Abstract
Purpose
Stage III melanoma is associated with a high risk of relapse and mortality. Nevertheless, follow-up guidelines have largely been empirical rather than evidence-based.
Patients and Methods
Clinical records of stage III patients with no evidence of disease seen at Memorial Sloan-Kettering Cancer Center (MSKCC) between 1992 and 2004, who ultimately relapsed, were reviewed retrospectively to evaluate date of first relapse, time to first relapse, method of first relapse detection, and survival. We also determined overall 5-year relapse-free survival (RFS) of all stage III patients seen at MSKCC during this period.
Results
The overall 5-year RFS for stage IIIA, IIIB, and IIIIC patients was 63%, 32%, and 11%, respectively. Among relapsing patients, 340 had adequate follow-up to be evaluable for all parameters. Site of first relapse was local/in-transit (28%), regional nodal (21%), or systemic (51%). First relapses were detected by the patient or family, physician, or by screening radiologic tests in 47%, 21%, and 32% of patients, respectively. Multivariate analysis revealed that better overall survival was associated with younger age and first relapse being local/in-transit or nodal, asymptomatic, or resectable. For each substage, we estimated site-specific risk of first relapse.
Conclusion
Patients detected almost half of first relapses. Our data suggest that routine physical examinations beyond 3 years for stage IIIA, 2 years for stage IIIB, and 1 year for stage IIIC patients and radiologic imaging beyond 3 years for stages IIIA and IIIB and 2 years for stage IIIC patients would be expected to detect few first systemic relapses.
INTRODUCTION
The risk of relapse for stage III melanoma patients is high and varies with substage.1,2 Despite this high risk, the optimal follow-up strategies have not been defined. The goal of follow-up is to detect first relapses as soon as possible. Some practitioners recommend frequent follow-up with abundant use of radiographic imaging such as computed tomography (CT) and positron emission tomography scans. Others question the value of follow-up altogether and take a more minimalist approach. The few published studies looking at follow-up of stage III patients have either been relatively small or have not reported sites of first relapse.3–6 This makes it difficult to know how best to follow these patients to optimize the chance of detecting the initial relapse. As a result, the National Comprehensive Cancer Network (NCCN) follow-up guidelines for these patients are largely empirical rather than data-based.7 Of note is that the NCCN follow-up guidelines for stage III patients do not distinguish between American Joint Committee on Cancer (AJCC) 2002 stages IIIA, IIIB, and IIIC.
We used our institutional melanoma database to study first relapses among stage III patients. This study is a retrospective analysis of 340 patients with AJCC (2002) stage III melanoma in which we evaluated, among other parameters, the time and site of first recurrence, method of detection, and the overall survival. This is the largest analysis of stage III patients to date, and these data suggest that follow-up strategies should be different for patients with stage IIIA, IIIB, and IIIC melanoma.
PATIENTS AND METHODS
Patient Selection
We identified 429 patients with AJCC (2002) stage III melanoma who presented to Memorial Sloan-Kettering Cancer Center (MSKCC) between December 1998 and January 2002 and who were rendered free of disease but later relapsed. Information required for the study was lacking in 149 patients who were thus considered not evaluable. The remaining 280 patients were considered evaluable for this analysis. Because this initial cohort yielded only 35 evaluable patients with stage IIIA, we expanded the interval of presentation for stage IIIA patients to 2004 by including 60 stage IIIA patients we previously identified.6 Most patients were followed at our institution before relapse. Our standard approach in medical oncology was a physical examination every 3 months for the first 2 years, then every 6 months. In addition to medical oncology visits, patients underwent surgical and dermatologic visits. CT scans were typically obtained before these follow-up visits as were CBCs, comprehensive panels, and lactate dehydrogenase (LDH). We extracted demographic information, characteristics of the primary melanoma such as site, stage III substage, and adjuvant treatments. Descriptive information relative to first recurrence was captured such as site, sign of first recurrence, person/method of its detection (ie, symptoms, physical examination by a physician or family/friends, radiographic examinations, or blood tests), number of clinical evaluations before recurrence, treatment administered for the recurrence and outcome, current disease, and survival status. Patients who first relapsed at several sites concomitantly were scored on the basis of the site that was most advanced (eg, systemic sites outranked nodal sites which outranked local/in-transit sites). This study was approved by the MSKCC institutional review board.
Statistical Analysis
The cumulative progression-free survival and overall survival curves were calculated according to the Kaplan-Meier product-limit method. A stepwise selection procedure was used to construct a multivariate Cox model, in which variables were entered into the model if the χ2 statistic achieved a P value of ≤ .05 and were removed from the model if the χ2 statistic achieved a P value of > .05. P values and 95% CIs are two-sided. A P value ≤ .05 was considered statistically significant. SAS 9.1.3 (SAS Institute, Cary, NC) and R 2.7.0 software packages were used for statistical analysis.
