Abstract
Dendritic cells (DCs) are integral to differentiation of T helper cells into Th1, Th2 and Th17 subsets. Interleukin-6 (IL-6) plays an important role in regulating these three arms of the immune response by limiting a Th1 response and promoting Th2 and Th17 responses. In this study, we investigated pathways in DCs that promote IL-6 production. We show that the allergen house dust mite (HDM) or the mucosal adjuvant cholera toxin (CT) promotes cell surface expression of c-kit and its ligand, stem cell factor (SCF), on DCs. This dual upregulation of c-kit and SCF results in sustained signaling downstream of c-kit promoting IL-6 secretion. Intranasal administration of antigen into c-kit mutant mice or neutralization of IL-6 in cultures established using lung-draining lymph nodes from immunized wild-type mice blunted the Th2 and Th17 response. DCs lacking functional c-kit or those unable to express membrane-bound SCF secreted lower levels of IL-6 in response to HDM or CT. DCs expressing non-functional c-kit were unable to induce a robust Th2 or Th17 response and elicited diminished allergic airway inflammation when adoptively transferred into mice. Expression of the Notch ligand, Jagged-2, which has been associated with Th2 differentiation, was blunted in DCs from c-kit mutant mice. c-kit upregulation was specifically induced by Th2/Th17-skewing stimuli since the Th1-inducing adjuvant, CpG oligodeoxynucleotide (ODN), did not promote either c-kit or Jagged-2 expression. DCs generated from mice expressing a catalytically inactive form of the p110δ (p110D910A) subunit of PI3 kinase secreted lower levels of IL-6 upon stimulation with CT. Collectively, these results highlight the importance of the c-kit-PI3 kinase-IL-6 signaling axis in DCs in regulating T cell responses.
INTRODUCTION
Dendritic cells (DCs) are efficient antigen presenting cells and can prime naïve CD4+ T cells towards a Th1, Th2 or the recently discovered Th17 response 1–3. Significant progress has been made in the last decade in our understanding of the molecular basis of T helper responses. We and others have identified GATA-3 as the master regulator of Th2 differentiation 4–7. The transcription factors T-bet and ROR-γt are now recognized as critical regulators of Th1 and Th17 differentiation respectively 8,9. Studies have shown that the nature of the antigen, antigen concentration, pattern recognition molecules and cytokine milieu are some of the key regulators that determine the outcome of an immune response 1.
Cytokines play a crucial role in regulating T helper cell differentiation 1. Previous studies have shown the Th2-skewing ability of IL-6 10,11. More recently, IL-6 has been shown to be the quintessential cytokine regulating Th17 development in both mice and humans 12,13. Interestingly in certain immunological responses such as those elicited by allergens in asthma, both Th2 and Th17 responses are evident 14. In these situations, IL-6 would be a central regulator in not only inhibiting the Th1 response 11, but in also promoting Th2 and Th17 responses 10,12,13. However, mechanisms that regulate IL-6 production in DCs have not been elucidated. Also, there have been very few studies 15 that have addressed the role of c-kit expressed by DCs in modulating immune responses.
Our goal was to determine molecular mechanisms by which a complex allergen such as house dust mite (HDM) or the mucosal adjuvant cholera toxin (CT) promotes IL-6 production in DCs, which in turn, would induce Th2 and Th17 responses. Using microarray analysis to identify novel genes regulated by CT in bone marrow-derived dendritic cells (BMDCs), we identified significant upregulation of IL-6 and c-kit expression in CT-treated DCs as compared to that in control DCs. Since cytokine expression is triggered by activation of cell surface receptors, we hypothesized that c-kit expression and activation in DCs regulates the production of IL-6 and thereby the induction of a Th2 or Th17 response subsequent to DC-T cell interaction. To elucidate the role of c-kit expressed by DCs in regulating IL-6 production, we used c-kit mutant mice (WBB6F1 kitW/W-v; kitW/W-v). These mice have a deletion in the transmembrane region of c-kit and also a missense mutation in the kinase domain 16–18. Our in vitro data utilizing CT- and HDM-stimulated BMDCs or lung DCs and in vivo data using two models of allergic airways disease in mice coupled with an adoptive transfer experiment with wild-type (WT) and c-kit-defective DCs show for the first time that functional c-kit signaling in DCs via upregulation of c-kit and membrane-bound SCF is critical for the induction of a Th2 and Th17 response. Interestingly, c-kit expression was also found to influence expression of the Notch ligand, Jagged-2, that has been associated with Th2 responses 19. Using p110δ mutant mice with defective PI3 kinase signaling, which constitutes a major signaling pathway downstream of c-kit, we show that the c-kit/PI3 kinase signaling axis positively regulates production of IL-6. Thus, we have identified a novel signaling axis in DCs that promotes the induction of Th2 and Th17 responses, while significantly inhibiting the Th1 arm of the immune response.
