Abstract
Background Post-operative hepatic dysfunction is a major cause of concern when undertaking a liver resection. The generation of reactive oxygen species (ROS) as a result of hepatic ischaemia/reperfusion (I/R) injury can result in hepatocellular injury. Experimental evidence suggests that N-acetylcysteine may ameliorate ROS-mediated liver injury.
Methods A cohort of 44 patients who had undergone a liver resection and receiving peri-operative N-acetylcysteine (NAC) were compared with a further cohort of 44 patients who did not. Liver function tests were compared on post-operative days 1, 3 and 5. Peri-operative outcome data were retrieved from a prospectively maintained database within our unit.
ResultsAdministration of NAC was associated with a prolonged prothrombin time on the third post-operative day (18.4 versus 16.4 s; P = 0.002). The incidence of grades B and C liver failure was lower in the NAC group although this difference did not reach statistical significance (6.9% versus 14%; P = 0.287). The overall complication rate was similar between groups (32% versus 25%; P = ns). There were two peri-operative deaths in the NAC group and one in the control group (P = NS).
ConclusionIn spite of promising experimental evidence, this study was not able to demonstrate any advantage in the routine administration of peri-operative NAC in patients undergoing a liver resection.
Introduction
Hepatic resection is increasingly utilized to treat a variety of both benign and malignant diseases. One of the major causes of post-operative morbidity is the development of post-hepatectomy liver failure (PHLF), the frequency of which increases as the remnant liver volume decreases.1 In addition, the presence of background abnormalities in the hepatic parenchyma (e.g. chemotherapy associated liver injury, steatohepatitis, fibrosis/cirrhosis) increases the risk of developing PHLF.2,3–5
Vascular clamping of the hepatic inflow (and on occasion outflow) is commonly utilized during major hepatic surgery to minimize intra-operative blood loss and to facilitate parenchymal transection. These periods of vascular exclusion can result in hepatic ischaemia/reperfusion (I/R) injury which is associated with oxidative stress and depletion of intracellular ATP.6–8
Glutathione (GSH) is a key anti-oxidant within the liver that is depleted after I/R injury.9 GSH can be synthesized by tissues in response to a variety of insults from the amino acids glycine, glutamic acid and cysteine. It is the availability of cysteine which is most often the rate limiting step in the de novo synthesis of GSH.10 N-acetylcysteine (NAC) is a synthetic thiol which is readily able to enter cells, increasing intracellular cysteine concentrations and thereby enabling increased GSH synthesis.11
It has been suggested in experimental studies that NAC administration may reduce the extent of I/R injury with reported benefits including reduced free radical formation, reduced bacterial translocation from the gut, less microcirculatory disturbance and less hepatic necrosis.12 Several clinical trials have examined the role of NAC in liver transplantation but there is a sparsity of information related to its use in patients undergoing a hepatic resection.13–15
The aim of this study was to determine what role, if any, NAC might have in patients undergoing a hepatic resection with the key outcome measures being post-operative morbidity and mortality.
Methods
Patient selection
Five surgeons perform liver resections within our unit, one of whom (S.A.W.) routinely uses NAC in the post-operative period in an attempt to reduce I/R injury. The treatment arm in this study therefore consisted of 44 patients operated by this surgeon with the control arm consisting of 44 patients operated by the other surgeons between January 2005 and May 2010. Patients in the control arm were selected using the prospectively maintained unit database and were matched with the treatment arm for extent of resection, use of pre-operative chemotherapy and histological diagnosis.
Patient management
Patients undergoing a right or left trisectionectomy received pre-operative bacterial gut decontamination (neomycin 1 g qds; nystatin 1 million units qds; colistin 500 mg qds). All procedures were performed under low CVP anaesthesia with intermittent portal triad clamping utilized as necessary to minimize blood loss during parenchymal transection. The arterial and portal venous inflow were ligated prior to commencing parenchymal transection. Neither total vascular occlusion nor ischaemic pre-conditioning were utilized on any patient included within this series. Parenchymal transection was performed in all cases using a CUSA EXcel device (Integra Life Science, St Priest, France).
