Abstract
Purpose
Doxorubicin and cyclophosphamide (AC) every 3 weeks has been associated with frequent asymptomatic declines in left ventricular ejection fraction (LVEF). Dose-dense (dd) AC followed by paclitaxel (P) is superior to the same regimen given every third week. Herein, we report the early cardiac safety of three sequential studies of ddAC alone or with bevacizumab (B).
Patients and Methods
Patients with HER2-positive breast cancer were treated on two trials: ddAC followed by P and trastuzumab (T) and ddAC followed by PT and lapatinib. Patients with HER2-normal breast cancer were treated with B and ddAC followed by B and nanoparticle albumin–bound P. Prospective LVEF measurement by multigated radionuclide angiography scan before and after every 2 week AC for 4 cycles and at month 6 from all three trials were aggregated to determine the early risks of cardiac dysfunction.
Results
From January 2005 to May 2008, 245 patients were enrolled. The median age was 47 years (range, 27 to 75 years). Median LVEF pre-ddAC was 68% (range, 52% to 82%). LVEF post-ddAC was available in 241 patients (98%) and the median was unchanged at 68% (range, 47% to 81%). Per protocol no patients were ineligible for subsequent targeted biologic therapy based on LVEF decline post-ddAC. In addition, LVEF was available in 222 patients (92%) at 6 months, at which time the median LVEF was similar at 65% (range, 24% to 80%). Within 6 months of initiating chemotherapy, three patients (1.2%; 95% CI, 0.25% to 3.54%) developed CHF, all of whom received T.
Conclusion
Dose-dense AC with or without concurrent bevacizumab is not associated with frequent acute or short-term declines in LVEF.
INTRODUCTION
The 2000 Early Breast Cancer Trialists Collaborative Group overview of polychemotherapy in breast cancer demonstrated that anthracycline-based regimens are superior to nonanthracycline-based therapies in terms of disease-free survival (DFS) and overall survival (OS).1,2 However, the risk of long-term cardiomyopathy and congestive heart failure (CHF), related to total anthracycline dose, remains an important clinical concern.3 In trials that have evaluated conventionally scheduled administration of doxorubicin and cyclophosphamide (AC) every 3 weeks for 4 cycles followed by a taxane (several schedules), the incidence of CHF and changes in left ventricular ejection fraction (LVEF) ranged up to 2.5%.4–6 Dose-dense (dd) chemotherapy with AC followed by paclitaxel (P) every other week improves DFS and OS when compared with conventionally scheduled AC every 3 weeks followed by P and had one half of the rate of grade 3 to 4 cardiac events.6–7
Cardiac toxicities were a concern when trastuzumab (T; Herceptin, Genentech, San Francisco, CA) was studied with conventionally scheduled chemotherapy in the adjuvant setting for patients with HER2-positive breast cancer.8–12 Thus, rigorous cardiac follow-up was required, which included LVEF monitoring after the completion of AC every 3 weeks for 4 cycles in National Surgical Adjuvant Breast and Bowel Project (NSABP) B-31, North Central Cancer Treatment Group N9831, and Breast Cancer International Research Group (BCIRG) 006. In the combined analysis of NSABP B-31 and N9831, there was a patient drop-out rate of 6.7% due to significant asymptomatic LVEF declines after AC every 3 weeks that, per protocol prohibited these patients from progressing to the taxane and T phase.8 In distinction to this experience, we showed that ddAC followed by P with T13 did not lead to patient drop-outs after ddAC and in our pilot trial, all enrolled patients were able to proceed to receive P with T. Furthermore, the overall CHF rate was 1.4% (one of 70 patients)13 as compared with the 2% to 4% rate reported with prior anthracycline and taxane regimens when given in an every 3 weeks schedule.8,11 Based on these results, we conducted a second trial which differed only in the substitution of a weekly schedule for paclitaxel administration and the addition of lapatinib (ddAC followed by weekly P with T and lapatinib).14
For patients with HER2-normal breast cancer, bevacizumab (B) (Avastin, Genentech, San Francisco, CA), a humanized monoclonal antibody to vascular endothelial growth factor (VEGF) with proven efficacy in combination with chemotherapy for metastatic breast cancer,15 is being tested in the adjuvant setting. In preparation, B was evaluated with ddAC followed by P in the Eastern Cooperative Oncology Group (ECOG) study 2104 and the incidence of symptomatic CHF with ddAC followed by P with B was 2%.16 We conducted a similar feasibility trial combining B with ddAC followed by nanoparticle albumin–bound (nab) paclitaxel.17 Because all three of our prospective studies delivered 4 cycles of every other week (dose-dense) AC (alone or with bevacizumab) with serial cardiac monitoring, we aggregated the baseline, post-AC (month 2) and month 6 ejection fraction assessments to address the short-term cardiac safety of ddAC for 4 cycles.
