Abstract
Emerging evidence points to monocarboxylates as key players in the pathophysiology of temporal lobe epilepsy (TLE) with hippocampal sclerosis (mesial temporal lobe epilepsy, MTLE). Monocarboxylate transporters (MCT) 1 and 2, which are abundantly present on brain endothelial cells and perivascular astrocyte endfeet, respectively, facilitate the transport of monocarboxylates and protons across cell membranes. Recently, we reported that the density of MCT1 protein is reduced on endothelial cells and increased on astrocyte plasma membranes in the hippocampal formation in patients with MTLE and in several animal models of the disorder. Because the perivascular astrocyte endfeet comprise an important part of the neurovascular unit we now assessed the distribution of the MCT2 in hippocampal formations in TLE patients with (MTLE) or without hippocampal sclerosis (non-MTLE). Light microscopic immunohistochemistry revealed significantly less perivascular MCT2 immunoreactivity in the hippocampal formation in MTLE (n=6) than in non-MTLE (n=6) patients, and to a lesser degree in non-MTLE than in non-epilepsy patients (n=4). Immunogold electron microscopy indicated that the loss of MCT2 protein occurred on perivascular astrocyte endfeet. Interestingly, the loss of MCT2 on astrocyte endfeet in MTLE (n=3) was accompanied by an upregulation of the protein on astrocyte membranes facing synapses in the neuropil, when compared with non-MTLE (n=3). We propose that the altered distribution of MCT1 and MCT2 in TLE (especially MTLE) limits the flux of monocarboxylates across the blood brain barrier and enhances the exchange of monocarboxylates within the brain parenchyma.
Keywords: Blood-brain barrier, astrocyte endfoot, hippocampal sclerosis, ketone bodies, ketongenic diet
INTRODUCTION
Emerging evidence points to monocarboxylates, i.e., lactate, pyruvate, β-hydroxybutyrate and acetoacetate, as key players in the pathophysiology of medication-refractory TLE (Lauritzen et al. 2011; Lauritzen et al. 2012). Monocarboxylates use a specific transport mechanism provided by proton-linked monocarboxylate transporters (MCTs) to cross cell membranes (Halestrap and Meredith 2004). Recently, we discovered that MCT1 protein is less densely expressed on the endothelial cell membranes of microvessels from surgically resected hippocampal formations from patients with medication-refractory TLE (Lauritzen et al. 2011). Similar findings were also evident in several animal models of TLE (Lauritzen et al. 2012). We proposed that the loss of MCT1 on endothelial cells is implicated in the increased seizure susceptibility of medication-refractory TLE by limiting the transport of ketone bodies or other monocarboxylates across the blood-brain barrier (Gjedde and Crone 1975). This hypothesis presumes that other MCT isoforms do not compensate for the altered expression of MCT1.
At least two additional isoforms are present in the brain: MCT2 and MCT4 (Halestrap and Meredith 2004). MCT2 has a higher affinity for monocarboxylates as well as a different cellular expression than MCT1. There are discrepant reports in the literature on the distribution of MCT2; however, most studies in rodents indicate that the protein is expressed along the post-synaptic density of excitatory synapses and on perivascular astrocyte endfeet (Baud et al. 2003; Bergersen et al. 2001; Bergersen et al. 2005; Gerhart et al. 1998; Hanu et al. 2000; Rafiki et al. 2003). Whether MCT2 is expressed in significant quantities in the human brain remains controversial (Chiry et al. 2008; Price et al. 1998).
The aim of this study was twofold: First, to assess whether MCT2 is expressed in significant quantities in the human hippocampal formation. Second, to evaluate possible changes in the distribution of MCT2 in the hippocampal formation in patients with two types of medication-refractory TLE vs. in non-epilepsy control subjects.
MATERIALS AND METHODS
Human subjects and tissue preparation
Patients with medication-refractory TLE underwent phased presurgical evaluation at the Yale-New Haven Hospital, and those elected for surgery had their hippocampus resected according to standard procedures (Spencer and Spencer 1991). Informed consent from each patient and institutional approval were obtained for the surgery and for the use of tissue for this study. Randomly selected hippocampal formations from 23 TLE patients were included in the study (Table 1). Six hippocampal formations obtained at autopsy from non-epilepsy subjects were included as additional comparison controls. The TLE patients were classified into two categories: Patients with TLE and concomitant hippocampal sclerosis (referred to as mesial temporal lobe epilepsy, MTLE, n=12) and patients with other types of TLE, i.e., non-MTLE (n=11). For complete characterizations of the two TLE-groups, please refer to Eid and colleagues (Eid et al. 2004). Tissue preparation was done as previously described (Lauritzen et al. 2011). The category to which each sample belonged was concealed from the investigators during stereological and quantitative immunogold analysis.
Table 1. Characteristics of patients selected for the study.