Post-operative care followed standard unit protocols in both groups other than for the addition of NAC in the treatment group. An infusion of NAC (10 g/24 h in 250 ml of 5% dextrose) was commenced at the time of parenchymal transection and continued post-operatively for 3 days or until a fall in the post-operative alanine aminotransferase (ALT).
Data collection
A prospectively maintained electronic database was used to retrieve demographic details, histological diagnosis and post-operative complications. The hospital laboratory database was searched to retrieve pre- and post-operative blood results i.e. prothrombin time (PT), bilirubin and ALT. Histology reports were reviewed for all patients to identify the presence of background parenchymal injury.
PHLF was defined as an elevation of bilirubin (range 0–21 mg/dl) and PT (range 10–13 s) above the upper limit of normal on post-operative day 5 or beyond. In patients where these values were elevated pre-operatively, a failure to return to pre-operative levels by day 5 was considered to be diagnostic of PHLF. The severity of PHLF was graded according to a recent consensus paper.16 Only grade B or C liver failure were considered to represent a complication of surgery as grade A PHLF does not require, by definition, any change to patient management.
A major hepatectomy was defined as a resection of 3 or more Couinaud segments (i.e. right or left hemi-hepatectomy and trisectionectomy). The presence of background parenchymal liver injury was determined from histology reports and was defined as the presence of steatosis >30%, steatohepatitis, sinusoidal injury (sinusoidal dilatation, nodular regenerative hyperplasia and hepatocyte atrophy) or fibrosis.
Statistical analysis
Discrete variables were assessed for statistical significance using the Chi-squared test. Continuous data are expressed as median (range) and were compared using a two-tailed Mann Whitney U-test.
Changes in post-operative ALT, bilirubin and PT were assessed by treatment group using a two-way anova as previously described by Clavien et al.17 Differences in values at individual time points were assessed using Bonferroni's post test. In the case of missing data for a given time point, those individuals were excluded from the analysis. All analysis was performed using GraphPad PRISM (GraphPad, La Jolla, CA, USA). A P-value of less than 0.05 was considered statistically significant.
Results
Patient characteristics
Both control and NAC treatment groups contained 44 patients which were well matched in regard to gender (n = 30 versus n = 34 males) and median age [69.5 (40–84) versus 66.5 (42–81) years]. In both groups the main indication for surgery was colorectal liver metastases (n = 38 per group). The proportion of patients who received pre-operative systemic chemotherapy (n = 31 versus n = 30) and underwent a major hepatectomy (n = 38 versus n = 39) was similar between groups. The median duration of portal triad clamping was similar between the groups [30.0 (15–60) versus 26.0 (8–65) min]. There was a numerically higher incidence of parenchymal injury overall in the NAC group (n = 21 versus n = 16) although this difference was not statistically significant. While there was a tendency to a longer hospital stay in the NAC group this difference was not statistically significant [13.5 (4–62) versus 11.0 (6–31) days; P = 0.0976] (Table 1). The time interval between cessation of chemotherapy and surgery was greater than 6 weeks for all patients.
Table 1.
