I. Synopsis
Diabetes and impaired glucose tolerance affect a substantial proportion of older adults. While the aging process can be associated with alterations in glucose metabolism, including both relative insulin resistance and islet cell dysfunction, abnormal glucose metabolism is not a necessary component of aging. Instead, older adults with diabetes and altered glucose status likely represent a vulnerable subset of the population at high-risk for complications and adverse geriatric syndromes such as accelerated muscle loss, functional disability, frailty, and early mortality. Goals for treatment of diabetes in the elderly include control of hyperglycemia, prevention and treatment of diabetic complications, avoidance of hypoglycemia and preservation of quality of life. Given the heterogeneity of the elderly population with regards to the presence of comorbidities, life expectancy, and functional status, an individualized approach to diabetes management is often appropriate. A growing area of research seeks to explore associations of dysglycemia and insulin resistance with the development of adverse outcomes in the elderly and may ultimately inform guidelines on the use of future glucose-lowering therapies in this population.
Keywords: Diabetes, aging, insulin resistance, islet cell dysfunction
II. Epidemiology of Diabetes and Impaired Glucose States with Aging
Diabetes in older adults is a growing public health concern with almost one-third of U.S. adults over the age of 60 years having diabetes, of which approximately half is undiagnosed, and an additional one-third of older adults have prediabetes (1). Diabetes prevalence in older adults is more than twice that of middle-aged adults (1). It is projected that the numbers of elderly persons will almost double by the year 2030 (2,3). In addition, the number of people in nursing homes with diabetes continues to increase (4). Consequently, the burden of diabetes in the elderly is significant and growing.
Glucose intolerance is associated with aging (1, 5-7). Aging has been associated with elevated levels of both glucose and insulin after oral glucose challenge testing (8). The 2-hour plasma glucose during an oral glucose tolerance test (OGTT) rises much more steeply than fasting glucose levels with aging (8-10). As a result, elderly individuals are more likely to be classified as having abnormal glucose status compared to younger adults using similar diagnostic criteria for diabetes (11). Some authors have suggested that the diagnosis of diabetes can be made many years earlier using OGTT versus fasting glucose levels alone in older persons (12). Data from the Baltimore Longitudinal Study of Aging (BLSA) demonstrate an age-related increase in progression rate from normal glucose status to impaired glucose tolerance (IGT) that is almost twice the progression rate from normal to impaired fasting glucose (IFG) after 20 years of follow-up (12). These findings suggest that oral glucose tolerance testing, in particular, is important to consider when characterizing abnormal glucose status in the elderly.
Tags: Glucose intolerance, diabetes, elderly, prediabetes, oral glucose tolerance testing
III. Altered Glucose Metabolism with Aging
Using hyperinsulinemic-euglycemic clamp methodology as a method for quantification of insulin effectiveness in regulating glucose transport into tissues, whole body insulin sensitivity is demonstrably reduced in older versus younger adults (13, 14). Impaired intracellular whole-body rates of glucose oxidation in elderly versus young adults have also been reported (15). Potential explanations for reduced insulin effectiveness with aging include: 1) increased abdominal fat mass, 2) decreased physical activity, 3) sarcopenia, 4) mitochondrial dysfunction, 5) hormonal changes (i.e., lower IGF-1 and DHEA), and 6) increased oxidative stress and inflammation (16). Nonetheless, insulin sensitivity decreases with age even after adjustment for differences in adiposity, fat distribution, and physical activity (17).
Islet cell dysfunction with aging is also a significant contributing factor to abnormal glucose metabolism with aging. Insulin secretion is most commonly tested using an oral glucose tolerance test (OGTT); it is standardized, simple to administer, and widely used in longitudinal studies. However, an oral load of 75 grams of glucose is delivered as a rapid bolus to the gut, and can trigger neural and incretin responses, over and beyond stimulus by the glucose per se, to the insulin-secreting beta-cells. Responses to physiological stimuli, such as a meal containing complex carbohydrates, fat and protein, may be different from that of a glucose load. With those limitations in mind, there is a gradual decline with aging in insulin secretion during the first hour in response to an oral glucose load, despite older adults actually having higher glucose levels after the glucose challenge (18, 19). Once plasma glucose levels reach the diabetic range, however, insulin secretion is severely compromised (20).
Beta-cell function has also been evaluated using the hyperglycemic clamp, in which plasma glucose levels are increased in a square wave fashion and maintained at this level for a fixed duration of time in a controlled manner. The final attained stable (or clamped) plasma glucose level can be varied and insulin secretion studied with clamped plasma glucose levels as high as 450 mg/dl (13). The beta-cell response to a hyperglycemic clamp is stereotypical in that there is a first phase insulin secretion that occurs within 2-3 minutes of the initiation of the square wave of infused glucose, followed by a slower plateau phase that reaches stability in about 100-120 minutes. In general, among older adults with normal glucose tolerance based on the OGTT, deficits in insulin secretion are seen only when high plasma glucose levels are achieved when compared to the young. But, as with all patients with type 2 diabetes, once an elderly patient has developed diabetes, the first phase insulin secretion in response to the square wave of a hyperglycemic clamp is defective to absent (13), and the plateau phase insulin secretion is less than in non-diabetic subjects.
Insulin secretion falls under essentially two types: constitutive (sometimes called basal) and stimulated (such as occurs after a meal, an OGTT, or hyperglycemic clamp) secretion, with constitutive insulin accounting for approximately 50% of the total 24-hour insulin output. Insulin secretion is pulsatile in nature. Using one-minute blood sampling and highly sensitive insulin assays, rapid, low-amplitude insulin pulses occurring approximately every 8-14 minutes and larger amplitude ultradian pulses occurring every 60-140 minutes can be elucidated (21). The rapid pulses persist even in isolated islets and are independent of circulating glucose levels while the ultradian pulses are tightly coupled to glucose, by which they are entrained. In the fasting state, elderly subjects without diabetes have disorderly pulsatile insulin secretion, with reduced amplitude and mass of the rapid pulses and decreased frequency of the ultradian pulses. Scheen, et al. (1996) (22) and Meneilly, et al. (1999) (23) both studied pulsatile insulin secretion in young and elderly subjects under conditions of sustained experimentally induced hyperglycemia. Overall, the findings were similar to the fasting state; specifically, older subjects displayed reduced amplitude and insulin mass of the rapid pulses and the ultradian pulses were more irregular with lower amplitude compared to younger subjects. The authors also found that glucose infusion for up to 53 hours (22) was not capable of ‘normalizing’ pulsatile insulin secretion in older individuals. Pulsatile insulin secretion is important for regulating glucose output from the liver and may be involved in maintaining skeletal muscle in a state of metabolic readiness, such as maintaining insulin receptors and glucose transporters in a ‘primed’ state. The disordered pulsatile insulin secretion seen in the elderly may, in fact, play a role in the previously described decrease in insulin sensitivity observed with aging. In type 2 diabetes, severe disorderliness is found as the oscillatory pattern of insulin secretion is almost totally disrupted. The rapid pulses are replaced by irregular pulses of short duration, and the ultradian pulses are disrupted, chaotic and uncoupled from glucose, as glucose fails to control the periodicity of the ultradian pulses (24).
