Abstract
BackgroundTwo-stage liver resections with portal vein occlusion have become standard in patients with low volume future liver remnants. Whether they are associated with more complications is unclear. The aim of this study was to compare complications of one- and two-stage resections in a retrospective study.
MethodsPatients with two-stage right liver resections with a previous portal vein occlusion were compared with patients with one-stage right liver resections between 2002 and 2010. Primary endpoints were the incidence of complications by severity. Secondary endpoints were mortality, post-operative liver- and kidney function tests, length of hospitalization and transfusion events. Logistic and linear regression analyses were performed to adjust for confounders.
ResultsThe groups were comparable except for right trisectionectomies, pre-operative chemotherapy and underlying liver disease. Overall complications occurred in 25 out of 35 patients with two-stage and 106 out of 163 in one-stage procedures. Severe complications were observed in 47 out of 163 patients versus 9 out of 35 patients, respectively. Two-stage procedures had no increased adjusted risk for complications [relative risk (RR) 0.9, P = 0.79]. Mortality (5.7% versus 3.7%) and post-operative liver failure rates (2.9% versus 3.1%) were low. Secondary endpoints showed no adjusted differences in risk.
ConclusionThis study suggests that liver resections in two stages are not associated with more post-operative complications than one-stage resections. These results should support the adoption of two-stage liver resections in selected patients.
Introduction
A two-stage liver resection was developed more than 10 years ago in patients with extensive hepatic tumour load localized in the liver to achieve complete (R0) resections. The aim was to prevent too extensive resection with one-stage surgery only, which might leave an insufficient remnant liver and a risk of post-operative liver failure and death.1 Subsequently, a number of modifications were described such as portal vein embolization as the first stage, after the first stage2 or portal vein ligation during the initial operation, both aiming at an increase of the volume of the future liver remnant (FLR) prior to the second operation.3,4 The FLR is the volume of liver remaining after a resection and is usually determined in absolute volume units by volumetry or in per cent of either the volumetric total liver volume (FLR) or in per cent of the standardized liver volume based on body surface area and weight (sFLR).5 Single-centre non-comparative studies focusing on colorectal cancer metastases have reported completion of the procedures in two stages in patients enrolled in stage one in the range of 69% to 81%. Disease-free and overall survival were comparable to one-stage resections for hepatic colorectal cancer metastases in patients with a lesser tumour load.6 The median interval between the hepatic resections in the reported series was between 6 weeks and 4.5 months.1,2,7,8,9 In the majority of the strategies used, the major liver resection is performed during the second stage, and thus associated with a longer operative time, more blood loss and more complications, as well as longer hospital stay, than the smaller liver resections performed during the first operation.2,6,8 The difference is likewise related to the more extensive liver surgery, but other factors such as the impact of operating in the same field for a second time may also contribute to the higher complication rate. On the other hand, portal vein manipulations and two-stage approaches are generally chosen to make right hepatectomies and right trisectionectomies safer by increasing the volume of the FLR in patients in which the FLR is judged too small with an increasing risk of post-operative liver failure. Therefore, major liver resections performed in two stages might actually be safer.
The goal of this study was to examine complications and resource utilization of major liver resections performed in two stages as compared with one-stage procedures. As the majority of procedures performed in two stages are on the right side, the analysis was limited to right hepatectomies or trisectionectomies.
Methods
Study population and design
Data were analysed from a prospectively collected liver surgery database of hepatectomies, performed between 2002 and 2010 in a single tertiary care centre [Swiss Hepato-Pancreatico-Biliary (HPB) Centre, University Hospital Zurich, Switzerland], thereby identifying right hepatectomies and right trisectionectomies. Patients with combined liver and extrahepatic resections were excluded. Of the remaining cases, those performed in one stage were segregated from those carried out in two stages with portal vein ligation in stage one. The study was approved by the institutional review board for human studies at the University Hospital Zurich (KEK-ZH-Nr. 2012-0386).