Summary of patient demographics
| Control (n = 44) | NAC (n = 44) | P-value | |
|---|---|---|---|
| Gender | |||
| Male | 30 (68.2%) | 34 (77.3%) (77.3%) | 0.473 |
| Female | 14 (31.8%) | 10 (22.7%) | |
| Age at time of surgery | |||
| Median age (years) | 69.5 | 66.5 | 0.960 |
| Indication for surgery | |||
| Colorectal liver metastases | 38 (86.4%) | 38 (86.4%) | |
| Hepatocellular carcinoma | 1 (2.3%) | 3 (6.8%) | |
| Adenocarcinoma gallbladder | 2 (4.5%) | 1 (2.3%) | 0.598 |
| Cholangiocarcinoma | 2 (4.5%) | 1 (2.3%) | |
| Neuroendocrine metastases | 1 (2.3%) | — | |
| Adenosquamous carcinoma | — | 1 (2.3%) | |
| Pre-operative chemotherapy | |||
| Yes | n = 31 (70.5%) | n = 30 (68.2%) | 1.00 |
| No | n = 13 (29.5%) | n = 14 (31.8%) | |
| Extent of surgery | |||
| Major hepatectomy | 38 (86.4%) | 39 (88.6%) | 1.00 |
| Minor hepatectomy | 6 (13.6%) | 5 (11.4%) | |
| Portal triad clamping | |||
| Median duration (min) | 30.0 | 26.0 | 0.600 |
| Parenchymal injury | |||
| All | 16 (36.4%) | 21 (47.7%) | 0.194 |
| Steatosis > 30% | 7 (15.9%) | 8 (18.2%) | 0.500 |
| Steatohepatitis | 0 (0%) | 1 (2.3%) | 0.500 |
| Sinusoidal injury | 13 (29.5%) | 9 (20.5%) | 0.230 |
| Fibrosis | 1 (2.3%) | 1 (2.3%) | 0.756 |
| Length of hospital stay | |||
| Median stay (days) | 11.0 | 13.5 | 0.098 |
NAC, N-acetylcysteine.
Post-operative liver function
Serum ALT, bilirubin and PT were recorded pre-operatively and on post-operative days 3, 5 and 7 as markers of hepatocellular injury (ALT) and hepatocyte synthetic and excretory functions (PT and bilirubin). The control and NAC treated groups did not differ significantly in baseline ALT [26.5 (10–105) versus 28.0 (9–104) u/l; P = 0.77; Fig. 1a], PT [12 (10–15) versus 11 (10–15) s; P = 0.877; Fig. 1b] or bilirubin (9 (4–53) versus 8 (3–28) mg/dl; P = 0.0666; Fig. 1c].
Figure 1.
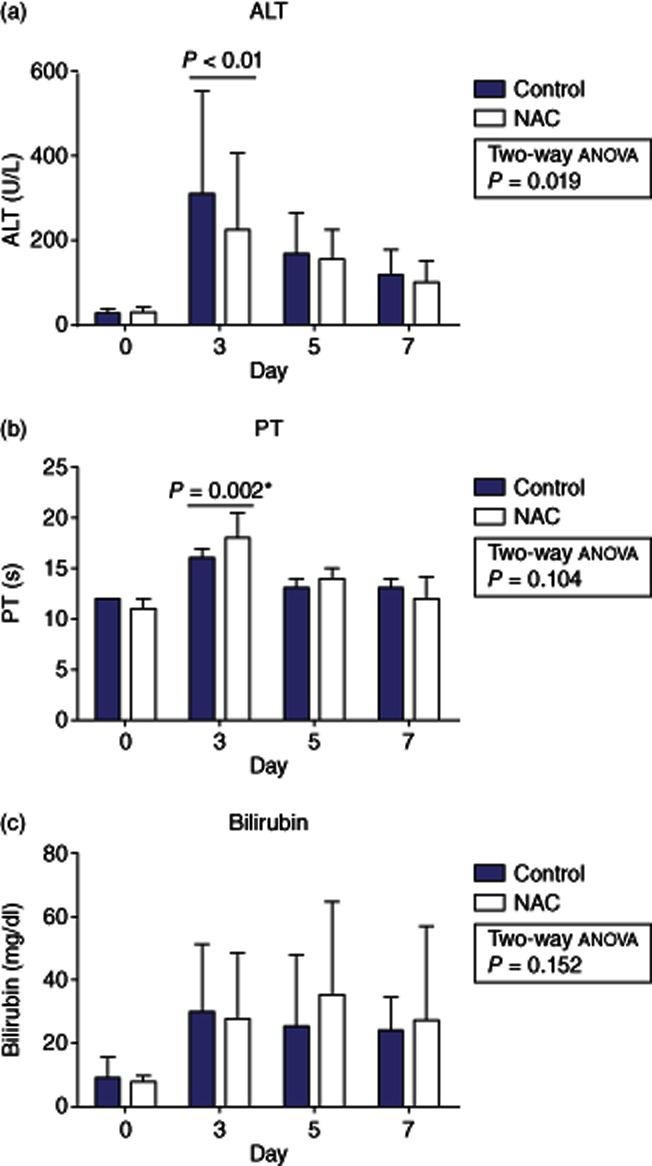
Changes in alanine aminotransferase (ALT) (a), prothrombin time (PT) (b) and bilirubin (c) from baseline after a liver resection in patients receiving peri-operative N-acetylcysteine (NAC) or matched controls. Data presented as median ± interquartile range. (* = Mann–Whitney U test)
In both patient groups, hepatocellular injury, as measured by a rise in serum ALT, peaked on post-operative day 3 with a decline towards baseline values on day 7. NAC treatment was associated with a lower post-operative ALT level as assessed by two-way anova (P = 0.019) with this difference being most marked on post-operative day 3 [308 (91–1599) versus 223 (74–764) u/l; P < 0.01].