Rising plasma glucose accounts for approximately 50% of the secreted insulin after a meal or OGTT; the remaining 50% is due to incretin hormones released from the enteroendocrine cells lining the gut (25). Incretins are peptide hormones of which there are two main types: gastric inhibitory polypeptide (GIP) and glucagon-like peptide-1 (GLP-1). Their effect on the beta-cell is to increase glucose-dependent insulin secretion. There is no evidence that secretion of incretins, as measured by plasma levels after OGTT, is defective in aging per se, although the beta-cell may be less responsive to their stimulatory effects. In an elegant study, Elahi, et al. (1984) (26) combined the hyperglycemic clamp with oral glucose (to stimulate endogenous GIP secretion) and found that, in older individuals, GIP secretion in response to the oral glucose was actually increased compared to younger participants but led to slightly lower insulin secretion in response to the endogenous GIP. Hyperglycemic clamps at 100 mg/dl and 230 mg/dl above basal have been performed in young and elderly subjects (27) with combined GIP infusions. At the lower clamped plasma glucose level, the potentiation of glucose-induced insulin secretion by GIP was reduced by half in the elderly compared to the young, while at the higher clamped plasma glucose level, insulin response to GIP was similar in both age groups. However, since the lower clamped plasma glucose level is more physiological, this probably reflects a true decline in GIP effectiveness with aging. In type 2 diabetes, GIP no longer potentiates glucose-induced insulin secretion and also increases glucagon secretion which further exacerbates hyperglycemia (28); however, GIP secretion is not lower in type 2 diabetes compared to non-diabetic subjects (25). The data on GLP-1secretion with increasing age and onset of type 2 diabetes is similar to GIP in that secretion of GLP-1 is not necessarily decreased with older age or the presence of diabetes but, unlike GIP, GLP-1 is still a powerful stimulus to insulin secretion – hence the robust development of a number of agents for treating type 2 diabetes that activate the GLP-1 receptor on beta-cells (i.e., exenatide, liragulatide). Also, unlike GIP, use of GLP-1 receptor agonists leads to a decrease in glucagon secretion (25).
The enzyme dipeptidyl peptidase 4 (DPP 4) inactivates both GIP and GLP-1 and has decreasing activity with older age (29), which may explain the higher GIP levels observed in the elderly (26). The relatively recent development of DPP 4 inhibitors (i.e. sitagliptin, saxagliptin, linagliptin) as therapeutic agents may also have a role for treatment of diabetes in the elderly.
Tags: hyperinsulinemic clamp, insulin resistance, islet cell function, insulin secretion, beta cell, hyperglycemic clamp, incretins, glucagon-like peptide-1, GLP-1, gastric inhibitory peptide, GIP, dipeptidyl-pepidase 4, DPP 4 enzyme
IV. Complications of Diabetes in the Elderly
Microvascular and macrovascular complications of diabetes occur in older patients, similar to younger persons, although the absolute risk of cardiovascular disease is much higher in older adults (30). However, diabetes in the older adult population is heterogeneous and includes individuals with both middle-age and elderly-onset diabetes (31), with the latter group accounting for up to one-third of older adults with diabetes. Middle-age onset adults may have worse glycemic control and are more likely to be taking glucose-lowering medications. Whereas the prevalence of macrovascular diseases (stroke, coronary heart disease, peripheral arterial disease) may be similar between the middle-age and elderly onset diabetes groups, the burden of microvascular disease (particularly retinopathy) may be greater in the former (31, 32). Thus, the age of diabetes onset may impact the burden of disease and diabetic complications present in the elderly patient with diabetes, but more studies need to be done.
Tags: microvascular complications, macrovascular complications, middle-age onset diabetes, elderly-age onset diabetes
V. Geriatric Syndromes Associated with Diabetes
Descriptions of otherwise healthy centenarians without impaired glucose uptake suggest that insulin resistance is not a necessary component of the aging process (33, 34). Instead, older adults with abnormal glucose status and diabetes likely represent a vulnerable subset at high risk for adverse outcomes. Geriatric syndromes that have been described to occur more frequently in persons with diabetes include loss of muscle function, functional limitations and disability, and frailty—all of which can significantly impact quality-of-life in the older patient—in addition to early mortality (Figure 1). Diabetes also increases the risk of other common geriatric syndromes such as depression, cognitive dysfunction, chronic pain, injurious falls, urinary incontinence, and polypharmacy (30) but will not be specifically discussed here.
Figure 1. Diabetes and the Pathway to Disability.
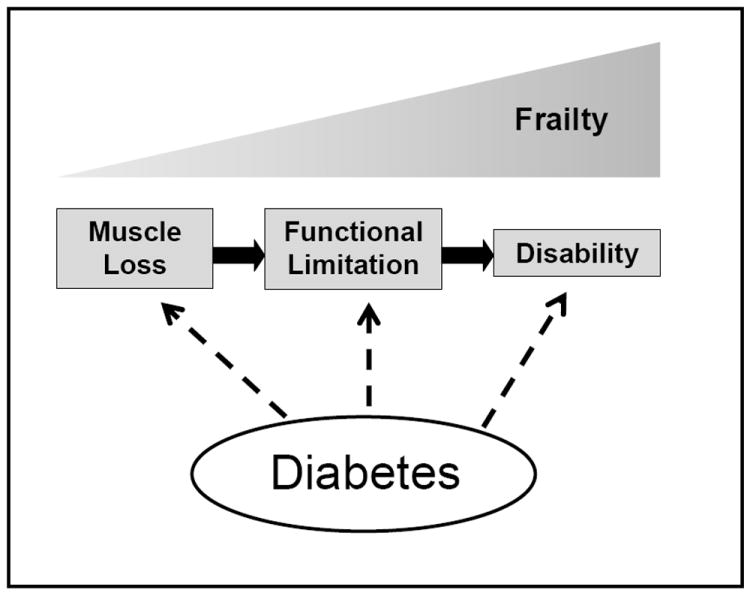
Figure 1 depicts proposed associations of diabetes with each step in the pathway to disability. Accelerated loss of muscle mass and strength, particularly in the lower extremities, may lead to functional limitations in routine tasks of daily living which may ultimately result in physical disability among older persons with diabetes. Frailty is a geriatric syndrome that encompasses the full spectrum of the disability process and is also more common with impaired glucose states and diabetes.