RESULTS
Patient Demographics
For this study, 340 patients with AJCC stage III melanoma who recurred after being free of disease were considered evaluable. Of these, 95 (28%) had stage IIIA, 155 (46%) had stage IIIB, and 90 (26%) had stage IIIC disease; 64% were men and 36% were women (Table 1). The median age was 57 years. Median follow-up for patients without relapse was 77 months (range, 5 to 148 months). Fifty-one percent had an extremity primary site, 26% truncal, 15% head and neck, and 8% unknown (Table 1). There were no mucosal melanomas in this cohort. Information relative to adjuvant treatment was available for 338 patients; 177 (52%) initially received adjuvant therapy after surgical resection. The most common form of adjuvant treatment was experimental vaccines (23%) and high-dose interferon alfa (16%).
Table 1.
Clinical and Pathologic Characteristics of Patients With Initial Diagnosis of AJCC Stage III Cutaneous Melanoma
| Characteristic | No. | % |
|---|---|---|
| Age, years | ||
| Median | 57 | |
| Range | 11-95 | |
| Sex | ||
| Male | 218 | 64 |
| Female | 122 | 36 |
| Primary site | ||
| Head and neck | 50 | 15 |
| Extremities | 175 | 51 |
| Trunk | 89 | 26 |
| Mucosal | 0 | 0 |
| Unknown primary | 26 | 8 |
| Substage III (AJCC, 2002) | ||
| IIIA | 95 | 28 |
| IIIB | 155 | 46 |
| IIIC | 90 | 26 |
| Adjuvant therapy* | ||
| Interferon | 55 | 16 |
| Vaccine | 79 | 23 |
| Chemotherapy | 21 | 6 |
| Radiotherapy | 22 | 6 |
| None | 161 | 47 |
Abbreviation: AJCC, American Joint Committee on Cancer.
For two patients, no information was available regarding adjuvant therapy.
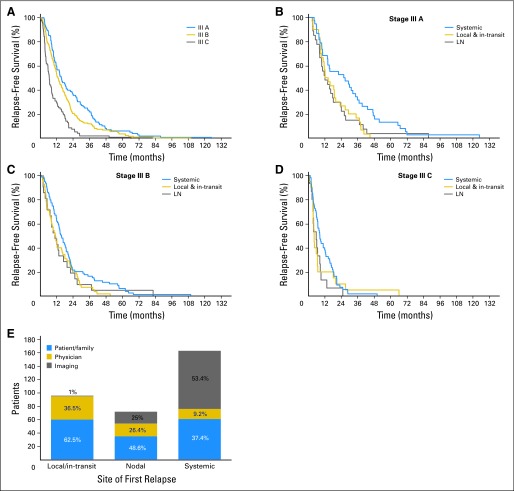
The cumulative time to first relapse was plotted for each stage III substage (Fig 1A). The site of first relapse is reported in Table 2 by substage. First relapses among stage IIIA patients were local/in-transit, nodal, and systemic sites in 32%, 28%, and 40% of patients, respectively. First relapses among stage IIIB and IIIC patients more frequently occurred at systemic sites. The time to first relapse event, either regional and/or systemic, was analyzed by substage. In stage IIIA, essentially all local/in-transit and nodal recurrences occurred by 40 months, whereas systemic recurrences occurred as late as 71 months (Fig 1B). First relapses in patients with stage IIIB occurred over similar time courses independent of site (Fig 1C). The group of stage IIIC patients showed the earliest recurrences (Fig 1D); indeed almost all first relapses occurred by 2 years.
Fig 1.
(A) Relapse-free survival of all 340 patients with melanoma substages IIIA, IIIB, and IIIC who relapsed. Relapse-free survival was estimated separately for patients with substages IIIA (B), IIIB (C), and IIIC (D) by anatomic site of first relapse. (E) Site of first relapse and method of its detection. Local and in-transit recurrences are combined. LN, lymph node.
Table 2.
Site of First Relapses by Substage
| Substage | Local/In-Transit (%) | Nodal (regional) (%) | Systemic (%) |
|---|---|---|---|
| IIIA | 32 | 28 | 40 |
| IIIB | 30 | 19 | 51 |
| IIIC | 22 | 17 | 61 |
| All stage III | 28 | 21 | 51 |
Method of Detection of First Relapse
We analyzed how each first relapse was discovered (Fig 1E). Patients and/or family members discovered 62% of local and in-transit recurrences and 49% of nodal recurrences. Systemic recurrences were less likely to be detected by the patients. Only 37% of patients whose first recurrence was systemic detected the recurrence themselves, either by noticing a new tumor or other symptoms that led to further evaluation. Physical examination by a physician accounted for the detection of 36% of the local and in-transit recurrences. Twenty-six percent of nodal recurrences were detected by physicians, whose capacity to detect systemic recurrences was even lower (9%). In only four patients (1.2%), the first sign of relapse was elevated LDH; none of the other blood tests were informative.
