory pathways involved in the antigen-presenting cells-T-cell interaction. Blood. 1999;93:1277–86. [PubMed] [Google Scholar]
- 54.Semenzato G. Immunology of interstitial lung diseases: cellular events taking place in the lung of sarcoidosis, hypersensitivity pneumonitis and HIV infection. Eur Respir J. 1991;4:94–102. [PubMed] [Google Scholar]
- 55.Shearer GM, Bernstein DC, Tung KSK, et al. A model for the selective loss of major histocompatibility complex self-restricted T cell immune response during the development of acquired immune deficiency syndrome (AIDS) J Immunol. 1986;137:2514–21. [PubMed] [Google Scholar]
- 56.Fiala M, Herman V, Gornbein J. Role of CD8+ in late opportunistic infections of patients with AIDS. Res Immunol. 1992;143:903–7. doi: 10.1016/0923-2494(92)80113-y. [DOI] [PubMed] [Google Scholar]
- 57.Barry SM, Johnson MA, Janossy G. Increased proportions of activated and proliferating memory CD8+ T lymphocytes in both blood and lung are associated with blood HIV viral load. J Acquir Immun Defic Synd. 2003;34:351–7. doi: 10.1097/00126334-200312010-00001. [DOI] [PubMed] [Google Scholar]
- 58.Levacher M, Hulstaert F, Tallet S, et al. The significance of activation markers on CD8 lymphocytes in human immunodeficiency syndrome: staging and prognostic value. Clin Exp Immunol. 1992;90:376–82. doi: 10.1111/j.1365-2249.1992.tb05854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lieberman J, Shankar P, Manjanath N, et al. Dressed to kill? A review of why antiviral CD8 T lymphocytes fail to prevent progressive immunodeficiency in HIV-1 infection. Blood. 2001;98:1667–77. doi: 10.1182/blood.v98.6.1667. [DOI] [PubMed] [Google Scholar]
- 60.Twigg HL, 3rd, Spain BA, Soliman DM, et al. Production of interferon-gamma by lung lymphocytes in HIV-infected individuals. Am J Physiol. 1999;276:L256–62. doi: 10.1152/ajplung.1999.276.2.L256. [DOI] [PubMed] [Google Scholar]
- 61.McMichael AJ, Rowland-Jones SL. Cellular immune responses to HIV. Nature. 2001;410:980–7. doi: 10.1038/35073658. [DOI] [PubMed] [Google Scholar]
- 62.Herberman RB. Adoptive therapy with purified CD8 cells in HIV infection. Semin Hematol. 1992;29:35–40. [PubMed] [Google Scholar]
- 63.Ho M, Armstrong J, McMahon D, et al. A phase 1 study of adoptive transfer of autologous CD8+ T lymphocytes in patients with acquired immunodeficiency syndrome (AIDS)-related complex or AIDS. Blood. 1993;81:2093–101. [PubMed] [Google Scholar]
- 64.Agostini C, Poletti V, Zambello R, et al. Phenotypical and functional analysis of bronchoalveolar lavage lymphocytes in patients with HIV infection. Am Rev Respir Dis. 1988;138:1609–15. doi: 10.1164/ajrccm/138.6.1609. [DOI] [PubMed] [Google Scholar]
- 65.Agostini C, Zambello R, Trentin L, et al. Cytotoxic events taking place in the lung of patients with HIV-1 infection: evidence of an intrinsic defect of the major histocompatibility complex-unrestricted killing partially restored by the incubation with rIL-2. Am Rev Respir Dis. 1990;142:516–22. doi: 10.1164/ajrccm/142.3.516. [DOI] [PubMed] [Google Scholar]
- 66.Poli G, Introna M, Zanaboni F, et al. Natural killer cells in intravenous drug abusers with lymphadenopathy syndrome. Clin Exp Immunol. 1985;62:128–35. [PMC free article] [PubMed] [Google Scholar]
- 67.Rao PV, Ramanavelan S, Rajasekaran S, et al. Natural-killer cell-derived cytolytic molecules in HIV-associated pulmonary tuberculosis-role of exogenous interleukins. J Cin Immunol. 2010;30:393–401. doi: 10.1007/s10875-010-9380-y. [DOI] [PubMed] [Google Scholar]