PATIENTS AND METHODS
Patients with HER2-positive breast cancer as 3+ by immunohistochemistry or amplified by fluorescent in situ hybridization, regardless of nodal status or tumor size, were eligible for trial 1 (adjuvant/neoadjuvant ddAC followed by PT) at Memorial Sloan-Kettering Cancer Center (MSKCC). Subsequently, similar patients were eligible for trial 2 (ddAC followed by weekly PT plus lapatinib) conducted jointly by MSKCC, Dana-Farber/Harvard Cancer Center (including Dana-Farber Cancer Institute, Massachusetts General Hospital, and Beth Israel Deaconess Medical Center). Patients with HER2-normal breast cancer (node positive or high risk node negative) were eligible for trial 3, a B-based adjuvant study at MSKCC and University of California, San Francisco (B and ddAC followed by B and nab-P). All patients had an absolute neutrophil count (ANC) ≥ 1,000/μL, platelet count ≥100,000/μL, normal total bilirubin and transaminases ≤ 2.5 upper limit of normal.
The LVEF by multigated radionuclide angiography (MUGA) scan had to be at or above 55% for trial 1 (ddAC followed by PT).13 The minimal enrollment LVEF was subsequently lowered to ≥ 50% for trial 2 (ddAC followed by weekly PT plus lapatinib)14 and trial 3 the B-containing study (B and ddAC followed by B and nab-P).17 Patients who received B were also required to have a normal serum creatinine or a creatinine clearance ≥ 60 mL/min by the Cockroft-Gault formula. Patients with a known history of unstable angina, myocardial infarction within 12 months, CHF, serious medical illnesses, inability to give consent, or who were pregnant were excluded. Patients with clinically significant peripheral vascular disease, a stroke within 12 months, or blood pressure higher than 150/100 mmHg were excluded from receiving B. Otherwise, patients were not excluded on the basis of controlled hypertension. All patients gave informed consent and all studies were reviewed and approved by the individual institutional review boards of each institution.
Treatment
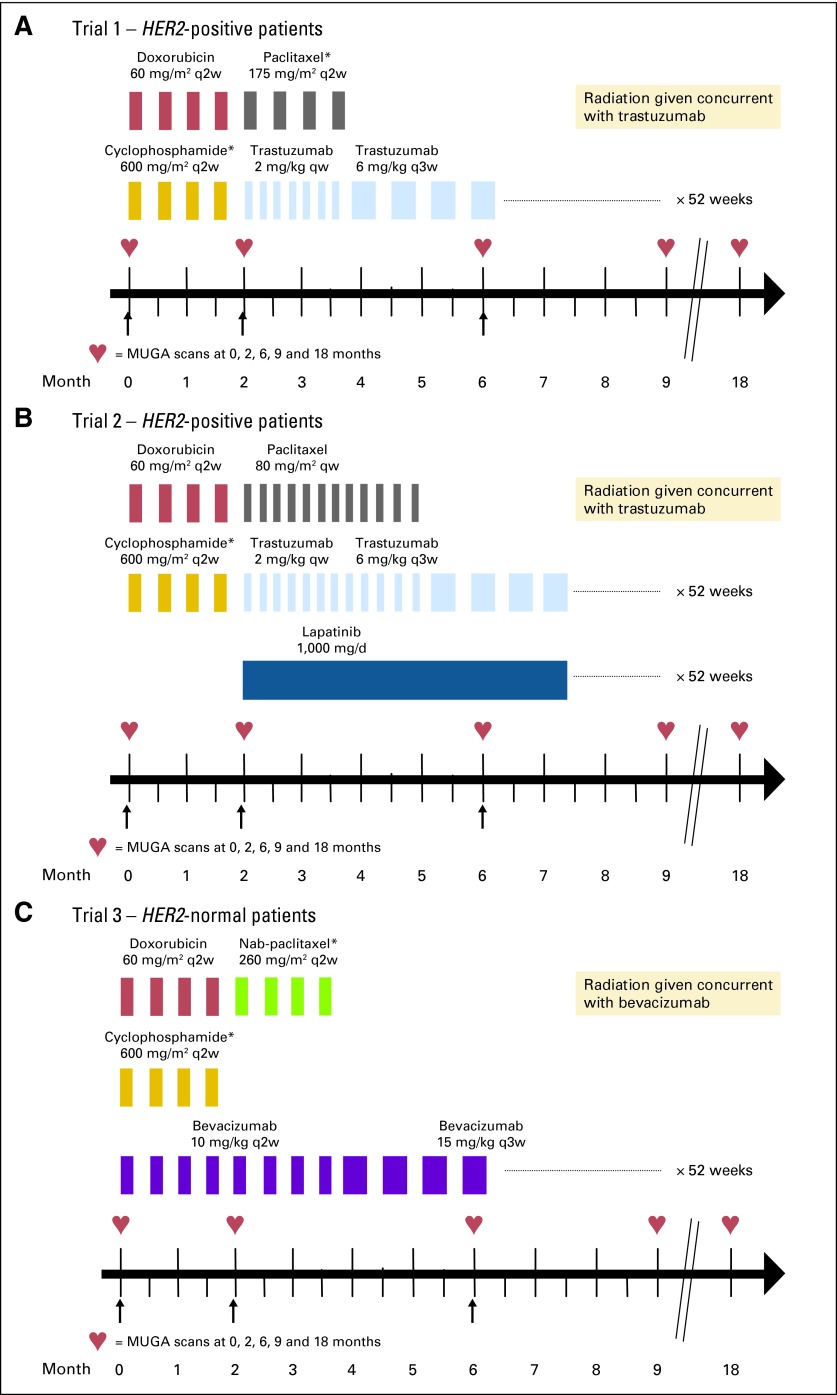
In all three studies AC (doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2) was given intravenously (IV) once every 14 days and supported by pegfilgrastim. In trial 1, this was followed by P (175 mg/m2) IV every 2 weeks for 4 cycles, with pegfilgrastim at 6 mg subcutaneously (SQ) on day 2. T was given IV weekly (4 mg/kg loading dose followed by 2 mg/kg) starting with paclitaxel and was continued every 3 weeks (6 mg/kg) after the completion of chemotherapy, for 1 year's duration (Fig 1A). In trial 2, AC was instead followed by P IV weekly (80 mg/m2) for 12 doses. T was given in the same fashion as in the pilot study above, but lapatinib (1,000 mg orally daily) was added concurrently with PT, for 1 year's duration (Fig 1B). In trial 3 (patients with HER2-normal breast cancer), AC was given concurrently with B (10 mg/kg) once every 2 weeks. This was followed by nab-P (260 mg/m2) IV every 2 weeks for 4 cycles, again with pegfilgrastim (6 mg SQ on day 2). After chemotherapy was completed, B was changed to 15 mg/kg once every third week for 12 cycles to complete 1 year of treatment (Fig 1C). Standard chemotherapy premedications were used. Patients with estrogen receptor– or progesterone receptor–positive tumors received tamoxifen or aromatase inhibitors as appropriate, and radiation therapy was recommended per institutional guideline.
Fig 1.
(A-C) Schemas of all three trials. Black arrows indicate timing of LVEF (left ventricular ejection fraction) measurement reported in this analysis. q2w, every 2 weeks; qw, weekly; q3w, every 3 weeks; MUGA, multigated radionuclide angiography. (*) Pegfilgrastim was given subcutaneously on day 2 after chemotherapy.
Cardiac Monitoring: LVEF Assessment
All patients underwent assessment of LVEF by MUGA scans at study enrollment, at month 2 (on completion of ddAC regardless of B usage), and at months 6, 9, and 18 (similar to NSABP B31 and N9831). In the HER2-positive group, T was held if the post-ddAC LVEF (month 2) declined by more than15% or ≤ 15% to less than the lower limit of normal. In the HER2-normal group, since B was initiated with ddAC, adjustments of B were recommended based on the LVEF results. Three categories of asymptomatic LVEF decline were defined across the B-based study to guide physicians on whether to hold or continue it based on absolute asymptomatic declines in LVEF and the absolute value of LVEF as presented in Table 1. These categories were absolute decreases of less than 10%, 10 to 15%, and ≥16% (Table 1).
Table 1.
Guidelines for Continuation or Discontinuation of Bevacizumab Based on LVEF Results for ddAC Plus Bevacizumab Followed by Nanoparticle Albumin–Bound Paclitaxel Plus Bevacizumab
| LVEF on MUGA | Absolute Decrease |
||
|---|---|---|---|
| < 10% | 10% to 15% | ≥ 16% | |
| ≥ 55% | Continue bevacizumab | Continue bevacizumab | Hold bevacizumab and repeat MUGA after 4 weeks |
| 50%-54% | Continue bevacizumab and repeat MUGA in 4 weeks | Hold bevacizumab and repeat MUGA after 4 weeks | Hold bevacizumab and repeat MUGA after 4 weeks |
| ≤ 49% | Hold bevacizumab and repeat MUGA after 4 weeks | Hold bevacizumab and repeat MUGA after 4 weeks | Hold bevacizumab and repeat MUGA after 4 weeks |
Abbreviations: LVEF, left ventricular ejection fraction; ddAC, dose-dense doxorubicin and cyclophosphamide; MUGA, multigated radionuclide angiography.