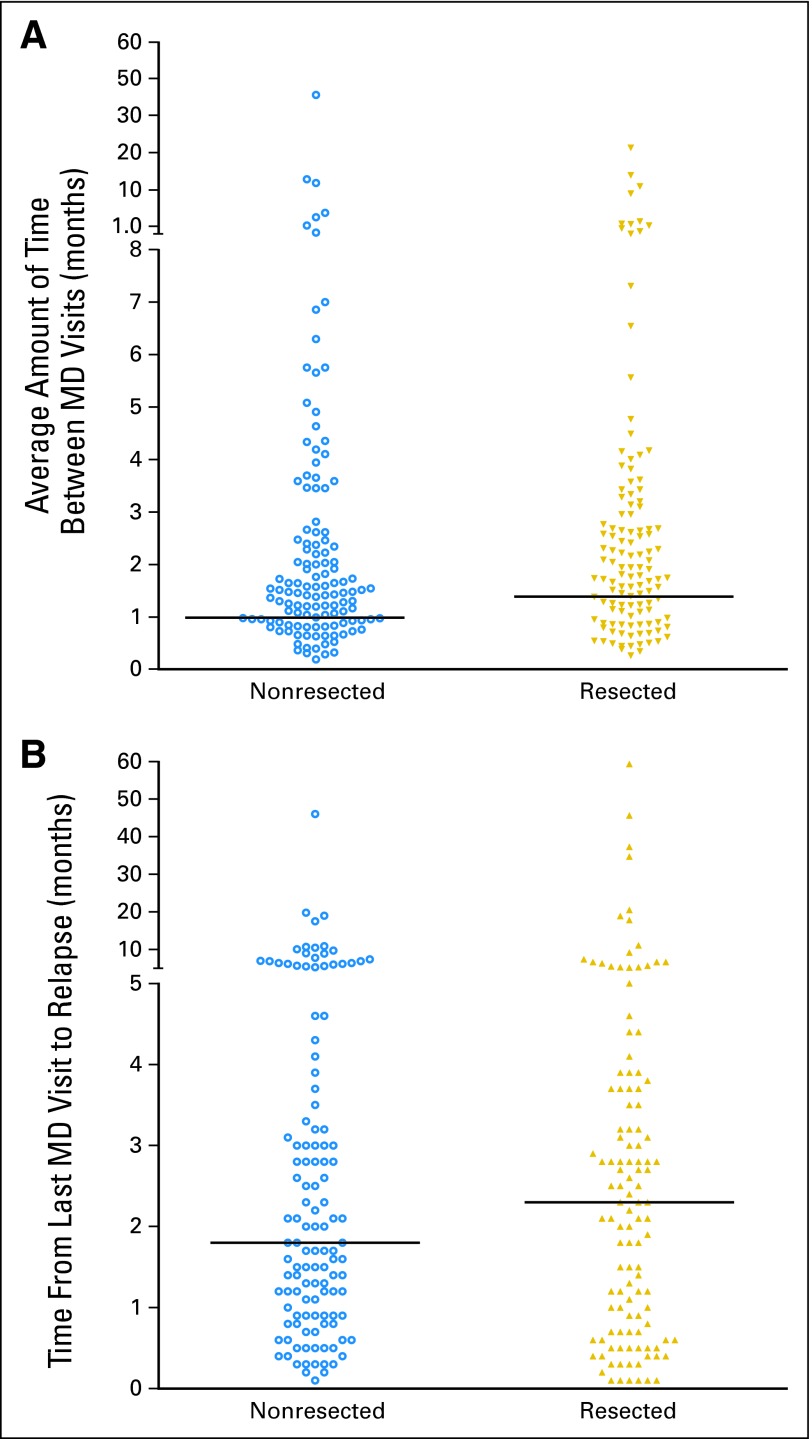
Medical oncologic visits were performed every 3 months for the first 2 years, then every 6 months. Surgical and dermatologic visits were also performed during this time. Intensity of follow-up, as indicated by the average number of months between visits to the physician (months of progression-free survival/number of medical oncology, surgical, or dermatologic visits) did not correlate with the likelihood of discovering a resectable relapse (Appendix Fig A1A, online only). Since this analysis may not account for long gaps in follow-up, we also analyzed the average time from last physician visit to first relapse (Appendix Fig A1B). There was no difference in time since last visit between resectable and nonresectable patients.