| Patient and classificatio n |
Sex | Age (years) |
Time since first unprovoked seizure (years) |
Antiepileptic drugs at surgery |
MRI findings | Pathology | Immunohistochemical labeling |
|---|---|---|---|---|---|---|---|
|
Non-MTLE 1 |
F | 18 | 3 | Valproate, lamotrigine | Open lip schizencephaly, R hemisphere | Marked gliosis in cortex and white matter |
LM: β-dystroglycan |
| 2 | F | 28 | 21 | Lamotrigine, zonisamide, phenytoin |
Nonspecific, focal T2 signal hyperintensities in subcortical white matter, R frontal lobe |
Mild to moderate neuronal loss with severe reactive gliosis. Abnormal lamination and clustering of neurons. |
LM: MCT2, β-dystroglycan |
| 3 | M | 4 | 2 | Oxcarbazepine | Abnormal signal of R amygdala, hippocampal head and parahippocampal gyrus with cyst near R hippocampal head |
Oligodendroglioma | LM:β-dystroglycan |
| 4 | F | 49 | 13 | Carbamazepine XR, phenytoin, lamotrigine |
Large mass in R frontal and R temporal lobes |
Oligodendroglioma and ganglioneurocytoma |
LM: MCT2, β-dystroglycan |
| 5 | F | 38 | <1 | Levetiracetam | Nonenhancing R hippocampal lesion | Glioma | LM: MCT2, β-dystroglycan |
| 6 | M | 13 | 9 | Phenytoin, levetiracetam, carbamazepine |
Large porencephalic cavity in the R MCA distribution with surrounding gliosis. Compatible with remote R MCA infarct. |
Frequent heterotopic neurons in molecular layer of cerebral cortex |
LM: MCT2 |
| 7 | M | 6 | Levetiracetam, lamotrigine | Abnormal gray matter with local mass- effect involving L frontal cortex. Possible localized hemimegalencephaly and cortical dysplasia. |
Cortical dysplasia | LM: MCT2, β-dystroglycan | |
| 8 | M | 69 | 7 | Phenytoin | Neoplastic lesion, R temporal lobe | Oligodendroglioma | LM: MCT2 |
| 9 | F | 10 | 5 | Lamotrigine | R temporal tumor | Low-grade astrocytoma | EM: MCT2 |
| 10 | M | 28 | 26 | Carbamazepine, acetazolamide |
Ectopic gray matter adjacent to L anterior horn. High-signal by FLAIR in R posterior temporal lobe with corresponding low T1 signal. Possible bilateral hippocampal atrophy. |
Heterotopic neurons in the molecular layer of the dentate gyrus. Subpial glial cell proliferation. |
EM: MCT2 |
| 11 | M | 44 | 26 | Carbamazepine, clonazepam |
Possible R mesial temporal sclerosis | Hippocampus w/mild, diffuse neuronal loss and mild to moderate increase in number of white matter glial cells. |
EM: MCT2 |
|
MTLE 12 |
F | 17 | 5 | Oxcarbazepine, lamotrigine, carbamazepine |
Heterotopic gray matter in posterior R temporal lobe white matter with extension to the atrium and body of the right lateral ventricle. |
Hippocampal sclerosis | LM: MCT2 |
| 13 | M | 57 | 39 | Phenytoin, carbamazepine, topiramate, levetiracetam |
Mesial temporal sclerosis | Hippocampal sclerosis | LM: β-dystroglycan |
| 14 | F | 51 | 48 | Levetiracetam, pregabalin | R mesial temporal sclerosis | Hippocampal sclerosis | LM: MCT2, β-dystroglycan |
| 15 | F | 40 | 28 | Felbamate, topiramate, gabapentin, carbamazepine |
L hippocampal sclerosis | Hippocampal sclerosis | LM: MCT2 |
| 16 | F | 42 | 7 | Levetiracetam, oxcarbazepine |
R hippocampal sclerosis | Hippocampal sclerosis | LM: MCT2, β-dystroglycan |
| 17 | F | 40 | 37 | Carbamazepine, levetiracetam, zonisamide |
L hippocampal sclerosis | Hippocampal sclerosis | LM: β-dystroglycan |
| 18 | M | 62 | 56 | Valproate, levetiracetam, phenytoin |
L mesial temporal sclerosis | Hippocampal sclerosis | LM: MCT2 |
| 19 | F | 40 | 27 | Carbamazepine, levetiracetam |
Increased T2 signal, L hippocampus | Hippocampal sclerosis | LM: MCT2, β-dystroglycan |
| 20 | F | 30 | 22 | Zonisamide, carbamazepine XR |
R mesial temporal sclerosis | Hippocampal sclerosis | LM: β-dystroglycan |
| 21 | F | 51 | 11 | Carbamazepine | L mesial temporal sclerosis | Hippocampal sclerosis | EM: MCT2 |
| 22 | F | 37 | 11 | Primidone, lamotrigine | R mesial temporal sclerosis | Hippocampal sclerosis | EM: MCT2 |
| 23 | F | 15 | 5 | Carbamazepine XR, lamotrigine |
L mesial temporal sclerosis | Hippocampal sclerosis | EM: MCT2 |
| Patient and classification | Sex | Age (years) | Cause of death | Immunohistochemical labeling |
|---|---|---|---|---|
| Autopsy 1 | M | 33 | Adenocarcinoma of lung with widespread metastases | LM: MCT2, β-dystroglycan |
| 2 | M | 52 | Myocardial infarct, coronary atherosclerosis | LM: MCT2, β-dystroglycan |
| 3 | F | 49 | Ruptured berry aneurysm, R posterior inferior cerebellar artery | LM: MCT2 |
| 4 | F | 76 | Peritonitis | LM: MCT2 |
| 5 | M | 61 | Myocardial infarct, cardiovascular atherosclerosis | LM: β-dystroglycan |
| 6 | F | 62 | Dementia, mixed type | LM: β-dystroglycan |
Abbreviations: L, left; MCA, middle cerebral artery; R, right; XR, extended release
Light microscopic immunohistochemistry and stereological point count analysis
Fifty μm thick Vibratome sections were incubated free floating in solutions of β-dystroglycan (B-DG-CE, Novocastra; 0.1 μg/ml) or MCT2 (AB1287, Millipore, Billerica, MA; diluted 1:100) antibodies for 72 h at 4 °C and processed according to the avidin biotin peroxidase method (Hsu et al. 1981) using the Vectastain Elite Kit (Vector Laboratories, Burlingame, CA). The immunostained sections were mounted on gelatin coated glass slides and examined in a light microscope. To quantify the density of β-dystroglycan and MCT2-positive structures on hippocampal formations, stereological point count analysis (Gundersen et al. 1988) was performed as follows: A test system with a set of regularly spaced points was created on a transparent A4-sized foil and superimposed on printed A4-sized pictures of β-dystroglycan or MCT2 labeled hippocampal sections. The areal density of positive profiles was estimated by counting points hitting β-dystroglycan- or MCT-labeled structures, divided by points hitting the hippocampal formation. Densities were expressed as positive counts per 100 counts.
Electron microscopic immunocytochemistry
The postembedding immunogold method was done as described by Bergersen and colleagues (Bergersen et al. 2008). Small tissue blocks (0.3 × 0.5 × 1 mm3) of area CA1 were dissected from 500-μm thick Vibratome sections and subjected to freeze substitution. Single immunogold labeling for MCT2 was performed on ultrathin sections from 3 non-MTLE and 3 MTLE patients using a rabbit anti-MCT2 primary antibody (kindly provided by A. Halestrap, University of Bristol, UK, (Jackson et al. 1997), 1:100) and a colloidal gold-conjugated secondary antibody (goat F (ab) anti-rabbit 10 nm, BB International, Cardiff; 1:20). Images were taken with a FEI Technai 12 transmission electron microscope (Hillsboro, OR). The specificity of the primary and secondary antibodies was tested by preabsorbtion with the peptide used for immunization and by omitting the primary antibody from the immunogold protocol, respectively, both of which prevented labeling of tissue components (Bergersen et al. 2008).
