Abstract
Dynamic analysis of oxygen (O2) has been limited by the lack of a real-time, quantitative, and biocompatible sensor. To address these demands, we designed a ratiometric optode matrix consisting of the phosphorescence quenching dye platinum (II) octaethylporphine ketone (PtOEPK) and nanocystal quantum dots (NQDs), which when embedded within an inert polymer matrix allows long-term pre-designed excitation through fluorescence resonance energy transfer (FRET). Depositing this matrix on various glass substrates allowed the development of a series of optical sensors able to measure interstitial oxygen concentration [O2] with several hundred millisecond temporal resolution in varying biological microdomains of active brain tissue.
The relationship between oxygen consumption and neuronal activity is critical for understanding the basis for normal and pathological brain functioning. To date this assumption has relied on various methodologies that either indirectly monitor hemodynamic (Ogawa et al. 1990, Vanzetta and Grinvald 1999, Wolf et al. 2007) and metabolic changes (LaManna et al. 1987, Lakowicz et al. 1992), or more direct sensing of O2 using polarography (Clark 1956),Silver 1973), binding of nitroimidazole-based compounds (Koch 2002), and electron paramagnetic resonance methods (Swartz and Clarkson 1998). To improve spatial and temporal resolution, more recent advances in direct [O2] sensing have relied on phosphorescence quenching dyes that linearly and reversibly change luminescence by single-photon (Kautsky and Hirsch 1931, Vanderkooi et al. 1987, Papkovsky and O'Riordan 2005) or two-photon excitation (Sakadzic et al. 2010), allowing intracellular (Xu et al. 2001, Koo et al. 2004, Park et al. 2005, O'Riordan et al. 2007, Zhdanov et al. 2008, Dmitriev et al. 2010, Koo Lee et al. 2010, Wang et al. 2011) or extracellular (Babilas et al. 2008, Finikova et al. 2008, Hynes et al. 2009, Lebedev et al. 2009, Ceroni et al. 2011, Esipova et al. 2011) measurements in biological samples. Conventionally, such dyes have been coupled with two-photon enhancing dendrimers (Brinas et al. 2005) or O2 insensitive organic reference dyes that are restricted by low luminescence lifetimes, dissimilar bleaching curves, and the requirement that each dye must be closely matched to the excitation and emission spectrum when using a single excitation source (Xu et al. 2001). To design a ratiometric sensor suited for greater functionality and ease of fabrication for accurate O2 sensing, we coupled the oxygen sensing dye platinum (II) octaethylporphine ketone (PtOEPK) with highly photostable nanocrystal quantum dots (NQDs) in an inert polymer matrix.
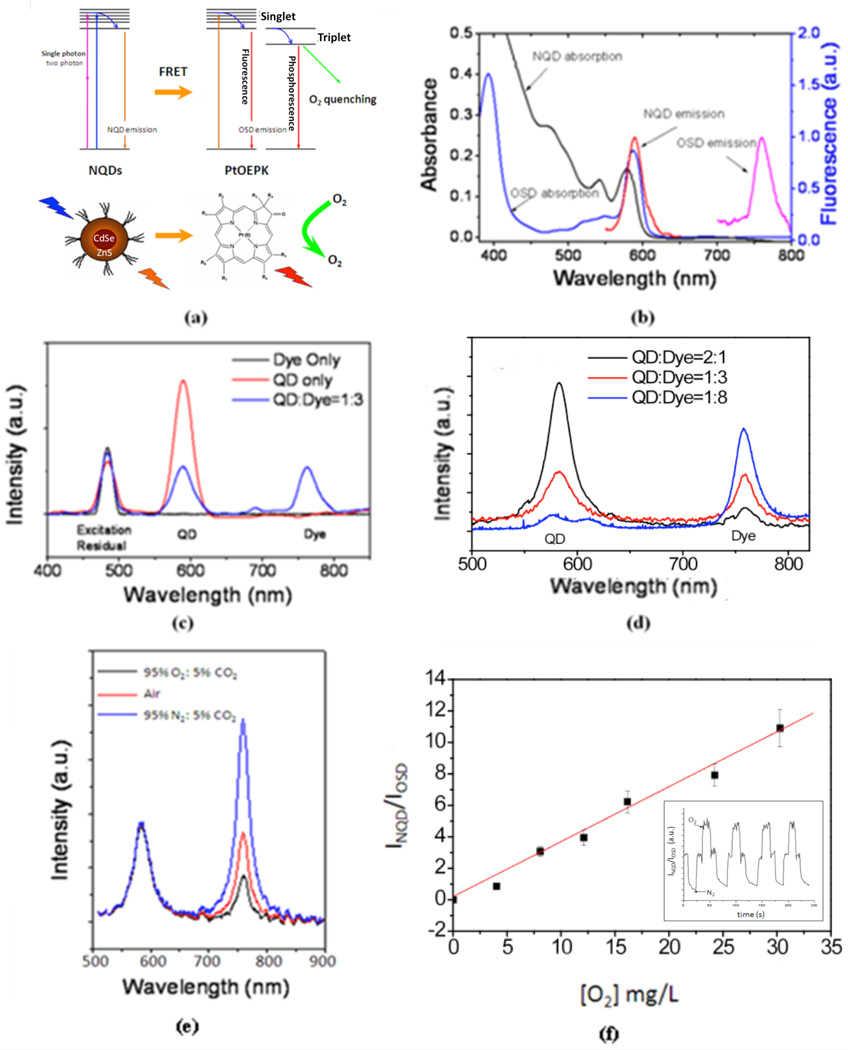
Fluorescent NQDs are excellent candidates for constructing biosensors due to their high quantum yields, photostability, broad excitation profiles, multiphoton capability, and color tuneability (Michalet et al. 2005). The NQDs we have chosen not only act as an O2 insensitive reference light emitter, but their narrow emission provides perfect spectral overlap at the absorption peak of PtOEPK, allowing optimized FRET excitation (Figure 1 a, 1 b). Previously, FRET O2 sensing has become promising for quantifying O2 in solution (McLaurin et al. 2009, Amelia et al. 2011), but this methodology has yet to show feasibility within biological tissue. In order to utilize FRET O2 sensing within a complex biological system, we entrapped the highly toxic dye mixture within a biocompatible and O2 permeable polyvinyl chloride (PVC) polymer matrix (Papkovsky 2004). This matrix could then be deposited on various glass substrates to simultaneously allow the interstitial monitoring of O2 and electrical recordings over various biological microdomains during spontaneous states of neuronal hyperexcitability including seizures.