Whereas ALT provides a useful measure of hepatocellular injury, it provides little information about hepatocyte function and for this reason we compared PT and bilirubin between groups on days 3, 5 and 7. Changes in PT mirrored those seen with ALT but on this occasion there was no overall difference in PT values according to treatment group as assessed by two-way anova (P = 0.104). Nonetheless, it was noted that the PT value on post-operative day 3 appeared to be markedly shorter in the control group as compared with those treated with NAC [16 (11–30) versus 18 (13–26) s] with this difference being statistically significant as judged by a Mann–Whitney U-test (P = 0.002).
Serum bilirubin was elevated to a similar degree in both the control and NAC treated groups on post-operative day 3 [30 (9–116) versus 28 (8–120) mg/dl; P = 0.781]. Day 3 represented the peak in serum bilirubin in the control group (Fig. 1c) whereas in the NAC group the elevation persisted on day 5 [25 (7–43) versus 36 (11–168) mg/dl; P = 0.192] and day 7 [24 (6–161) versus 27 (6–212) mg/dl; P = 0.3661] although the individual differences were not significant at any time point. There was no overall effect of NAC on bilirubin as judged by two-way anova (P = 0.152).
Complications
The incidence of PHLF of all grades was greater in those patients receiving NAC (n = 17; 38.6%) as compared with controls (n = 10; 22.7%; P = 0.165) although this difference did not reach statistical significance. There was a preponderance of grade A PHLF in the NAC group (n = 14, 31.8%) as compared with the controls (n = 4, 9%; P = 0.025) which appeared to be a result of the tendency towards a prolonged PT in this patient group. The incidence of clinically relevant grade B or C fistulae did not differ significantly between the NAC (n = 3, 6.9%) and control (n = 6; 13.6%; P = 0.484) groups (see Table 2). Limiting the analysis to only those patients undergoing a major hepatic resection had no effect on results.
Table 2.
Grade of post-hepatectomy liver failure according to the ISGLS consensus statement
| Grade of PHLF | Control (n = 44) | NAC (n = 44) | P-value |
|---|---|---|---|
| None | 34 (77.3%) | 27 (61.3%) | 0.165 |
| A | 4 (9.1%) | 14 (31.8) | 0.025 |
| B or C | 6 (13.6%) | 3 (6.9%) | 0.484 |
NAC, N-acetylcysteine; PHLF, post-hepatectomy liver failure.
There was no statistically significant difference in the incidence of patients experiencing clinically important complications (i.e. Dindo-Clavien grade 2 or greater) between the NAC group (n = 11, 25%) and controls (n = 14, 31.8%; P = 0.318). There were two peri-operative deaths in the NAC group as compared (Table 3) with one in the control group (P = NS). All three deaths were a consequence of multi-organ failure secondary to sepsis in patients who developed grade C liver failure.
Table 3.
Complications classified according to the Dindo-Clavien method
| Clavien-Dindo grade | Control (n = 44) | NAC (n = 44) |
|---|---|---|
| 1 | 2 (4.5%) | 12 (27.3%) |
| 2 | 4 (9.1%) | 4 (9.1%) |
| 3 | 6 (13.6%) | 4 (9.1%) |
| 4 | 3 (6.8%) | 1 (2.3%) |
| 5 | 1 (2.3%) | 2 (4.5%) |
Grade 1 complications in each group consisted of exclusively grade A PHLF which did not result in a change to patient management.