Surgical procedure
The one-stage procedures were performed in livers with a volume of >30% of future liver remnant/total liver volume (FLR/TLV). The clamp crush technique for parenchymal transection and selective inflow occlusion (Pringle manoeuvre) were used as necessary. The two-stage procedures were chosen for livers with a FLR/TLV < 30% using a combination of portal vein ligation with cleaning of the future liver remnant in stage one. The second major resection followed once the FLR/TLV reached at least 30% according to a previously published approach.4 Volumes of sFLR were calculated by subtracting the resected specimen weight from the standardized total liver volume based on weight and body surface area as described by Vauthey et al.5
Endpoints
The primary endpoint was post-operative complications. This parameter was assessed using the Clavien–Dindo classification.9 Complications >IIIA were considered as severe complications according to this previous publication.10 Secondary endpoints were defined as follows: mortality during hospitalization (grade V Clavien–Dindo), liver failure as defined by the ‘50-50’ criteria as assessed by bilirubin levels and prothrombin time at day 5,11 as well as post-operative peak levels of aspartate transaminase (AST), alanine transaminase (ALT), bilirubin and creatinine, intra-operative blood loss and the percentage of patients undergoing intra-operative blood transfusions (packed red blood cells and fresh frozen plasma) by review of anaesthesia records, length of stay on a special care unit and length of hospitalization.
Statistical analysis
The distribution of variables was analysed using means and standard deviation (SD) for normally distributed, and median and interquartile ranges (IQR) for non-normally distributed data. Data were tested for normality using the Kolmogorov–Smirnov test and performed quantile–quantile plots of dependent variables.
The primary endpoint (the percentage of patients with overall and severe complications) was compared between the two groups using univariate logistic regression, and in the main analysis, a multivariable logistic regression model with complications as the dependent, and group allocation as the independent variable. Potential confounders for which we adjusted in the multivariate logistic regression analysis were gender (male/female), pre-operative chemotherapy (yes/no), the American Society of Anesthesiologists (ASA) physical status classification (≥2/<2), macrovesicular liver steatosis (<30%, >30%), liver fibrosis (yes/no), baseline ALT and AST, pre-operative bilirubin and creatinine levels, extent of resection by description of a right trisectionectomy or right hepatectomy in the operative reports and blood loss and intra-operative transfusions.
This study did not include enough patients for a multivariate analysis for the endpoints mortality during hospitalization, 90-day mortality and liver failure according to the ‘50/50’ criteria.
Uni- and multivariate linear regression analyses were performed for further secondary endpoints such as peak levels of transaminases, total bilirubin and creatinine after surgery, blood loss and intra-operative blood transfusions, length of stay on a special care unit and hospital stay. Data were reported as point estimates, 95% confidence intervals (CI) and P-values (≤0.05 considered as significant).
Data were analysed using the statistical program STATA (Version 11; Stata Corp., College Station, TX, USA).
Results
Figure 1 represents a flow sheet of the patient population identified in the database. One hundred and sixty-three one-stage procedures were compared with 35 operations performed in two stages.
Figure 1.
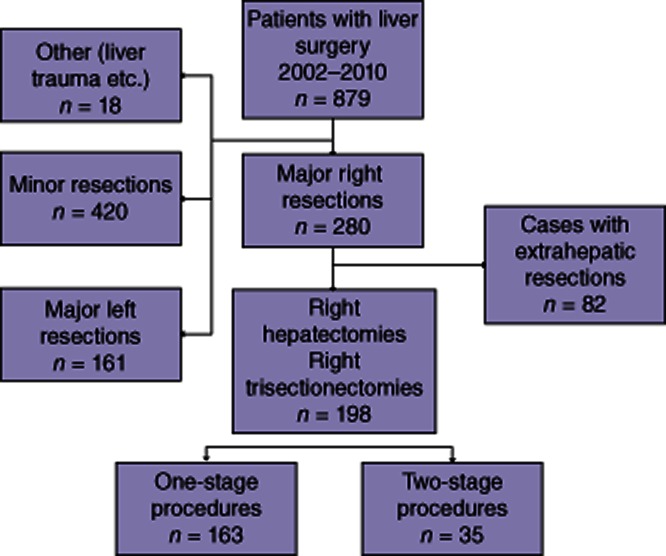
Flow chart of our liver resection database to compare a cohort undergoing right liver resections in one stage with a cohort undergoing right liver resections as a second stage of a two-stage procedure
Patient characteristics are shown in Table 1. Patients undergoing two-stage resections had a higher ASA score, higher Charlson index and more metastatic liver tumours than those undergoing one-stage resections. Along with this there was more use of chemotherapy prior to resections in two-stage resections. The presence of >30% steatosis or fibrosis was more frequent in the two-stage resections. No differences were found in the size of sFLR at the time of resection.