RESULTS
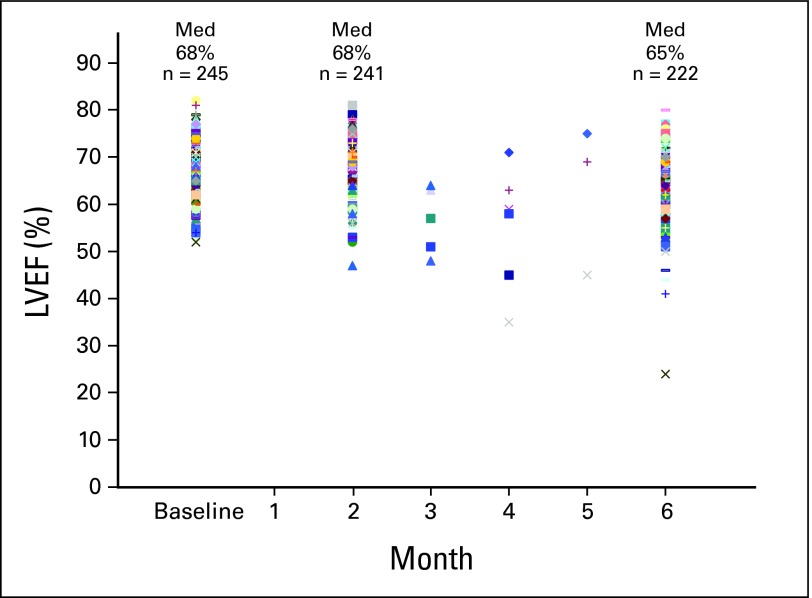
From January 2005 to May 2008, 245 patients were enrolled in the three trials. The median age was 47 years (range, 27 to 75 years; Table 2). There were 165 patients on the T-containing studies (70 in trial 1 and 95 in trial 2) and 80 patients on the B-containing study. Overall, 231 patients (94%) received chemotherapy in the adjuvant setting and 14 (6%) were treated in the neoadjuvant setting. The median baseline LVEF was 68% (range, 52% to 82%; Fig 2). This was similar for the groups treated with bevacizumab (median, 68%; range, 53% to 82%) or not (median, 68%; range, 52% to 81%).
Table 2.
Baseline Ages of Patients
| AC-PT |
AC, PT, and Lapatinib |
AC + B |
Overall |
|||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | |
| No. of patients | 70 | 95 | 80 | 245 | ||||
| Median age, years | 49 | 46 | 48 | 47 | ||||
| Range | 27-72 | 28-73 | 27-75 | 27-75 | ||||
| < 40 | 14 | 20 | 20 | 21 | 15 | 19 | 49 | 20 |
| 40-49 | 23 | 33 | 37 | 39 | 30 | 38 | 90 | 37 |
| 50-59 | 22 | 31 | 29 | 31 | 24 | 30 | 75 | 31 |
| 60-69 | 9 | 13 | 8 | 8 | 10 | 12 | 27 | 11 |
| 70+ | 2 | 3 | 1 | 1 | 1 | 1 | 4 | 1 |
| Surgery | ||||||||
| Lumpectomy | 36 | 51 | 36 | 38 | 23 | 29 | 94 | 38 |
| Modified radical mastectomy | 34 | 49 | 59 | 62 | 57† | 71 | 151 | 62 |
| Pre-existing hypertension | 11 | 16 | 17 | 18 | 10 | 13 | 37 | 15 |
| Radiation | ||||||||
| Breast/chest | 50 | 71 | 74 | 78 | 56 | 70 | 172 | 69 |
| Left sided | 21 | 42 | 38* | 51 | 23/56 | 41 | 81/172 | 47 |
| Right sided | 29 | 58 | 37* | 50 | 33/56 | 59 | 91/172 | 53 |
Abbreviations: AC, doxorubicin and cyclophosphamide; PT, paclitaxel and trastuzumab; B, bevacizumab.
Includes one patient who received bilateral radiotherapy.
Includes one patients with prior mastectomy and subsequent ipsilateral recurrence.
Fig 2.
Absolute LVEF (left ventricular ejection fraction) values over time. Med, median.
Cardiac Safety After ddAC Alone or With B
At month 2.
Results on LVEFs were available in 241 of 245 patients (98%) at month 2 (one withdrew consent and declined both treatment and MUGA scans, one discontinued treatment and was lost to follow-up, one developed progressive breast cancer, and one developed viral pneumonia and multisystem organ failure with sepsis and died after AC cycle 2; on review CHF did not contribute to her death).