PHLF, post-hepatectomy liver failure.
Discussion
It has long been known that hepatic I/R injury is, in part, mediated by the generation of reactive oxygen species (ROS) which is associated with the depletion of hepatic GSH.18 The ability of NAC to replenish hepatic GSH levels has led to its use in several experimental models of hepatic I/R where it has been demonstrated to reduce the extent of liver injury.9,19,20 In particular, the use of NAC in experimental I/R results in less ROS-mediated DNA damage,21 less lipid peroxidation21 and a reduction in ROS-mediated activation of the pro-inflammatory transcription factor NF-κB.22,23
At a functional level, NAC has been shown to improve the hepatic microcirculation after experimental I/R injury with a significant reduction in the number of non-perfused sinusoids.24–26 Similar findings have also been reported in experimental models of orthotopic liver transplantation (OLT).27 It has been proposed that this may be a result of the direct protective effects of NAC on the sinusoidal endothelium which results in endothelial shedding of selectins and reduced circulating levels of the adhesion molecules ICAM-1 and VCAM-1.15,28
In spite of the considerable experimental evidence suggesting a potential role for NAC in reducing the complications associated with hepatic resection this is the first publication to explore its role in this setting.12 Several small trials have explored the role of NAC in reducing graft dysfunction after OLT. The most consistently reported end point in these previous studies is liver function and in most studies NAC is associated with a lower peak in serum transaminase levels after OLT29,30 although this is not reflected in all studies.31 It has also been observed that the use of NAC may be associated with a lower incidence of primary non-function29 and less severe rejection as compared with controls.30
In this study, we have shown that in patients undergoing an elective hepatic resection the use of NAC does not appear to be associated with a reduction in hepatocellular injury as measured by serum ALT levels. Overall, NAC-treated patients experienced a higher frequency of PHLF which was predominantly of grade A and appeared to be a consequence of the more exaggerated increases in post-operative serum bilirubin in this group. There was a trend towards a lower incidence of clinically significant PHLF (grades B & C) and overall complications in NAC-treated patients although these differences did not reach statistical significance.
NAC administration was associated with a statistically significant prolongation of the PT on post-operative day 3 which returned to match that of controls beyond this point. This resolution is consistent with the cessation of drug infusion in the majority of patients. NAC has been previously reported to result in a prolonged PT in healthy volunteers through direct effects on the vitamin K-dependent clotting factors II, VII, IX and X.32,33 This may occur as a result of NAC-mediated interruption of disulphide bonds necessary for the function of these clotting factors.34 As a result of these and other studies, caution has been advised in making the assumption that a prolonged PT equates to impaired liver function in patients being treated with NAC.35
Patients in this study did not undergo a formal assessment of the functional reserve of the liver such as the LiMAx test or measurement of the indiocyanine green retention rate.36,37 Patients who have received pre-operative chemotherapy therapy are more likely to have an elevated indiocyanine green retention rate, particularly in the context of oxaliplatin-induced sinusoidal injury.38,39 It is known that oxaliplatin-based chemotherapy can result in depletion of intracellular GSH perhaps thereby increasing the susceptibility of these patients to ROS-mediated liver injury.40 It may be that the focused use of peri-operative NAC in this cohort may prove to be beneficial.
In summary, in this study we were not able to demonstrate any evidence in support of the routine administration of NAC after a liver resection. While it may be possible that our study was underpowered to detect a true protective effect of NAC, it may also be that our inclusion criteria were too broad. Further studies may be better served to focus on those patients at a higher risk of PHLF such as those undergoing a more extensive resection, particularly with a background of significant parenchymal disease.
Conflicts of interest
The authors have no relevant conflicts of interest to declare.