Table 1.
Characteristics of patients undergoing right liver resections either in one stage or as a second stage of a two-stage procedure
| Right liver resection | P-value | |||
|---|---|---|---|---|
| One-stage procedure n = 163 | Two-stage procedure n = 35 | |||
| Age (years) | 57 (46–67) | 59 (48–64) | 0.82 | |
| Gender male/female | 86/77 | 22/13 | 0.28 | |
| BMI (kg/m2) | 24 (22–27) | 23.4 (21.1–28) | 0.72 | |
| Charlson Index | 6 (2–9) | 8 (6–9) | <0.001 | |
| ASA score | 2 (2–2) | 2 (2–3) | 0.036 | |
| – ≤2 | 133 | 26 | ||
| – >2 | 30 | 9 | 0.32 | |
| Malignant disease | 125 | 31 | 0.24 | |
| Pre-operative chemotherapy | 64 | 21 | 0.025 | |
| Liver tumour | 0.008 | |||
| – None | 7 | 0 | ||
| – Primary | 74 | 9 | ||
| – Secondary | 82 | 26 | ||
| Portal vein embolization/portal vein ligation | 0 | 9/26 | – | |
| sFLR (%) | 47% (19–69%) | 47% (19–64%) | 0.96 | |
| Liver cirrhosis | 8 | 2 | 0.85 | |
| Liver steatosis | 70 | 16 | 0.79 | |
| – ≥30% | 23 | 11 | 0.006 | |
| Liver fibrosis | 33 | 10 | 0.29 | |
| AST preoperative (U/l) | 30.5 (23–41) | 37.5 (28–47) | 0.037 | |
| ALT preoperative (U/l) | 29 (19–45) | 35 (25–60) | 0.07 | |
| Bilirubin pre-operative (μmol/l) | 9 (6–13) | 8.5 (6–11) | 0.36 | |
| Creatinine pre-operative (μmol/l) | 77 (67–89) | 74 (65–79) | 0.24 | |
All results are presented in median (interquartile range).
ASA, American Society of Anesthesiologists score; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BMI, body mass index; sFLR, standardized future liver remnant at the time of resection (weight of standardized total liver volume minus weight of the resected specimen).
Intra-operative parameters are presented in Table 2. There were more trisectionectomies in the two-stage group.
Table 2.
Intra-operative parameters of patients undergoing right liver resections either in one stage or as a second stage of a two-stage procedure
| Right liver resection | |||
|---|---|---|---|
| One-stage procedure n = 163 | Two-stage procedure n = 35 | P-value | |
| Trisectionectomy | 51 | 19 | 0.01 |
| Surgery time (min) | 310 (260–370) | 320 (300–405) | 0.16 |
| Pringle ( no. of patients) | 139 | 27 | 0.45 |
| – Pringle time (min) | 30 (28–37) | 30 (27–38) | 0.18 |
| Blood loss (ml) | 500 (300–800) | 600 (400–1000) | 0.07 |
| Red blood cell transfusion | 18 | 7 | 0.19 |
| Fresh frozen plasma transfusion | 6 | 2 | 0.63 |
All results are presented in median and interquartile range.
Uncorrected post-operative outcomes are compared in Table 3 (univariate analysis) and relative risks (RR) for outcome endpoints in the multivariate regression model in Table 4. Taking into account the possible confounders, the two-stage group was not at risk for more overall complications [RR 0.9, with a 95% confidence interval (CI) 0.36–2.17, P = 0.79] or severe complications (RR 0.5, 95% CI 0.17–1.3, P = 0.14). The incidence of liver failure by the ‘50/50’ criteria could not be statistically evaluated because of the small number of events (less than 5). Mortality during hospitalization (=Grade V complication) and 30-day mortality could also not be compared owing to the small number of events. There was a trend towards a higher 90-day mortality in the two-stage group in the model [adjusted odds ratio (OR) 3.7, P = 0.06]. The two-stage group was not at risk for increased levels of post-operative transaminases, bilirubin or creatinine, or for blood loss or blood transfusions in the multivariate model.