Immunogold quantification
The MCT2-gold particle (gp) densities were determined on astrocyte membranes facing microvessels, i.e. endfeet specializations, and on plasma membranes of astrocyte processes in the neuropil. Morphological criteria according to Peters and colleagues were used to identify the profile types (Peters et al. 1991). The MCT2 labeling densities were quantified using the following procedures: i) Astrocyte endfeet. Ten random microvessels were examined from each patient. Since astrocyte endfeet form an essentially continuous cover around the microvessels in the brain (Mathiisen et al. 2010), all membranes facing the basal lamina were included in the analysis. The length of each membrane profile was measured using Image J (National Institutes of Health, Bethesda, MD), and associated gp recorded, including gp with their centers located on the membrane itself or within 30 nm on the intracellular side. As about 90 % of the gp that represent a membrane protein are found within 40 nm of the midline of the membrane profile (Chaudhry et al. 1995), the 30 nm distance includes most of the relevant gp, at the same time minimizing the contribution of any immunoreactivity elsewhere than in the membrane; gp within 30 nm on the extracellular side were excluded to minimize the contribution of gp representing antigenic sites in neighboring cells. The areal densities of membrane-associated gp were calculated by dividing the number of gp by the “membrane area” (membrane length in nm × 30 nm). Densities were expressed as gp/μm2. ii) Plasma membranes of astrocyte processes in the neuropil. Fifteen random astrocyte processes localized in the neuropil were examined for each patient. Gold particle densities were calculated and expressed as described for i).
Statistical analysis
A one-way ANOVA followed by a Tukey HSD post-hoc test were used for multiple comparisons (point count analysis of β-dystroglycan and MCT2 in non-epilepsy controls, non-MTLE and MTLE). A student’s T-test was used for comparison of immunogold labeling of MCT2 between non-MTLE and MTLE patients. A p value of < 0.05 was considered a statistically significant difference. Unless otherwise stated, the data are presented as mean ± SD.
RESULTS
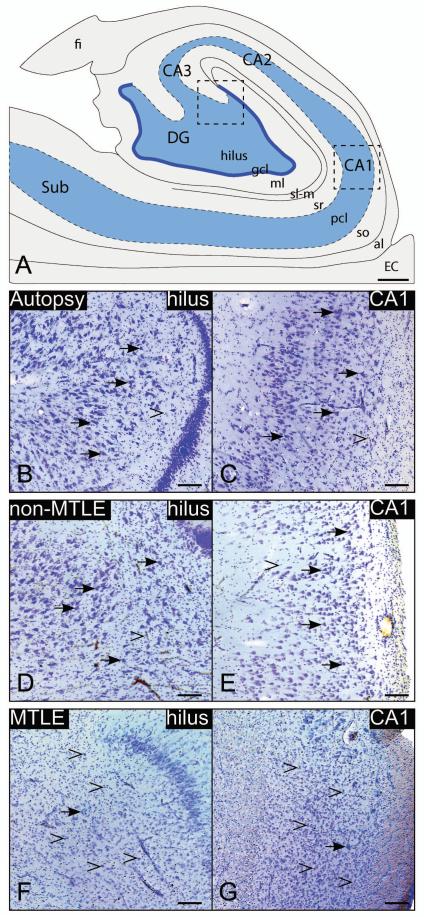
Consistent with earlier reports (Eid et al. 2004; Lauritzen et al. 2011), the histopathology of hippocampal formations from autopsy controls (n=6) (Figs. 1B, C) and non-MTLE patients (n=11) (Figs. 1D, E) were impossible to distinguish visually, and did not exhibit significant neuronal loss or reactive gliosis. However, patterned neuronal cell loss and proliferation of glial cells were prominent in sections from MTLE patients (n=12), particularly in the dentate hilus and CA1 (Fig. 1F, G respectively).
Fig. 1. Nissl-stained coronal sections from representative cases.
High-power images from the dentate hilus (B, D, F) and CA1 (C, E, G) of the hippocampal formation (A) are presented. Hippocampal formations from autopsy and non-MTLE subjects are characterized by abundant neurons (arrows) in the hilus (B, D) and CA1 (C, E). In contrast, there is marked neuronal loss in the hilus (F) and CA (G) in the hippocampal formation in MTLE subjects. The loss of neurons in these areas is accompanied by reactive gliosis (open arrowheads in F, G). Little or no reactive gliosis is present in autopsy (B, C) or non-MTLE (D, E). Abbreviations: al, alveus; EC, entorhinal cortex; CA1-3, cornu ammonis 1-3 of the hippocampus; DG, dentate gyrus; fi, fimbria; gcl, granular cell layer; ml, molecular layer of the DG; sl-m, stratum lacunosum-moleculare; so, stratum oriens; pcl, stratum pyramidale; sr, stratum radiatum; SUB, subiculum. Bars: A, 1 mm: B-G, 200 um.