Figure 1.
(a) Scheme of FRET transfer between NQDs and the phosphorescence quenching oxygen dye PtOEPK. (b) Excitation and emission spectra of NQDs and PtOEPK. Excitation at 450 nm caused narrow NQD emission at the same peak absorption of PtOEPK (590 nm). (c) Single-photon excitation of NQDs, PtOEPK, and dye mixture. 480 nm excitation of a PVC matrix containing only PtOEPK caused minimal emission intensity (black trace) unless coupled with NQDs (blue trace), (d) Excitation of an optode thin film using two-photon excitation at varying NQD:OSD ratios. 860 nm excitation and a mass ratio of 1:3 of QD:PtOEPK produced a balanced emission intensity ratio at 590 nm and 757 nm. e) Emission response of NQDs and PtOEPK in varying [O2]. (f) Linearity and reversibility (inset) of FRET matrix in various [O2] at 34°C, (R2=0.9983).
Methods
Animals
Experiments were performed on male Sprague-Dawley rats (P21-P35) with Pennsylvania State University Institutional Animal Care and Use Committee approved protocol.
Electrophysiology
Animals were anesthetized using diethyl-ether and decapitated, brains were removed, the left hippocampus was isolated, and transverse 350 – 400 µm sections were cut in cold dissection buffer (in mM: 2.6 KCL, 1.23 NaH2PO4, 24 NaHCO3, 0.1CaCI2, 2MgCI2, 205 sucrose, 20 glucose, 4°C) using a vibratome. Slices were then incubated for a minimum of 1 hour in artificial cerebral spinal fluid (ACSF: in mM: 130 NaCI, 2 MgSO4, 3.5 KCI, 2 CaCI2, 10 glucose, 2.5 NaH2PO4, 24 NaHCO3 at pH 7.3, 30°C) oxygenated with 95% O2: 5% CO2. Recordings were taken using (ACSF: in mM: 130 NaCI, 0.6 MgSO4, 3.5 KCI, 1 CaCI2, 10 glucose, 2.5 NaH2PO4, 24 NaHCO3 at pH 7.3, 32–34°C) oxygenated with 95% O2: 5% CO2. Extracellular recordings were performed using pulled borosilicate glass micropipettes (1–3 MΩ 0.9% NaCI) and current clamp recordings (4–7 MΩ) containing (in mM: 116 K gluconate, 6 KCI, 0.5 EGTA, 20 HEPES, 10 phosphocreatine, 0.3 NaGTP, 2 NaCI, 4 MgATP, and 0.3% Neurobiotin at pH 7.25, 295 mOsm). Electrical recordings were performed using an Axoclamp 2B MCC700 (Molecular devices) amplifier filtered (5 kHz whole cell, 1 kHz extracellular) and digitized at 10 kHz (Digidata and Pclamp#7, Molecular Devices).
Seizures were initiated by bath application of the potassium channel blocker 4-aminopyridine (4-AP, 100 – 200 µM) and raising the bath temperature to 32–34°C (Perreault et al. 1991). Alignment of seizure onset was determined electrically by the identifiable fast positive extracellular shift (FPES, 1–5 mV, Figure 4.3). Termination was determined by the cessation of spike activity and a return to baseline membrane potential. Single cell recordings were performed on cells showing electrical signatures (step and ramp current) and identifiable morphology of pyramidal neurons. Optical and electrical recordings were performed on an Olympus IX71 inverted microscope (2D thin film experiments), Zeiss Axioskop2 FSPIus (optode coated microelectrode experiments), and an Olympus BX51WI (backfilled micro-optode experiments), equipped with a dual emission beam splitter (Optical Insights, Santa Fe, New Mexico) and Sensicam EM camera (Cooke Instruments, Inc.) driven with IPLab software (BioVision Technologies) at an acquisition rate of 5 frames/second. Optical and electrical data were taken simultaneously by triggering electrical activity to start with each optical recording.
Two-dimensional optode sensor
An O2 sensitive optode matrix was fabricated by dissolving a 2:1 ratio of polyvinyl chloride (PVC) and bis(2-ethylhexyl)sebacate (DOS) with a 3:1 ratio of PtOEPK and CdSe/ZnS NQDs (6nm, Ocean NanoTeach LLC) dissolved in tetrahydrofuran (THF). The optode matrix was prepared by slowly dissolving 22 mg of PVC and 44 mg of DOS in 1.5 mL THF. Then 10 ng of PtOEPK and 3.3 ng of NQDs was added, thoroughly mixed, and the matrix was evaporated to 1 mL. Thin films (~40 nm) were deposited on microscope cover glass (24×60 no.1) by spin coating 20 µl of matrix at 3000 rotations per minute for 30 seconds. Films were then thoroughly rinsed with deionized (DI) water and placed in a desiccator overnight to remove any excess THF. Before each use the optode coated cover slips were mounted and sealed within a perfusion-type electrophysiology platform. Hippocampal slices (350 µm) were placed directly on top of the thin film and perfused with 95% O2: 5% CO2 saturated artificial cerebral spinal fluid (ACSF). Epifluorescence was focused on the thin film coating using an Olympus IX71 Inverted Microscope equipped with Hg bulb illumination and an UPlanSApo (4 ×/0.16 w) objective (Olympus).