- 68.Rankin JA, Walzer PD, Dwyer JM, et al. Immunologic alterations in bronchoalveolar lavage fluid in the acquired immunodeficiency syndrome (AIDS) Am Rev Respir Dis. 1983;128:189–94. doi: 10.1164/arrd.1983.128.1.189. [DOI] [PubMed] [Google Scholar]
- 69.Twigg HL, 3rd, Spain BA, Soliman DM, et al. Impaired IgG production in the lungs of HIV-infected individuals. Cell Immunol. 1996;170:127–33. doi: 10.1006/cimm.1996.0142. [DOI] [PubMed] [Google Scholar]
- 70.Breen EC, Rezai AR, Nakajima K, et al. Infection with HIV is associated with elevated IL-6 levels and production. J Immunol. 1990;144:480–4. [PubMed] [Google Scholar]
- 71.Lane HC, Masur H, Edgar LC, et al. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1983;309:453–8. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- 72.Eagan R, Twigg HL, French N, et al. Lung fluid immunoglobulin from HIV-infected subjects has impaired opsonic function against pneumococci. Clin Infect Dis. 2007;44:1632–8. doi: 10.1086/518133. [DOI] [PubMed] [Google Scholar]
- 73.Poli G, Bottazzi B, Acero R, et al. Monocyte function in intravenous drug abusers with lymphadenopathy syndrome and in patients with acquired immunodeficiency syndrome: selective impairment of chemotaxis. Clin Exp Immunol. 1985;62:136–42. [PMC free article] [PubMed] [Google Scholar]
- 74.Nielsen H, Kharazmi A, Faber V. Blood monocyte and neutrophil functions in the acquired immune deficiency syndrome. Scand J Immunol. 1986;24:291–6. doi: 10.1111/j.1365-3083.1986.tb02096.x. [DOI] [PubMed] [Google Scholar]
- 75.Musher DM, Watson DA, Nickeson D, et al. The effect of HIV infection on phagocytosis and killing of Staphylococcus aureus by human pulmonary alveolar macrophages. Am J Med. 1990;299:158–63. doi: 10.1097/00000441-199003000-00003. [DOI] [PubMed] [Google Scholar]
- 76.Ezekowitz RA, Williams DJ, Koziel H, et al. Uptake of Pneumocystis carinii mediated by the macrophage mannose receptor. Nature. 1991;351:155–8. doi: 10.1038/351155a0. [DOI] [PubMed] [Google Scholar]
- 77.Koziel H, Eichbaum Q, Kruskal BA, et al. Reduced binding and phagocytosis of Pneumocystis carinii by alveolar macrophages from persons infected with HIV-1 correlates with mannose receptor downregulation. J Clin Invest. 1998;102:1332–44. doi: 10.1172/JCI560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Elssner A, Carter JE, Yunger TM, et al. HIV-1 infection does not impair human alveolar macrophage phagocytic function unless combined with cigarette smoking. Chest. 2004;125:1071–6. doi: 10.1378/chest.125.3.1071. [DOI] [PubMed] [Google Scholar]
- 79.Murray HW, Rubin BY, Masur H, et al. Impaired production of lymphokines and immune (gamma) interferon in the acquired immunodeficiency syndrome. N Engl J Med. 1984;310:883–9. doi: 10.1056/NEJM198404053101404. [DOI] [PubMed] [Google Scholar]
- 80.Rich EA, Toossi Z, Fujiwara H, et al. Defective accessory function of monocytes in human immunodeficiency virus-related disease syndromes. J Lab Clin Med. 1988;112:174–81. [PubMed] [Google Scholar]
- 81.Twigg HL, 3rd, Lipscomb MF, Yoffe B, et al. Enhanced accessory cell function by alveolar macrophages from patients infected with the human immunodeficiency virus: potential role for depletion of CD4+ cells in the lung. Am J Respir Cell Mol Biol. 1989;1:391–400. doi: 10.1165/ajrcmb/1.5.391. [DOI] [PubMed] [Google Scholar]