A) Loss of Muscle Function
Previous studies of older adults with diabetes have demonstrated decreased muscle strength and mass, especially in the lower extremities (35). Older adults with type 2 diabetes have ~50% more rapid decline in knee extensor strength than those without diabetes over a 3-year period (36) suggesting that decreased muscle strength is a consequence of type 2 diabetes, with similar findings for muscle mass (37). Disease duration and severity may have a role in the decreased muscle strength observed among persons with diabetes. Park, et al. (2006) reported that leg muscle quality was lowest in older adults with diabetes (mean age 74 years) who had the longest diabetes duration (≥6 years) or most severe hyperglycemia (hemoglobin A1c >8%) (35). Even among persons without diabetes, associations between hyperglycemia and insulin resistance with decreased muscle mass and strength, have been described (38, 39, 40).
In older adults, skeletal muscle protein synthesis may be resistant to the anabolic action of insulin (41, 42). Insulin resistance is also associated with activation of muscle proteolysis pathways (43) which may further lead to muscle loss. In turn, muscle is the primary site for insulin-dependent glucose uptake and reduced muscle surface area for insulin-mediated glucose uptake may further aggravate peripheral insulin resistance, leading to a vicious cycle. Oral insulin sensitizers have been reported to preserve muscle mass (44) although similar associations for muscle strength have yet to be investigated. Interestingly, skeletal muscle mitochondrial function is reduced in type 2 diabetes and may potentially be improved with peripheral insulin sensitization (45, 46).
Potential pathways underlying the association of diabetes with reduced muscle function include the presence of comorbidities such as peripheral neuropathy which may mediate these associations (47, 48), however, decreased leg muscle strength is present even after accounting for lower extremity nerve dysfunction in diabetes (49). Diabetes is associated with inflammatory markers, which in turn may also lead to impaired muscle function (50, 51).
B) Functional Limitations and Physical Disability
In the general population, muscle strength is a predictor of functional limitations and disability (52). Persons with diabetes perform worse on objective measures of lower extremity physical performance such as walking, chair stands, and tandem stand (53). Slower walking speed in persons with type 2 diabetes has been demonstrated (53, 54). Interestingly, the greatest differences have been observed at maximal walking speeds (55). There is some evidence that impaired muscle function mediates the association of diabetes with lower gait speed in older adults (54). Of note, severe hyperglycemia and insulin resistance have also been associated with walking difficulties and relatively poorer performance based measures of lower extremity function both in persons with and without diabetes (53, 56, 57).
Similarly, diabetes can have a significant impact on physical functioning such as lower extremity mobility, potentially mediated through effects on muscle function (58). Functional disability, or difficulty in performing routine physical tasks, is more common in older adults with diabetes compared to those without diabetes (57-64). Older adults with diabetes have significantly greater difficulty in a range of routine physical activities including walking a quarter mile, climbing stairs, reaching overhead, doing housework, bathing, eating and participating in leisure activities compared to their counterparts. Up to 70% of adults with diabetes have difficulties performing these tasks of everyday living (64).
The higher prevalence of functional disability in older adults with diabetes may be related to the presence of comorbidities such as cardiovascular disease, vision loss, obesity, and arthritis (59, 63, 64). These comorbidities may be associated with decreased cardiopulmonary reserve or restricted physical movement which contribute to physical disability. However, these factors do not consistently explain the association of diabetes with disability; of note, the degree of glycemic control is also related to disability among older adults with diabetes (57, 64, 65). Support for an association between hyperglycemia and disability comes from previous studies reporting a significant correlation between higher A1c levels and disability (57, 65). Alternatively, physical and cognitive impairment may affect ability to self-manage diabetes and lead to poorer glycemic control; thus, the relationship between hyperglycemia and disability is likely bidirectional and requires further investigation.
C) Frailty Syndrome
Diabetes has been associated with the presence of frailty, a geriatric condition of physiological vulnerability to stressors associated with adverse outcomes such as disability and mortality (66-69). Frailty increases with age and is distinguished by a characteristic phenotype. A common definition includes the presence of three or more of the following criteria: unintentional weight loss, self-reported exhaustion, muscle weakness (poor grip strength), slow walking speed, or low physical activity (67).
Recent literature has suggested an association between insulin resistance and frailty (16, 70) in cross-sectional studies of persons with and without diabetes. Altered glucose-insulin dynamics has also been reported in frail women compared to their counterparts with 120-minute post oral glucose tolerance test (OGTT) levels of both glucose and insulin better discriminating frailty status compared to fasting values (71, 72). Similar alterations in glucose dynamics have also been described in frail older adults who underwent a mixed meal test, and had a more exaggerated and prolonged glucose response after 2 hours compared to nonfrail older adults (73). These studies suggest relative insulin resistance in frail compared to non-frail older adults.
The presence of hyperglycemia and insulin resistance is also temporally related to the subsequent development of frailty in longitudinal cohort studies (53, 74). In the Cardiovascular Health Study (CHS), homeostasis model-insulin resistance (HOMA-IR) was calculated based on fasting glucose and insulin levels. For every standard deviation increment in HOMA-IR, the adjusted hazard ratio for frailty was 1.15 (95% CI 1.02–1.31) (74). Individuals who eventually developed frailty were also more likely, in parallel, to develop diabetes compared to older adults who never developed frailty (8.6% versus 4.2%). Thus, the association between frailty and abnormal glucose status is likely bidirectional. In the Women’s Health and Aging Study, older women in the highest HbA1c category (≥8%) compared to lowest (<5.5%) had a significant three-fold increased risk for the development of frailty, with most events occurring in the highest HbA1c category (53). These findings suggest that hyperglycemia, particularly in the diabetic range, can predict the onset of incident frailty less than a decade later (53).
The underlying physiological mechanisms relating insulin resistance to the development of frailty remain unclear. Frail older adults have a higher burden of inflammatory markers that may also affect glucose metabolism (71, 75, 76). Hyperglycemia may further activate inflammatory pathways that subsequently cause muscle catabolism and disability as part of the frailty process (77). Frail women on average are also more likely to be obese which may be associated with chronic inflammation (72, 78). Interestingly, one study found that only frail obese, but not frail lean adults, had reduced insulin sensitivity versus non-frail counterparts (16). However, another study found that associations of insulin resistance and frailty are independent of obesity (72). In addition, chronic hyperglycemia may be a risk factor for cardiovascular disease, which in turn has been associated with frailty (79); however, the presence of cardiovascular disease does not fully explain these associations. Thus, underlying mechanisms linking hyperglycemia with frailty remain unclear but are likely multifactorial (80).