The post-ddAC LVEF at month 2 was normal in 240 of 241 patients (99%; 95% CI, 97.7% to 99.9%). The LVEF was similar whether patients received B (median, 68%; range, 53% to 80%) or not (median, 68%; range, 47% to 81%). In both the HER2-positive and HER2-normal groups (after ddAC and B-ddAC, respectively), no patients stopped treatment at month 2 because of a significant asymptomatic LVEF decline. Only one patient in the entire three-study cohort had an asymptomatic LVEF decline to below the lower limit of normal immediately after ddAC for 4 cycles (Fig 2). After ddAC, this patient (in trial 2) had an LVEF decline from 68% to 47% on a MUGA scan at an outside institution. Per protocol, this patient did not begin anti-HER2 therapy immediately. A MUGA at MSKCC 1 month later revealed normal LVEF (64%) and anti-HER2 therapy was initiated per protocol, with no further LVEF declines to date. One other patient (in trial 3) had an asymptomatic LVEF decline exceeding 15% after B and ddAC (71% to 53%). After the interruption in B, this improved to 64% 4 weeks later and B was reintroduced; per protocol, this patient remained on study.
An asymptomatic LVEF decline of more than 15% after ddAC occurred in only two patients of 241 evaluable (0.8%; 95% CI, 0.1% to 3.0%). One of these patients received B and ddAC and the other ddAC alone as detailed above. An asymptomatic LVEF decline of 10% to 15% occurred in seven (3%; 95% CI, 1.1% to 5.9%) of 241 patients (three had ddAC and four had B and ddAC); five of these patients had subsequent improvement in LVEFs and continued treatment, one withdrew consent and one developed progressive breast cancer. Of the 241 patients, 96 (40%, 95% CI, 33.6% to 46.3%) had LVEF declines of less than 10%. The majority of patients, 136 of 241 (56%; 95% CI, 49.9% to 62.8%) had stable or improved LVEFs compared to baseline values (Table 3).
Table 3.
Change in LVEF After Dose-Dense AC With or Without Bevacizumab (months 2 and 6)
| Change in LVEF | Month 2 |
Month 6 |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Total patients | 241 | 222 | ||
| Decrease | ||||
| ≥ 16% | 2 | 0.8 | 7* | 3 |
| 10%-15% | 7 | 3 | 24 | 11 |
| < 10% | 96 | 40 | 102 | 46 |
| 5-< 10% | 35 | 15 | 46 | 21 |
| < 5% | 61 | 25 | 56 | 25 |
| Stable or improved | 136 | 56 | 89 | 40 |
Abbreviations: LVEF, left ventricular ejection fraction; AC, doxorubicin and cyclophosphamide.
This includes one patient with congestive heart failure and LVEF decline ≥ 16% who had month 6 LVEF measured per protocol.
At month 6.
Results were available in 222 of 241 patients (92%) at 6 months (one expired from hepatitis B reactivation, five withdrew consent, 11 had toxicities [noncardiac] and two had CHF and did not have month 6 LVEF assessments). At month 6, the LVEF was normal in 217 of 222 patients (98%; 95% CI, 94.8% to 99.3%) and the median LVEF was 65% (range, 24% to 80%; Fig 2). This was similar whether patients received bevacizumab (median, 64%; range, 51% to 77%) or not (median, 65%; range, 24% to 80%). Three patients had an asymptomatic LVEF decline to below the normal limit. An asymptomatic decline of more than 15% occurred in six of 222 evaluable patients (3%; 95% CI, 1% to 5.8%) and all six had subsequent improvement in LVEF; a symptomatic decline of more than 15% occurred in one of 222 evaluable patients (0.5%, 95% CI, 0.01% to 2.5%) who developed CHF at 6 months of evaluation. An asymptomatic LVEF decline of 10% to 15% occurred in 24 of 222 patients (11%; 95% CI, 7.1% to 15.7%). LVEF declines of less than 10% were common and occurred in 102 of 222 patients (46%; 95% CI, 39.3% to 52.7%). The LVEF was stable or improved in 89 of 222 patients (40%; 95% CI, 33.6% to 46.9%; Table 3).
Within 6 months of initiating chemotherapy, a total of three of 245 patients (1.2%; 95% CI, 0.25% to 3.54%) had developed CHF, all of whom received anti-HER2 therapy (one in trial 1 and two in trial 2; Table 4). One additional patient treated on trial 2 developed CHF at month 12. Therefore, at 1 year, the respective CHF rates for trials 1, 2, and 3 were 1.4%, 3%, and 0% (median follow-up of 28, 12, and 18 months).13,14 In addition, one (1.3%, 95% CI, 0.03% to 6.8%) of 80 patients in trial 3 developed CHF at month 15 (3 months after completing therapy).17
Table 4.