Optode coated microelectrodes
Using a previously described dip coating method (Park et al. 2005) with the optode matrix as described above, pulled borosilicate microelectrodes (1–3 MΩ., 1–2 µm tip diameter) were mounted onto a micromanipulator and submerged 10× within the dye and polymer matrix to deposit a thin (<50 nm) homogeneous coating on the distal portion of the electrode (Barker et al. 1998). To ensure the tip remained unclogged and functional for local field potential recordings, each electrode was prefilled with 0.9% NaCI prior to dipping. The electrode was then thoroughly rinsed with distilled water and dried under a stream of N2 to ensure complete removal of THF. The optode coated microelectrode was mounted onto a micromanipulator and placed at a depth of ~100 µm into extracellular matrix of the CA1 stratum pyramidale and simultaneous optical and electrical recordings were taken. Luminescence was monitored using a Zeiss Axioscope 2 upright microscope with Hg bulb illumination and an Achroplan (10 ×/ 0.30 w) objective (Zeiss).
Backfilled micro-optodes
Using a 4:1 ratio of PtOEPK:NQDs and 2:1 ratio of PVC:DOS in THF, pulled borosilicate micropipettes (tip diameter 3 µm) were submerged into the optode matrix to allow capillary action to backfill each micro-optode. Using a syringe, positive pressure was applied to the filled micro-optode to ensure the matrix was concentrated to the tip. Filled micro-optodes were then dried under a stream of N2, thoroughly rinsed in distilled water, dried again, and stored in a desiccator until use. To allow measurements around single cell microdomains, a ratiometrically balanced backfilled micro-optode was first placed juxtacellular to the target cell of interest using differential infrared contrast optics. A second intracellular electrode was used for whole cell patch clamping. Optical measurements were taken from a 3 µm×3 µm region of interest from the micro-optode tip using an Olympus BX51W1 equipped with Hg bulb illumination and a LUMPIanFI (40 ×/ 0.80 w) objective (Olympus).
Time-resolved photoluminescence
Femtoseond laser pulses with temporal duration of 80 fs from an optical parametric amplifier (TOPAS-C, Coherent) pumped by a regenerative Tksapphire amplifier (Libra HE, Coherent) were employed to excite the sensor sample for dynamical studies of the emission. The temporal traces were recorded with sub-10 ns resolution by a photomultiplier (PMT, Hamamatsu) and fast oscilloscope.
Results
FRET excitation of phosphorescence quenching dye
In order to induce FRET, we utilized a PVC polymer matrix to promote short distance (~10 nm) dipole-dipole interactions. The average donor-acceptor separation distance between NQDs and OSD molecules was determined by their concentrations embedded in the polymer matrix. FRET excitation was confirmed by time-resolved photoluminescence (TRPL) studies where NQDs were excited using a femto-second laser and the emission relaxation rate increased with an increase in OSD concentration [supplementary data]. FRET was also confirmed with single and two-photon excitation of thin film optode coatings with varying NQD and PtOEPK concentrations (Figure 1 c, d). At the same absorption intensity, PtOEPK emission was dependent of the presence of NQD.
Quantitative calibration of the thin film optode sensors was performed before and after every experiment by measuring the ratiometric luminescence intensity in varying [O2] within artificial cerebral spinal fluid (ACSF) at 34°C. Varying [O2] were obtained by adding mixtures of 95% O2: 5% CO2 and 95% N2: 5% CO2 within the ACSF perfusate. The ratio (R) of the emission luminescence from NQDs and OSDs was calculated with the form R = INQD / Iosd Where INQD and Iosd are the NQDs and OSD luminescent intensities at a given [O2]. From 0 to 100% oxygen saturation at 34°C, the OSD phosphorescence quenching is reversible and approximately linearly dependent on dissolved O2 level as described by the Stern-Volmer equation:
where Io is the emission of the OSD in N2 saturated solution, Ic is the emission in a given dissolved [O2], and Ksv is the Stern-Volmer quenching constant determined by the slope of luminescence vs. [O2] (Stern and Volmer 1919)(Figure 1 e, 1 f).
The response time of our sensors was estimated by the Einstein diffusion equation:
where R2 is the diffusion length, D is the diffusion constant (which is estimated to be 2 × 10 -9 m2/s from the diffusion constant of O2 in water), and τ is the response time (Einstein 1905). This produces a theoretical response time of <100 µs for a ~ 100 nm thick film and <200 ms for a 3 µm2 backfilled micro-optode. The actual response times from our FRET optodes were evaluated manually by switching 95% N2: 5% CO2 saturated ACSF buffer with 95% O2: 5% CO2 saturated buffer and measuring the time to achieve 90% maximum intensity. This method confirmed an upper limit response time of less than 200 ms.
The reproducibility of each optode sensor was determined using a constant excitation intensity and recording similar emission intensities with the same camera settings. The same recipe was followed for each set of experiments and errors <5 % were found between calibration tests of fresh sensors. Before each experiment, the luminescence of each optode was checked to ensure a balanced OSD and NQD emission intensity. Optodes where balanced luminescence was not observed were discarded.