References
- Shoup M, Gonen M, D'Angelica M, Jarnagin WR, DeMatteo RP, Schwartz LH, et al. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003;7:325–330. doi: 10.1016/s1091-255x(02)00370-0. [DOI] [PubMed] [Google Scholar]
- Karoui M, Penna C, Amin-Hashem M, Mitry E, Benoist S, Franc B, et al. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases.[see comment. Ann Surg. 2006;243:1–7. doi: 10.1097/01.sla.0000193603.26265.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrns KE, Tsiotos GG, DeSouza NF, Krishna MK, Ludwig J, Nagorney DM. Hepatic steatosis as a potential risk factor for major hepatic resection. J Gastrointest Surg. 1998;2:292–298. doi: 10.1016/s1091-255x(98)80025-5. [DOI] [PubMed] [Google Scholar]
- Gomez D, Malik HZ, Bonney GK, Wong V, Toogood GJ, Lodge JP, et al. Steatosis predicts postoperative morbidity following hepatic resection for colorectal metastasis. Br J Surg. 2007;94:1395–1402. doi: 10.1002/bjs.5820. [DOI] [PubMed] [Google Scholar]
- van den Broek MA, Olde Damink SW, Dejong CH, Lang H, Malago M, Jalan R, et al. Liver failure after partial hepatic resection: definition, pathophysiology, risk factors and treatment. Liver Int. 2008;28:767–780. doi: 10.1111/j.1478-3231.2008.01777.x. [DOI] [PubMed] [Google Scholar]
- Belghiti J, Noun R, Malafosse R, Jagot P, Sauvanet A, Pierangeli F, et al. Continuous versus intermittent portal triad clamping for liver resection: a controlled study. Ann Surg. 1999;229:369–375. doi: 10.1097/00000658-199903000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardanian AJ, Busuttil RW, Kupiec-Weglinski JW. Molecular mediators of liver ischemia and reperfusion injury: a brief review. Mol Med. 2008;14:337–345. doi: 10.2119/2007-00134.Vardanian. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klune JR, Tsung A. Molecular biology of liver ischemia/reperfusion injury: established mechanisms and recent advancements. Surg Clin North Am. 2010;90:665–677. doi: 10.1016/j.suc.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Fukuzawa K, Emre S, Senyuz O, Acarli K, Schwartz ME, Miller CM. N-acetylcysteine ameliorates reperfusion injury after warm hepatic ischemia. Transplantation. 1995;59:6–9. doi: 10.1097/00007890-199501150-00002. [DOI] [PubMed] [Google Scholar]
- Davis W, Jr, Ronai Z, Tew KD. Cellular thiols and reactive oxygen species in drug-induced apoptosis. J Pharmacol Exp Ther. 2001;296:1–6. [PubMed] [Google Scholar]
- Zafarullah M, Li WQ, Sylvester J, Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci. 2003;60:6–20. doi: 10.1007/s000180300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jegatheeswaran S, Siriwardena AK. Experimental and clinical evidence for modification of hepatic ischaemia-reperfusion injury by N-acetylcysteine during major liver surgery. HPB. 2011;13:71–78. doi: 10.1111/j.1477-2574.2010.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay A, Cassidy D, Sutherland F, Dixon E. Clinical results of N-acetylcysteine after major hepatic surgery: a review. J Hepatobiliary Pancreat Surg. 2008;15:473–478. doi: 10.1007/s00534-007-1306-6. [DOI] [PubMed] [Google Scholar]
- Santiago FM, Bueno P, Olmedo C, Muffak-Granero K, Comino A, Serradilla M, et al. Effect of N-acetylcysteine administration on intraoperative plasma levels of interleukin-4 and interleukin-10 in liver transplant recipients. Transplant Proc. 2008;40:2978–2980. doi: 10.1016/j.transproceed.2008.08.103. [DOI] [PubMed] [Google Scholar]
