Abstract
Vγ2Vδ2 T (also known as Vγ9Vδ2 T) cells exist only in primates, and in humans represent a major γδ T-cell sub-population in the total population of circulating γδ T cells. Results from recent studies suggest that while (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP) phosphoantigen from Mycobacterium tuberculosis (Mtb) and other microbes activates and expands primate Vγ2Vδ2 T cells, the Vγ2Vδ2 T-cell receptor (TCR) recognizes and binds to HMBPP on antigen-presenting cells (APC). In response to HMBPP stimulus, Vγ2Vδ2 TCRs array to form signaling-related nanoclusters or nanodomains during the activation of Vγ2Vδ2 T cells. Primary infections with HMBPP-producing pathogens drive the evolution of multieffector functional responses in Vγ2Vδ2 T cells, although Vγ2Vδ2 T cells display different patterns of responses during the acute and chronic phases of Mtb infection and in other infections. Expanded Vγ2Vδ2 T cells in primary Mtb infection can exhibit a broader TCR repertoire and a greater clonal response than previously assumed, with different distribution patterns of Vγ2Vδ2 T-cell clones in lymphoid and non-lymphoid compartments. Emerging in vivo data suggest that HMBPP activation of Vγ2Vδ2 T cells appears to impact other immune cells during infection.
Keywords: (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate, γδ T cell, HMBPP, human infection, phosphoantigen, T cell receptor, T-cell response, tuberculosis
Introduction
Human γδ T cells appear to function as non-classical T cells and contribute to both innate and adaptive immune responses in infections with Mycobacterium tuberculosis (Mtb) and other pathogens.1,2,3,4 In humans, there is a unique γδ T-cell sub-population, termed Vγ2Vδ2 T cells (Vγ9Vδ2 T cells), which express T-cell receptor (TCR) comprising Vγ2 and Vδ2 chains. Vγ2Vδ2 T cells exist only in primates (both human and non-human) and represent a major circulating γδ T-cell subset that typically constitutes up to 65%–90% of total peripheral blood γδ T cells.
Vγ2Vδ2 T cells can be activated by metabolites from isoprenoid synthesis, such as isopentenyl pyrophosphate and (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP), which are usually referred to as phosphoantigens.5,6 Isoprenoids are produced by one of two major pathways: the classical mevalonate pathway and the alternative, non-mevalonate pathway. Isopentenyl pyrophosphate is an intermediate metabolite that is present in both pathways, whereas HMBPP is only produced in the non-mevalonate pathway by certain microbes including Mtb and Listeria monocytogenes (LM).5,6 HMBPP is approximately 1000-fold more potent than isopentenyl pyrophosphate for the in vitro activation of Vγ2Vδ2 T cells.6 Infections with Mtb and other selected microbes that produce phosphoantigens have been shown to activate or expand Vγ2Vδ2 T cells in humans and in non-human primates.7 This article reviews the recent progress in characterizing the responses of Vγ2Vδ2 T cells to infection with Mtb and other microbes at the molecular and cellular levels.
Vγ2Vδ2 TCR-dependent recognition of HMBPP and TCR-driven activation of Vγ2Vδ2 T cells
Vγ2Vδ2 T cells in humans and non-human primates are the only known γδ T-cell subset capable of recognizing a microbial phosphoantigen, as there appears to be no convincing evidence that γδ T cells from mice and other species can recognize or bind HMBPP or other phospholigands.1,2,3,4 Work over the past decade has elucidated the chemistry of HMBPP and other phospholigands and has characterized the ability of these molecules to activate Vγ2Vδ2 T cells.6,8 However, some aspects of the molecular interaction between HMBPP and Vγ2Vδ2 T cells remain to be described. Specifically, additional studies are required to test the hypothesis that the Vγ2Vδ2 TCR recognizes HMBPP and drives the activation of Vγ2Vδ2 T cells.5,9,10,11,12,13,14,15 Molecular imaging and TCR binding visualization may provide the novel approaches that are necessary to understanding the molecular aspects of the Vγ2Vδ2 T-cell interaction with HMBPP.
Our TCR knowledge and major histocompatibility complex (MHC) tetramer technology16,17 have been employed to develop a soluble, high-affinity-binding Vγ2Vδ2 TCR tetramer for detailed studies of γδ TCR function.18 Soluble Vγ2Vδ2 TCR tetramer, once labeled fluorescently, permits the visualization of TCR binding to HMBPP presented on the surface of an antigen-presenting cell (APC).18 The Vγ2Vδ2 TCR tetramer binds exogenous HMBPP on the APC membrane with an appreciable affinity18 (Figure 1). Binding of the Vγ2Vδ2 TCR tetramer is specific, as membrane HMBPP can be recognized only by the Vγ2Vδ2 TCR but not the Vγ2Vδ1 TCR tetramer.18 A similar specificity is observed for Vγ2Vδ2 TCR tetramer binding to ‘endogenous' phospholigand presented on the membrane of mycobacterium-infected dendritic cells (DCs).18 These results suggest that HMBPP phosphoantigen activation of Vγ2Vδ2 T cells is TCR-dependent18 and that infected DCs or macrophages can rapidly present HMBPP to Vγ2Vδ2 TCRs, resulting in the early activation and expansion of Vγ2Vδ2 T cells during infections with HMBPP-producing pathogens.
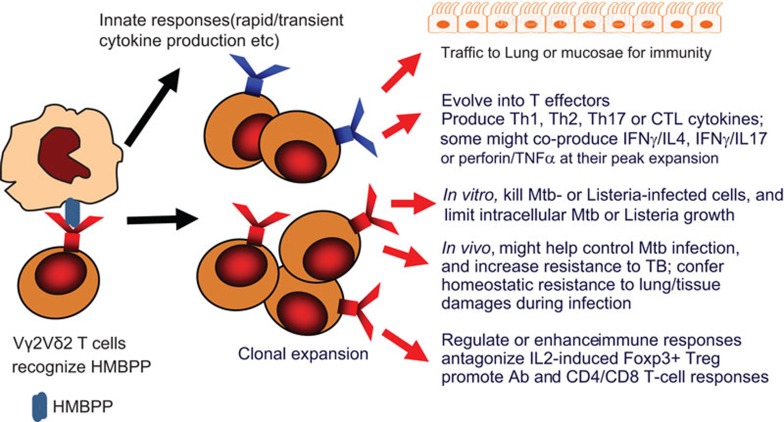
Figure 1.