D) Early Mortality
Diabetes in older adults is associated with increased disability, frailty, and accelerated muscle loss. These adverse geriatric syndromes can be associated with both increased healthcare expenditures (81, 82) and early mortality (66, 69, 83). On average, persons with diabetes have an almost two-fold increased risk of death from any cause, with 40% of this difference in survival due to non-vascular deaths (84). Results from the Baltimore Longitudinal Study of Aging demonstrate higher total and cardiovascular mortality even in older adults with impaired glucose tolerance compared to those with normal glucose metabolism (85, 86). A J-shaped association of hemoglobin A1c (HbA1c) with mortality has also been described, with increased mortality rates seen at higher levels of HbA1c but also, to a lesser degree, at lower HbA1c levels (87, 88). Interestingly, reduced length and activity of the telomerase enzyme, which maintains stability of chromosomes with aging has been linked to impaired glucose-stimulated insulin islet secretion and may also underlie these epidemiological associations but is currently still an area of active investigation (89).
Tags: geriatric syndromes, quality-of-life, skeletal muscle mass, skeletal muscle strength, leg function, functional disability, mobility, walking, gait speed, frailty, inflammation, obesity, hyperglycemia, insulin resistance, mortality, hemoglobin A1c, telomerase enzyme
VI. Treatment of diabetes in the elderly
A) Guidelines
The goals of diabetes care in the older patient with diabetes include: 1) control of hyperglycemia; 2) prevention and treatment of macrovascular and microvascular complications of diabetes; 3) avoidance of hypoglycemia; and 4) preservation of quality-of-life. Although the goals are similar to younger adults, older adults with diabetes are heterogeneous in their physical and cognitive functioning capacity, multiple comorbidities, and life expectancy. Otherwise robust older adults with expected life expectancy over 10 years might benefit from similar glycemic goals as younger adults (i.e. HbA1c <7%) to prevent diabetic complications. However, for frail older adults with multiple comorbidities and limited life expectancy, the avoidance of hypoglycemia and symptomatic hyperglycemia along with preservation of quality-of-life are arguably just as important, and less stringent targets (i.e. HbA1c <8%) may be more appropriate. Further, patient preferences must also be recognized in decisions related to diabetes management. Thus, treatment often requires an individualized approach for the older patient with diabetes.
Current glycemic targets of HbA1c<7% are based on older studies (e.g., United Kingdom Prospective Diabetes Study) that showed a significantly reduced risk of developing diabetic microvascular complications with intensive versus standard glucose control (90). Long-term follow-up of UKPDS demonstrated further benefits of early and intensive glucose control on reducing long-term risk of developing macrovascular disease (90). However, elderly individuals were excluded from UKDPS and there have been few other clinical trials examining the benefits of intensive glycemic control in older individuals; thus, extrapolation of previous results of clinical trials to elderly patients is challenging (91). Recent clinical trials studying the effect of intensive glucose control that included older patients with diabetes failed to show a clear benefit of intensive glucose control on mortality and, in fact, demonstrated potential harm with too aggressive glucose lowering (i.e. HbA1c <6%) (92-94). As a result, recent recommendations from the American Diabetes Association and the American Geriatrics Society recognize that a patient-centered approach may be more appropriate for older adults with diabetes (11, 95, 96). Cardiovascular risk factor control (i.e. lowering blood pressure, treating dyslipidemia, smoking cessation, aspirin therapy) is also recommended for the majority of adults with diabetes based on health status.
Management of the older patient with diabetes is complex and often individualized. Avoiding hypoglycemia and preserving functional independence are additional considerations for the older patient with diabetes. On the other hand, an evolving area of research is exploring the degree to which hyperglycemia may be associated with the presence of adverse geriatric syndromes such as physical disability, accelerated muscle loss, and frailty and may ultimately impact glycemic goals in the elderly. Current guidelines recognize the need to account for the presence of limited life expectancy and/or multiple comorbidities in treatment decisions for this vulnerable and growing population of individuals with diabetes.
B) Treatment
Lifestyle recommendations, for older adults with prediabetes or diabetes can be tailored to physical function ability and are more appropriate for obese elderly individuals than those who are underweight. Encouragingly, the oldest age group in the Diabetes Prevention Program (>60 years at of age at baseline) had the most dramatic improvement in glycemic control over time, with lifestyle modification programs and was associated with better adherence to lifestyle programs compared to younger age groups (97, 98). In prescribing dietary modification, additional factors to consider in the elderly patient with diabetes include social factors such as whether patients are eating alone (easier to eat precooked meals) and the presence of depression. Impaired perception to sweet and salty occurs with age, so using potassium chloride and using sucralose or stevia for sweetness may be preferred because they are perceived to be saltier and sweeter than sodium chloride or sucrose (99). A sedentary lifestyle in an older patient might signify the need for a reduced intake of total calories. Alcohol consumption is also associated with a significant number of calories and should be ascertained when formulating a lifestyle modification program.
Daily exercise, as part of activities of daily living, is necessary for the older patient with diabetes. Weight bearing exercises can improve insulin sensitivity. In the older patient with diabetes, usual recommendations for aerobic and resistance exercise can be prescribed as tolerated in the context of other medical conditions (i.e. arthritis, heart disease) that may otherwise limit exercise tolerance or participation in physical activities (100).
The choice of pharmacologic therapy for the older patient with diabetes may be affected by changes in renal and hepatic functions with aging, and the physical and cognitive abilities of each patient, in addition to other co-existing comorbidities. Figure 2 outlines the sites of action for common glucose-lowering therapies used in diabetes management. Special considerations in the elderly include the need to monitor renal function using both creatinine and glomerular filtration rate when prescribing metformin due to risk of lactic acidosis; hypoglycemia with sulfonylureas, which work by increasing endogenous insulin secretion, often require dose reductions when used with insulin and in patients with renal insufficiency (short-acting glimepiride and glipizide may be preferred); and weight-neutral agents such as DPP 4 inhibitors that increase endogenous levels of incretins may be appropriate in cachetic older adults. Thiazolidenediones, which are PPAR gamma activators, may exacerbate underlying heart failure and are associated with increased risk of bone fractures, so they should be avoided in persons with underlying bone disease but they are less likely to result in hypoglycemia. GLP-1 receptor agonists are injectable agents that can slow gastric motility and lead to weight loss. Thus, these agents may be useful for obese older patients with diabetes but not in those with complications such as gastroparesis. Endogenous insulin clearance may also be decreased with aging (101, 102) and, perhaps, exogenous insulin clearance as well although this has not been consistently described (103). Insulin clearance is particularly affected by changes in renal function, which may affect dosing.
Figure 2. Sites of Action for Common Glucose-Lowering Therapies.