- Weigand MA, Plachky J, Thies JC, Spies-Martin D, Otto G, Martin E, et al. N-acetylcysteine attenuates the increase in alpha-glutathione S-transferase and circulating ICAM-1 and VCAM-1 after reperfusion in humans undergoing liver transplantation. Transplantation. 2001;72:694–698. doi: 10.1097/00007890-200108270-00023. [DOI] [PubMed] [Google Scholar]
- Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS) Surgery. 2011;149:713–724. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Selzner M, Rudiger HA, Sindram D, Madden J, Clavien PA. Mechanisms of ischemic injury are different in the steatotic and normal rat liver. Hepatology. 2000;32:1280–1288. doi: 10.1053/jhep.2000.20528. [DOI] [PubMed] [Google Scholar]
- Stein HJ, Oosthuizen MM, Hinder RA, Lamprechts H. Oxygen free radicals and glutathione in hepatic ischemia/reperfusion injury. J Surg Res. 1991;50:398–402. doi: 10.1016/0022-4804(91)90209-5. [DOI] [PubMed] [Google Scholar]
- Sener G, Tosun O, Sehirli AO, Kacmaz A, Arbak S, Ersoy Y, et al. Melatonin and N-acetylcysteine have beneficial effects during hepatic ischemia and reperfusion. Life Sci. 2003;72:2707–2718. doi: 10.1016/s0024-3205(03)00187-5. [DOI] [PubMed] [Google Scholar]
- Galhardo MA, Junior CQ, Riboli Navarro PG, Morello RJ, De Jesus Simoes M, De Souza Montero EF. Liver and lung late alterations following hepatic reperfusion associated to ischemic preconditioning or N-acetylcysteine. Microsurgery. 2007;27:295–299. doi: 10.1002/micr.20359. [DOI] [PubMed] [Google Scholar]
- Keles MS, Demirci N, Yildirim A, Atamanalp SS, Altinkaynak K. Protective effects of N-acetylcysteine and Ginkgo biloba extract on ischaemia-reperfusion-induced hepatic DNA damage in rats. Clin Exp Med. 2008;8:193–198. doi: 10.1007/s10238-008-0005-1. [DOI] [PubMed] [Google Scholar]
- Hur GM, Ryu YS, Yun HY, Jeon BH, Kim YM, Seok JH, et al. Hepatic ischemia/reperfusion in rats induces iNOS gene transcription by activation of NF-kappaB. Biochem Biophys Res Commun. 1999;261:917–922. doi: 10.1006/bbrc.1999.1143. [DOI] [PubMed] [Google Scholar]
- Robinson SM, Mann DA. Role of nuclear factor kappaB in liver health and disease. Clin Sci (Lond) 2010;118:691–705. doi: 10.1042/CS20090549. [DOI] [PubMed] [Google Scholar]
- Koeppel TA, Thies JC, Lehmann T, Gebhard MM, Herfarth C, Otto G, et al. Improvement of hepatic microhemodynamics by N-acetylcysteine after warm ischemia. Eur Surg Res. 1996;28:270–277. doi: 10.1159/000129466. [DOI] [PubMed] [Google Scholar]
- Glantzounis GK, Yang W, Koti RS, Mikhailidis DP, Seifalian AM, Davidson BR. Continuous infusion of N-acetylcysteine reduces liver warm ischaemia-reperfusion injury. Br J Surg. 2004;91:1330–1339. doi: 10.1002/bjs.4694. [DOI] [PubMed] [Google Scholar]
- Fusai G, Glantzounis GK, Hafez T, Yang W, Quaglia A, Sheth H, et al. N-Acetylcysteine ameliorates the late phase of liver ischaemia/reperfusion injury in the rabbit with hepatic steatosis. Clin Sci (Lond) 2005;109:465–473. doi: 10.1042/CS20050081. [DOI] [PubMed] [Google Scholar]
- Koeppel TA, Lehmann TG, Thies JC, Gehrcke R, Gebhard MM, Herfarth C, et al. Impact of N-acetylcysteine on the hepatic microcirculation after orthotopic liver transplantation. Transplantation. 1996;61:1397–1402. doi: 10.1097/00007890-199605150-00020. [DOI] [PubMed] [Google Scholar]