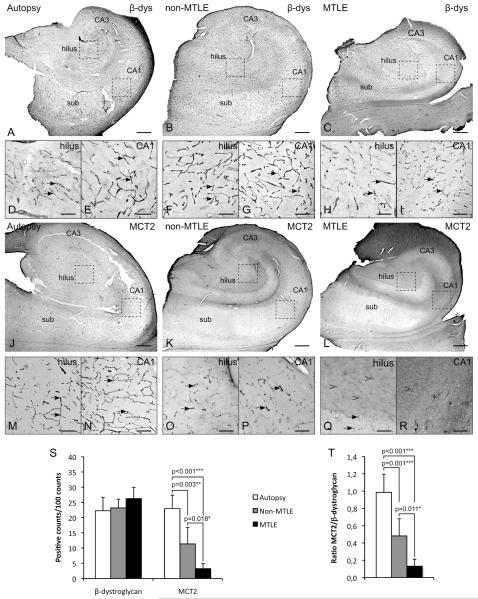
We next used immunohistochemistry and quantitative stereological analysis to compare the expression of MCT2 to that of β-dystroglycan in hippocampal formations from autopsy (n=4 and 4 respectively), non-MTLE (n=6 and 6) and MTLE patients (n=6 and 6) (Fig. 2). β-dystroglycan, a member of the dystrophin associated protein complex has been hypothesized to play a key role in the integrity of the blood brain barrier (Zaccaria et al. 2001) and was used is a marker for perivascular endfeet (Tian et al. 1996). As indicated also by Heuser and colleagues (Loss of perivascular Kir4.1 potassium channels in the sclerotic hippocampus of patients with mesial temporal lobe epilepsy. Unpublished manuscript), there was no difference in the density of β-dystroglycan labeled microvessels between the patient groups (p = 0.159) (Fig. 2A-I, S). In contrast, such homogeneity among groups was not present on sections labeled for MCT2 (Fig. 2J-R). The labeling pattern for MCT2 in non-epilepsy control (Fig. 2J, M, N) was similar to that for β-dystroglycan (Fig. 2A-I), indicating that MCT2 and β-dystroglycan are expressed on the same microvessels. However, in TLE patients (Fig. 2K-R) the MCT2 labeling along microvessels was reduced compared with the labeling of β-dystroglycan. Densities of MCT2 labeled structures were reduced by 52 % in non-MTLE (11±5 positive counts per 100 counts, p=0.003), and by 87 % in MTLE (3±2, p<0.001) compared to non-epilepsy autopsy specimens (23±5). A 73 % reduction in MCT2-density was found in MTLE vs. non-MTLE (p=0.018). The greatest loss of labeling was seen in areas of MTLE exhibiting neuronal loss and reactive gliosis, such as the dentate hilus (Fig. 2Q) and CA1 (Fig. 2R). In those areas, the microvessel associated labeling of MCT2 was replaced by a diffuse, granular labeling of MCT2 in the neuropil (Fig. 2Q-R). The ratios between MCT2 and β-dystroglycan labeling densities were 1.0±0.2, 0.5±0.2 and 0.1±0.1 for autopsy, non-MTLE and MTLE respectively, showing that essentially all microvessels expressed MCT2 protein in non-epilepsy hippocampal formations (ratio = 1), whereas many microvessels expressed β-dystroglycan but not MCT2 in non-MTLE vs. autopsy (P=0.001) and in MTLE vs. autopsy (p<0.001) (Fig. 2T). We discovered a smaller but significant reduction in the MCT2/β-dystroglycan ratio in MTLE vs. non-MTLE (p=0.011), consistent with the hypothesis of significantly fewer MCT2-positive microvessels in the MTLE than in the non-MTLE hippocampal formation.
Fig. 2. Immunohistochemistry quantitation of microvessels labeled with β-dystroglycan and monocarboxylate transporter 2 (MCT2).
(A-I) β-dystroglycan is abundantly expressed on microvessels (arrows) throughout the hippocampal formation in autopsy (A, D, E), non-MTLE (B, F, G) and MTLE (C, H, I) subjects. There is no difference in the density of β-dystroglycan positive microvessels in the hippocampal formation among autopsy, non-MTLE or MTLE subjects (p = 0.159) (S). The diagrams depict mean±SD. (J-R) Microvessels are densely labeled with MCT2 in autopsy hippocampal formations (J, M, N). The labeling pattern and density are similar to that of β-dystroglycan (S). However, there is a significant loss of MCT2-positive microvessels in non-MTLE (K, O, P) vs. autopsy (J, M, N, S) and in MTLE (L, Q, R) vs. non-MTLE and autopsy (S). The reduction of MCT2-positive microvessels is particularly evident in areas with considerable neuronal loss and reactive gliosis, such as the dentate hilus (Q) and CA1 (R). Here, the transporter is completely lost (asterisk) or strongly reduced (open arrowhead), compared to similar regions in autopsy (M, N) and non-MTLE (O, P). The ratios of the labeling densities in MCT2 vs. β-dystroglycan confirm that the loss of MCT2 on astrocyte endfeet is not due to a loss of β-dystroglycan associated microvessels. Bars: Large images (A-C, J-L), 1 mm; insets (D-I, M-R), 200 μm.
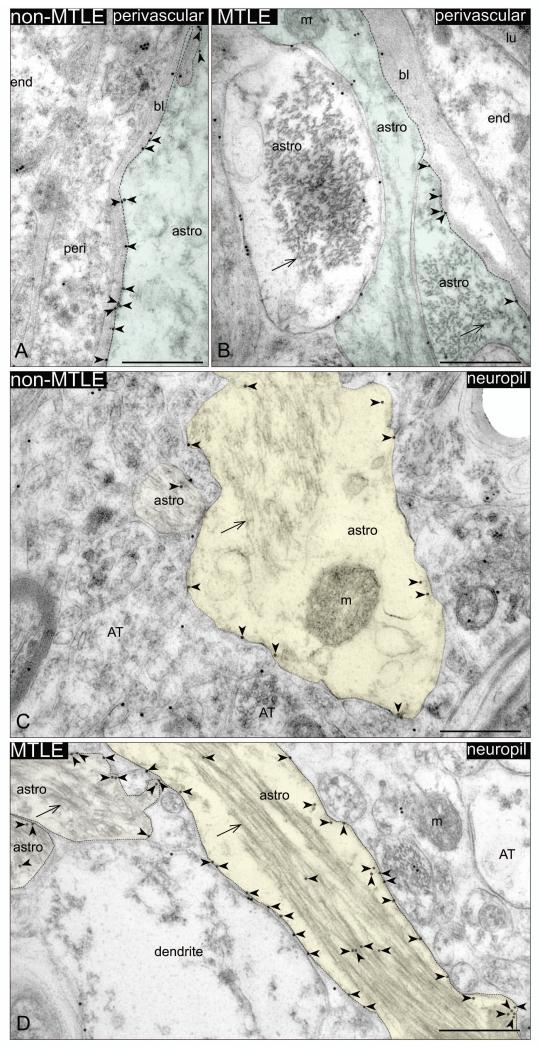
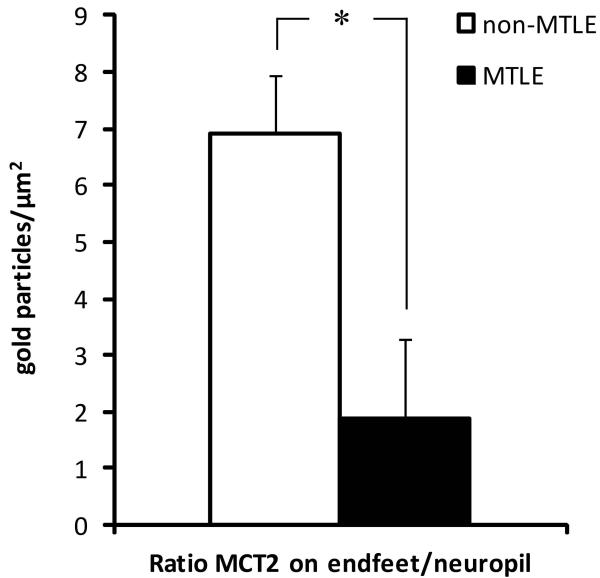
Immunogold electron microscopy revealed that MCT2 is primarily present on the plasma membranes of perivascular astrocyte endfeet and of astrocyte processes in the neuropil, in non-MTLE as well as in MTLE hippocampal formations (Fig. 3). Unlike MCT1 (Lauritzen et al. 2011), MCT2 was not detected on the endothelium of microvessels. Due to the poor ultrastructural quality of the autopsy tissue, the electron microscopic analysis was limited to non-MTLE and MTLE brains, which were chemically fixed immediately after surgical resection. In non-MTLE hippocampal formations, dense MCT2-labeling was present along membranes of astrocyte endfeet (126±44 gp/um2) (Fig. 3A), whereas scant labeling was present on astrocyte plasma membranes in the neuropil (8±5 gp/um2) (Fig. 3B). A reverse pattern was found in MTLE, in which there was a substantial decrease in labeling on astrocyte endfeet compared to non-MTLE (73±26 gp/um2) (Fig. 3C). In addition, MCT2 was strongly upregulated on the plasma membrane of astrocytes in the neuropil in MTLE, (49±24) (Fig. 3D). As expected, the labeling ratio of MCT2 on astrocyte perivascular endfeet vs. astrocyte processes in the neuropil was significantly lower in MTLE compared to in non-MTLE (1.9±1.2 vs. 6.9±1.4, p=0.01) (Fig. 4).