Patients With CHF
| Patient No. | Age (years) | Treatment | LVEF |
|||
|---|---|---|---|---|---|---|
| At Baseline | At Month 2 | At CHF Diagnosis | At Follow-Up | |||
| 1* | 56 | ddAC → P-trastuzumab | > 75% | 79% | CHF at month 4 ↓ 45% (ECHO) | 50% 5 months and 58% 22 months later |
| 2† | 44 | ddAC → weekly P- trastuzumab-lapatinib | 68% | 68% | CHF at month 3 ↓ 48% (ECHO) | 62% (ECHO) 6 months later |
| 3‡ | 35 | ddAC → weekly P- trastuzumab-lapatinib | 52% | 56% | CHF at month 6 ↓ 24% (ECHO) | 24% 2 months later |
Abbreviations: CHF, congestive heart failure; LVEF, left ventricular ejection fraction; ECHO, echocardiogram; ddAC, dose-dense doxorubicin and cyclophosphamide; P, paclitaxel.
Patient 1 developed shortness of breath with clinical CHF at month 4. She was treated with a diuretic, a beta blocker, and an angiotensin-converting enzyme (ACE) inhibitor. Trastuzumab was discontinued per protocol. Her LVEF subsequently improved and she is currently clinically well.
Patient 2 developed pleuritic chest pain and acute respiratory distress at month 3 and was diagnosed with CHF. She was stabilized on a diuretic and an ACE inhibitor. Per-protocol P-trastuzumab-lapatinib was discontinued. Six months after this event, she is clinically stable and off all cardiac medications
Patient 3 had exertional dyspnea at month 6 and was found to have an LVEF of 24%. She came off P-trastuzumab-lapatinib and was treated with a beta blocker, digoxin, and a diuretic with improvement in symptoms. Four months after this event she has completed right-sided chest radiotherapy and is considering a permanent pacemaker.
DISCUSSION
To our knowledge, this is the first report of the acute cardiac safety of ddAC with or without B measured prospectively. We did not confirm the previously reported high rate (6.7%) of significant asymptomatic declines in LVEF with conventionally scheduled AC.8 In both trials for the HER2-positive patients, only one patient (in trial 2) had a treatment delay after ddAC due to an asymptomatic LVEF decline. Repeat MUGA at the parent institution was normal and allowed this patient to commence anti-HER2 therapy. Unlike trial 1 where all patients were required to have serial MUGA scans performed at only one institution, patients in trial 2 were permitted to have MUGAs done at various local facilities and it was possible that this might have added variability in the LVEF results. In the HER2-normal group where ddAC was given with concurrent bevacizumab, only one (1.2%) of 80 patients had a significant (> 15%) asymptomatic (and temporary) LVEF decline after ddAC. In this study, four (5%) of 78 evaluable patients receiving concurrent B and ddAC had asymptomatic absolute LVEF declines of more than 10% from baseline after 4 cycles of B and ddAC. This compared favorably to ECOG 2104 in which 13 (14%) of 93 patients receiving concurrent B and ddAC had asymptomatic LVEF declines more than 10%. In comparison to ECOG 2104 in which CHF occurred in 2% of patients receiving concurrent B and ddAC with a median follow-up of 14.6 months, the CHF rate in our B-based study was 1.3% with a median follow-up of 18 months.17
Of note, there were other studies that have evaluated the combination of B with an anthracycline. One example was a study by Wedam et al18 on the combination of B with doxorubicin and docetaxel (50 mg/m2 and 75 mg/m2) for 6 cycles every 3 weeks in the preoperative setting. In this study of 21 patients, no patient had a decline in LVEF of greater than 15%. Thus, an anthracycline combined with B appeared to be feasible in these studies which was consistent with our trial 3, in which B was combined with ddAC.
The LVEF monitoring in our three studies was similar to that in NSABP B-31 and N 9831.8 Across all three of our studies asymptomatic declines in LVEF were more common at month 6 compared to month 2. This likely reflected the predicted, additional effect of anti-HER2 therapy on LVEF. The majority of these declines occurred in patients treated with T with or without lapatinib, again suggesting that HER2-targeting agents contributed to these asymptomatic declines, likely due to the interaction with the prior anthracycline. Notably, the patients in our studies who developed CHF during treatment all received anti-HER2 therapies. Similarly, in the B-31 study, 28 of 31 patients developed CHF during the year of T (like our patients).19 In addition in N9831 the cumulative 1-, 2-, and 3-year CHF rates were unchanged at 3.3%.20 Thus, we now have long-term data out to at least a year and do not expect these rates to increase with longer follow-up. We see no early signal of higher rates of CHF when incorporating HER2-targeting agents into ddAC compared with every 3-weekly AC.