Immune responses and functions of HMBPP-specific Vγ2Vδ2 T cells in infections. Shown are drawings describing the following immune events: (i) Vγ2Vδ2 TCR recognition of HMBPP phosphoantigen on the APC surface; (ii) innate immune responses of HMBPP-activated Vγ2Vδ2 T cells; (iii) clonal expansion of Vγ2Vδ2 T cells in response to signals from HMBPP/TCR and cytokines; (iv) trafficking of Vγ2Vδ2 T cells to lungs or mucosal surfaces; (v) evolving T effector functions of producing/coproducing cytokines; (vi) anti-TB or anti-Listeria activities of Vγ2Vδ2 T cells in vitro; (vii) potential in vivo functions of Vγ2Vδ2 T cells against TB; (viii) immune enhancing function of Vγ2Vδ2 T cells. APC, antigen-presenting cell; HMBBP, (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate; TB, tuberculosis; TCR, T-cell receptor.
With regard to immune recognition, Vγ2Vδ2 TCR binding to HMBPP on APCs may trigger HMBPP-engaged TCR events that drive the activation and subsequent expansion of Vγ2Vδ2 T cells early in infection. To evaluate this hypothesis, HMBPP-triggered TCR molecular responses were visualized using an innovative approach coupling near-field optical microscopy and fluorescent quantum dot nanotechnology, which allows for nanoscale molecular imaging (<50 nm) of events in immune cells at the best optical resolution.19 Such a best-optical-resolution molecular imaging system demonstrated that non-stimulating Vγ2Vδ2 TCR molecules in resting γδ T cells are evenly distributed across the cell surface at a mean distance of less than 50–70 nm.19 In contrast, HMBPP pulsation of APCs rapidly induces the formation of Vγ2Vδ2 TCR nanoclusters approximately 120–300 nm in diameter.19 These TCR molecular events coincide with the translocation of PKCè to the membrane for signal transduction and activation of Vγ2Vδ2 T cells (Zhong et al., 2012). Surprisingly, Vγ2Vδ2 TCR nanoclusters can be sustained on the membrane during the in vivo clonal expansion of Vγ2Vδ2 T cells following HMBPP/IL-2 treatment and mycobacterial infection. TCR nanoclusters can array to form nanodomains or microdomains on the membranes of clonally expanded Vγ2Vδ2 T cells.19 Interestingly, expanded Vγ2Vδ2 T cells bearing TCR nanoclusters or nanodomains were able to recognize phosphoantigen a second time and display an improved effector function.19 These novel findings suggest that the Vγ2Vδ2 TCR plays a fundamental role in the recognition of HMBPP and the subsequent activation and expansion of Vγ2Vδ2 T cells during infection with HMBPP-producing pathogens.
Patterns of Vγ2Vδ2 T-cell immune responses during acute and chronic phases of Mtb infection and other infections
Major expansion of Vγ2Vδ2 T cells in primary infections with Mtb and other pathogens
Infections of humans and non-human primates with HMBPP-producing microbes can induce a major expansion of Vγ2Vδ2 T cells.7 Typically, M. bovis BCG and Mtb both produce HMBPP, and early infections of macaques with these mycobacteria can induce a remarkable expansion of Vγ2Vδ2 T cells (1, Figure 1). Surprisingly, reinfection with BCG or BCG immunization followed by Mtb challenge can induce a rapid recall expansion or adaptive immune response in macaques.1 The recurrence of clonotypic TCR sequences in Vγ2Vδ2 T cells is also evident during rapid recall expansion, which suggests a memory-like response.1 In addition, an appreciable expansion of Vγ2Vδ2 T cells can be detected in the blood and lung tissues of macaques with primary pneumonic plague following inhalational Yersinia pestis infection (20, and data not shown). Furthermore, new studies have provided additional in vivo evidence that the rapid recall expansion or adaptive immune response of Vγ2Vδ2 T cells can be induced during reinfection of macaques with LM capable of producing HMBPP.21 In fact, increases in human γδ T cells and Vγ2Vδ2 T cells were reported in leprosy granulomatous disease and in tuberculous meningitis, respectively (see review 22). Additional examples of infection-driven expansion of human γδ T cells include salmonellosis, brucellosis, legionellosis, tularemia, malaria, toxoplasmosis and leishmaniasis, among others (see review 22). The notion that human Vγ2Vδ2 T cells can mount an adaptive immune response during infection is further supported by data from other human studies.7,8,23,24,25,26,27
Role of cytokines in HMBPP expansion of Vγ2Vδ2 T cells during infection
Previous work has shown that IL-2, IL-15 or IL-21 is needed for HMBPP-mediated expansion of Vγ2Vδ2 T cells in culture.1,28,29 Consistent with this phenomenon, in vivo studies have demonstrated that only HMBPP plus IL-2 cotreatment, but not IL-2 or HMBPP alone, can induce major expansion of Vγ2Vδ2 T cells in macaques,30,31 although IL-2 treatment of macaques expands CD4+CD25+Foxp3+ Treg cells.32,33 Primary infection of macaques or humans with a number of viral or bacterial pathogens incapable of producing HMBPP does not induce an appreciable expansion of Vγ2Vδ2 T cells, despite the fact that these primary infections can lead to the production of high levels of cytokines1,34 (Ryan et al., 2012). This finding argues that HMBPP, but not other antigens, is responsible for the expansion of Vγ2Vδ2 T cells in infections with HMBPP-producing pathogens.
Other cytokines may also contribute to the HMBPP-stimulated expansion of Vγ2Vδ2 T cells in infections with HMBPP-producing microbes. Interestingly, infection of macaques with Mtb and BCG induced a major expansion of Vγ2Vδ2 T cells and the coincident expression of variant IL-4 (VIL-4) mRNA encoding a protein comprising the N-terminal 97 amino acids (a.a.) of IL-4 and a unique C-terminal 96 a.a. domain that includes a signaling-related proline-rich motif. Upon expression and purification, VIL-4 acts as a novel cytokine to induce the expansion of HMBPP-stimulated Vγ2Vδ2 T cells in a dose- and time-dependent manner.2 Surprisingly, upon HMBPP stimulation, VIL-4-expanded Vγ2Vδ2 T cells appear to be heterologous effector cells capable of producing IL-4, interferon (IFN)-γ and tumor necrosis factor (TNF)-α.2 Thus, mycobacterial infection in macaques induces a variant IL-4 transcript that functions as a growth factor promoting the expansion of HMBPP-specific Vγ2Vδ2 T effector cells.2 It is likely that other cytokines may exert similar effects on Vγ2Vδ2 T cells during mycobacterial infection and function as a ‘second or third signal' for sustaining HMBPP/TCR-mediated activation and clonal expansion during infection.