Of the 163 patients whose first site of relapse was systemic, the method of detection could not be determined from the records in nine patients. As noted above, 37% of patients whose first relapse was systemic detected the relapse either by noticing a new tumor or new symptoms such as pain, bleeding, or seizure. In the remaining 63% of patients whose first detectable relapse was systemic, the relapse was asymptomatic. Radiographic tests, largely CT scans (72%), detected asymptomatic systemic relapses in 53% (n = 87) of these patients.
Recurrences in Stage III Substages in Which First Relapse Was a Systemic Site
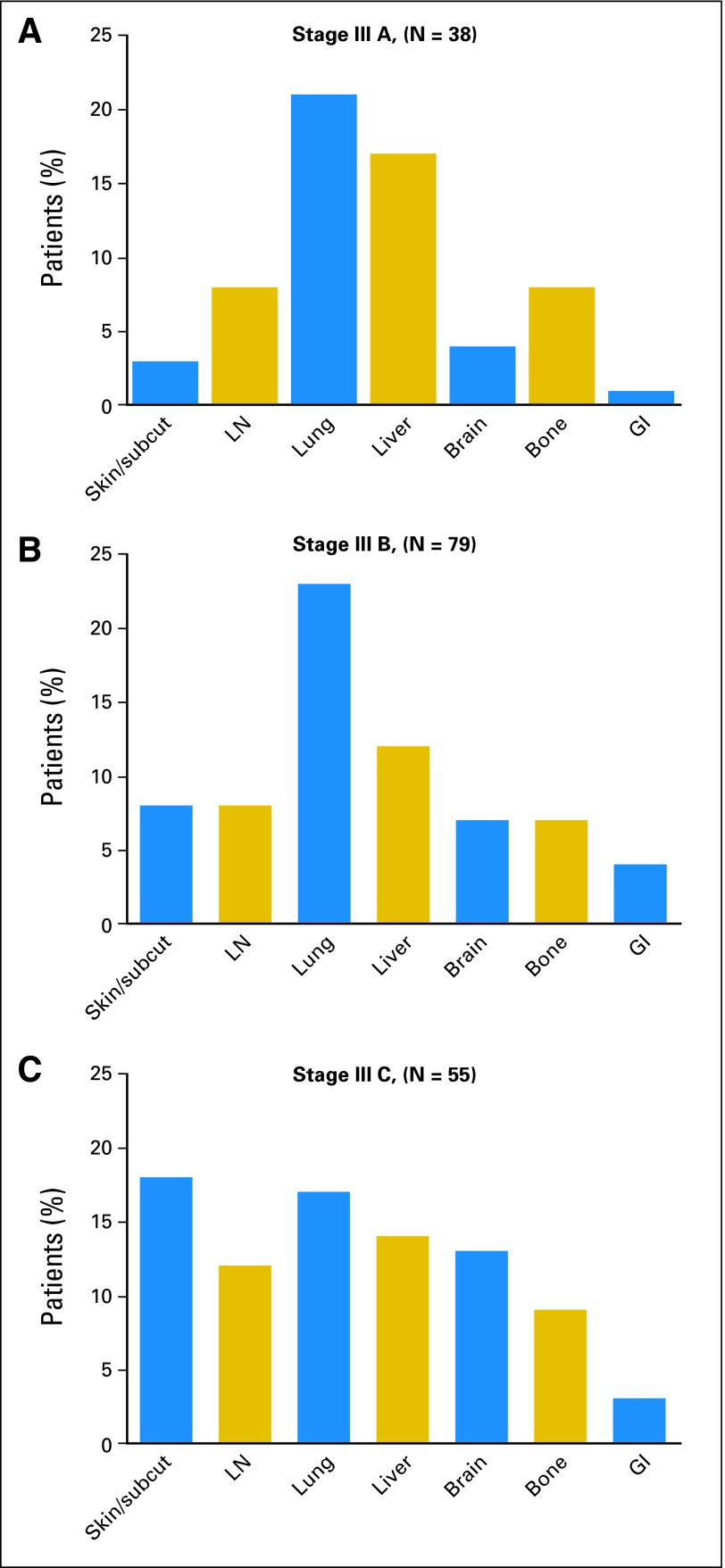
For stage IIIA and IIIB patients, lung and liver were the most common first sites of systemic relapse (Figs 2A and 2B); in stage IIIC patients, first sites of systemic relapses were evenly distributed among skin/subcutaneous, nodal, lung, liver, brain, and bone (Fig 2C). GI sites as first site of recurrence were uncommon in all substages.