Table 3.
Post-operative outcome of patients undergoing a right liver resection either in one stage or as a second stage of a two-stage procedure
| Right liver resection | |||
|---|---|---|---|
| One-stage procedure n = 163 | Two-stage procedure n = 35 | ||
| Post-operative complications overalla (incl. mortality during hospitalization) | 106 | 25 | |
| Post-operative complicationsa | |||
| – None | 57 | 10 | |
| – Grade I | 31 | 7 | |
| – Grade II | 28 | 9 | |
| – Grade IIIA | 22 | 3 | |
| – Grade IIIB | 9 | 0 | |
| – Grade IVA | 7 | 2 | |
| – Grade IVB | 3 | 2 | |
| – Grade V (=mortality during hospitalization) | 6 | 2 | |
| Severe complications ≥IIIAa | 47 | 9 | |
| Post-operative liver failureb | 5 | 1 | |
| 30 days mortality | 5 | 0 | |
| 90 days mortality | 9 | 5 | |
| AST peak (U/l) | 505 (334–807) | 543 (370–986) | |
| ALT peak (U/l) | 461 (306–737) | 476 (293–1030) | |
| Bilirubin peak (μmol/l) | 45 (33–63) | 38 (20–63) | |
| Creatinine peak (μmol/l) | 82 (68–96) | 79 (69–107) | |
| Length of hospital stay (days) | 12 (10–15) | 12 (11–22) | |
| Length of special care unit stay (days) | 1 (1–2) | 1 (1–1) | |
All results are presented in median and interquartile range.25
Complication classification according to the Clavien–Dindo classification system.
‘Complications ≥ III A’ includes mortality during hospitalization.
Post-operative liver failure as defined by the ‘50-50’ criteria.
AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Table 4.
Primary endpoint complications as well as secondary endpoints after liver resection as the second stage of a two-stage hepatectomy compared with resection in one stage expressed as an odds ratio or difference between values, adjusted in a multivariate regression model
| Odds ratio or difference for two-stage hepatectomy versus one-stage hepatectomy in multivariate regression model | ||
|---|---|---|
| Unadjusted odds ratio (95% CI, P-value) | Adjusted odds ratio (95% CI, P-value) | |
| Post-operative complications overalla | 1.3 (0.6–3.0, P = 0.47) | 0.9 (0.36–2.17, P = 0.79) |
| Severe complications ≥ IIIAa | 0.9 (0.37–1.96, P = 0.71) | 0.5 (0.17–1.30, P = 0.14) |
| Post-operative liver failureb | – | – |
| Mortality during hospitalization (Grade V)b | – | – |
| 30 day mortalityb | – | – |
| 90 day mortality | 2.8 (0.9–9.0, P = 0.079) | 3.7 (0.9–15.2, P = 0.066) |
| Unadjusted difference (95% CI, P-value) | Adjusted difference (95% CI, P-value) | |
| AST peak (U/l) | 86.6 (−89.8–263.1, P = 0.33) | −11.4 (−204.5–181.6, P = 0.91) |
| ALT peak (U/l) | −1.54 (−171.4–174.5, P = 0.99) | −78.0 (−269.0–113.0, P = 0.42) |
| Bilirubin peak (μmol/l) | 5.3 (−19.5–30.1, P = 0.68) | 11.8 (−16.8–40.4, P = 0.42) |
| Creatinine peak (μmol/l) | 9.4 (−6.8–25.6, P = 0.25) | 3.6 (−13.9–21.2, P = 0.68) |
| Length of hospital stay (days) | 4.1 (1.2–6.9, P = 0.005) | 1.9 (−1.0–4.9, P = 0.20) |
| Special care unit stay (days) | 0.86 (−0.8–2.5, P = 0.30) | 0.3 (−1.4–2.1, P = 0.70) |
| Blood loss (ml) | 51.0 (−254.9–357.0, P = 0.74) | −54.7 (−395.3–285.8, P = 0.75) |
| Blood transfusion (%) | 2.0 (0.77 −5.27, P = 0.15) | 2.3 (0.42–12.98, P = 0.34) |
All results are presented in median (interquartile range).