- 82.Macatonia SE, Lau R, Patterson S, et al. Dendritic cell infection, depletion and dysfunction in HIV-infected individuals. Immunology. 1990;71:38–45. [PMC free article] [PubMed] [Google Scholar]
- 83.Macatonia SE, Taylor PM, Knight SC, et al. Primary stimulation by dendritic cells induces antiviral proliferative and cytotoxic T cell responses in vitro. J Exp Med. 1989;169:1255–64. doi: 10.1084/jem.169.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lähdevirta J, Maury CP, Teppo AM, et al. Elevated levels of circulating cachectin/tumor necrosis factor in patients with acquired immunodeficiency syndrome. Am J Med. 1988;85:289–91. doi: 10.1016/0002-9343(88)90576-1. [DOI] [PubMed] [Google Scholar]
- 85.Wright SC, Jewett A, Mitsuyasu R, et al. Spontaneous cytotoxicity and tumor necrosis factor production by peripheral blood monocytes from AIDS patients. J Immunol. 1988;141:99–104. [PubMed] [Google Scholar]
- 86.Ammann AJ, Palladino MA, Volberding P, et al. Tumor necrosis factor alpha and beta in acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. J Clin Immunol. 1987;7:481–5. doi: 10.1007/BF00915059. [DOI] [PubMed] [Google Scholar]
- 87.Molina J-M, Scaden DT, Amirault C, et al. Human immunodeficiency virus does not induce interleukin-1, interleukin-6, or tumor necrosis factor in mononuclear cells. J Virol. 1990;64:2901–6. doi: 10.1128/jvi.64.6.2901-2906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Israël-Biet D, Cadranel J, Beldjord K, et al. Tumor necrosis factor production in HIV-seropositive subjects: relationship with lung opportunistic infections and HIV expression in alveolar macrophages. J Immunol. 1991;147:490–4. [PubMed] [Google Scholar]
- 89.Gordon MA, Gordon SB, Musaya L, et al. Primary macrophages from HIV-infected adults show dysregulated cytokine responses to Salmonella, but normal internalization and killing. AIDS. 2007;21:2399–408. doi: 10.1097/QAD.0b013e3282f25107. [DOI] [PubMed] [Google Scholar]
- 90.Tachado SD, Zhang J, Zhu J, et al. HIV impairs TNF-alpha release in response to toll-like receptor 4 stimulation in human macrophages in vitro. Am J Resp Cell Mol Biol. 2005;33:610–21. doi: 10.1165/rcmb.2004-0341OC. [DOI] [PubMed] [Google Scholar]
- 91.Han H, Li X, Yue SC, et al. Epigenetic regulation of tumor necrosis factor (TNFα) release in human macrophages by HIV-1 single-stranded RNA (ssRNA) is dependent on TLR8 signaling. J Biol Chem. 2012;287:13778–86. doi: 10.1074/jbc.M112.342683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tamburrini E, De Luca A, Ventura G, et al. Pneumocystis carinii stimulates in vitro production of tumor necrosis factor-alpha by human macrophages. Med Microbiol Immunol. 1991;180:15–20. doi: 10.1007/BF00191696. [DOI] [PubMed] [Google Scholar]
- 93.Krishnan VL, Meager A, Mitchell DM, et al. Alveolar macrophages in AIDS patients: Increased spontaneous tumour necrosis factor-alpha production in Pneumocystis carinii pneumonia. Clin Exp Immunol. 1990;80:156–60. doi: 10.1111/j.1365-2249.1990.tb05225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rayment N, Miller RF, Ali N, et al. Synthesis of tumor necrosis factor-alpha mRNA in bronchoalveolar lavage cells from human immunodeficiency virus-infected persons with Pneumocystis carinii pneumonia. J Infect Dis. 1996;174:654–9. doi: 10.1093/infdis/174.3.654. [DOI] [PubMed] [Google Scholar]
- 95.Huang ZB, Eden E. Effect of corticosteroids on IL1 beta and TNF alpha release by alveolar macrophages from patients with AIDS and Pneumocystis carinii pneumonia. Chest. 1993;104:751–5. doi: 10.1378/chest.104.3.751. [DOI] [PubMed] [Google Scholar]