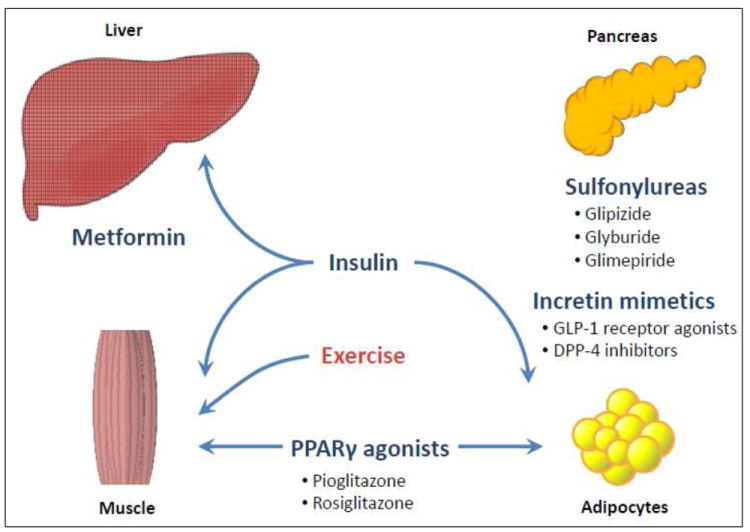
Figure 2 demonstrates the sites of action for common glucose-lowering therapies used in persons with type 2 diabetes. The different mechanisms by which these drugs improve blood glucose, along with potential benefits and side effects, are important considerations in the management of the older patient with diabetes. Exercise, particularly muscle-strengthening or resistance activities, can have additional benefits on glucose uptake by skeletal muscle.
Monitoring of blood glucose in older patients with diabetes is similar to younger adults. However, hemoglobin A1c may rise by ~0.1% with each decade of age independent of changes in blood glucose and potentially affect the interpretability of HbA1c in older patients (104). Self-monitoring of blood glucose can be considered based on the patient’s cognitive ability, functional status, and risk of hypoglycemia.
Tags: guidelines, treatment, hypoglycemia, comorbidities, life expectancy, lifestyle, taste, alcohol, pharmacologic therapy, self-monitoring blood glucose
VII. Summary
Diabetes and altered glucose metabolism commonly occur with aging. Oral glucose tolerance testing may help characterize abnormal glucose status in the elderly population. Diabetes in this population is heterogeneous, with middle-age onset versus elderly-onset individuals possibly representing groups at different risk for the development of microvascular complications. Geriatric syndromes such as muscle loss, disability, and frailty are more prevalent in older patients with diabetes and may be related to the presence of hyperglycemia or insulin resistance but more research is needed. Treatment of diabetes in the elderly includes lifestyle recommendations when appropriate and the use of pharmacologic therapies which account for the presence of comorbidities, especially renal and hepatic impairment, as well as the physical and cognitive abilities of the patient while seeking to minimize hypoglycemia. Ultimately, goals of care need to be individualized for the elderly patient with diabetes.
Key Points.
Adults aged 60 and over have more than twice the prevalence of diabetes compared to younger age groups. The number of older persons with diabetes will continue to grow as the population ages.
Abnormal glucose metabolism is associated with aging but not a necessary component.
Older persons with diabetes and/or abnormal glucose metabolism may be at higher risk of developing adverse geriatric syndromes such as accelerated muscle loss, functional disability and frailty.
Goals of care for older persons with diabetes need to be individualized and consider treatment of symptomatic hyperglycemia, prevention of long-term complications, avoidance of hypoglycemia, and preservation of quality of life.
Lifestyle modifications, in particular regular exercise as tolerated, and pharmacological therapies that account for the presence of comorbid renal and hepatic impairments or physical and cognitive limitations, are important components of diabetes management in older adults.
Acknowledgments
This work is supported in part by the IRP/NIH, National Institute on Aging and also the National Institute of Diabetes and Digestive and Kidney Diseases (K23DK093583). We thank David Liu (NIA) for help with Figure 2 illustration.
Footnotes
Disclosures:
Conflict of interest (RK, JE): None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988-1994 and 2005-2006. Diabetes Care. 2009;32:287–294. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) Public Health and Aging: Trends in Aging --- United States and Worldwide. JAMA. 2003;289:1371–3. [PubMed] [Google Scholar]
- 3.Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Decker FH, Luo H, et al. Trends in the prevalence and comorbidities of diabetes mellitus in nursing home residents in the United States: 1995-2004. J Am Geriatr Soc. 2010;58:724–30. doi: 10.1111/j.1532-5415.2010.02786.x. [DOI] [PubMed] [Google Scholar]
- 5.Shimokata H, Muller DC, Fleg JL, et al. Age as an independent determinant of glucose tolerance. Diabetes. 1991;40:44–51. doi: 10.2337/diab.40.1.44. [DOI] [PubMed] [Google Scholar]
- 6.DeFronzo RA. Glucose intolerance and aging. Diabetes Care. 1981;4:493–501. doi: 10.2337/diacare.4.4.493. [DOI] [PubMed] [Google Scholar]
- 7.Ferrannini E, Vichi S, Beck-Nielsen H, et al. Insulin action and age. European Group for the Study of Insulin Resistance (EGIR) Diabetes. 1996;45:947–53. doi: 10.2337/diab.45.7.947. [DOI] [PubMed] [Google Scholar]
- 8.Davidson MB. The effect of aging on carbohydrate metabolism. Metabolism. 1979;28:688–705. doi: 10.1016/0026-0495(79)90024-6. [DOI] [PubMed] [Google Scholar]
- 9.Elahi D, Muller DC, Egan JM, et al. Glucose tolerance, glucose utilization and insulin secretion in ageing. Novartis Found Symp. 2002;242:222–42. [PubMed] [Google Scholar]
- 10.Elahi D, Muller DC. Carbohydrate metabolism in the elderly. Eur J Clin Nutr. 2000;54(Suppl 3):S112–20. doi: 10.1038/sj.ejcn.1601032. [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes Association. Standards of medical care in diabetes--2012. Diabetes Care. 2012;35(Suppl 1):S11–63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meigs JB, Muller DC, Nathan DM, et al. The natural history of progression from normal glucose tolerance to type 2 diabetes in the Baltimore Longitudinal Study of Aging. Diabetes. 2003;52:1475–84. doi: 10.2337/diabetes.52.6.1475. [DOI] [PubMed] [Google Scholar]
- 13.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–23. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 14.Defronzo RA. Glucose intolerance and aging: evidence for tissue insensitivity to insulin. Diabetes. 1979;28:1095–101. doi: 10.2337/diab.28.12.1095. [DOI] [PubMed] [Google Scholar]
- 15.Gumbiner B, Thorburn AW, Ditzler TM, et al. Role of impaired intracellular glucose metabolism in the insulin resistance of aging. Metabolism. 1992;41:1115–21. doi: 10.1016/0026-0495(92)90296-m. [DOI] [PubMed] [Google Scholar]
- 16.Goulet ED, Hassaine A, Dionne IJ, et al. Frailty in the elderly is associated with insulin resistance of glucose metabolism in the postabsorptive state only in the presence of increased abdominal fat. Exp Gerontol. 2009;44:740–44. doi: 10.1016/j.exger.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Elahi D, Muller DC, McAloon-Dyke M, et al. The effect of age on insulin response and glucose utilization during four hyperglycemic plateaus. Exp Gerontol. 1993;28:393–409. doi: 10.1016/0531-5565(93)90066-m. [DOI] [PubMed] [Google Scholar]