Probing interstitial oxygen during spontaneous SLE
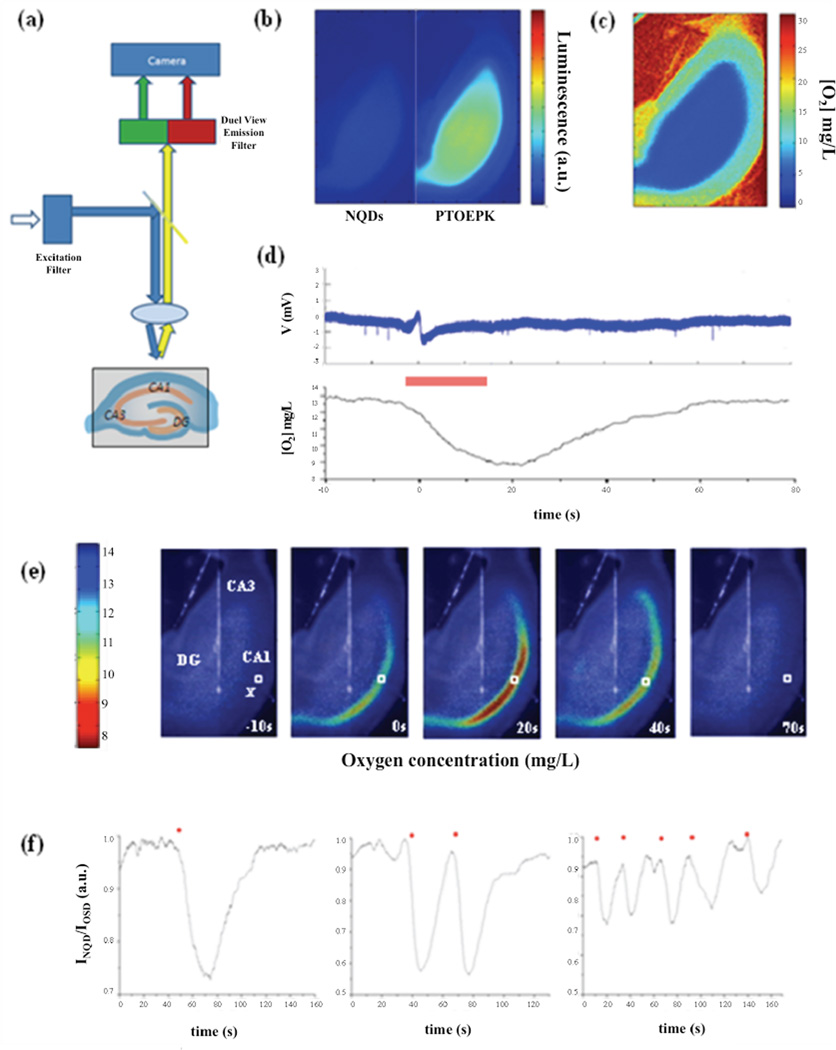
We first monitored O2 dynamics by recording the ratiometric intensity of an optode film in direct contact with an entire hippocampal slice (Figure 2 a). In the absence of tissue, the NQD and PtOEPK luminescence was balanced when continuously perfused with saturated 95% O2: 5% CO2 ACSF. The addition of a hippocampal slice produced a marked increase in PtOEPK luminescence while NQD luminescence showed no intensity change (Figure 2 b). This initial increase represents a marked decrease in [O2] due to the metabolic activity of the tissue and O2 diffusion limitations. For a 350 µm thick slice, the [O2] gradually decreased horizontally from ~18 mg/L to ~5 mg/L 250 µm from the slice edge even though 31 ± 1 mg/L [O2] perfused over the slice at a rate of 1 mL/min at 34°C (Figure 2 c).
Figure 2.
(a) Experimental setup used to record luminescence from a FRET based optode. Absorption at 450 ± 30 nm was used to excite a 40 nm thin film optode matrix in direct contact with a hippocampal slice. The emission was then split using a dual emission beam splitter and a ratiometric image was captured in the camera, (b) Optode emission from NQDs (600 ± 30 nm) and OSD (750 ± 30 nm) as oxygen saturated ACSF perfused the slice, (c) Calibrated ratiometric optode film under a slice perfused at a rate of 1 mL/min at 34°C. (d) Simultaneous local field potential and [O2] recorded from a 50 µm×50 µm region of interest within the CA1 st. pyramidale during a spontaneous SLE (seizure duration indicated by red bar), (e) Calibrated ratiometric images were overlaid with a bright field image to visualize real-time spatiotemporal changes in O2 dynamics over the entire slice. Changes in O2 were localized primarily within the CA1 that preceded FPES onset and outlasted the electrical seizure duration. X = local field potential electrode position, □= 50 µm × 50 µm region of interest, (f) Ratiometric imaging within the CA1 st. pyramidale during single or multiple SLEs. Seizure onset was associated with a decrease in INQD/IOSD hat returned to baseline values if not interrupted by a subsequent SLE. Seizure onset denoted by *.
To induce spontaneous hyperexcitability, 200 µm of the potassium channel blocker 4-aminopyridine (4-AP) was administered in the bath solution. Electrical activity was recorded using a local field potential electrode within the CA1 st. pyramidale. Within minutes spontaneous seizure-like events (SLEs) resulted in focal increases of O2 consumption localized primarily within the CA1 st. pyramidale (Figure 2 d, e, Movie [supplementary data]). In all observed slices (n=33), a decrease in [O2] occurred prior to the fast positive excitatory spike (FPES), an activity marker associated with seizure onset (Ziburkus et al. 2006), and the hypoxic duration outlasted the electrical seizure termination. Each hypoxic episode would then slowly return back to baseline values if not interrupted by a subsequent SLE (Figure 2 f).