- 96.Wallace JM, Barbers RG, Oishi JS, et al. Cellular and T-lymphocyte subpopulation profiles in bronchoalveolar lavage fluid from patients with acquired immunodeficiency syndrome and pneumonitis. Am Rev Respir Dis. 1984;130:786–90. doi: 10.1164/arrd.1984.130.5.786. [DOI] [PubMed] [Google Scholar]
- 97.Meddows-Taylor S, Pendle S, Tiemessen CT. Altered expression of CD88 and associated impairment of complement 5a-induced neutrophil responses in human immunodeficiency virus type 1-infected patients with and without pulmonary tuberculosis. J Infect Dis. 2001;183:662–5. doi: 10.1086/318532. [DOI] [PubMed] [Google Scholar]
- 98.Bandres JC, Trial J, Musher DM, et al. Increased phagocytosis and generation of reactive oxygen products by neutrophils and monocytes of men with stage 1 human immunodeficiency virus infections. J Infect Dis. 1993;168:75–83. doi: 10.1093/infdis/168.1.75. [DOI] [PubMed] [Google Scholar]
- 99.Baldwin GC, Gasson JC, Quan SG, et al. Granulocyte-macrophage colony-stimulating factor enhances neutrophil function in acquired immunodeficiency syndrome patients. Proc Natl Acad Sci USA. 1988;85:2763–6. doi: 10.1073/pnas.85.8.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Roilides E, Walsh TJ, Pizzo PA, et al. Granulocyte colony-stimulating factor enhances the phagocytic and bactericidal activity of normal and defective human neutrophils. J Infect Dis. 1991;163:579–83. doi: 10.1093/infdis/163.3.579. [DOI] [PubMed] [Google Scholar]
- 101.Armbruster C, Krugluger W, Huber M, et al. Immunoglobulin G Fc(gamma) receptor expression on polymorphonuclear cells in bronchoalveolar lavage fluid of HIV-infected and HIV-seronegative patients with bacterial pneumonia. Clin Chem Lab Med. 2004;42:192–7. doi: 10.1515/CCLM.2004.035. [DOI] [PubMed] [Google Scholar]
- 102.Limper AH, Offord KP, Smith TF, et al. Pneumocystis carinii pneumonia: differences in lung parasite number and inflammation in patients with and without AIDS. Am Rev Respir Dis. 1989;140:1204–9. doi: 10.1164/ajrccm/140.5.1204. [DOI] [PubMed] [Google Scholar]
- 103.Mason GR, Hashimoto CH, Dickman PS, et al. Prognostic implications of bronchoalveolar lavage neutrophilia in patients with Pneumocystis carinii pneumonia and AIDS. Am Rev Respir Dis. 1989;139:1336–42. doi: 10.1164/ajrccm/139.6.1336. [DOI] [PubMed] [Google Scholar]
- 104.Azoulay E, Parrot A, Flahault A, et al. AIDS-related Pneumocystis carinii pneumonia in the era of adjunctive steroids: implication of BAL neutrophilia. Am J Respir Crit Care Med. 1999;160:493–9. doi: 10.1164/ajrccm.160.2.9901019. [DOI] [PubMed] [Google Scholar]
- 105.Laursen AL, Obel N, Rungby J, et al. Phagocytosis and stimulation of the respiratory burst in neutrophils by Pneumocystis carinii. J Infect Dis. 1993;168:1466–71. doi: 10.1093/infdis/168.6.1466. [DOI] [PubMed] [Google Scholar]
- 106.Laursen AL, Obel NS, Holmskov U, et al. Activation of the respiratory burst by Pneumocystis carinii Efficiency of different antibody isotypes, complement, lung surfactant protein D, and mannan-binding lectin. APMIS. 2003;111:405–15. doi: 10.1034/j.1600-0463.2003.t01-1-1110205.x. [DOI] [PubMed] [Google Scholar]
- 107.Benfield TL, Vestbo J, Junge J, et al. Prognostic value of interleukin-8 in AIDS-associated Pneumocystis carinii pneumonia. Am J Respir Crit Care Med. 1995;151:1058–62. doi: 10.1164/ajrccm/151.4.1058. [DOI] [PubMed] [Google Scholar]