Effector response of Vγ2Vδ2 T cells is ‘depressed' in chronically active tuberculosis (TB) or HIV disease but quite potent in latent Mtb infection
Similar to CD4+ or CD8+ T cells, Vγ2Vδ2 T cells appear to be depressed in frequency or effector function during chronically active tuberculosis or in the chronic phase of HIV infection.34,35 It is argued that these depressed Vγ2Vδ2 T cells may contribute to the development of active TB or be a result of immune dysfunction in chronic tuberculosis. It is also possible that the prolonged exposure of Vγ2Vδ2 T cells to significant levels of Mtb HMBPP and to cytokines in chronic TB leads to phenotypic and functional changes that in turn alter the responses and effector function of these cells. This hypothesis is supported by data indicating that Vγ2Vδ2 T cells remain competent in latent Mtb infection.36 To further address this issue, we investigated the immune responses of Vγ2Vδ2 T cells in the context of HIV-1/Mtb coinfection in humans. We demonstrated that HIV-infected TB patients exhibited a similar number or fewer Vγ2Vδ2 T cells or IFNγ-producing Vγ2Vδ2 T cells when compared to healthy individuals.37 Interestingly, there was a significantly greater number of HMBPP-driven IFNγ+ Vγ2Vδ2 T cells in HIV-1+ individuals with a latent Mtb infection than in HIV-1+ TB patients and in healthy individuals. The potent response of Vγ2Vδ2 T effector cells in HIV-1 infection coincides with the maintenance of latent Mtb infection in those HIV-1+ persons whose CD4+ T-cell count is >200/μl.37 This finding suggests that Vγ2Vδ2 T cells are involved in the host responses to latent Mtb infection, even in HIV-1-infected individuals.
‘Depressed' Vγ2Vδ2 T cells in SHIV-infected individuals appear to undergo in vivo expansion and mount effector function in response to phosphoantigen/IL-2 treatment
While previous studies have suggested that Vδ2 or Vγ2 T cells in HIV-1-infected individuals are susceptible to deletion or dysfunction, it was not known whether Vγ2Vδ2 T cells could maintain some degree of functional response to HMBPP phosphoantigen stimulation in vivo. To address this question, chronically simian-human immunodeficiency virus (SHIV)-infected macaques with CD4+ T-cell counts <500/μl were treated with HMBPP phosphoantigen and IL-2 and the expansion and effector function of Vγ2Vδ2 T cells was assessed.31 HMBPP/IL-2 administration during both the acute and chronic phases of SHIV infection induced massive activation and expansion of Vγ2Vδ2 T cells and stimulated Vγ2Vδ2 T-cell effector functions including trafficking to the lungs and the production of antimicrobial cytokines.31 These results are consistent with the restoration of ‘depressed' Vγ2Vδ2 T-cell function by antiretroviral treatment of SIVmac-infected macaques.34 Additional data suggest that HMBPP/IL-2 immune stimulation could overcome potentially ‘depressed' Vγ2Vδ2 T cells in HIV infection and allow Vγ2Vδ2 T cells to mount an antimicrobial response. However, detailed comparisons may reveal some differences in magnitude and length of responses between normal and SHIV/HIV-infected individuals. Nevertheless, these findings may temporally be applicable to combating HMBPP-producing opportunistic pathogens in individuals chronically infected with HIV.
TCR repertoire and clonal responses of HMBPP-specific Vγ2Vδ2 T cells in lymphoid and non-lymphoid compartments during Mtb infection
Compared to αβ T cells, γδ T cells constitute a minor T-cell population in the total T-cell pool. In this context, the TCR repertoire of γδ T cells is considered to be smaller than that of αβ T cells. However, it is not known whether the HMBPP phosphoantigen-specific Vγ2Vδ2 T-cell sub-population has a comparable or a smaller TCR repertoire than an epitope-specific CD4+ or CD8+ T-cell sub-population. Over the past two decades, we have defined the TCR repertoire of a dominant CD8+ T-cell sub-population specific for peptide presented by a protective MHC class I allele (Mamu-A*01) in SIVmac-infected rhesus macaques.16,38,39 Using the same approach, we have recently characterized the TCR repertoire of purified protein derivative (PPD)/epitope-specific CD4+/CD8+ T cells and of an HMBPP-specific Vγ2Vδ2 T-cell sub-population in Mtb-infected rhesus macaques.40,41 These comprehensive studies have allowed us to estimate the size of the TCR repertoire of the HMBPP-specific Vγ2Vδ2 T-cell sub-population in comparison to that of peptide-specific αβ T cells in macaques.
Approximately 80 dominant clonotypic TCR sequences representing approximately 10 Vβ families have been identified in the SIVmac peptide-specific CD8+ T-cell sub-population sorted either by MHC class I/peptide tetramer binding or by isolation of CTL clones from 11 MamuA*01+, SIVmac-infected macaques.16,38,39 Similarly, approximately 90 dominant clonotypic sequences representing approximately 12 Vβ families were detected in PPD-specific CD4+ or CD8+ T cells during Mtb infection in five macaques.40 Interestingly, these same five Mtb-infected macaques exhibited >200 dominant clonotypic TCR sequences representing unique HMBPP-specific Vγ2Vδ2 T-cell clones.41 These data suggest that expanded HMBPP-specific Vγ2Vδ2 T cells employ an unusually broad TCR repertoire in TB. In fact, the majority of TCR clones that were found in the lymphoid system, lung, kidney and liver were unique, despite the fact that a small number of clonotypes detected in the blood could be repeatedly identified in the spleen, kidney or lung tissues.41 Notably, all three Jδ segments were employed by Vγ2Vδ2 T cells.41
These results are consistent with our previous observation that even during advanced SIVmac infection, the TCR repertoire of macaque Vγ2Vδ2 T cells remained broad, with surprisingly large pools of distinct TCR clonotypes.34 Broad TCR repertoires may be attributed to the repeated DN–DN regions and to the unlimited selection of three Jδ segments during TCR development. Functionally, the broad TCR repertoires of Vγ2Vδ2 T cells appear to permit massive proliferation and expansion of these γδ T cells without constraint. Either treatment with HMBPP plus IL-2 or mycobacterial infection can rapidly induce an up to 400-fold expansion of Vγ2Vδ2 T cells—expanding to comprise 80% of total CD3+ T cells from a baseline level of 1%.19,42 These results therefore suggest that the HMBPP-driven Vγ2Vδ2 T-cell sub-population that arises during Mtb infection in macaques displays an unexpectedly broad TCR repertoire. In addition, these findings appear to argue for a paradigm that an HMBPP-specific Vγ2Vδ2 T-cell sub-population may not have a smaller or narrower TCR repertoire than an αβ T-cell sub-population that is specific for a peptide epitope.