To ensure luminescence changes were derived from changes in PtOEPK and not intrinsic factors (i.e. cell swelling), the OSD was replaced with the oxygen insensitive dye HITC iodide (excitation 590 nm, emission 750 nm). During spontaneous SLEs, no change in HITC iodide luminescence was observed [supplementary data].
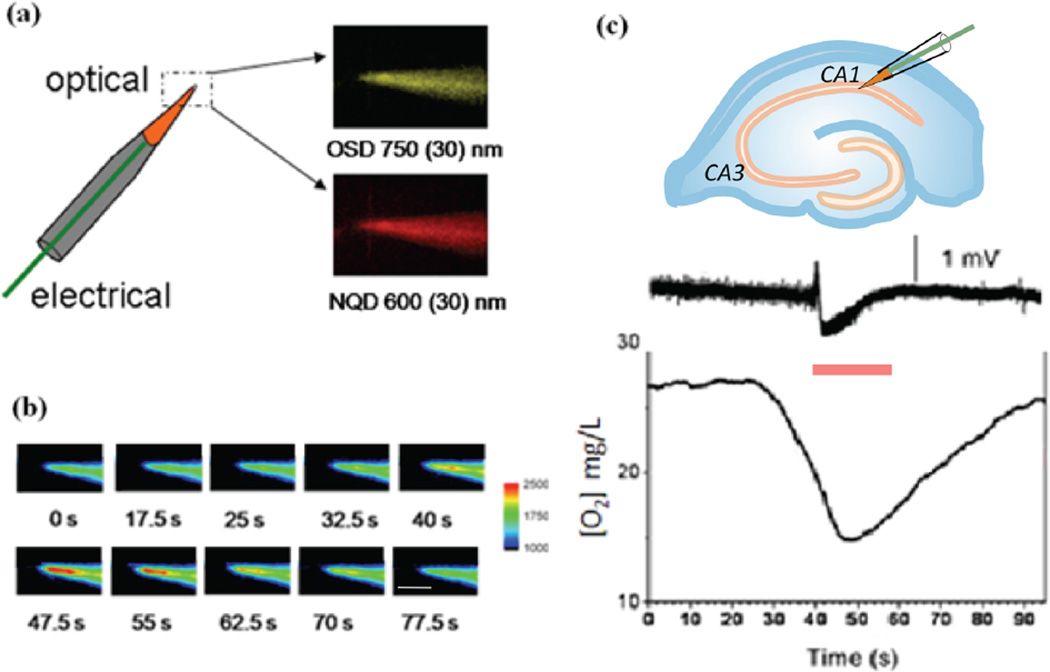
To measure more localized changes in [O2] associated with hyperexcitability, we then inserted optode coated local field potential electrodes within the CA1 st. pyramidale (Figure 3 a, c). The ratiometric luminescence from a 25 µm×25 µm region of interest was recorded from the microelectrode tip that again revealed a decrease in [O2] during spontaneous SLEs (Figure 3 c). Similar to the 2D films, an increase in O2 consumption was found prior to FPES onset within the st. pyramidale followed by prolonged hypoxic periods that outlasted the duration of the SLE.
Figure 3.
(a) Schematic of an optode coating on the tip of a functional local field potential microelectrode. The electrode was first prefilled with 0.9% NaCI and the distal portion of the electrode was dipped into the optode matrix to deposit a homogeneous coating on the tip. (b) Luminescence changes from OSD emission (750 ± 30 nm) recorded during a SLE. FPES onset occurred starting at timeframe 40 s. Scale bar 100µm (c) An optode coated microelectrode allowed simultaneous [O2] and electrical activity to be monitored within the CA1 st. pyramidale. During an individual SLE (red bar) a marked decrease in [O2] was observed prior to FPES onset that outlasted the duration of the electrical seizure event.
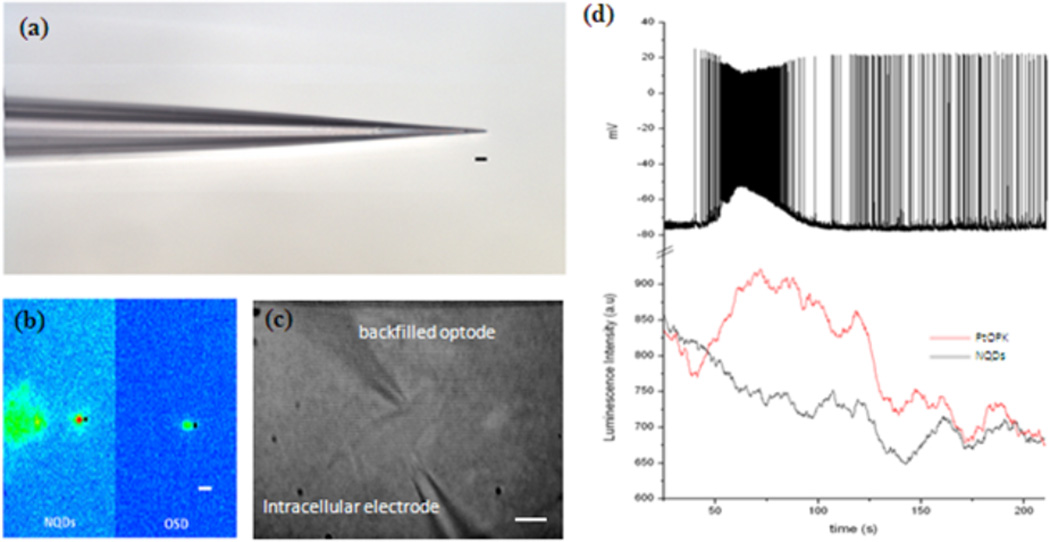
To further improve the spatial limitations of biological O2 sensing we positioned a backfilled micro-optode (Figure 4 a) juxtacellular to an outlying CA1 pyramidal neuron outside the dense cell body layer. A second intracellular electrode was then inserted to allow whole cell recordings from the same target cell (Figure 4 c). Excitation at 450nm generated NQD and OSD emission that was recorded from a 3 µm×3 µm region of interest within the distal portion of the electrode tip (Figure 4 b). In all stable recordings, spontaneous SLEs resulted in an increase in OSD luminescence (decrease in [O2]) around individual CA1 pyramidal cells (Figure 4 d), while no change in NQD luminescence was observed. Control experiments were recorded from backfilled micro-optodes placed at least 50 µm from any specific cell in the CA1 st. oriens with a local field potential electrode in the st. pyramidale. During a seizure event, no change in luminescence was observed unless the micro optode was positioned juxtacellular within 50 µM to a single cell [supplemental data].