Fig 2.
Site of first systemic relapses in patients with initial diagnosis of melanoma substages IIIA (A), IIIB (B), and IIIC (C). Subcut, subcutaneous; LN, lymph node.
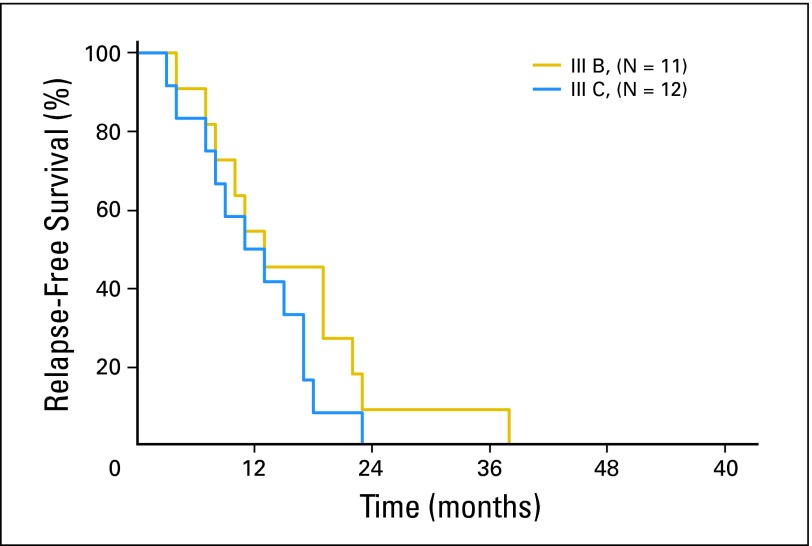
Only four stage IIIA patients (4%) experienced first relapse in the CNS. In stage IIIB and IIIC patients, metastases to CNS accounted for 7% and 13% of first relapses, respectively (Table 3). Almost all of these events occurred by 23 months in the stage IIIB patients and by 18 months in the stage IIIC patients (Appendix Fig A2, online only). Notably, of the 25 patients whose first systemic relapse was in the brain, nine (36%) presented with seizures.
Table 3.
Risk of First Relapse by Site
| Site of First Relapse | Proportion Among Relapsing Patients | Site-Specific Risk of First Relapse* | Months Until Site-Specific Relapse Risk ≤ 5%† |
|---|---|---|---|
| Stage IIIA | |||
| Local/in-transit | 0.32 | 0.16 | 31 |
| Lymph node | 0.28 | 0.15 | 24 |
| Systemic, non-brain | 0.36 | 0.14 | 32 |
| Brain | 0.04 | 0.02 | —‡ |
| Stage IIIB | |||
| Local/in-transit | 0.30 | 0.21 | 22 |
| Lymph node | 0.19 | 0.14 | 14 |
| Systemic, non-brain | 0.44 | 0.31 | 40 |
| Brain | 0.07 | 0.05 | —‡ |
| Stage IIIC | |||
| Local/in-transit | 0.22 | 0.19 | 7 |
| Lymph node | 0.17 | 0.14 | 6 |
| Systemic, non-brain | 0.48 | 0.40 | 21 |
| Brain | 0.13 | 0.11 | 13 |
Site-specific risk of first relapse adjusted for the proportion of patients never relapsing (see Site-Specific Risk of First Relapse).
Calculated based on absolute risk and time to relapse in Figure 1 and Appendix Figure A2 (online only).
Site-specific risk for brain metastases as first site of relapse in stage IIIA and IIIB patients was ≤ 5% at time of surgical resection.
Site-Specific Risk of First Relapse
These data provide the opportunity to develop follow-up guidelines for detection of first relapses in patients with stage III melanoma who are free of disease. We analyzed our entire database from this time period and found the overall 5-year risk of relapse at any site was 48% for stage IIIA, 71% for stage IIIB, and 85% for stage IIIC patients (data not shown). Site-specific risk of first relapse for local/in-transit skin, lymph node, visceral (non-brain), or brain relapse was calculated to adjust for the proportion of patients who would never relapse by multiplying the proportion of first relapses at a given site by the overall risk of relapse anywhere (Table 3). Using the data in Figure 1(panels B, C, and D) and Appendix Figure A2, we were able to estimate the time point after which the site-specific risk of first relapse at a given site was ≤ 5% (Table 3). For stage IIIA patients, the site-specific risk of a first relapse at local/in-transit, nodal, or systemic (non-brain) sites did not drop to ≤ 5% until 31, 24, and 32 months, respectively. For stage IIIB patients, risk of first relapses at these sites decreased to ≤ 5% at 22, 14, and 40 months, respectively. The site-specific risk of first relapses in the brain was ≤ 5% at the time of surgical resection in stage IIIA and IIIB patients.