Studies of the TCR repertoire and clonal responses of the Vγ2Vδ2 T-cell sub-population in TB also yield interesting information that has implications for cellular migration patterns and potential immunity in non-lymphoid tissues. While polyclonally expanded Vγ2Vδ2 T-cell clones from lymphoid tissues appear to localize to lung granulomas at the end point of Mtb infection by aerosol,41 some TCR clones appear to be more dominant than others in lymphocytes from liver or kidney tissues without apparent TB lesions. TCR CDR3 spectratyping revealed such clonal dominance, and the accumulation of dominant Vγ2Vδ2 T-cell clones in kidney and liver tissues was associated with undetectable or low-level TB burdens. Consistently, Vγ2Vδ2 T cells producing the anti-TB cytokine IFNγ were present in ‘lesion-free' kidney and liver tissue following a late or subtle infection in these distal organs.43 These findings raise the question as to whether a timely response by Vγ2Vδ2 T effector cells might indeed contribute to the resistance to TB lesions observed during late or subtle Mtb infection of liver and kidney tissues following the dissemination of pulmonary Mtb infection. Notably, unlike vaccine-protected macaques, unvaccinated animals infected by the pulmonary route with approximately 500 CFU Mtb exhibited significant delays in development and in pulmonary trafficking of Ag-specific αβ and γδ T effector cells producing IFNγ these animals develop severe TB lesions in the lungs.1,40,44
Severe TB was also associated with transient extrathoracic Mtb dissemination at approximately 10–20 days after pulmonary Mtb infection43 and resulted in the late and subtle infection of remote organs (kidney and liver). Activation of Vγ2Vδ2 T cells may be initiated sometime after pulmonary Mtb infection and may be augmented appreciably at the time when a late or subtle infection is occurring in the kidney or liver (days 10–20). The clonal dominance of expanded Vγ2Vδ2 T cells in kidney or liver tissues suggests that these γδ T cells might efficiently traffic to these tissues and limit Mtb-mediated damage and the formation of lesions. This notion is consistent with earlier findings that Vγ2Vδ2 T effector cells are able to confer homeostatic protection against Y. pestis lesions in the lung.20 Although the current study was not conclusive, the findings provide a rationale for future studies to determine if the timely response of Vγ2Vδ2 T effectors plays a role in limiting TB lesions during a late or subtle Mtb infection of the kidney and liver after an initial pulmonary exposure to Mtb.
Multieffector-functional immune responses of HMBPP-specific Vγ2Vδ2 T cells in infection
Evolving Vγ2Vδ2 T effectors producing or coproducing Th1, Th2, Th17 and CTL cytokines in infection
Recent studies suggest that Vγ2Vδ2 T cells appear to be plastic, evolving into effector cells that produce different cytokines in response to phosphoantigen and/or ‘second/third' signals. To understand the evolution of γδ T cells, the functional response of these cells was studied in the contexts of microbial infection and HMBPP/IL-2 treatment. Primary mycobacterial infection or HMBPP/IL-2 administration stimulated a major expansion of Vγ2Vδ2 T cells, and significant numbers of expanded Vγ2Vδ2 T cells can produce the anti-TB cytokines IFN-γ, TNF-α and perforin after HMBPP engagement in vitro.9,31,41 Th1-like γδ T effector cells can be directly detected even without in vitro HMBPP stimulation.9,31
It is hypothesized that the evolution of Vγ2Vδ2 T cells could be readily evaluated during LM infection of non-human primates, as LM is the only pathogen known to possess both the mevalonate and non-mevalonate isoprenoid biosynthesis pathways that produce the metabolic phosphates or phosphoantigens that activate primate Vγ2Vδ2 T cells. While subclinical systemic infection and reinfection of macaques with attenuated LM appears to mimic the immunological aspects of human systemic LM infection, this method of infection demonstrates the ability of Vγ2Vδ2 T cells to mount an expansion or recall-like expansion and to traffic to and accumulate in the pulmonary compartment and intestinal mucosa. Interestingly, expanded Vγ2Vδ2 T cells exhibit broad effector functions and produce or coproduce Th1, Th2, Th17 or cytotoxic cytokines during the adaptive immune response to LM infection.21 LM-expanded Vγ2Vδ2 T cells are able to produce IFN-γ, TNF-α, IL-4, IL-17 and perforin, with some coproducing IL-17 and IFN-γ, IL-4 and IFN-γ or TNF-α and perforin during peak γδ T-cell expansion following LM reinfection (21, Figure 1). It is noteworthy that such coproduction of Th1/Th2, Th1/Th17 or Th1/CTL cytokines can be detected by direct intracellular cytokine staining without prior in vitro HMBPP stimulation,21 resembling an in vivo effector function for spontaneous cytokine production. Simultaneous coproduction of these cytokines by T-helper cells appears to be uncommon, but not impossible, as the development of T-helper subsets that produce Th1, Th2 and Th17 cytokines is controlled tightly by single, unique master transcriptional factors such as T-bet, GATA-3 and RORγT.45,46
Effector function for killing infected target cells and inhibiting intracellular Mtb or LM bacterial replication
It is reasonable to propose that a wide range of cytokines produced by Vγ2Vδ2 T cells may allow γδ T effector cells to limit Mtb or LM infection and therefore contribute to immune protection against microbes,1,21 (Figure 1, and Chen et al., 2012). In fact, Listeria-infected target cells can be directly killed by in vivo-expanded Vγ2Vδ2 T cells in the absence of in vitro HMBPP stimulation.21 This novel finding appears to be linked to the perforin-producing effector function of Vγ2Vδ2 T cells.21 Importantly, these in vivo-expanded Vγ2Vδ2 T cells can inhibit intracellular LM replication.21 This study provides the first experimental evidence demonstrating the direct lysis of microbe-infected cells and the inhibition of intracellular microbes by in vivo-expanded Vγ2Vδ2 T cells without prior in vitro Ag stimulation. These data are indeed consistent with other studies demonstrating that extensive in vitro activation/stimulation of Vγ2Vδ2 T cells by phosphoantigen/pathogen and IL-2 allows cultured Vγ2Vδ2 T cells to lyse mycobacterium-infected cells or inhibit intracellular mycobacteria47,48 (Chen, 2012).