The apparent relative short-term cardiac safety of dose-dense AC in our three consecutive studies may not be expected by many in the medical oncology community. We believe that there are several possible explanations for our favorable outcomes. First, in terms of cardiotoxicity ddAC is at least as safe as every 3-weekly AC.6–7 With a long-term follow-up of 6.5 years, the Cancer and Leukemia Group B study 9741 showed that in patients treated with ddAC followed by P, the grade 3 to 4 cardiac event rate was one half of that noted in those treated with the every 3-weekly schedule of the same chemotherapy.6–7 Second, within the limited number of centers participating in these studies, there may have been greater standardization of the interpretation of MUGA scans based on the use of consistent equipment and readers. This is further supported by the two patients (both in trial 2), who had LVEF declines to below the lower limit of normal at outside institutions with normalization of LVEF at the main center. If this hypothesis is correct, the reproducibility of LVEF measurement by MUGA in different institutions is a potential problem in larger multicenter studies. Furthermore, there is some evidence that LVEF measurement with MUGA, as used in these trials, may be more reproducible than that with echocardiograms.21 This, too, could have profound implications for the routine use and management of anthracyclines and T in much of the world. Albeit low, the LVEF changes that were noted in our three studies may be a concern later, and the changes that were not seen could be due to variable LVEF readings when dealing with multicenter trials. Third, the inherent subtle investigator bias in patient selection within our three studies may have excluded patients at a higher risk of cardiac dysfunction. In this case, the large multicenter trials may be more representative of a less selected patient population. However, we see no evidence of a differential protocol-enrollment selection bias in our trials as compared to the cooperative group trials. As an example, patients on the NSABP B-31 and N98318 trials had similar ages at baseline as our patients in trial 1 and the studies had similar cardiac exclusion criteria.13
Given the cardiotoxicity risks of anthracyclines, T, and possibly B, adjuvant trials involving these drugs have incorporated rigorous cardiac monitoring for asymptomatic declines in LVEF. This has led to the widespread recognition and concern about asymptomatic short-term declines in ejection fraction measured by MUGA and/or echocardiogram because these have been used as surrogates for the long-term risk of CHF.3 The true significance is, however, unclear as many more patients have been noted to have these changes in the prospective randomized trials than those who actually ever develop clinical CHF. With the large numbers of patients treated with anthracyclines, there are no clear available data to confirm a significant long-term impact of early asymptomatic LVEF change.
Our combined analysis of three trials adds new information on the risk of early asymptomatic ejection fraction change related to a more effective (dd) schedule of administration for AC. In patients receiving ddAC alone or with B, we did not identify a significant incidence of short-term cardiac toxicity. Longer-term follow-up of cardiac toxicity of ddAC with and without B, T, and lapatinib is ongoing. The assessment of biomarkers for cardiac injury in ongoing and future trials may allow us to see if there is any correlation with asymptomatic declines in LVEF.
Footnotes
See accompanying editorial on page 6073
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00591851, NCT00482391, NCT00436709.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Maura Dickler, Genentech (C), Roche (C); Nancy Lin, GlaxoSmithKline (C), Genentech (U); Carol Chen, Herceptin National Cardiology Advisory Board (C); Clifford A. Hudis, Genentech (C); Chau T. Dang, Genentech (C), GlaxoSmith Kline (C) Stock Ownership: None Honoraria: Patrick G. Morris, Genentech, Bristol-Myers Squibb; Maura Dickler, Roche; Heather L. McArthur, Genentech; Beverly Moy, Genentech, GlaxoSmith Kline; Chau T. Dang, Genentech, GlaxoSmithKline Research Funding: Maura Dickler, Genentech, Abraxis; Heather L. McArthur, Genentech, Abraxis; Tiffany Traina, Genentech, Roche; Nancy Lin, GlaxoSmithKline, Genentech; Eric Winer, Genentech; Chau T. Dang, Genentech, GlaxoSmithKline Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Patrick G. Morris, Larry Norton, Clifford A. Hudis, Chau T. Dang
Administrative support: Patrick G. Morris, Laura Godfrey, Benjamin Nulsen
Provision of study materials or patients: Patrick G. Morris, Maura Dickler, Heather L. McArthur, Tiffany Traina, Steven Sugarman, Nancy Lin, Beverly Moy, Steven Come, Hope Rugo, Larry Norton, Eric Winer, Clifford A. Hudis, Chau T. Dang
Collection and assembly of data: Patrick G. Morris, Heather L. McArthur, Laura Godfrey, Benjamin Nulsen, Carol Chen, Richard Steingart, Chau T. Dang