- 18.Chen M, Halter JB, Porte D., Jr The role of dietary carbohydrate in the decreased glucose tolerance of the elderly. J Am Geriatr Soc. 1987;35:417–24. doi: 10.1111/j.1532-5415.1987.tb04663.x. [DOI] [PubMed] [Google Scholar]
- 19.Muller DC, Elahi D, Tobin JD, et al. The effect of age on insulin resistance and secretion: a review. Semin Nephrol. 1996;16:289–98. [PubMed] [Google Scholar]
- 20.Tabák AG, Jokela M, Akbaraly TN, et al. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet. 2009;373:2215–21. doi: 10.1016/S0140-6736(09)60619-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polonsky KS, Given BD, Van Cauter E. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J Clin Invest. 1988;81:442–8. doi: 10.1172/JCI113339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheen AJ, Sturis J, Polonsky KS, et al. Alterations in the ultradian oscillations of insulin secretion and plasma glucose in aging. Diabetologia. 1996;39:564–72. doi: 10.1007/BF00403303. [DOI] [PubMed] [Google Scholar]
- 23.Meneilly GS, Veldhuis JD, Elahi D. Disruption of the pulsatile and entropic modes of insulin release during an unvarying glucose stimulus in elderly individuals. J Clin Endocrinol Metab. 1999;84:1938–43. doi: 10.1210/jcem.84.6.5753. [DOI] [PubMed] [Google Scholar]
- 24.O’Meara NM, Sturis J, Van Cauter E, et al. Lack of control by glucose of ultradian insulin secretory oscillations in impaired glucose tolerance and in non-insulin-dependent diabetes mellitus. J Clin Invest. 1993;92:262–71. doi: 10.1172/JCI116560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim W, Egan JM. The Role of Incretins in Glucose Homeostasis and Diabetes Treatment. Pharmacol Rev. 2008;60:470–512. doi: 10.1124/pr.108.000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elahi D, Andersen DK, Muller DC, et al. The enteric enhancement of glucose-stimulated insulin release. The role of GIP in aging, obesity, and non-insulin-dependent diabetes mellitus. Diabetes. 1984;33:950–7. doi: 10.2337/diab.33.10.950. [DOI] [PubMed] [Google Scholar]
- 27.Meneilly GS, Ryan AS, Minaker KL, et al. The effect of age and glycemic level on the response of the beta-cell to glucose-dependent insulinotropic polypeptide and peripheral tissue sensitivity to endogenously released insulin. J Clin Endocrinol Metab. 1998;83:2925–32. doi: 10.1210/jcem.83.8.5003. [DOI] [PubMed] [Google Scholar]
- 28.Chia CW, Carlson OD, Kim W, et al. Exogenous glucose-dependent insulinotropic polypeptide worsens post prandial hyperglycemia in type 2 diabetes. Diabetes. 2009;58:1342–9. doi: 10.2337/db08-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meneilly GS, Demuth HU, McIntosh CH, et al. Effect of ageing and diabetes on glucose-dependent insulinotropic polypeptide and dipeptidyl peptidase IV responses to oral glucose. Diabet Med. 2000;17:346–50. doi: 10.1046/j.1464-5491.2000.00236.x. [DOI] [PubMed] [Google Scholar]
- 30.Chiniwala N, Jabbour S. Management of diabetes mellitus in the elderly. Curr Opin Endocrinol Diabetes Obes. 2011;18:148–52. doi: 10.1097/MED.0b013e3283444ba0. [DOI] [PubMed] [Google Scholar]
- 31.Selvin E, Coresh J, Brancati FL. The burden and treatment of diabetes in elderly individuals in the U.S. Diabetes Care. 2006;29:2415–9. doi: 10.2337/dc06-1058. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Qin MZ, Liu Q, et al. Clinical analysis of elderly patients with elderly-onset type 2 diabetes mellitus in China: assessment of appropriate therapy. J Int Med Res. 2010;38:1134–41. doi: 10.1177/147323001003800342. [DOI] [PubMed] [Google Scholar]
- 33.Barbieri M, Rizzo MR, Manzella D, et al. Age-related insulin resistance: is it an obligatory finding? The lesson from healthy centenarians. Diabetes Metab Res Rev. 2001;17:19–26. doi: 10.1002/dmrr.178. [DOI] [PubMed] [Google Scholar]
- 34.Paolisso G, Gambardella A, Ammendola S, et al. Glucose tolerance and insulin action in healty centenarians. Am J Physiol. 1996;270:E890–4. doi: 10.1152/ajpendo.1996.270.5.E890. [DOI] [PubMed] [Google Scholar]
- 35.Park SW, Goodpaster BH, Strotmeyer ES, et al. Decreased muscle strength and quality in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes. 2006;55:1813–8. doi: 10.2337/db05-1183. [DOI] [PubMed] [Google Scholar]
- 36.Park SW, Goodpaster BH, Strotmeyer ES, et al. Health, Aging, and Body Composition Study. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes Care. 2007;30:1507–1512. doi: 10.2337/dc06-2537. [DOI] [PubMed] [Google Scholar]
- 37.Park SW, Goodpaster BH, Lee JS, et al. Health, Aging, and Body Composition Study. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care. 2009;32:1993–1997. doi: 10.2337/dc09-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barzilay JI, Cotsonis GA, Walston J, et al. Health ABC Study. Insulin resistance is associated with decreased quadriceps muscle strength in nondiabetic adults aged >or=70 years. Diabetes Care. 2009;32:736–8. doi: 10.2337/dc08-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lazarus R, Sparrow D, Weiss ST. Handgrip strength and insulin levels: cross-sectional and prospective associations in the Normative Aging Study. Metabolism. 1997;46:1266–9. doi: 10.1016/s0026-0495(97)90228-6. [DOI] [PubMed] [Google Scholar]
- 40.Kalyani RR, Metter EJ, Ramachandran R, et al. Glucose and insulin measurements from the oral glucose tolerance test and relationship to muscle mass. J Gerontol A Biol Sci Med Sci. 2012;67:74–81. doi: 10.1093/gerona/glr022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rasmussen BB, Fujita S, Wolfe RR, et al. Insulin resistance of muscle protein anabolism in aging. FASEB. 2006;20:768–9. doi: 10.1096/fj.05-4607fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Volpi E, Mittendorfer B, Rasmussen BB, et al. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab. 2000;85:4481–90. doi: 10.1210/jcem.85.12.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X, Hu Z, Hu J, et al. Insulin resistance accelerates muscle protein degradation: Activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology. 2006;147:4160–8. doi: 10.1210/en.2006-0251. [DOI] [PubMed] [Google Scholar]