All results are adjusted for possible confounders such as gender, pre-operative chemotherapy (no/yes), ASA score (≤2/ > 2), liver steatosis (<30%/≥30%), liver fibrosis (no/yes), baseline ALT/AST and bilirubin level, trisectionectomy, blood loss.
ASA, American Society of Anesthesiologists score; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Complication classification according to the Clavien–Dindo classification system.
Less than five events: therefore no statistical calculation was performed.
A subgroup analysis of outcomes for patients undergoing trisectionectomies (n = 70) is shown in Table 5. No mortalities in the procedures performed in the context of two-stage operations could be observed. As a result of low numbers, a multivariate regression model could not be performed.
Table 5.
Subgroup analysis of right trisectionectomies performed in one stage compared with those performed as a second stage of a two-stage procedure
| Right trisectionectomy | |||
|---|---|---|---|
| One-stage procedure n = 51 | Two-stage procedure n = 19 | P-value | |
| Complications overall | 34 | 13 | 0.96 |
| Severe complications ≥ IIIAa | 20 | 5 | 0.51 |
| Mortality during hospitalization | 3 | 0 | a |
All results are presented in median (interquartile range).
Less than five events: therefore no statistical calculation was performed.
Discussion
This study suggests that major resections in two stages with previous resections and portal vein embolization or ligation with the intent to induce future liver remnant volumetric growth are not associated with a higher degree of overall or severe post-operative complications than can be observed in one-stage major liver resections. The number of mortalities in both groups is within the expected range for complex major liver surgery.12 These results compare with previously published data that have demonstrated a mortality of 0–9% and an overall complication rate of 26% to 59% after two-stage liver resections.2,8,9,13 The 65–71% incidence of overall complications in this series is 10% higher than in past studies. This might be as a result of the prospectively collected complications database in Zurich with several levels of quality control. It has been demonstrated in the past that clinical data collection may lead to underreporting of complications.14
One of the two mortalities after the two-stage resections in this series might have been because of a small sFLR of 40%. The second patient had a sFLR of 47% and died because of a bile leak and progressive sepsis. Five of the six mortalities after one-stage resections were reported in the medical documentation to be as a result of post-operative liver failure, one was related to a post-operative stroke event. However, in no case was the size of sFLR less than 40%.
A subgroup analysis for extended right trisectionectomies showed that there was no mortality in 19 procedures performed as a second stage compared with 6% of one-stage right trisectionectomies. The incidence of severe complications also appeared reduced (Table 5). As a result of the low incidence of events, a multivariate analysis for this subgroup could not be performed. However, larger series of right trisectionectomies reported peri-operative mortalities between 6% and 12%15,16
None of the clinical markers suggesting a higher risk such as increased post-operative creatinine or bilirubin values, and the duration of special care unit and hospital stay were the same. Also blood loss and transfusions were not significantly increased in the two-stage resection group, once the analysis was corrected for possible confounders. It could be postulated that portal vein manipulation makes major liver resections safer by increasing the size of the FLR outweighing the potential risks of liver surgery after previous portal vein manipulation.
This study is timely because of the recently presented data of new approaches to two-stage resections such as the Associating Liver Partition with Portal vein Ligation for Staged Hepatectomy (ALPPS) approach.17–19 In this operation, rapid hypertrophy of the volume of FLR is induced by parenchymal transection with portal vein ligation as a first stage. Stage two is then performed in rapid succession 1 or 2 weeks after stage one. The relatively high overall mortality rate of 10% or more reported by the pioneers and other early experience of this approach17 has led to a discussion about the clinical safety to the new rapid hypertrophy approach, as compared with the traditional approach of two-stage hepatectomies. From the data presented here, it appears that the second stage, at least in traditional two-stage strategies, is comparable in risk to standard major right liver resections. However, careful prospective monitoring using validated systems for recording complications such as the Clavien–Dindo classification shows that severe complications in right liver resections occur in 25–28%. The incidence for severe complications (>IIIA) in liver resections overall including a minor resection is 24% in previous studies using the same methodology.20
A limitation of the current study is that the groups differ in certain characteristics. The results were therefore adjusted for possible confounders to increase the validity of the results. More pre-operative chemotherapy might put the two-stage hepatectomy cohort at a disadvantage. There were also more fibrotic livers and more livers with macrosteatosis over 30% selected for the two-stage approach, which also potentially puts the two-stage hepatectomy patients at a disadvantage. The second stage of the two-stage hepatectomy cohort also encompassed more right trisectionectomies and the need for increased use of blood transfusions, which might all be direct and indirect markers for more extensive resections.