For stage IIIC patients, the site-specific risk of a first relapse at local/in-transit sites or nodal sites dropped to ≤ 5% by 7 months. Risk of first relapse in a systemic site other than brain was 40%, which dropped to ≤ 5% by 21 months. The risk that a stage IIIC patient would have brain as the site of first relapse was 11% initially. This dropped to ≤ 5% by 13 months.
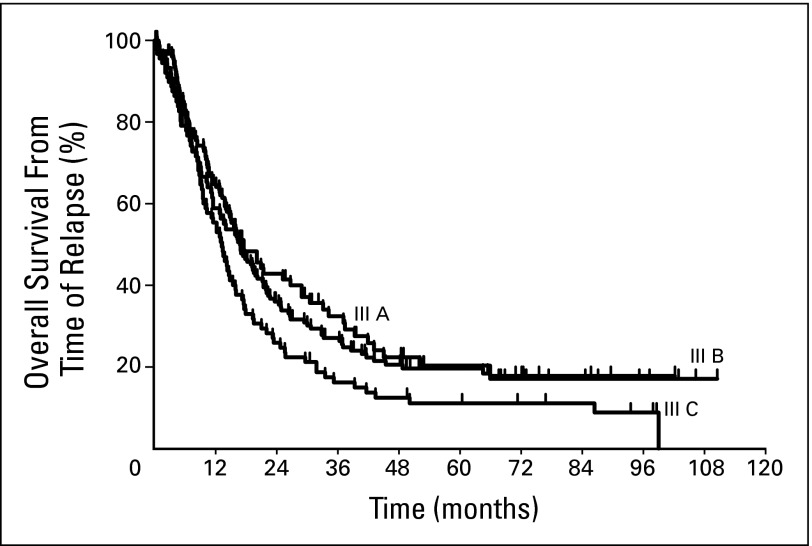
Overall Survival From Time of Relapse
We calculated the overall survival for each substage from time of first relapse. The estimated 5-year survivals for stages IIIA, IIIB, and IIIC from time of first relapse were 20%, 20%, and, 11%, respectively (Appendix Fig A3, online only). Survival curves showed a plateau at around 50 months in all substages. To assess how individual variables correlate with overall survival, we analyzed each of them in a multivariate model (Table 4). Regional site of relapse, either in-transit or nodal, was associated with longer overall survival compared with systemic sites of disease (P < .001). Symptomatic relapses, as opposed to relapses discovered by physical examination or radiographic imaging, were associated with shorter survival. Alternatively, having a recurrence that could be completely resected was associated with longer survival (relative risk = 2.31; 95% CI, 1.68 to 3.18; P < .001). Age was an independent variable that inversely correlated with survival (relative risk = 1.02; 95% CI, 1.01 to 1.03; P < .001). Sex, stage III substage, site of primary, adjuvant therapy, treatment for recurrence, median follow-up, and number of physical examinations were not independently associated with overall survival.
Table 4.
Multivariate Model for Overall Survival
| Variable | Relative Risk | 95% CI | P |
|---|---|---|---|
| Type of metastasis | |||
| In-transit v systemic | 0.42 | 0.29 to 0.61 | < .001 |
| Nodal v systemic | 0.64 | 0.44 to 0.92 | |
| Detection method | |||
| Physician or tests v symptoms | 0.67 | 0.50 to 0.88 | .004 |
| Complete resection of recurrence | |||
| No v yes | 2.31 | 1.68 to 3.18 | < .001 |
| Age | |||
| Young v elderly | 1.02 | 1.01 to 1.03 | .001 |
DISCUSSION
Patients who are free of disease after resection of stage III melanoma constitute a large cohort at high risk for relapse. As a result, most physicians recommend close follow-up, yet there is uncertainty about the precise frequency and duration of follow-up required. The NCCN guidelines7 recommend a physical examination every 3 to 6 months for 3 years and then every 4 to 12 months for two more years. In the absence of specific signs or symptoms, chest x-rays, CT scans, and blood tests are considered optional. The wide range of acceptable follow-up approaches in the guidelines reflects the lack of data driving these recommendations.
Garbe3 reported on prospective follow-up of 209 stage III melanoma patients after surgery, testing an aggressive follow-up regimen. All patients were examined every 3 months for 5 years after surgery and then every 6 months for 5 more years. Every 6 months patients also had blood tests, a chest x-ray, and ultrasound examination of the appropriate lymph node basin. CT or positron emission tomography scans were used to investigate findings suspicious for recurrence. They found that 48% of relapses were detected by physical examination, 28% by CT scan, and 9.5% by lymph node ultrasound. Relapses were rarely discovered by chest x-ray, blood tests, or by patients outside of the scheduled physical examination. They did not report whether there were differences among substages, the time to recurrence, or the site of first recurrence. Consistent with our results, they found that patients who had potentially resectable relapse showed a better estimated 5-year survival (60%) than patients with unresectable relapse (15%).