- 44.Lee CG, Boyko EJ, Barrett-Connor E, et al. Osteoporotic Fractures in Men (MrOS) Study Research Group. Insulin sensitizers may attenuate lean mass loss in older men with diabetes. Diabetes Care. 2011;34:2381–2386. doi: 10.2337/dc11-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Phielix E, Schrauwen-Hinderling VB, Mensink M, et al. Lower intrinsic ADP-stimulated mitochondrial respiration underlies in vivo mitochondrial dysfunction in muscle of male type 2 diabetic patients. Diabetes. 2008;57:2943–2949. doi: 10.2337/db08-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rabøl R, Boushel R, Almdal T, et al. Opposite effects of pioglitazone and rosiglitazone on mitochondrial respiration in skeletal muscle of patients with type 2 diabetes. Diabetes Obes Metab. 2010;12:806–14. doi: 10.1111/j.1463-1326.2010.01237.x. [DOI] [PubMed] [Google Scholar]
- 47.Strotmeyer ES, de Rekeneire N, Schwartz AV, et al. Health ABC Study. Sensory and motor peripheral nerve function and lower-extremity quadriceps strength: the health, aging and body composition study. J Am Geriatr Soc. 2009;57:2004–2010. doi: 10.1111/j.1532-5415.2009.02487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andersen H, Nielsen S, Mogensen CE, et al. Muscle strength in type 2 diabetes. Diabetes. 2004;53:1543–1548. doi: 10.2337/diabetes.53.6.1543. [DOI] [PubMed] [Google Scholar]
- 49.Volpato S, Bianchi L, Lauretani F, et al. Role of Muscle Mass and Muscle Quality in the Association Between Diabetes and Gait Speed. Diabetes Care. 2012;35:1672–9. doi: 10.2337/dc11-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Hall G, Steensberg A, Fischer C, et al. Interleukin-6 markedly decreases skeletal muscle protein turnover and increases nonmuscle amino acid utilization in healthy individuals. J Clin Endocrinol Metab. 2008;7:2851–58. doi: 10.1210/jc.2007-2223. [DOI] [PubMed] [Google Scholar]
- 51.Duncan BB, Schmidt MI, Pankow JS, et al. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2003;52:1799–1805. doi: 10.2337/diabetes.52.7.1799. [DOI] [PubMed] [Google Scholar]
- 52.Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- 53.Kalyani RR, Tian J, Xue QL, et al. Hyperglycemia and Incidence of Frailty and Lower Extremity Mobility Limitations in Older Women. J Am Geriatr Soc. 2012;60:1701–7. doi: 10.1111/j.1532-5415.2012.04099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Volpato S, Blaum C, Resnick H, et al. Women’s Health and Aging Study. Comorbidities and impairments explaining the association between diabetes and lower extremity disability: The Women’s Health and Aging Study. Diabetes Care. 2002;25:678–83. doi: 10.2337/diacare.25.4.678. [DOI] [PubMed] [Google Scholar]
- 55.Ko SU, Stenholm S, Chia CW, et al. Gait pattern alterations in older adults associated with type 2 diabetes in the absence of peripheral neuropathy--results from the Baltimore Longitudinal Study of Aging. Gait Posture. 2011;34:548–552. doi: 10.1016/j.gaitpost.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuo HK, Leveille SG, Yen CJ, et al. Exploring how peak leg power and usual gait speed are linked to late-life disability: data from the National Health and Nutrition Examination Survey (NHANES), 1999-2002. Am J Phys Med Rehabil. 2006;85:650–658. doi: 10.1097/01.phm.0000228527.34158.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Rekeneire N, Resnick HE, Schwartz AV, et al. Health, Aging, and Body Composition study. Diabetes is associated with subclinical functional limitation in nondisabled older individuals: the Health, Aging, and Body Composition study. Diabetes Care. 2003;26:3257–63. doi: 10.2337/diacare.26.12.3257. [DOI] [PubMed] [Google Scholar]
- 58.Sinclair AJ, Conroy SP, Bayer AJ. Impact of diabetes on physical function in older people. Diabetes Care. 2008;31:233–235. doi: 10.2337/dc07-1784. [DOI] [PubMed] [Google Scholar]
- 59.Gregg EW, Beckles GL, Williamson DF, et al. Diabetes and physical disability among older U.S. adults. Diabetes Care. 2000;23:1272–7. doi: 10.2337/diacare.23.9.1272. [DOI] [PubMed] [Google Scholar]
- 60.Volpato S, Ferrucci L, Blaum C, et al. Progression of lower-extremity disability in older women with diabetes: the Women’s Health and Aging Study. Diabetes Care. 2003;26:70–75. doi: 10.2337/diacare.26.1.70. [DOI] [PubMed] [Google Scholar]
- 61.Ryerson B, Tierney EF, Thompson TJ, et al. Excess physical limitations among adults with diabetes in the U.S. population, 1997-1999. Diabetes Care. 2003;26:206–10. doi: 10.2337/diacare.26.1.206. [DOI] [PubMed] [Google Scholar]
- 62.Egede LE. Diabetes, major depression, and functional disability among U.S. adults. Diabetes Care. 2004;27:421–8. doi: 10.2337/diacare.27.2.421. [DOI] [PubMed] [Google Scholar]
- 63.Maty SC, Fried LP, Volpato S, et al. Patterns of disability related to diabetes mellitus in older women. J Gerontol A Biol Sci Med Sci. 2004;59:148–53. doi: 10.1093/gerona/59.2.m148. [DOI] [PubMed] [Google Scholar]
- 64.Kalyani RR, Saudek CD, Brancati FL, et al. The Association of Diabetes, Comorbidities, and Hemoglobin A1c with Functional Disability in Older Adults: Results from the National Health and Nutrition Examination Survey (NHANES), 1999-2006. Diabetes Care. 2010;33:1055–1060. doi: 10.2337/dc09-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bossoni S, Mazziotti G, Gazzaruso C, et al. Relationship between instrumental activities of daily living and blood glucose control in elderly subjects with type 2 diabetes. Age Ageing. 2008;37:222–5. doi: 10.1093/ageing/afm158. [DOI] [PubMed] [Google Scholar]
- 66.Boyd CM, Xue QL, Simpson CF, et al. Frailty, hospitalization, and progression of disability in a cohort of disabled older women. Am J Med. 2005;118:1225–31. doi: 10.1016/j.amjmed.2005.01.062. [DOI] [PubMed] [Google Scholar]
- 67.Fried LP, Tangen CM, Walston J, et al. Cardiovascular Health Study. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 68.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–6. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 69.Wolinsky FD, Callahan CM, Fitzgerald JF, et al. Changes in functional status and the risks of subsequent nursing home placement and death. J Gerontol. 1993;48:S94–101. [PubMed] [Google Scholar]