The association of the size of FLR to post-operative outcomes has been demonstrated in several studies in the past; however, the cut-off of safer liver surgery based on volumetry and histological changes remains controversial.4,15,21–24 Liver weight, recorded in pathology reports of a resected specimen, were used and subtracted from the standardized total liver volume to estimate the sFLR at the time of resection. Calculations demonstrate that sFLR volumes between the two groups were similar and ranged from 19% to over 60% in both groups, presumably as a result of portal vein manipulation prior to resection. None of the mortalities occurred in a patient with a sFLR < 40%.
Lastly, complications were chosen as a global outcome including mortality as the small number of patients with two-stage resections, even in large centres, makes it difficult to choose mortality or liver failure, as a primary endpoint. Prospective studies will have to be performed to evaluate the two-stage resections with portal vein ligation especially given the recent technical innovations such as the ALPPS procedure.
In conclusion, evidence is provided that two-stage liver resections with portal vein occlusion are not associated with a higher peri-operative risk compared with one-stage liver resections in spite of the high-risk population, in which they are used.
Conflicts of interest
All authors declare that they have no competing interests related to the present manuscript.
References
- Adam R, Laurent A, Azoulay D, Castaing D, Bismuth H. Two-stage hepatectomy: a planned strategy to treat irresectable liver tumors. Ann Surg. 2000;232:777–785. doi: 10.1097/00000658-200012000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeck D, Oussoultzoglou E, Rosso E, Greget M, Weber JC, Bachellier P. A two-stage hepatectomy procedure combined with portal vein embolization to achieve curative resection for initially unresectable multiple and bilobar colorectal liver metastases. Ann Surg. 2004;240:1037–1049. doi: 10.1097/01.sla.0000145965.86383.89. discussion 49–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aussilhou B, Lesurtel M, Sauvanet A, Farges O, Dokmak S, Goasguen N, et al. Right portal vein ligation is as efficient as portal vein embolization to induce hypertrophy of the left liver remnant. J Gastrointest Surg. 2008;12:297–303. doi: 10.1007/s11605-007-0410-x. [DOI] [PubMed] [Google Scholar]
- Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545–1559. doi: 10.1056/NEJMra065156. [DOI] [PubMed] [Google Scholar]
- Vauthey JN, Abdalla EK, Doherty DA, Gertsch P, Fenstermacher MJ, Loyer EM, et al. Body surface area and body weight predict total liver volume in Western adults. Liver Transpl. 2002;8:233–240. doi: 10.1053/jlts.2002.31654. [DOI] [PubMed] [Google Scholar]
- Brouquet A, Abdalla EK, Kopetz S, Garrett CR, Overman MJ, Eng C, et al. High survival rate after two-stage resection of advanced colorectal liver metastases: response-based selection and complete resection define outcome. J Clin Oncol. 2011;29:1083–1090. doi: 10.1200/JCO.2010.32.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kianmanesh R, Farges O, Abdalla EK, Sauvanet A, Ruszniewski P, Belghiti J. Right portal vein ligation: a new planned two-step all-surgical approach for complete resection of primary gastrointestinal tumors with multiple bilateral liver metastases. J Am Coll Surg. 2003;197:164–170. doi: 10.1016/S1072-7515(03)00334-X. [DOI] [PubMed] [Google Scholar]