Figure 4.
(a) Pulled glass electrodes were dipped into the FRET optode matrix and capillary action concentrated a small amount of dye and polymer at the tip. Scale bar 10 µm. (b) Excitation at 450 ± 30 nm resulted in bright OSD and NQD emission from the tip of the backfilled electrode. Optical recordings were taken from the 3 µm×3 µm region of interest (■) at the distal portion of the electrode. Scale bar 10 µm. (c) Bright field image of backfilled juxtacellular optode {top) and whole cell patch clamp electrode (bottom) attached to a pyramidal neuron just outside the dense cell body layer of the stratum pyramidale. Scale bar 10 µm. (d) Intracellular electrical and juxtacellular optode recordings during an individual SLE. A backfilled electrode placed juxtacellular to a pyramidal neuron (red trace) increased luminescence during SLE onset while NQDs (black trace) revealed no change in luminescence with the SLE.
Discussion
Real-time monitoring of O2 is of great interest due to its fundamental role in virtually every aspect of cellular metabolism. Here we present a ratiometric and multifunctional O2 sensing technology to allow simultaneous optical and electrical recordings from large scale neural population networks to individual extracellular microdomains. To show functionality and biocompatibility, we monitored the fast interstitial [O2] fluctuations during spontaneous states of hyperexcitability in living tissue. These real-time metabolic responses offer a critical insight into the cellular demands associated with hyperexcitability and its associated hypermetabolism.
Although previous methods of measuring [O2] have extensively been used, the development of fast quantitative optical methods will improve the spatiotemporal resolution for measuring [O2] and cellular activity within living tissue. Our single photon excitation FRET based methodology offers several advantages. First, the sensor is self-referencing, as the ratio of the reference to the sensing dye represents a parameter that is independent of dye concentration or variations with instrumentation. Second, coupling the OSD and NQD mixture within a biologically inert and O2 permeable polymer matrix improves luminescent stability and provides possibilities for alternative sensing modalities using multiphoton microcopy techniques (McLaurin et al. 2009, Achatz et al. 2011, Amelia et al. 2011). Third, in contrast with polarographic techniques, our molecular sensor does not consume the O2 it seeks to measure. Lastly, this alternative sensing method can be easily fabricated and deposited on various glass substrates depending on the desired application.
Although our FRET sensors were designed to provide limited interactions with biological macromolecules, a detailed investigation of long-term biocompatibility and toxicity was beyond the scope of this study. A potential safety concern with any plasticized PVC matrix is leaching (Tickner et al. 2001). Although we did encounter a baseline luminescence change (<20 % over 200 s) in the backfilled micro-optode experiments, the drift is most likely due to photobleaching, as an increased light intensity was required to excite the microliter amounts of OSD/NQDs/plastizier. Even if leaching were to occur, we were able to reliably record the electrical activity from a single cell for tens of minutes, even though a plasticized probe was located juxtacelluar to the same cell.
As with all types of sensors, selectivity is also a potential concern. Oxygen sensing dyes may also be quenched by nitric oxide (NO) (Koo et al. 2004). In our experimental setup we cannot rule out the possibility of NO production, but the nanomolar concentrations reported within the literature (Brown 1995) are minimal in comparison to the millimolar oxygen changes we observe at rest and during individual seizures. Another possible contributor to changes in OSD signaling is the production of other oxygen species, as intense hyperactivity undoubtedly generates some forms of reactive oxygen species (ROS), especially from within the mitochondrial electron transport chain (Murphy 2009). The rapid production of ROS in the brain can be catalyzed with a variety of enzymes (superoxide dismutase, catalase, glutathione peroxidase) or antioxidants to neutralize the unpaired electrons. These free radical scavengers are usually sufficient to deal with the toxic threat of ROS production but we cannot rule out the possibility that ROS are generated and contribute to our measurements during seizure activity (Schiff and Somjen 1985).
Conclusions
To improve the spatiotemporal resolution, selectivity, and functionality of direct O2 measurement techniques, we have designed a FRET based oxygen sensor to allow real-time extracellular O2 measurements in living tissue. Using new developments in molecular imaging combined with intracellular and extracellular recordings, we were able to localize, quantify, and assess the interstitial oxygen demands associated with changes in neuronal activity. This ratiometric and multifunctional O2 sensor allows simultaneous optical and electrical recordings from larger-scale brain structures to smaller-scale microdomains localized around single cells.
Supplementary Material
Highlights.
We report how to fabricate a series of FRET based optical oxygen sensors.