- Taut FJ, Schmidt H, Zapletal CM, Thies JC, Grube C, Motsch J, et al. N-acetylcysteine induces shedding of selectins from liver and intestine during orthotopic liver transplantation. Clin Exp Immunol. 2001;124:337–341. doi: 10.1046/j.1365-2249.2001.01531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thies JC, Teklote J, Clauer U, Tox U, Klar E, Hofmann WJ, et al. The efficacy of N-acetylcysteine as a hepatoprotective agent in liver transplantation. Transpl Int. 1998;11(Suppl. 1):S390–S392. doi: 10.1007/s001470050505. [DOI] [PubMed] [Google Scholar]
- Bucuvalas JC, Ryckman FC, Krug S, Alonso MH, Balistreri WF, Kotagal U. Effect of treatment with prostaglandin E1 and N-acetylcysteine on pediatric liver transplant recipients: a single-center study. Pediatr Transplant. 2001;5:274–278. doi: 10.1034/j.1399-3046.2001.005004274.x. [DOI] [PubMed] [Google Scholar]
- Steib A, Freys G, Collin F, Launoy A, Mark G, Boudjema K. Does N-acetylcysteine improve hemodynamics and graft function in liver transplantation? Liver Transpl Surg. 1998;4:152–157. doi: 10.1002/lt.500040204. [DOI] [PubMed] [Google Scholar]
- Knudsen TT, Thorsen S, Jensen SA, Dalhoff K, Schmidt LE, Becker U, et al. Effect of intravenous N-acetylcysteine infusion on haemostatic parameters in healthy subjects. Gut. 2005;54:515–521. doi: 10.1136/gut.2004.043505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsen S, Teisner A, Jensen SA, Philips M, Dalhoff K, Bendtsen F. Effect of N-acetylcysteine on the accuracy of the prothrombin time assay of plasma coagulation factor II+VII+X activity in subjects infused with the drug. Influence of time and temperature. Scand J Clin Lab Invest. 2009;69:643–650. doi: 10.3109/00365510902943262. [DOI] [PubMed] [Google Scholar]
- Koterba AP, Smolen S, Joseph A, Basista MH, Brecher AS. Coagulation protein function. II. Influence of thiols upon acetaldehyde effects. Alcohol. 1995;12:49–57. doi: 10.1016/0741-8329(94)00069-p. [DOI] [PubMed] [Google Scholar]
- Schmidt LE, Knudsen TT, Dalhoff K, Bendtsen F. Effect of acetylcysteine on prothrombin index in paracetamol poisoning without hepatocellular injury. Lancet. 2002;360:1151–1152. doi: 10.1016/S0140-6736(02)11194-9. [DOI] [PubMed] [Google Scholar]
- Stockmann M, Lock JF, Malinowski M, Niehues SM, Seehofer D, Neuhaus P. The LiMAx test: a new liver function test for predicting postoperative outcome in liver surgery. HPB. 2010;12:139–146. doi: 10.1111/j.1477-2574.2009.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris-Stiff G, Gomez D, Prasad R. Quantitative assessment of hepatic function and its relevance to the liver surgeon. J Gastrointest Surg. 2009;13:374–385. doi: 10.1007/s11605-008-0564-1. [DOI] [PubMed] [Google Scholar]
- Krieger PM, Tamandl D, Herberger B, Faybik P, Fleischmann E, Maresch J, et al. Evaluation of chemotherapy-associated liver injury in patients with colorectal cancer liver metastases using indocyanine green clearance testing. Ann Surg Oncol. 2011;18:1644–1650. doi: 10.1245/s10434-010-1494-1. [DOI] [PubMed] [Google Scholar]
- Nakano H, Oussoultzoglou E, Rosso E, Casnedi S, Chenard-Neu MP, Dufour P, et al. Sinusoidal injury increases morbidity after major hepatectomy in patients with colorectal liver metastases receiving preoperative chemotherapy. Ann Surg. 2008;247:118–124. doi: 10.1097/SLA.0b013e31815774de. [DOI] [PubMed] [Google Scholar]
- Kweekel DM, Gelderblom H, Guchelaar HJ. Pharmacology of oxaliplatin and the use of pharmacogenomics to individualize therapy. Cancer Treat Rev. 2005;31:90–105. doi: 10.1016/j.ctrv.2004.12.006. [DOI] [PubMed] [Google Scholar]