In vivo effector function of Vγ2Vδ2 T cells contributing to host resistance to pathogen-induced lung damage during acute pulmonary infection
One of the remarkable immune features of HMBPP-specific Vγ2Vδ2 T cells is their capability to traffic to the lungs and other mucosal surfaces upon activation and expansion42,43 (Figure 1). The possibility that Vγ2Vδ2 T effector cells can confer protection against pulmonary TB is currently under investigation (Crystal et al., 2012). Recent studies have extended the observation that HMBPP plus IL-2 treatment induces the prolonged accumulation of Vγ2Vδ2 T effector cells in lungs19,20,32,42 and have investigated whether HMBPP/IL-2-expanded Vγ2Vδ2 T cells mediate protection against acutely fatal pneumonic plague in the macaque model of inhalational Y. pestis infection. A delayed HMBPP/IL-2 administration after inhalational Y. pestis infection overcame the acute infection and induced a marked expansion of Vγ2Vδ2 T cells.20 However, expanded Vγ2Vδ2 T cells failed to control extracellular Y. pestis replication and infection, leading to extrathoracic dissemination, septicemia and fatal shock. This outcome is understandable, as an extracellular bacterial infection would typically be controlled by neutralizing antibodies and not T effector cells. Surprisingly, despite the absence of infection control, expansion of Vγ2Vδ2 T cells after HMBPP/IL-2 treatment led to the attenuation of lesions in the lungs.20 Consistent with this observation, HMBPP-activated Vγ2Vδ2 T cells accumulated in pulmonary interstitial spaces surrounding small blood vessels and airway mucosa in lung tissues with no or mild lesions.20 These infiltrating Vγ2Vδ2 T cells produced FGF-7, a homeostatic mediator against tissue damage.49 In contrast, control macaques treated with glucose plus IL-2 or glucose alone exhibited severe hemorrhaging and necrosis in most lung lobes, with no or very few Vγ2Vδ2 T cells detectable in lung tissues. These data support the paradigm that circulating Vγ2Vδ2 T cells can traffic to the lungs for homeostatic protection against tissue damage during infection. This study provides the first in vivo evidence that Vγ2Vδ2 T cells can contribute to antimicrobial immunity in humans and primates.
In vivo evidence suggesting that expansion of Vγ2Vδ2 T cells potentially downregulates Foxp3+ Treg cells and enhances Ag-specific Ab response and αβ CD4+ or CD8+ T-cell responses in infections
HMBPP/IL-2 manipulation system19,42 was also utilized to examine if HMBPP/IL-2 expansion of Vγ2Vδ2 T cells can regulate immune cells and promote Ab and T-cell responses in infections (Figure 1).
Expansion of Vγ2Vδ2 T cells can antagonize Foxp3+ Tregs and overreacting proinflammatory Th22 cells
Foxp3+ Treg cells control immune responses to self- and foreign antigens and play a major role in maintaining the balance between immunity and tolerance.50,51,52 Hyperresponsive Tregs during infection may lead to the suppression of host antimicrobial immunity.50 To examine the interplay between Treg and Vγ2Vδ2 T cells, HMBPP/IL-2-expanded Vγ2Vδ2 T cells30,32,42 were assessed for their ability to antagonize IL-2-expanded CD4+CD25+ regulatory T cells53,54,55,56 in macaques. A short-term IL-2 treatment regimen induced a marked expansion of CD4+CD25+Foxp3+ Treg cells and the subsequent suppression of the mycobacterium-induced expansion of Vγ2Vδ2 T cells during BCG infection in macaques. Surprisingly, the expansion of Vγ2Vδ2 T cells induced by the addition of the phosphoantigen Picostim (which is similar to HMBPP) to the IL-2 treatment regimen appeared to downregulate the IL-2-induced expansion of CD4+CD25+Foxp3+ T cells.32 This downregulated expansion of Tregs led to a sustained increase in the number of Vγ2Vδ2 T cells at 42 days following Picostim/IL-2 treatment of BCG-infected macaques. Consistent with this observation, the in vitro activation of Vγ2Vδ2 T cells in PBMCs by phosphoantigen plus IL-2 could downregulate the IL-2-induced expansion of CD4+CD25+Foxp3+ T cells, though this HMBPP-mediated antagonism appears to require APCs (monocytes) or other lymphocytes.32
Because activated Vγ2Vδ2 T cells alone did not directly depress the expansion of CD4+CD25+Foxp3+ T cells in culture in the absence of APCs and other PBMCs, specific cytokines produced by Vγ2Vδ2 T effector cells were assessed for the ability to downregulate the IL-2-induced proliferation of Tregs. In the proliferation assay, cytokines were neutralized using anti-IFN-γ, anti-IL-4 or anti-TGF-β neutralizing antibodies, as HMBPP phosphoantigen stimulation is able to upregulate many genes, including genes encoding these cytokines (Wang et al., data not shown). Surprisingly, while anti-TGF-β and anti-IL-4 neutralizing antibodies did not affect the HMBPP-mediated downregulation of Tregs, treatment with an anti-IFN-γ neutralizing antibody significantly reduced the ability of HMBPP-activated Vγ2Vδ2 T cells to antagonize Treg expansion.32
These results suggest that the Th1 network contributes to the ability of Vγ2Vδ2 T cells to antagonize Tregs. Furthermore, the activation of Vγ2Vδ2 T cells by Picostim+IL-2 treatment appears to reverse the Treg-driven suppression of immune responses of phosphoantigen-specific IFNγ+ or perforin+ Vγ2Vδ2 T cells and PPD-specific IFNγ+ αβ T cells.32 It is notable that HMBPP-mediated activation of Vγ2Vδ2 T cells also results in the downregulation of overreacting proinflammatory Th22 cells during Mtb infection, and that such regulatory effects appear to be mediated by the Th1 network of Vγ2Vδ2 T cells as well.9 This constraining or antagonizing of Tregs by γδ T cells has likewise been reported by other groups.57,58 Altogether, these data demonstrate that phosphoantigen activation/expansion of Vγ2Vδ2 T cells can antagonize Tregs and the Treg-associated suppression of and antimicrobial T-cell responses in mycobacterial infections. Results from studies analyzing HMBPP/IL-2 treatment of macaques in the context of mycobacterial infection provide the first evidence suggesting that certain T-cell subsets in the immune system can antagonize overreacting Th22 cells, Foxp3+ Tregs and the Treg-associated suppression of Ag-specific T-cell responses in infections.
HMBPP/IL-2 expansion of Vγ2Vδ2 T cells might promote Ab and T-cell responses in infections
Production or coproduction of Th1, Th2 and Th17 cytokines by Vγ2Vδ2 T effector cells during infection might help to activate a variety of other immune cells such as DCs/macrophages, CD4+ T subsets, CD8+ T cells and B cells, and therefore may regulate both innate and adaptive immunity. In the context of HMBPP/IL-2 immune stimulation, the massive activation/expansion of Vγ2Vδ2 T cells during chronic SHIV infection did not exacerbate SHIV disease, but instead led to a decrease or subtle change in the viral load.