RESULTS
DC Cytokine balance is critical for induction of Th2 and Th17 response by the mucosal adjuvant CT or the allergen HDM
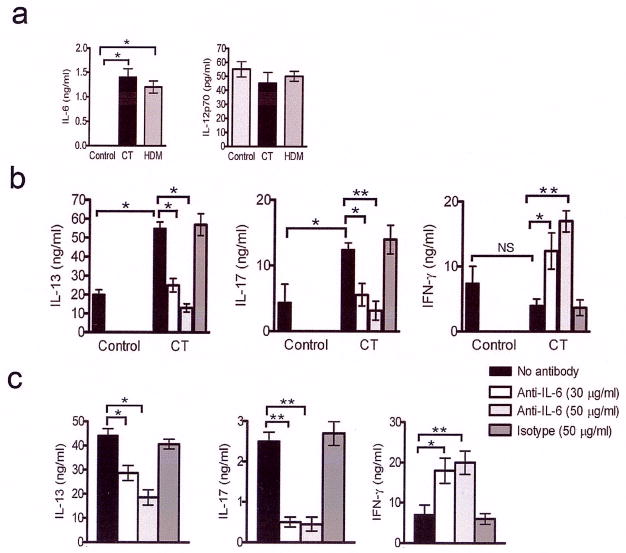
The mucosal adjuvant CT has been associated with IL-6 production 20. Recent studies have implicated IL-6 in IL-17 production 12,13,21, which is also involved in allergic airway inflammation 14. To dissect the importance of cytokines secreted by DCs in influencing T cell response, we analyzed cytokine levels in culture supernatants of BMDCs stimulated with CT or the allergen HDM that causes asthma in humans 22. As shown in Fig. 1a, both CT and HDM promoted IL-6 production from BMDCs. The level of secretion of the Th1 skewing cytokine IL-12p70 was not significantly different between HDM- and CT-treated DCs and control DCs. A similar cytokine profile of high IL-6 and low IL-12 production by lung DCs was associated with inhibition of Th1 and promotion of Th2 response 11. Neutralization of IL-6 resulted in a cytokine profile that showed a switch in the Th response with a dominant Th1 response with elevated levels of IFN-γ coupled with inhibition of IL-13 and IL-17 production (Fig 1b). Furthermore, addition of IL-12 to CT-stimulated BMDCs switched the response of T cells in co-culture to a Th1 response by promoting IFN-γ production (data not shown). These results showed that altering the cytokine balance by neutralizing IL-6 or adding IL-12 diverted the dominant Th2 and Th17 -skewing property of CT to a more Th1 response. We next investigated the role of IL-6 in the shaping of the immune outcome in vivo in response to the natural allergen HDM. Mice were immunized intranasally with the allergen and the lung-draining lymph nodes (LNs) were harvested. This protocol of immunization has been shown to prime the T cells from the lung-draining lymph towards a Th2 response 23. Immunization with the allergen increased the Th2 and Th17 response while neutralization of IL-6 crippled the dominant Th2+Th17 immune response (Fig 1c). Intranasal immunization of IL-6−/−mice with OVA/CT or HDM resulted in significant attenuation of both Th2 and Th17 responses (Supplementary Fig. 1 online). These results show that whether T cell responses are triggered by BMDCs or lung DCs, IL-6 plays an important role in regulating T cell responses.
Figure 1. CT and HDM-stimulate IL-6 secretion from DCs in vitro and in vivo.