Data analysis and interpretation: Patrick G. Morris, Maura Dickler, Heather L. McArthur, Carol Chen, Richard Steingart, Clifford A. Hudis, Chau T. Dang
Manuscript writing: Patrick G. Morris, Maura Dickler, Clifford A. Hudis, Chau T. Dang
Final approval of manuscript: Patrick G. Morris, Maura Dickler, Heather L. McArthur, Tiffany Traina, Steven Sugarman, Nancy Lin, Beverly Moy, Steven Come, Laura Godfrey, Benjamin Nulsen, Carol Chen, Richard Steingart, Hope Rugo, Larry Norton, Eric Winer, Clifford A. Hudis, Chau T. Dang
REFERENCES
- 1.Early Breast Cancer Trialists' Collaborative Group. Polychemotherapy for early breast cancer: An overview of the randomized trials. Lancet. 1998;352:930–942. [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomized trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 3.Carver JR, Shapiro CL, Ng A, et al. ASCO Cancer Survivorship Expert Panel: American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: Cardiac and pulmonary late effects. J Clin Oncol. 2007;25:3991–4008. doi: 10.1200/JCO.2007.10.9777. [DOI] [PubMed] [Google Scholar]
- 4.Henderson IC, Berry DA, Demetri GD, et al. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976–983. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- 5.Mamounas EP, Bryant J, Lembersky BC, et al. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: Results from NSABP-B 28. J Clin Oncol. 2005;23:3686–3696. doi: 10.1200/JCO.2005.10.517. [DOI] [PubMed] [Google Scholar]
- 6.Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of Intergroup trial C9741/Cancer and Leukemia Group B trial 9741. J Clin Oncol. 2003;21:1431–1439. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 7.Hudis C, Citron ML, Berry D, et al. Five year follow-up of INT C9741: Dose dense (DD) chemotherapy (CRx) is safe and effective. Breast Cancer Res. 2005;94:S20. suppl 1. [Google Scholar]
- 8.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 9.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 10.Smith I, Procter M, Gelber RD, et al. HERA study team: 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: A randomised controlled trial. Lancet. 2007;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 11.Slamon DJ, Eiermann W, Robert NJ, et al. Phase III trial comparing doxorubicin and cyclophosphamide followed by docetaxel with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab with docetaxel, carboplatin and trastuzumab in HER2 positive early breast cancer patients: Second interim analysis of the BCIRG 006 study. San Antonio Breast Cancer Symposium; December 14-17, 2006; San Antonio, TX. [Google Scholar]
- 12.Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354:809–820. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 13.Dang C, Fornier M, Sugarman S, et al. The safety of dose-dense doxorubicin and cyclophosphamide followed by paclitaxel with trastuzumab in HER2/neu overexpressed/amplified breast cancer. J Clin Oncol. 2008;26:1216–1222. doi: 10.1200/JCO.2007.12.0733. [DOI] [PubMed] [Google Scholar]
- 14.Dang C, Lin N, Moy B, et al. Dose-dense doxorubicin and cyclophosphamide followed by weekly paclitaxel with trastuzumab and lapatinib in HER2/neu-positive breast cancer is not feasible due to excessive diarrhea: Updated results. San Antonio Breast Cancer Symposium; December 10-14, 2008; San Antonio, TX. abstr 2108. [Google Scholar]
- 15.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 16.Miller K, O'Neill A, Perez E, et al. Phase II feasibility trial bevacizumab incorporating into dose-dense AC>T in patients with lymph node positive breast cancer: A trial of the Eastern Cooperative Oncology Group (E2104) J Clin Oncol. 2008;26:11s. doi: 10.1093/annonc/mdr344. abstr 520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McArthur H, Rugo H, Nulsen B, et al. Updated results: Cardiac safety of adjuvant bevacizumab plus dose-dense doxorubicin/cyclophosphamide followed by nanoparticle albumin-bound paclitaxel in patients with early stage breast cancer. San Antonio Breast Cancer Symposium; December 10-14, 2008; San Antonio, TX. abstr 4104. [Google Scholar]
- 18.Wedam SB, Low JA, Yang JA, et al. Antiangiogenic and antitumor effects of bevacizumab in patients with inflammatory and locally advanced breast cancer. J Clin Oncol. 2006;24:769–777. doi: 10.1200/JCO.2005.03.4645. [DOI] [PubMed] [Google Scholar]
- 19.Tan-Chiu E, Yothers G, Romond E, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab, as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol. 2005;23:7811–7819. doi: 10.1200/JCO.2005.02.4091. [DOI] [PubMed] [Google Scholar]
- 20.Perez EA, Suman VJ, Davidson NE, et al. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J Clin Oncol. 2008;26:1231–1238. doi: 10.1200/JCO.2007.13.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takuma S, Ota T, Muro T, et al. Left ventricular function by real-time 3-dimensional echocardiography compared with conventional noninvasive methods. J Am Soc Echocardiogr. 2001;14:275–284. doi: 10.1067/mje.2001.111158. [DOI] [PubMed] [Google Scholar]