Surprisingly, although the viral antigen load was decreased upon HMBPP/IL-2 treatment during chronic SHIV infection, HMBPP activation of Vγ2Vδ2 T cells boosted HIV Env-specific Ab titers. Such increases in Ab titers were sustained for >170 days and were immediately preceded by the increased production of IFN-γ, TNF-α, IL-4 and IL-10 during peak expansion of Vγ2Vδ2 T cells that displayed memory phenotypes and by the short-term increased effector function of Vγ2Vδ2 T cells and CD4+ and CD8+ αβ T cells producing antimicrobial cytokines. The significance for these findings appears to be twofold: (i) HMBPP/Vγ2Vδ2 T cell-based intervention may be useful for combating neoplasms and HMBPP-producing opportunistic pathogens in chronically HIV-infected individuals; and (ii) HMBPP-activated Vγ2Vδ2 T cells may function as an adjuvant and facilitate the production of Abs by B cells and enhance the function of CD4+ and CD8+ αβ T cells producing antimicrobial cytokines during infection.
Notably, these observations in macaques are consistent with the in vitro findings from human studies. It has been shown that the activation of human γδ T cells can stimulate DC maturation.59,60 The HMBPP-dependent cross-talk between monocytes and human γδ T cells can drive rapid inflammatory cytokine responses and monocyte differentiation to DCs and can facilitate the development CD4 T helper cells in the presence of additional microbial stimulants.61 Activated human Vγ2Vδ2 T cells appear to function as APCs in vitro, presenting peptide antigens to CD4+ and CD8+ T cells.62,63 Vγ2Vδ2 T cells have also been shown to improve the ability of B cells to produce antibodies in vitro.59 Future definitive studies will seek to show that Vγ2Vδ2 T effector cells may potentially enhance Ag-specific Ab responses and αβ CD4+ or CD8+ T-cell responses in infections.
Acknowledgments
The author thanks the Chen Lab staff for technical support and suggestions. This work was supported by the National Institutes of Health R01 grants HL64560 and RR13601 (both to ZWC).
References
- Shen Y, Zhou D, Qiu L, Lai X, Simon M, Shen L, et al. Adaptive immune response of Vgamma2Vdelta2+ T cells during mycobacterial infections. Science. 2002;295:2255–2258. doi: 10.1126/science.1068819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z, Wang R, Lee Y, Chen CY, Yu X, Wu Z, et al. Tuberculosis-induced variant IL-4 mRNA encodes a cytokine functioning as growth factor for (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate-specific Vgamma2Vdelta2 T cells. J Immunol. 2009;182:811–819. doi: 10.4049/jimmunol.182.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien RL, Roark CL, Jin N, Aydintug MK, French JD, Chain JL, et al. Gammadelta T-cell receptors: functional correlations. Immunol Rev. 2007;215:77–88. doi: 10.1111/j.1600-065X.2006.00477.x. [DOI] [PubMed] [Google Scholar]
- Thedrez A, Sabourin C, Gertner J, Devilder MC, Allain-Maillet S, Fournié JJ, et al. Self/non-self discrimination by human gammadelta T cells: simple solutions for a complex issue. Immunol Rev. 2007;215:123–135. doi: 10.1111/j.1600-065X.2006.00468.x. [DOI] [PubMed] [Google Scholar]
- Belmant C, Espinosa E, Poupot R, Peyrat MA, Guiraud M, Poquet Y, et al. 3-Formyl-1-butyl pyrophosphate A novel mycobacterial metabolite-activating human gammadelta T cells. J Biol Chem. 1999;274:32079–32084. doi: 10.1074/jbc.274.45.32079. [DOI] [PubMed] [Google Scholar]
- Eberl M, Hintz M, Reichenberg A, Kollas AK, Wiesner J, Jomaa H. Microbial isoprenoid biosynthesis and human gammadelta T cell activation. FEBS Lett. 2003;544:4–10. doi: 10.1016/s0014-5793(03)00483-6. [DOI] [PubMed] [Google Scholar]
- Chen ZW, Letvin NL. Adaptive immune response of Vgamma2Vdelta2 T cells: a new paradigm. Trends Immunol. 2003;24:213–219. doi: 10.1016/s1471-4906(03)00032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita CT, Mariuzza RA, Brenner MB. Antigen recognition by human gamma delta T cells: pattern recognition by the adaptive immune system. Springer Semin Immunopathol. 2000;22:191–217. doi: 10.1007/s002810000042. [DOI] [PubMed] [Google Scholar]
- Yao S, Huang D, Chen CY, Halliday L, Zeng G, Wang RC, et al. Differentiation, distribution and gammadelta T cell-driven regulation of IL-22-producing T cells in tuberculosis. PLoS Pathog. 2010;6:e1000789. doi: 10.1371/journal.ppat.1000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vani J, Shaila MS, Rao MK, Krishnaswamy UM, Kaveri SV, Bayry J. B lymphocytes from patients with tuberculosis exhibit hampered antigen-specific responses with concomitant overexpression of interleukin-8. J Infect Dis. 2009;200:481–482; author reply 482–484. doi: 10.1086/599843. [DOI] [PubMed] [Google Scholar]
- Eberl M, Altincicek B, Kollas AK, Sanderbrand S, Bahr U, Reichenberg A, et al. Accumulation of a potent gammadelta T-cell stimulator after deletion of the lytB gene in Escherichia coli. Immunology. 2002;106:200–211. doi: 10.1046/j.1365-2567.2002.01414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarikonda G, Wang H, Puan KJ, Liu XH, Lee HK, Song Y, et al. Photoaffinity antigens for human gammadelta T cells. J Immunol. 2008;181:7738–7750. doi: 10.4049/jimmunol.181.11.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verreck FA, de Boer T, Langenberg DM, Hoeve MA, Kramer M, Vaisberg E, et al. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci USA. 2004;101:4560–4565. doi: 10.1073/pnas.0400983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukowski JF, Morita CT, Tanaka Y, Bloom BR, Brenner MB, Band H. V gamma 2V delta 2 TCR-dependent recognition of non-peptide antigens and Daudi cells analyzed by TCR gene transfer. J Immunol. 1995;154:998–1006. [PubMed] [Google Scholar]
- Wang H, Lee HK, Bukowski JF, Li H, Mariuzza RA, Chen ZW, et al. Conservation of nonpeptide antigen recognition by rhesus monkey V gamma 2V delta 2 T cells. J Immunol. 2003;170:3696–3706. doi: 10.4049/jimmunol.170.7.3696. [DOI] [PubMed] [Google Scholar]
- Chen ZW, Li Y, Zeng X, Kuroda MJ, Schmitz JE, Shen Y, et al. The TCR repertoire of an immunodominant CD8+ T lymphocyte population. J Immunol. 2001;166:4525–4533. doi: 10.4049/jimmunol.166.7.4525. [DOI] [PubMed] [Google Scholar]