Fig. 3. Immunogold electron microscopy of monocarboxylate transporter 2 (MCT2) on perivascular astrocyte endfeet membranes (green) and on astrocyte plasma membranes in the neuropil (yellow).
Dense labeling of membrane associated MCT2 (red arrowheads) is present along the perivascular astrocyte endfoot facing the basal lamina (stippled line) in non-MTLE (A), whereas labeling of the same compartment is significantly reduced in MTLE (C). MCT2 labeling is also found on astrocytes plasma membranes in the neuropil, and to a lesser extent on organelles in the cytoplasm of these cells (B, D). Compared to non-MTLE (B), the density of MCT2 on plasma membranes of astrocytes in the neuropil is significantly upregulated in MTLE (stippled line in D). Abbreviations: astro, astrocytes; AT, axon terminal; bl, basal lamina; end, endothelial cell; lu, lumen; m, mitochondria. Arrow, bundle of intermediate filaments. Bars: 500 nm.
Fig. 4. Quantitation of immunogold labeling for monocarboxylate transporter 2 (MCT2) on astrocytes in CA1 of the hippocampal formation.
The redistribution of MCT2 in MTLE resulted in a reduced ratio of MCT2 labeling on perivascular endfeet and plasma membrane of astrocytes in the neuropil in these subjects compared to in non-MTLE (p=0.01).
DISCUSSION
This is the first study of the cellular and ultrastructural distribution of MCT2 protein in the hippocampal formation in patients with medically intractable TLE. MCT2 protein was abundantly expressed in the hippocampal formation in all subject categories. Patients with TLE were characterized by a loss of MCT2 on perivascular astrocyte endfeet membranes, and an upregulation of the protein on astrocyte plasma membranes in the neuropil. The redistribution of MCT2 was most prominent in patients with TLE and concomitant hippocampal sclerosis (i.e., MTLE).
There is increasing evidence to suggest that astrocytes contribute to the development of neurological disorders such as epilepsy (de Lanerolle et al. 2010; Harris 1975; Pollen and Trachtenberg 1970). Notably, the sclerotic hippocampal formation in MTLE is characterized by expanded populations of phenotypically and functionally altered (often termed “reactive”) astrocytes. Reactive astrocytosis has been proposed to facilitate the development of epileptic seizures via a variety of mechanisms (de Lanerolle et al. 2010). Our observations that the distribution of MCT2 protein are altered on astrocytes in the human epileptogenic hippocampal formation further define the concept and possible consequences of reactive astrocytosis in TLE. The potential significance of the aberrant expression of MCT2 on reactive astrocytes will be discussed below.
MCT2 deficiency on perivascular endfeet
As the astrocyte endfeet cover most of the circumference of the blood vessels (Mathiisen et al. 2010), the majority of the monocarboxylate flux into and out of the brain is facilitated by astrocytes. The loss of MCT2 on perivascular endfeet in the human epileptogenic hippocampal formation suggests that both influx and the efflux of monocarboxylates to the brain, are perturbed. An adequate exchange of monocarboxylates across the blood brain barrier appears to be required for normal brain function (Ivanov and Zilberter 2011; Suzuki et al. 2011) and a deficiency of monocarboxylate fuels, especially ketone bodies, may promote hyperexcitability (Holmgren et al. 2010). Ketone bodies are metabolized in the normal human brain, even during euglycemic, non-ketonemic conditions (Pan et al. 2002). It is plausible that ketone bodies, even in small quantities, are necessary for normal brain activity, such as excitatory and inhibitory neurotransmission. Notably, Juge and colleagues recently discovered that acetoacetate modulates vesicular glutamate release and suppresses seizures evoked with 4-aminopyridine (Juge et al. 2010). Furthermore, ketone bodies enhance the brain energy stores (Pan et al. 1999), increase the number of mitochondria and render hippocampal synapses more resilient to synaptic depression following mild hypoglycemia (Bough et al. 2006). Ketone bodies also enhance GABA-mediated inhibition (Williamson et al. 2005), thus resulting in more sustained GABA-mediated inhibition (Cantello et al. 2007).