- 70.Blaum CS, Xue QL, Tian J, et al. Is hyperglycemia associated with frailty status in older women? J Am Geriatr Soc. 2009;57:840–47. doi: 10.1111/j.1532-5415.2009.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walston J, McBurnie MA, Newman A, et al. Cardiovascular Health Study. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162:2333–41. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 72.Kalyani RR, Varadhan R, Weiss CO, et al. Frailty Status and Altered Glucose-Insulin Dynamics. J Gerontol A Biol Sci Med Sci. 2011 Aug 26; doi: 10.1093/gerona/glr141. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Serra-Prat M, Palomera E, Clave P, et al. Effect of age and frailty on ghrelin and cholecystokinin responses to a meal test. Am J Clin Nutr. 2009;89:1410–7. doi: 10.3945/ajcn.2008.27076. [DOI] [PubMed] [Google Scholar]
- 74.Barzilay JI, Blaum C, Moore T, et al. Insulin resistance and inflammation as precursors of frailty: the Cardiovascular Health Study. Arch Intern Med. 2007;167:635–41. doi: 10.1001/archinte.167.7.635. [DOI] [PubMed] [Google Scholar]
- 75.Senn JJ, Klover PJ, Nowak IA, et al. IL-6 induces cellular insulin resistance in hepatocytes. Diabetes. 2002;51:3391–99. doi: 10.2337/diabetes.51.12.3391. [DOI] [PubMed] [Google Scholar]
- 76.Lee CC, Adler AI, Sandhu MS, et al. Association of C-reactive protein with type 2 diabetes: prospective analysis and meta-analysis. Diabetologia. 2009;52:1040–7. doi: 10.1007/s00125-009-1338-3. [DOI] [PubMed] [Google Scholar]
- 77.Barbieri M, Ferrucci L, Ragno E, et al. Chronic inflammation and the effect of IGF-I on muscle strength and power in older persons. Am J Physiol Endocrinol Metab. 2003;284:E481–7. doi: 10.1152/ajpendo.00319.2002. [DOI] [PubMed] [Google Scholar]
- 78.Hubbard RE, Lang IA, Llewellyn DJ, et al. Frailty, body mass index, and abdominal obesity in older people. J Gerontol A Biol Sci Med Sci. 2010;65:377–381. doi: 10.1093/gerona/glp186. [DOI] [PubMed] [Google Scholar]
- 79.Newman AB, Gottdiener JS, Mcburnie MA, et al. Cardiovascular Health Study Research Group. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56:M158–66. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 80.Fried LP, Xue QL, Cappola AR, et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Sci Med Sci. 2009;64:1049–57. doi: 10.1093/gerona/glp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fried TR, Bradley EH, Williams CS, et al. Functional disability and health care expenditures for older persons. Arch Intern Med. 2001;161:2602–7. doi: 10.1001/archinte.161.21.2602. [DOI] [PubMed] [Google Scholar]
- 82.Janssen I, Shepard DS, Katzmarzyk PT, et al. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52:80–85. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- 83.Newman AB, Kupelian V, Visser M, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61:72–77. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 84.Emerging Risk Factors Collaboration. Seshasai SR, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Metter EJ, Windham BG, Maggio M, et al. Glucose and insulin measurements from the oral glucose tolerance test and mortality prediction. Diabetes Care. 2008;31:1026–30. doi: 10.2337/dc07-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sorkin JD, Muller DC, Fleg JL, et al. The relation of fasting and 2-h postchallenge plasma glucose concentrations to mortality: data from the Baltimore Longitudinal Study of Aging with a critical review of the literature. Diabetes Care. 2005;28:2626–32. doi: 10.2337/diacare.28.11.2626. [DOI] [PubMed] [Google Scholar]
- 87.Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362:800–811. doi: 10.1056/NEJMoa0908359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang ES, Liu JY, Moffet HH, et al. Glycemic control, complications, and death in older diabetic patients: the diabetes and aging study. Diabetes Care. 2011;34:1329–1336. doi: 10.2337/dc10-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kuhlow D, Florian S, von Figura G, et al. Telomerase deficiency impairs glucose metabolism and insulin secretion. Aging (Albany NY) 2010;2:650–8. doi: 10.18632/aging.100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 91.Finucane TE. “Tight control” in geriatrics: the emperor wears a thong. J Am Geriatr Soc. 2012;60:1571–5. doi: 10.1111/j.1532-5415.2012.04057.x. [DOI] [PubMed] [Google Scholar]
- 92.Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Patel A, MacMahon S, Chalmers J, et al. ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 94.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 95.Durso SC. Using clinical guidelines designed for older adults with diabetes mellitus and complex health status. JAMA. 2006;295:1935–40. doi: 10.1001/jama.295.16.1935. [DOI] [PubMed] [Google Scholar]
- 96.Brown AF, Mangione CM, Saliba D, et al. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc. 2003;51:S265–S280. doi: 10.1046/j.1532-5415.51.5s.1.x. [DOI] [PubMed] [Google Scholar]
- 97.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wing RR, Hamman RF, Bray GA, et al. Diabetes Prevention Program Research Group. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obes Res. 2004;12:1426–34. doi: 10.1038/oby.2004.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shin YK, Cong WN, Cai H, Kim W, et al. Age-related changes in mouse taste bud morphology, hormone expression, and taste responsivity. J Gerontol A Biol Sci Med Sci. 2012;67:336–44. doi: 10.1093/gerona/glr192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Colberg SR, Sigal RJ, Fernhall B, et al. American College of Sports Medicine; American Diabetes Association. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement executive summary. Diabetes Care. 2010;33:2692–2696. doi: 10.2337/dc10-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McGuire EA, Tobin JD, Berman M, et al. Kinetics of native insulin in diabetic, obese, and aged men. Diabetes. 1979;28:110–20. doi: 10.2337/diab.28.2.110. [DOI] [PubMed] [Google Scholar]
- 102.Fink RI, Revers RR, Kolterman OG, et al. The metabolic clearance of insulin and the feedback inhibition of insulin secretion are altered with aging. Diabetes. 1985;34:275–80. doi: 10.2337/diab.34.3.275. [DOI] [PubMed] [Google Scholar]
- 103.Mooradian AD. Special considerations with insulin therapy in older adults with diabetes mellitus. Drugs Aging. 2011;28:429–38. doi: 10.2165/11590570-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 104.Pani LN, Korenda L, Meigs JB, et al. Effect of aging on A1C levels in individuals without diabetes: evidence from the Framingham Offspring Study and the National Health and Nutrition Examination Survey 2001-2004. Diabetes Care. 2008;31:1991–6. doi: 10.2337/dc08-0577. [DOI] [PMC free article] [PubMed] [Google Scholar]