- Wei H, Wang R, Yuan Z, Chen CY, Huang D, Halliday L, et al. DR*W201/P65 tetramer visualization of epitope-specific CD4 T-cell during M. tuberculosis infection and its resting memory pool after BCG vaccination. PLoS One. 2009;4:e6905. doi: 10.1371/journal.pone.0006905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Huang D, Lai X, Chen M, Zhong W, Wang R, et al. Definition of APC presentation of phosphoantigen (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate to Vgamma2Vdelta 2 TCR. J Immunol. 2008;181:4798–4806. doi: 10.4049/jimmunol.181.7.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Shao L, Ali Z, Cai J, Chen ZW. NSOM/QD-based nanoscale immunofluorescence imaging of antigen-specific T-cell receptor responses during an in vivo clonal V{gamma}2V{delta}2 T-cell expansion. Blood. 2008;111:4220–4232. doi: 10.1182/blood-2007-07-101691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Chen CY, Ali Z, Shao L, Shen L, Lockman HA, et al. Antigen-specific Vgamma2Vdelta2 T effector cells confer homeostatic protection against pneumonic plaque lesions. Proc Natl Acad Sci USA. 2009;106:7553–7558. doi: 10.1073/pnas.0811250106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan-Payseur B, Frencher J, Shen L, Chen CY, Huang D, Chen ZW. Multieffector-functional immune responses of HMBPP-specific Vgamma2Vdelta2 T cells in nonhuman primates inoculated with Listeria monocytogenes {Delta}actA prfA*. J Immunol. 2012;189:1285–1293. doi: 10.4049/jimmunol.1200641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZW, Letvin NL. Vgamma2Vdelta2+ T cells and anti-microbial immune responses. Microbes Infect. 2003;5:491–498. doi: 10.1016/s1286-4579(03)00074-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieli F, Poccia F, Lipp M, Sireci G, Caccamo N, di Sano C, et al. Differentiation of effector/memory Vdelta2 T cells and migratory routes in lymph nodes or inflammatory sites. J Exp Med. 2003;198:391–397. doi: 10.1084/jem.20030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitard V, Roumanes D, Lafarge X, Couzi L, Garrigue I, Lafon ME, et al. Long-term expansion of effector/memory Vdelta2-gammadelta T cells is a specific blood signature of CMV infection. Blood. 2008;112:1317–1324. doi: 10.1182/blood-2008-01-136713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poccia F, Agrati C, Castilletti C, Bordi L, Gioia C, Horejsh D, et al. Anti-severe acute respiratory syndrome coronavirus immune responses: the role played by V gamma 9V delta 2 T cells. J Infect Dis. 2006;193:1244–1249. doi: 10.1086/502975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abate G, Eslick J, Newman FK, Frey SE, Belshe RB, Monath TP, et al. Flow-cytometric detection of vaccinia-induced memory effector CD4(+), CD8(+), and gamma delta TCR(+) T cells capable of antigen-specific expansion and effector functions. J Infect Dis. 2005;192:1362–1371. doi: 10.1086/444423. [DOI] [PubMed] [Google Scholar]
- Hoft DF, Brown RM, Roodman ST. Bacille Calmette-Guerin vaccination enhances human gamma delta T cell responsiveness to mycobacteria suggestive of a memory-like phenotype. J Immunol. 1998;161:1045–1054. [PubMed] [Google Scholar]
- Vermijlen D, Ellis P, Langford C, Klein A, Engel R, Willimann K, et al. Distinct cytokine-driven responses of activated blood gammadelta T cells: insights into unconventional T cell pleiotropy. J Immunol. 2007;178:4304–4314. doi: 10.4049/jimmunol.178.7.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraviglia S, Caccamo N, Salerno A, Sireci G, Dieli F. Partial and ineffective activation of V gamma 9V delta 2 T cells by Mycobacterium tuberculosis-infected dendritic cells. J Immunol. 2010;185:1770–1776. doi: 10.4049/jimmunol.1000966. [DOI] [PubMed] [Google Scholar]
- Sicard H, Ingoure S, Luciani B, Serraz C, Fournié JJ, Bonneville M, et al. In vivo immunomanipulation of V gamma 9V delta 2 T cells with a synthetic phosphoantigen in a preclinical nonhuman primate model. J Immunol. 2005;175:5471–5480. doi: 10.4049/jimmunol.175.8.5471. [DOI] [PubMed] [Google Scholar]
- Ali Z, Yan L, Plagman N, Villinger F, Chen ZW. γδ T cell immune manipulation during chronic but not acute phase of simian-human immunodeficiency virus infection confers immunological benefits. J Immunol. 2009. [DOI] [PMC free article] [PubMed]
- Gong G, Shao L, Wang Y, Chen CY, Huang D, Yao S, et al. Phosphoantigen-activated V gamma 2V delta 2 T cells antagonize IL-2-induced CD4+CD25+Foxp3+ T regulatory cells in mycobacterial infection. Blood. 2009;113:837–845. doi: 10.1182/blood-2008-06-162792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Huang D, Yao S, Halliday L, Zeng G, Wang RC, et al. IL-2 simultaneously expands Foxp3+ T regulatory and T effector cells and confers resistance to severe tuberculosis (TB): implicative Treg-T effector cooperation in immunity to TB. J Immunol. 2012;188:4278–4288. doi: 10.4049/jimmunol.1101291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Shen Y, Huang D, Qiu L, Sehgal P, Du GZ, et al. Development of Vgamma2Vdelta2+ T cell responses during active mycobacterial coinfection of simian immunodeficiency virus-infected macaques requires control of viral infection and immune competence of CD4+ T cells. J Infect Dis. 2004;190:1438–1447. doi: 10.1086/423939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZW. Immunology of AIDS virus and mycobacterial co-infection. Curr HIV Res. 2004;2:351–355. doi: 10.2174/1570162043351147. [DOI] [PubMed] [Google Scholar]
- Dieli F, Sireci G, Caccamo N, di Sano C, Titone L, Romano A, et al. Selective depression of interferon-gamma and granulysin production with increase of proliferative response by Vgamma9/Vdelta2 T cells in children with tuberculosis. J Infect Dis. 2002;186:1835–1839. doi: 10.1086/345766. [DOI] [PubMed] [Google Scholar]
- Shao L, Zhang W, Zhang S, Chen CY, Jiang W, Xu Y, et al. Potent immune responses of Ag-specific Vgamma2Vdelta2+ T cells and CD8+ T cells associated with latent stage of Mycobacterium tuberculosis coinfection in HIV-1-infected humans. AIDS. 2008;22:2241–2250. doi: 10.1097/QAD.0b013e3283117f18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZW, Shen L, Regan JD, Kou Z, Ghim SH, Letvin NL. The T cell receptor gene usage by simian immunodeficiency virus gag-specific cytotoxic T lymphocytes in rhesus monkeys. J Immunol. 1996;156:1469–1475. [PubMed] [Google Scholar]