The transport of monocarboxylates between cells is bidirectional and dependent on the concentration gradient of these molecules and of protons across the plasma membrane. At rest, the brain exports small amounts of lactate, but imports lactate when plasma levels rise above brain levels, such as during physical exercise (van Hall et al. 2009). The loss of MCT1 and MCT2 associated with microvessels in TLE suggests that the clearance of brain lactate by the blood, as well as import of lactate from the blood to the brain, may be impaired in this condition. Microdialysis studies have indeed demonstrated that extracellular lactate is chronically elevated in the epileptogenic hippocampal formation in patients with TLE, hours to days away from a seizure episode (Cavus et al. 2005). Moreover, extracellular brain lactate increases above the basal levels during a seizure episode in similar patient populations (During et al. 1994). Impaired export of lactate and protons from the brain to the blood when the rate glycolysis is higher than the oxidative metabolism of glucose could acidify the astrocyte cytoplasm, slow the rate of glycolysis through inhibition of phosphofructokinase, and thus inhibit the synthesis of ATP (Leite et al. 2011). A deficiency in ATP in astrocytes is likely to impair the uptake of extracellular glutamate (Danbolt 2001). Numerous studies in laboratory animals have demonstrated that an excess of extracellular brain glutamate can cause seizures and neuronal death (Olney et al. 1986). Notably, increased extracellular glutamate concentrations are present in the epileptogenic hippocampal formation in patients with TLE, even several hours away from a seizure, and the extracellular glutamate levels are particularly high when hippocampal sclerosis is present (Cavus et al. 2005; During and Spencer 1993). Thus, it is possible that the accumulation of lactate in the brain in TLE indicates a slowed synthesis of ATP by astrocytes with an impaired uptake of extracellular glutamate by these cells.
Another possibility is that the downregulation of MCT2 on perivascular endfeet is adaptive changes that prevent loss of locally produced lactate to the blood, thereby increasing the seizure threshold via one of several possible mechanisms. Firstly, the lactate metabolite, pyruvate, inhibits vesicular glutamate uptake with an affinity comparable to that of acetoacetate (Juge et al. 2010, supplemental information). Secondly, lactate interacts with the G-protein coupled receptor, GPR81 (HCA1), to lower cAMP (Offermanns et al. 2011). As β-adrenergic receptors elevate cAMP levels, elevated lactate would therefore be expected to mimic the known anticonvulsive effect of β-adrenoceptor blockers (De Sarro et al. 2002; Luchowska et al. 2002). An analogous mechanism may contribute to the antiepileptic effect of ketone bodies through the HCA2 receptor, which binds β-hydroxybutyrate (Offermanns et al. 2011). Finally, increased levels of extracellular lactate may also have an inhibitory effect on neuronal excitability due to acidification of the extracellular compartment at the site of seizure activity (During et al. 1994), which can inhibit receptor activity, including NMDA receptors.
Increased MCT2 on astrocyte processes in neuropil
The upregulation of MCT2 on astrocyte profiles in the neuropil in the hippocampal formation in TLE may represent a compensatory response to the increased extracellular lactate in epileptogenic brain areas. Such upregulation could facilitate the influx of lactate from areas of high concentrations in the extracellular space to the astrocyte compartment. Once taken up by the astrocyte compartment, lactate may diffuse throughout the astrocyte syncytium to areas of low lactate concentrations (Dienel and Cruz 2003; Rouach et al. 2008). Because MCT2 is rapidly saturated, an upregulation of the transporter on astrocyte membranes in the neuropil might be a response to the increased lactate buffering demands.
Astrocytes play an important role in the brain energy metabolism. According to astrocyte-neuron lactate shuttle hypothesis, glutamate released from nerve terminals stimulates metabolism of glucose in astrocytes via activation of Na+/K+-ATPases on astrocyte membranes (Pellerin and Magistretti 1994). Astroctyes then release lactate metabolized from glucose to the extracellular space which can be taken up an oxidized by neurons. The increased levels of MCT2 on astrocyte membranes in the neuropil might be a compensatory mechanism to buffer the increased levels of lactate and protons that accumulates in the astrocyte cytosol following a high glycolytic rate mediated by seizing neurons.
MCT2 expression and species differences
Different cellular localizations of MCT2 have been reported in different species. In the rodent brain, MCT2 protein has been detected on neurons (Debernardi et al. 2003; Pierre et al. 2002; Pierre et al. 2000), more specifically on post-synaptic densities (Bergersen et al. 2001; Bergersen et al. 2005), and on perivascular astrocyte endfeet (Gerhart et al. 1998; Hanu et al. 2000). Little is known about the MCT2 expression in the human brain. Price and colleagues (1998) detected only small amounts of MCT2 mRNA levels in human brain tissues. In contrast, Chiry and colleagues (Chiry et al. 2008) reported a strong and widespread expression of MCT2 mainly on neurons but also on astrocytes in the cerebral cortex from non-epilepsy controls. We were unable to detect MCT2 on neurons in CA1 of the hippocampal formation in TLE patients. The discrepancies between studies may be related to species differences and to methodological issues and the use of antibodies from different sources.
ACKNOWLEDGMENTS
We thank Ms. Ilona Kovacs, Ms. Bjørg Riber and Ms. Karen Marie Gujord for excellent technical assistance; Dr. Max Larsson for developing the software used to quantify the immunogold labeling; Ms. Jennifer Bonito for managing the epilepsy patient database and Drs. Jullie Pan and Jon Storm-Mathisen for helpful discussions. This work was supported by grants from the Medical Faculty of the University of Oslo (to F.L.), the Research Council of Norway (to F.L. and L.H.B.), the National Institutes of Health (NS058674 and NS070824 to T.E.), and the Swebelius Trust (to D.D.S.). Part of this work was also made possible by CTSA Grant Number UL1 RR024139 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. The contents of the publication are solely the responsibility of the authors and do not necessarily represent the official view of NCATS or NIH.