FRET sensing can monitor extracellular oxygen dynamics in living tissue.
Seizure events are associated with layer specific O2 changes in the hippocampus.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achatz D, Meier R, Fischer L, Wolfbeis O. Luminescent Sensing of Oxygen Using a Quenchable Probe Along with Upconverting Nanoparticles/Angew. Chem. 2011;123:274–277. doi: 10.1002/anie.201004902. [DOI] [PubMed] [Google Scholar]
- Amelia M, Lavie-Cambot A, McClenaghan N, Credi A. A ratiometric luminescent oxygen sensor based on a chemically functionalized quantum dot. Chem. Commun. 2011;47:325–327. doi: 10.1039/c0cc02163f. [DOI] [PubMed] [Google Scholar]
- Babilas P, Lamby P, Prantl L, Schreml S, Jung EM, Liebsch G, Wolfbeis OS, Landthaler M, Szeimies RM, Abels C. Transcutaneous pO2 imaging during tourniquet-induced forearm ischemia using planar optical oxygen sensors. Skin Res Technol. 2008;14:304–311. doi: 10.1111/j.1600-0846.2008.00295.x. [DOI] [PubMed] [Google Scholar]
- Barker SLR, Thorsrud BA, Kopelman R. Nitrite- and Chloride-Selective Fluorescent Nano-Optodes and in vitro application to rat conceptuses. Anal. Chem. 1998;70:100–104. doi: 10.1021/ac970912s. [DOI] [PubMed] [Google Scholar]
- Briñas RP, Troxler T, Hochstrasser RM, Vinogradov SA. Phosphorescent Oxygen Sensor with Dendritic Protection and Two-Photon Absorbing Antenna. J. Am. Chem. Soc. 2005;127:11851–11862. doi: 10.1021/ja052947c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GC. Nitric oxide regulates mitochondrial respiration and cell functions by inhibiting cytochrome oxidase. FEBS Lett. 1995;369:136–139. doi: 10.1016/0014-5793(95)00763-y. [DOI] [PubMed] [Google Scholar]
- Clark LC. Monitor and control of blood and tissue oxygen tension. Trans. Am. Soc. Artif Internal Organs. 1956;2:41–48. [Google Scholar]
- Ceroni P, Lebedev AY, Marchi E, Yuan M, Esipova TV, Bergamini G, Wilson DF, Busch TM, Vinogradov SA. Evaluation of Phototoxicity of Dendritic Porphyrin-Based Phosphorescent Oxygen Probes: An in Vitro Study. Photochem. Photobiol. Sci. 2011;10:1056–1065. doi: 10.1039/c0pp00356e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitriev RI, Zhdanov AV, Ponomarev GV, Yashunski DV, Papkovsky DB. Intracellular oxygen-sensitive phosphorescent probes based on cell-penetrating peptides. Anal Biochem. 2010;398(1):24–33. doi: 10.1016/j.ab.2009.10.048. [DOI] [PubMed] [Google Scholar]
- Einstein A. Ann. Phys. 1905;17:549. [Google Scholar]
- Esipova TV, Karagodov A, Miller J, Wilson DF, Busch TM, Vinogradov SA. Two new “protected” oxyphors for biological oximetry: properties and application in tumor imaging. Analytical Chemistry. 2011;83:8756–8765. doi: 10.1021/ac2022234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finikova OS, Lebedev AY, Aprelev A, Troxler T, Gao F, Garnacho C, Muro S, Hochstrasser RM, Vinogradov SA. Oxygen microscopy by two-photon-excited phosphorescence. Chem physchem. 2008;9:1673–1679. doi: 10.1002/cphc.200800296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes J, Natoli E, Jr., Will Y. Fluorescent pH and Oxygen Probes of the Assessment of Mitochondrial Toxicity in Isolated Mitochondria and Whole Cells. Curr. Protoc. Tox. 2009;40:2.16.1–2.16.22. doi: 10.1002/0471140856.tx0216s40. [DOI] [PubMed] [Google Scholar]
- Kautsky H, Hirsch A. Interactions of excited dye molecules and oxygen. Ber Dtsch Chem Ges. 1931;64:2677–2686. [Google Scholar]
- Koch CJ. Measurement of absolute oxygen levels in cells and tissues using oxygen sensors and 2-nitroimidazole EF5. Methods Enzymol. 2002;352:3–31. doi: 10.1016/s0076-6879(02)52003-6. [DOI] [PubMed] [Google Scholar]
- Koo Y-E, Cao LY, Kopelman R, Koo SM, Brasuel M, Philbert MA. Real-time measurements of dissolved oxygen inside live cells by organically modified silicate fluorescent nanosensors. Anal. Chem. 2004;76:2498–2505. doi: 10.1021/ac035493f. [DOI] [PubMed] [Google Scholar]
- Koo Lee YE, Ulbrich EE, Kim G, Hah H, Strollo C, Fan W, Gurjar R, Koo S, Kopelman R. Near Infrared Luminescent Oxygen Nanosensors with Nanoparticle Matrix Tailored Sensitivity. Anal. Chem. 2010;82:8446–8455. doi: 10.1021/ac1015358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowicz JR, Szmacinski H, Nowaczyk K, Johnson ML. Fluorescence lifetime imaging of free and protein-bound NADH. Proc. Natl. Acad. Sci. 1992;89(4):1271–1275. doi: 10.1073/pnas.89.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaManna JC, Sick TJ, Pikarsky SM, Rosenthal M. Detection of an oxidizable fraction of cytochrome oxidase in intact rat brain. Am J Physiol. 1987;253:C477–C483. doi: 10.1152/ajpcell.1987.253.3.C477. [DOI] [PubMed] [Google Scholar]