- Chen ZW, Yamamoto H, Watkins DI, Levinson G, Letvin NL. Predominant use of a T-cell receptor V beta gene family in simian immunodeficiency virus Gag-specific cytotoxic T lymphocytes in a rhesus monkey. J Virol. 1992;66:3913–3917. doi: 10.1128/jvi.66.6.3913-3917.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du G, Chen CY, Shen Y, Qiu L, Huang D, Wang R, et al. TCR repertoire, clonal dominance, and pulmonary trafficking of mycobacterium-specific CD4+ and CD8+ T effector cells in immunity against tuberculosis. J Immunol. 2010;185:3940–3947. doi: 10.4049/jimmunol.1001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Chen CY, Zhang M, Qiu L, Shen Y, Du G, et al. Clonal immune responses of Mycobacterium-specific gammadelta T cells in tuberculous and non-tuberculous tissues during M. tuberculosis infection. PLoS One. 2012;7:e30631. doi: 10.1371/journal.pone.0030631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali Z, Shao L, Halliday L, Reichenberg A, Hintz M, Jomaa H, et al. Prolonged (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate-driven antimicrobial and cytotoxic responses of pulmonary and systemic Vgamma2Vdelta2 T cells in macaques. J Immunol. 2007;179:8287–8296. doi: 10.4049/jimmunol.179.12.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Shen Y, Qiu L, Chen CY, Shen L, Estep J, et al. Immune distribution and localization of phosphoantigen-specific Vgamma2Vdelta2 T cells in lymphoid and nonlymphoid tissues in Mycobacterium tuberculosis infection. Infect Immun. 2008;76:426–436. doi: 10.1128/IAI.01008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Huang D, Wang RC, Shen L, Zeng G, Yao S, et al. A critical role for CD8 T cells in a nonhuman primate model of tuberculosis. PLoS Pathog. 2009;5:e1000392. doi: 10.1371/journal.ppat.1000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Hegazy AN, Peine M, Helmstetter C, Panse I, Fröhlich A, Bergthaler A, et al. Interferons direct Th2 cell reprogramming to generate a stable GATA-3(+)T-bet(+) cell subset with combined Th2 and Th1 cell functions. Immunity. 2010;32:116–128. doi: 10.1016/j.immuni.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Martino A, Casetti R, Sacchi A, Poccia F. Central memory Vgamma9Vdelta2 T lymphocytes primed and expanded by bacillus Calmette-Guerin-infected dendritic cells kill mycobacterial-infected monocytes. J Immunol (Baltimore, MD: 1950) 2007;179:3057–3064. doi: 10.4049/jimmunol.179.5.3057. [DOI] [PubMed] [Google Scholar]
- Dieli F, Troye-Blomberg M, Ivanyi J, Fournié JJ, Krensky AM, Bonneville M, et al. Granulysin-dependent killing of intracellular and extracellular Mycobacterium tuberculosis by Vgamma9/Vdelta2 T lymphocytes. J Infect Dis. 2001;184:1082–1085. doi: 10.1086/323600. [DOI] [PubMed] [Google Scholar]
- Jameson J, Ugarte K, Chen N, Yachi P, Fuchs E, Boismenu R, et al. A role for skin gammadelta T cells in wound repair. Science. 2002;296:747–749. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Riley JL, June CH, Blazar BR. Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity. 2009;30:656–665. doi: 10.1016/j.immuni.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y, Tarbell KV. Arming Treg cells at the inflammatory site. Immunity. 2009;30:322–323. doi: 10.1016/j.immuni.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Zhang H, Chua KS, Guimond M, Kapoor V, Brown MV, Fleisher TA, et al. Lymphopenia and interleukin-2 therapy alter homeostasis of CD4+CD25+ regulatory T cells. Nat Med. 2005;11:1238–1243. doi: 10.1038/nm1312. [DOI] [PubMed] [Google Scholar]
- Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107:2409–2414. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn E, Nelson EA, Mohseni M, Porcheray F, Kim H, Litsa D, et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. . Blood. 2006;108:1571–1579. doi: 10.1182/blood-2006-02-004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S, Kryczek I, Edwards RP, Zou L, Szeliga W, Banerjee M, et al. Interleukin-2 administration alters the CD4+FOXP3+ T-cell pool and tumor trafficking in patients with ovarian carcinoma. Cancer Res. 2007;67:7487–7494. doi: 10.1158/0008-5472.CAN-07-0565. [DOI] [PubMed] [Google Scholar]
- Petermann F, Rothhammer V, Claussen MC, Haas JD, Blanco LR, Heink S, et al. gammadelta T cells enhance autoimmunity by restraining regulatory T cell responses via an interleukin-23-dependent mechanism. Immunity. 2010;33:351–363. doi: 10.1016/j.immuni.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castella B, Riganti C, Fiore F, Pantaleoni F, Canepari ME, Peola S, et al. Immune modulation by zoledronic acid in human myeloma: an advantageous cross-talk between Vgamma9Vdelta2 T cells, alphabeta CD8+ T cells, regulatory T cells, and dendritic cells. J Immunol. 2011;187:1578–1590. doi: 10.4049/jimmunol.1002514. [DOI] [PubMed] [Google Scholar]
- Caccamo N, Battistini L, Bonneville M, Poccia F, Fournié JJ, Meraviglia S, et al. CXCR5 identifies a subset of Vgamma9Vdelta2 T cells which secrete IL-4 and IL-10 and help B cells for antibody production. J Immunol. 2006;177:5290–5295. doi: 10.4049/jimmunol.177.8.5290. [DOI] [PubMed] [Google Scholar]
- Ismaili J, Olislagers V, Poupot R, Fournie JJ, Goldman M. Human gamma delta T cells induce dendritic cell maturation. Clin Immunol. 2002;103:296–302. doi: 10.1006/clim.2002.5218. [DOI] [PubMed] [Google Scholar]
- Eberl M, Roberts GW, Meuter S, Williams JD, Topley N, Moser B. A rapid crosstalk of human gammadelta T cells and monocytes drives the acute inflammation in bacterial infections. PLoS Pathog. 2009;5:e1000308. doi: 10.1371/journal.ppat.1000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes M, Willimann K, Bioley G, Lévy N, Eberl M, Luo M, et al. Cross-presenting human gammadelta T cells induce robust CD8+ alphabeta T cell responses. Proc Natl Acad Sci USA. 2009;106:2307–2312. doi: 10.1073/pnas.0810059106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuter S, Eberl M, Moser B. Prolonged antigen survival and cytosolic export in cross-presenting human gammadelta T cells. Proc Natl Acad Sci USA. 2010;107:8730–8735. doi: 10.1073/pnas.1002769107. [DOI] [PMC free article] [PubMed] [Google Scholar]