REFERENCES
- Baud O, Fayol L, Gressens P, Pellerin L, Magistretti P, Evrard P, Verney C. Perinatal and early postnatal changes in the expression of monocarboxylate transporters MCT1 and MCT2 in the rat forebrain. Journal of Comparative Neurology. 2003;465(3):445–54. doi: 10.1002/cne.10853. [DOI] [PubMed] [Google Scholar]
- Bergersen L, Waerhaug O, Helm J, Thomas M, Laake P, Davies AJ, Wilson MC, Halestrap AP, Ottersen OP. A novel postsynaptic density protein: the monocarboxylate transporter MCT2 is co-localized with delta-glutamate receptors in postsynaptic densities of parallel fiber-Purkinje cell synapses. Experimental Brain Research. 2001;136(4):523–34. doi: 10.1007/s002210000600. [DOI] [PubMed] [Google Scholar]
- Bergersen LH, Magistretti PJ, Pellerin L. Selective postsynaptic co-localization of MCT2 with AMPA receptor GluR2/3 subunits at excitatory synapses exhibiting AMPA receptor trafficking. Cerebral Cortex. 2005;15(4):361–70. doi: 10.1093/cercor/bhh138. [DOI] [PubMed] [Google Scholar]
- Bergersen LH, Storm-Mathisen J, Gundersen V. Immunogold quantification of amino acids and proteins in complex subcellular compartments. Nat Protoc. 2008;3(1):144–52. doi: 10.1038/nprot.2007.525. [DOI] [PubMed] [Google Scholar]
- Bough KJ, Wetherington J, Hassel B, Pare JF, Gawryluk JW, Greene JG, Shaw R, Smith Y, Geiger JD, Dingledine RJ. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Annals of Neurology. 2006;60(2):223–35. doi: 10.1002/ana.20899. [DOI] [PubMed] [Google Scholar]
- Cantello R, Varrasi C, Tarletti R, Cecchin M, D’Andrea F, Veggiotti P, Bellomo G, Monaco F. Ketogenic diet: electrophysiological effects on the normal human cortex. Epilepsia. 2007;48(9):1756–63. doi: 10.1111/j.1528-1167.2007.01156.x. [DOI] [PubMed] [Google Scholar]
- Cavus I, Kasoff WS, Cassaday MP, Jacob R, Gueorguieva R, Sherwin RS, Krystal JH, Spencer DD, Abi-Saab WM. Extracellular metabolites in the cortex and hippocampus of epileptic patients. Annals of Neurology. 2005;57(2):226–35. doi: 10.1002/ana.20380. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Lehre KP, van Lookeren Campagne M, Ottersen OP, Danbolt NC, Storm-Mathisen J. Glutamate transporters in glial plasma membranes: highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron. 1995;15(3):711–20. doi: 10.1016/0896-6273(95)90158-2. [DOI] [PubMed] [Google Scholar]
- Chiry O, Fishbein WN, Merezhinskaya N, Clarke S, Galuske R, Magistretti PJ, Pellerin L. Distribution of the monocarboxylate transporter MCT2 in human cerebral cortex: an immunohistochemical study. Brain Research. 2008;1226:61–9. doi: 10.1016/j.brainres.2008.06.025. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Progress in Neurobiology. 2001;65(1):1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- de Lanerolle NC, Lee TS, Spencer DD. Astrocytes and epilepsy. Neurotherapeutics. 2010;7(4):424–38. doi: 10.1016/j.nurt.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sarro G, Di Paola ED, Ferreri G, De Sarro A, Fischer W. Influence of some beta-adrenoceptor antagonists on the anticonvulsant potency of antiepileptic drugs against audiogenic seizures in DBA/2 mice. European Journal of Pharmacology. 2002;442(3):205–13. doi: 10.1016/s0014-2999(02)01536-4. [DOI] [PubMed] [Google Scholar]
- Debernardi R, Pierre K, Lengacher S, Magistretti PJ, Pellerin L. Cell-specific expression pattern of monocarboxylate transporters in astrocytes and neurons observed in different mouse brain cortical cell cultures. Journal of Neuroscience Research. 2003;73(2):141–55. doi: 10.1002/jnr.10660. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF. Neighborly interactions of metabolically-activated astrocytes in vivo. Neurochemistry International. 2003;43(4-5):339–54. doi: 10.1016/s0197-0186(03)00021-4. [DOI] [PubMed] [Google Scholar]
- During MJ, Fried I, Leone P, Katz A, Spencer DD. Direct measurement of extracellular lactate in the human hippocampus during spontaneous seizures. Journal of Neurochemistry. 1994;62(6):2356–61. doi: 10.1046/j.1471-4159.1994.62062356.x. [DOI] [PubMed] [Google Scholar]
- During MJ, Spencer DD. Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet. 1993;341(8861):1607–10. doi: 10.1016/0140-6736(93)90754-5. [DOI] [PubMed] [Google Scholar]
- Eid T, Thomas MJ, Spencer DD, Runden-Pran E, Lai JC, Malthankar GV, Kim JH, Danbolt NC, Ottersen OP, de Lanerolle NC. Loss of glutamine synthetase in the human epileptogenic hippocampus: possible mechanism for raised extracellular glutamate in mesial temporal lobe epilepsy. Lancet. 2004;363(9402):28–37. doi: 10.1016/s0140-6736(03)15166-5. [DOI] [PubMed] [Google Scholar]
- Gerhart DZ, IEnerson BE, Zhdankina OY, Leino RL, Drewes LR. Expression of the monocarboxylate transporter MCT2 by rat brain glia. Glia. 1998;22(3):272–81. [PubMed] [Google Scholar]
- Gjedde A, Crone C. Induction processes in blood-brain transfer of ketone bodies during starvation. American Journal of Physiology. 1975;229(5):1165–9. doi: 10.1152/ajplegacy.1975.229.5.1165. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988;96(5):379–94. doi: 10.1111/j.1699-0463.1988.tb05320.x. others. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Meredith D. The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Archiv European Journal of Physiology. 2004;447(5):619–28. doi: 10.1007/s00424-003-1067-2. [DOI] [PubMed] [Google Scholar]
- Hanu R, McKenna M, O’Neill A, Resneck WG, Bloch RJ. Monocarboxylic acid transporters, MCT1 and MCT2, in cortical astrocytes in vitro and in vivo. Am J Physiol Cell Physiol. 2000;278(5):C921–30. doi: 10.1152/ajpcell.2000.278.5.C921. [DOI] [PubMed] [Google Scholar]
- Harris AB. Cortical neuroglia in experimental epilepsy. Experimental Neurology. 1975;49(3):691–715. doi: 10.1016/0014-4886(75)90052-7. [DOI] [PubMed] [Google Scholar]
- Holmgren CD, Mukhtarov M, Malkov AE, Popova IY, Bregestovski P, Zilberter Y. Energy substrate availability as a determinant of neuronal resting potential, GABA signaling and spontaneous network activity in the neonatal cortex in vitro. Journal of Neurochemistry. 2010;112(4):900–12. doi: 10.1111/j.1471-4159.2009.06506.x. [DOI] [PubMed] [Google Scholar]
- Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. Journal of Histochemistry and Cytochemistry. 1981;29(4):577–80. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Ivanov A, Zilberter Y. Critical state of energy metabolism in brain slices: the principal role of oxygen delivery and energy substrates in shaping neuronal activity. Front Neuroenergetics. 2011;3:9. doi: 10.3389/fnene.2011.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson VN, Price NT, Carpenter L, Halestrap AP. Cloning of the monocarboxylate transporter isoform MCT2 from rat testis provides evidence that expression in tissues is species-specific and may involve post-transcriptional regulation. Biochemical Journal. 1997;324(Pt 2):447–53. doi: 10.1042/bj3240447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juge N, Gray JA, Omote H, Miyaji T, Inoue T, Hara C, Uneyama H, Edwards RH, Nicoll RA, Moriyama Y. Metabolic control of vesicular glutamate transport and release. Neuron. 2010;68(1):99–112. doi: 10.1016/j.neuron.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen F, de Lanerolle NC, Lee TS, Spencer DD, Kim JH, Bergersen LH, Eid T. Monocarboxylate transporter 1 is deficient on microvessels in the human epileptogenic hippocampus. Neurobiology of Disease. 2011;41(2):577–84. doi: 10.1016/j.nbd.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen F, Perez EL, Melillo ER, Roh JM, Zaveri HP, Lee TS, Wang Y, Bergersen LH, Eid T. Altered expression of brain monocarboxylate transporter 1 in models of temporal lobe epilepsy. Neurobiology of Disease. 2012;45(1):165–76. doi: 10.1016/j.nbd.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite TC, Coelho RG, Da Silva D, Coelho WS, Marinho-Carvalho MM, Sola-Penna M. Lactate downregulates the glycolytic enzymes hexokinase and phosphofructokinase in diverse tissues from mice. FEBS Letters. 2011;585(1):92–8. doi: 10.1016/j.febslet.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Luchowska E, Luchowski P, Wielosz M, Kleinrok Z, Czuczwar SJ, Urbanska EM. Propranolol and metoprolol enhance the anticonvulsant action of valproate and diazepam against maximal electroshock. Pharmacology, Biochemistry and Behavior. 2002;71(1-2):223–31. doi: 10.1016/s0091-3057(01)00654-2. [DOI] [PubMed] [Google Scholar]
- Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia. 2010;58(9):1094–103. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- Offermanns S, Colletti SL, Lovenberg TW, Semple G, Wise A, AP IJ. International Union of Basic and Clinical Pharmacology. LXXXII: Nomenclature and Classification of Hydroxy-carboxylic Acid Receptors (GPR81, GPR109A, and GPR109B) Pharmacological Reviews. 2011;63(2):269–90. doi: 10.1124/pr.110.003301. [DOI] [PubMed] [Google Scholar]
- Olney JW, Collins RC, Sloviter RS. Excitotoxic mechanisms of epileptic brain damage. Advances in Neurology. 1986;44:857–77. [PubMed] [Google Scholar]
- Pan JW, Bebin EM, Chu WJ, Hetherington HP. Ketosis and epilepsy: 31P spectroscopic imaging at 4.1 T. Epilepsia. 1999;40(6):703–7. doi: 10.1111/j.1528-1157.1999.tb00766.x. [DOI] [PubMed] [Google Scholar]
- Pan JW, de Graaf RA, Petersen KF, Shulman GI, Hetherington HP, Rothman DL. [2,4-13 C2]-beta-Hydroxybutyrate metabolism in human brain. Journal of Cerebral Blood Flow and Metabolism. 2002;22(7):890–8. doi: 10.1097/00004647-200207000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(22):10625–9. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster H. The Fine Structure of the Nervous System. Neurons and Their Supporting Cells. Oxford University Press; New York: 1991. [Google Scholar]
- Pierre K, Magistretti PJ, Pellerin L. MCT2 is a major neuronal monocarboxylate transporter in the adult mouse brain. Journal of Cerebral Blood Flow and Metabolism. 2002;22(5):586–95. doi: 10.1097/00004647-200205000-00010. [DOI] [PubMed] [Google Scholar]
- Pierre K, Pellerin L, Debernardi R, Riederer BM, Magistretti PJ. Cell-specific localization of monocarboxylate transporters, MCT1 and MCT2, in the adult mouse brain revealed by double immunohistochemical labeling and confocal microscopy. Neuroscience. 2000;100(3):617–27. doi: 10.1016/s0306-4522(00)00294-3. [DOI] [PubMed] [Google Scholar]
- Pollen DA, Trachtenberg MC. Neuroglia: gliosis and focal epilepsy. Science. 1970;167(922):1252–3. doi: 10.1126/science.167.3922.1252. [DOI] [PubMed] [Google Scholar]
- Price NT, Jackson VN, Halestrap AP. Cloning and sequencing of four new mammalian monocarboxylate transporter (MCT) homologues confirms the existence of a transporter family with an ancient past. Biochemical Journal. 1998;329(Pt 2):321–8. doi: 10.1042/bj3290321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiki A, Boulland JL, Halestrap AP, Ottersen OP, Bergersen L. Highly differential expression of the monocarboxylate transporters MCT2 and MCT4 in the developing rat brain. Neuroscience. 2003;122(3):677–88. doi: 10.1016/j.neuroscience.2003.08.040. [DOI] [PubMed] [Google Scholar]
- Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322(5907):1551–5. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- Spencer DD, Spencer SS. Surgery for Epilepsy. Blackwell Scientific Publishers; Boston: 1991. [Google Scholar]
- Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144(5):810–23. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian M, Jacobson C, Gee SH, Campbell KP, Carbonetto S, Jucker M. Dystroglycan in the cerebellum is a laminin alpha 2-chain binding protein at the glial-vascular interface and is expressed in Purkinje cells. European Journal of Neuroscience. 1996;8(12):2739–47. doi: 10.1111/j.1460-9568.1996.tb01568.x. [DOI] [PubMed] [Google Scholar]
- van Hall G, Stromstad M, Rasmussen P, Jans O, Zaar M, Gam C, Quistorff B, Secher NH, Nielsen HB. Blood lactate is an important energy source for the human brain. Journal of Cerebral Blood Flow and Metabolism. 2009;29(6):1121–9. doi: 10.1038/jcbfm.2009.35. [DOI] [PubMed] [Google Scholar]
- Williamson A, Patrylo PR, Pan J, Spencer DD, Hetherington H. Correlations between granule cell physiology and bioenergetics in human temporal lobe epilepsy. Brain. 2005;128(Pt 5):1199–208. doi: 10.1093/brain/awh444. [DOI] [PubMed] [Google Scholar]
- Zaccaria ML, Di Tommaso F, Brancaccio A, Paggi P, Petrucci TC. Dystroglycan distribution in adult mouse brain: a light and electron microscopy study. Neuroscience. 2001;104(2):311–24. doi: 10.1016/s0306-4522(01)00092-6. [DOI] [PubMed] [Google Scholar]