- Tsai S, Marques HP, de Jong MC, Mira P, Ribeiro V, Choti MA, et al. Two-stage strategy for patients with extensive bilateral colorectal liver metastases. HPB. 2010;12:262–269. doi: 10.1111/j.1477-2574.2010.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicherts DA, Miller R, de Haas RJ, Bitsakou G, Vibert E, Veilhan LA, et al. Long-term results of two-stage hepatectomy for irresectable colorectal cancer liver metastases. Ann Surg. 2008;248:994–1005. doi: 10.1097/SLA.0b013e3181907fd9. [DOI] [PubMed] [Google Scholar]
- Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, et al. The ‘50-50 criteria’ on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824–828. doi: 10.1097/01.sla.0000189131.90876.9e. discussion 8–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406. doi: 10.1097/01.SLA.0000029003.66466.B3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun YS, Ribero D, Abdalla EK, Madoff DC, Mortenson MM, Wei SH, et al. Comparison of two methods of future liver remnant volume measurement. J Gastrointest Surg. 2008;12:123–128. doi: 10.1007/s11605-007-0323-8. [DOI] [PubMed] [Google Scholar]
- Dindo D, Hahnloser D, Clavien PA. Quality assessment in surgery: riding a lame horse. Ann Surg. 2010;251:766–771. doi: 10.1097/SLA.0b013e3181d0d211. [DOI] [PubMed] [Google Scholar]
- Kishi Y, Abdalla EK, Chun YS, Zorzi D, Madoff DC, Wallace MJ, et al. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg. 2009;250:540–548. doi: 10.1097/SLA.0b013e3181b674df. [DOI] [PubMed] [Google Scholar]
- Lang H, Sotiropoulos GC, Brokalaki EI, Radtke A, Frilling A, Molmenti EP, et al. Left hepatic trisectionectomy for hepatobiliary malignancies. J Am Coll Surg. 2006;203:311–321. doi: 10.1016/j.jamcollsurg.2006.05.290. [DOI] [PubMed] [Google Scholar]
- Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405–414. doi: 10.1097/SLA.0b013e31824856f5. [DOI] [PubMed] [Google Scholar]
- de Santibanes E, Clavien PA. Playing Play-Doh to prevent postoperative liver failure: the ‘ALPPS’ approach. Ann Surg. 2012;255:415–417. doi: 10.1097/SLA.0b013e318248577d. [DOI] [PubMed] [Google Scholar]
- de Santibanes E, Alvarez FA, Ardiles V. How to avoid postoperative liver failure: a novel method. World J Surg. 2012;36:125–128. doi: 10.1007/s00268-011-1331-0. [DOI] [PubMed] [Google Scholar]
- Breitenstein S, DeOliveira ML, Raptis DA, Slankamenac K, Kambakamba P, Nerl J, et al. Novel and simple preoperative score predicting complications after liver resection in noncirrhotic patients. Ann Surg. 2010;252:726–734. doi: 10.1097/SLA.0b013e3181fb8c1a. [DOI] [PubMed] [Google Scholar]
- Giraudo G, Greget M, Oussoultzoglou E, Rosso E, Bachellier P, Jaeck D. Preoperative contralateral portal vein embolization before major hepatic resection is a safe and efficient procedure: a large single institution experience. Surgery. 2008;143:476–482. doi: 10.1016/j.surg.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Shoup M, Gonen M, D'Angelica M, Jarnagin WR, DeMatteo RP, Schwartz LH, et al. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003;7:325–330. doi: 10.1016/s1091-255x(02)00370-0. [DOI] [PubMed] [Google Scholar]
- Ferrero A, Vigano L, Polastri R, Muratore A, Eminefendic H, Regge D, et al. Postoperative liver dysfunction and future remnant liver: where is the limit? Results of a prospective study. World J Surg. 2007;31:1643–1651. doi: 10.1007/s00268-007-9123-2. [DOI] [PubMed] [Google Scholar]
- Schindl MJ, Redhead DN, Fearon KC, Garden OJ, Wigmore SJ. The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005;54:289–296. doi: 10.1136/gut.2004.046524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makuuchi M, Thai BL, Takayasu K, Takayama T, Kosuge T, Gunven P, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521–527. [PubMed] [Google Scholar]