An earlier study by Hoffmann8 reported on 93 stage III melanoma patients who were followed prospectively in a manner similar to that described by Garbe; 60 patients relapsed. Similarly, they found that half the relapses were detected by physical examination and that radiographic imaging was less useful.
Poo-Hwu4 reported on the prospective follow-up of 63 stage III melanoma patients. Although stage III–specific results were not reported, the authors recommended physical examinations every 3 months the first year, every 4 months the second year, and then every 6 months up to 5 years along with CBC and liver function tests for stage III patients. They also recommended a chest x-ray and LDH level annually. In our study, the first site of relapse was rarely detected by chest-x-ray or blood tests.
Other investigators have reported retrospective data but have included few stage III patients and therefore do not provide guidance regarding follow-up in this patient cohort. Our data set, although retrospective, is unique in that it is the largest stage III data set to date focused on relapsing stage III patients with attention to substage, site and time of first relapse, and overall survival. These data allow us to make some tentative follow-up guidelines designed to detect first relapses.
For stage IIIA, the risk of initial relapse at a local/regional or lymph node site was ≤ 5% after 3 years from stage IIIA diagnosis, suggesting that frequent physical examinations beyond this point would be unlikely to detect many first relapses (Table 3). First systemic relapses were rarely discovered by physical examinations (Fig 1E). The risk of initial relapse at a systemic, non-brain site in this cohort of patients was ≤ 5% after 32 months from stage IIIA diagnosis. This suggests that CT scans after this time point would be unlikely to detect first relapses. In fact, prior reports have questioned the utility of screening CT scans at all in following stage IIIA patients, proposing that a physical examination would be sufficient.6,9,10
For stage IIIB patients, the risk of initial relapse at a local/regional or lymph node site dropped to ≤ 5% after 2 years from stage IIIB diagnosis, indicating that extending physical examinations beyond this time point would be unlikely to detect many first relapses. The risk of initial relapse at a systemic, non-brain site became ≤ 5% after 40 months from stage IIIB diagnosis. This suggests that scans after this time point would be unlikely to detect first relapses.
In stage IIIC patients, the risk of initial relapse at local/regional or lymph node site was ≤ 5% after 7 months from stage IIIC diagnosis, which suggests that frequent physical examinations beyond this time point would be unlikely to yield many new first relapses. Among the stage IIIC patients whose first recurrence was a systemic site, the risk became ≤ 5% by 21 months. Thus, CT scans beyond this time point would be expected to have low yield. Stage IIIC patients were the only subset that had an initial risk > 5% of first relapse being in the brain, although this risk dropped to ≤ 5% by 13 months. We noted that 36% of patients whose first relapse occurred in the brain presented with seizures. Byrne et al11 had previously reported that in 21% of melanoma patients with brain metastases, the first sign of brain metastases was seizure, but many of those patients already had known metastatic disease at the time of developing brain metastases. Given that early detection of brain metastases might spare patients from experiencing a proportion of these seizures, it would be reasonable for stage IIIC patients to have brain imaging initially.
Although our data do not address how frequently physical examinations should be done, they suggest that frequent physical examinations beyond 3 years for stage IIIA, 2 years for stage IIIB, and 1 year for stage IIIC patients are unlikely to detect a first relapse. Our data indicated that neither more intense nor more frequent follow-up is associated with discovery of resectable first relapses. A multivariate analysis failed to show an association between number of screening physical examinations and overall survival. The value of screening CT scans remains debatable, but our data show that beyond 3 years in stage IIIA and IIIB patients, and beyond 2 years in stage IIIC patients, the incidence of first relapses that are systemic is ≤ 5%, suggesting that CT scans after these time points would have a low probability of detecting first relapses. Routine brain imaging would be expected to have a low yield except perhaps in stage IIIC patients in the first year.
Another open question is whether early detection is associated with improved survival. We cannot address this directly, but our multivariate analysis showed that among stage III patients who relapsed, shorter survival was associated with systemic relapse and detection of the relapse by symptoms.
One weakness of the study is that it is a retrospective analysis. Although this allowed us to ensure long-term follow-up for each patient, there was a substantial proportion of patients for whom data were incomplete and who could not be included in this analysis. We also realize that, because our center is a melanoma referral center, our patients could represent a slightly skewed population. This report represents an initial attempt to formulate data-based follow-up guidelines for stage III patients by substage. Our guidelines should not be considered definitive but only as a first step. Future studies should pool data from several cancer centers to generate larger numbers representative of stage III melanoma patients throughout the world.