- Lebedev AY, Cheprakov AV, Sakadžić S, Boas DA, Wilson DF, Vinogradov SA. Dendritic Phosphorescent Probes for Oxygen Imaging in Biological Systems. ACS Applied Materials & Interfaces. 2009;l(6):1292–1304. doi: 10.1021/am9001698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin EJ, Greytak AB, Bawendi M, Nocera DG. Two-Photon absorbing nanocrystal sensors for ratiometric detection of oxygen. J. Am. Chem. Soc. 2009;131(36):12994–13001. doi: 10.1021/ja902712b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalet X, Pinaud F, Bentolila A, Tsay M, Doose S, Li J, Sundaresan G, Wu M, Gambhir S, Weiss S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MP. How mitochondria produce reactive oxygen species. J. Biochem. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Riordan TC, Fitzgerald K, Ponomarev GV, et al. Sensing intracellular oxygen using near-infrared phosphorescent probes and live-cell fluorescence imaging. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1613. doi: 10.1152/ajpregu.00707.2006. [DOI] [PubMed] [Google Scholar]
- Papkovsky DB. Methods in optical oxygen sensing: Protocols and critical analyses. Method Enzymol. 2004;381:715–735. doi: 10.1016/S0076-6879(04)81046-2. [DOI] [PubMed] [Google Scholar]
- Papkovsky DB, O'Riordan TC. Emerging Applications of Phosphorescent Metalloporphyrins. J. Fluorescence. 2005;vol. 15:569–584. doi: 10.1007/s10895-005-2830-x. [DOI] [PubMed] [Google Scholar]
- Park EJ, Reid KR, Tang W, Kennedy RT, Kopelman R. Ratiometric fiber optic sensors for the detection of inter-and intra-cellular dissolved oxygen J. Mater. Chem. 2005;15:2913–2919. [Google Scholar]
- Perreault P, Avoli M. Physiology and pharmacology of epileptiform activity induced by 4-aminopyridine in rat hippocampal slices. J Neurophysiol. 1991;65:771–785. doi: 10.1152/jn.1991.65.4.771. [DOI] [PubMed] [Google Scholar]
- Sakadzic S, Roussakis E, Yaseen MA, Mandeville ET, Srinivasan VJ, Arai K, Ruvinskaya S, Devor A, Lo E, Vinogradov S, Boas D. Two-photon high-resolution measurement of partial pressure of oxygen in cerebral vasculature and tissue. Nat Methods. 2010;7:755–759. doi: 10.1038/nmeth.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff SJ, Somjen GG. Overshoot of oxygen pressure in post hypoxic brain tissue: A reevaluation. Brain Res. 1985;344:150–153. doi: 10.1016/0006-8993(85)91200-4. [DOI] [PubMed] [Google Scholar]
- Silver A. The oxygen micro-electrode. Adv. Exp. Med. Biol. 1973;37A:7–15. doi: 10.1007/978-1-4684-3288-6_2. [DOI] [PubMed] [Google Scholar]
- Stern O, Volmer M. Physik. Z. 1919;2:183–188. [Google Scholar]
- Swartz HM, Clarkson RB. The measurement of oxygen in vivo using EPR techniques. Phys. Med. Biol. 1998;43:1957–1975. doi: 10.1088/0031-9155/43/7/017. [DOI] [PubMed] [Google Scholar]
- Tickner J, Schettler T, Guidotti T, McCally M, Rossi M. Health risks posed by the use of di-2-ethylhexyl phthalate in PVC medical devices: a critical review. Am J Ind Med. 2001;39:100–111. doi: 10.1002/1097-0274(200101)39:1<100::aid-ajim10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Vanderkooi JM, Maniara G, Green TJ, Wilson DF. An optical method for measurement of dioxygen concentration based upon quenching of phosphorescence. J. Biol. Chem. 1987;262:5476–5482. [PubMed] [Google Scholar]
- Vanzetta I, Grinvald A. Increased cortical oxidative metabolism due to sensory stimulation: implications for functional brain imaging. Science. 1999;286:1555–1558. doi: 10.1126/science.286.5444.1555. [DOI] [PubMed] [Google Scholar]
- Wang X, Gorris HH, Stolwijk JA, Meier RJ, Groegel DBM, Wegener J, Wolfbeis OS. Self-Referenced RGB Colour Imaging of Intracellular Oxygen. Chem. Sci. 2011;2:901–906. [Google Scholar]
- Wolf M, Ferrari M, Quaresima V. Progress of near-infrared spectroscopy and topography for brain and muscle clinical applications. J. Biomed. Opt. 2007;12:062104. doi: 10.1117/1.2804899. [DOI] [PubMed] [Google Scholar]
- Xu H, Aylott JW, Kopelman R, Miller TJ, Philbert MA. A real-time ratiometric method for the determination of molecular oxygen inside living cells using sol-gel-based spherical optical nanosensors with applications to rat C6 glioma. Anal. Chem. 2001;73:4124–413. doi: 10.1021/ac0102718. [DOI] [PubMed] [Google Scholar]
- Zhdanov AV, Ward MW, Prehn JH, Papkovsky DB. Dynamics of intracellular oxygen in PC12 cells upon stimulation of neurotransmission. J Biol Chem. 2008;283:5650–5661. doi: 10.1074/jbc.M706439200. [DOI] [PubMed] [Google Scholar]
- Žiburkus J, Cressman JR, Barreto E, Schiff SJ. Interneuron and pyramidal cell interplay during in vitro seizure-like events. J Neurophysiol. 2006;95:3948–3954. doi: 10.1152/jn.01378.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.