Acknowledgment
We thank Dr. Katherine S. Panageas for help with multivariate analysis and Drs. Jedd D. Wolchok, Mary S. Brady, and Richard D. Carvajal for critical comments on the manuscript.
Appendix
Fig A1.
(A) Dot plot of the average number of months between physician (MD) visits (months of progression-free survival [PFS]/number of medical oncology, surgical, or dermatologic visits in patients who had melanoma with resectable versus unresectable first relapses; P = .63, not significant). Data are calculated as total number of medical oncology, surgical, or dermatologic visits divided by total months of PFS. (B) Dot plot of the number of months from last MD visit until first relapse (P = .4, not significant). First recurrences before the next scheduled MD visit were detected by symptoms. Data were available for 72% of patients. Horizontal lines indicate median values.
Fig A2.
Time to first relapse among patients with melanoma substages IIIB and IIIC whose first relapse was in the brain.
Fig A3.
Kaplan-Meier estimates of overall survival for patients with melanoma substages IIIA, IIIB, and IIIC from time of first relapse. Tick marks represent censored patients.
Footnotes
Presented in part at the 45th Annual Meeting of the American Society of Clinical Oncology, May 30-June 3, 2009, Chicago, IL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Emanuela Romano, Paul B. Chapman
Provision of study materials or patients: Daniel G. Coit
Collection and assembly of data: Emanuela Romano, Michael Scordo, Paul B. Chapman
Data analysis and interpretation: Emanuela Romano, Michael Scordo, Stephen W. Dusza, Daniel G. Coit, Paul B. Chapman
Manuscript writing: Emanuela Romano, Paul B. Chapman
Final approval of manuscript: Emanuela Romano, Michael Scordo, Stephen W. Dusza, Daniel G. Coit, Paul B. Chapman
REFERENCES
- 1.Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–3648. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 2.Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: Validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19:3622–3634. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 3.Garbe C, Paul A, Kohler-Spath H, et al. Prospective evaluation of a follow-up schedule in cutaneous melanoma patients: Recommendations for an effective follow-up strategy. J Clin Oncol. 2003;21:520–529. doi: 10.1200/JCO.2003.01.091. [DOI] [PubMed] [Google Scholar]
- 4.Poo-Hwu WJ, Ariyan S, Lamb L, et al. Follow-up recommendations for patients with American Joint Committee on Cancer Stages I-III malignant melanoma. Cancer. 1999;86:2252–2258. [PubMed] [Google Scholar]
- 5.Francken AB, Shaw HM, Accortt NA, et al. Detection of first relapse in cutaneous melanoma patients: Implications for the formulation of evidence-based follow-up guidelines. Ann Surg Oncol. 2007;14:1924–1933. doi: 10.1245/s10434-007-9347-2. [DOI] [PubMed] [Google Scholar]
- 6.Moore Dalal K, Zhou Q, Panageas KS, et al. Methods of detection of first recurrence in patients with stage I/II primary cutaneous melanoma after sentinel lymph node biopsy. Ann Surg Oncol. 2008;15:2206–2214. doi: 10.1245/s10434-008-9985-z. [DOI] [PubMed] [Google Scholar]
- 7.Coit DG, Andtbacka R, Bichakjian CK, et al. Melanoma. J Natl Compr Canc Netw. 2009;7:250–275. doi: 10.6004/jnccn.2009.0020. [DOI] [PubMed] [Google Scholar]
- 8.Hofmann U, Szedlak M, Rittgen W, et al. Primary staging and follow-up in melanoma patients: Monocenter evaluation of methods, costs and patient survival. Br J Cancer. 2002;87:151–157. doi: 10.1038/sj.bjc.6600428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gold JS, Jaques DP, Busam KJ, et al. Yield and predictors of radiologic studies for identifying distant metastases in melanoma patients with a positive sentinel lymph node biopsy. Ann Surg Oncol. 2007;14:2133–2140. doi: 10.1245/s10434-007-9399-3. [DOI] [PubMed] [Google Scholar]
- 10.Buzaid AC, Sandler AB, Mani S, et al. Role of computed tomography in the staging of primary melanoma. J Clin Oncol. 1993;11:638–643. doi: 10.1200/JCO.1993.11.4.638. [DOI] [PubMed] [Google Scholar]
- 11.Byrne TN, Cascino TL, Posner JB. Brain metastasis from melanoma. J Neurooncol. 1983;1:313–317. doi: 10.1007/BF00165714. [DOI] [PubMed] [Google Scholar]