(a) BMDCs were incubated with or without CT for 24 h. The cell supernatants were analyzed for IL-6 and IL-12p70 levels by ELISA. Cytokine levels shown are mean ± s.e.m. of triplicates and are representative of three independent experiments *, P<0.001. (b) Anti-IL-6 antibody inhibits Th2 and Th17 response but promotes Th1 response. BMDCs were incubated with or without CT for 24 h and then co-cultured with DO11.10 T cells in the presence of OVA peptide. As shown, some wells containing BMDCs+CT also contained anti-IL-6 antibody or an isotype control. The cytokines present in the culture supernatants were assayed for IL-13, IL-17 and IFN-γ. The values represent means of triplicates ± s.d. and are representative of two independent experiments. *, P<0.05 and ** P<0.001.(c) Lymph node cells derived from WT mice show diminished Th2 and Th17 response but increased Th1 response upon treatment with anti-IL-6 antibody. Lymph node cells from WT mice following HDM instillation were cultured with anti-IL-6 antibody or an isotype control and IL-13 and IFN-γ levels in the culture supernatant were measured. Results shown represent means of triplicates ± s.e.m. and are representative of three independent experiments. *,P<0.05 **, P<0.001.
CT- and HDM-stimulated DCs upregulate c-kit expression
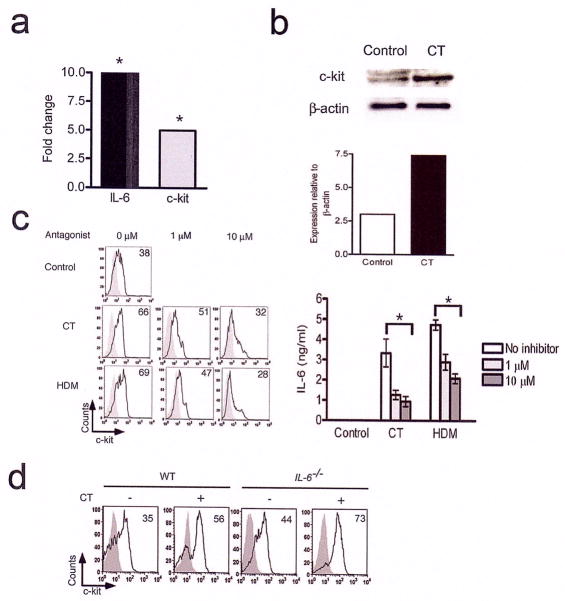
To further dissect and identify molecular mechanisms underlying IL-6 upregulation by CT, we undertook a microarray approach to identify genes differentially regulated in BMDCs upon CT treatment since this approach had allowed us to previously identify Ym1 as a statin-induced gene involved in Th2 differentiation 24. The microarray data analysis (details to be published elsewhere) revealed the c-kit gene to be significantly upregulated along with IL-6 in BMDCs upon CT treatment (Fig. 2a). Immunoblotting of total cell lysates revealed greater c-kit expression in CT-stimulated BMDCs compared to that in control DCs (Fig. 2b). As determined by flow cytometry, the frequency of cells expressing c-kit on the cell surface was increased by CT treatment (Fig. 2c) and this increase was dose-dependent (data not shown). We also investigated whether c-kit was induced by specific agents such as CT or an allergen such as HDM could also do the same. As shown in Fig. 2c, exposure of BMDCs to HDM increased the frequency of c-kit expressing-cells comparably. Stimulation of human monocytes with CT has been shown to suppress IL-12 levels via a cAMP-dependent pathway 25. We, therefore, examined the role of cAMP in c-kit upregulation by CT using the specific cAMP antagonist Rp-cAMPS that blocks cAMP-dependent downstream pathways such as protein kinase A 26. As shown in Fig. 2c, Rp-cAMPS caused a significant decrease in c-kit-expressing cells in both CT- and HDM-stimulated DCs in a dose-dependent fashion with a concomitant decrease in IL-6 production (Fig. 2c). It is presently unclear how HDM triggers cAMP/PKA-dependent mechanisms in DCs. c-kit expression on DCs derived from IL-6−/− mice was comparable to that on cells derived from WT mice suggesting that IL-6 is a downstream target of c-kit (Fig. 2d).
Figure 2. Increased expression of c-kit in CT- and HDM-stimulated BMDCs.
(a) Increased IL-6 and c-kit mRNA expression in CT-stimulated BMDCs as revealed by microarray analysis and confirmed by real-time PCR (shown). The fold change in c-kit and IL-6 mRNA expression in CT-stimulated BMDCs with respect to control DCs incubated in medium is shown. *, P<0.005. (b) c-kit expression in CT-stimulated BMDCs as revealed by immunoblotting. Total cell lysates prepared from CT-stimulated BMDCs were probed for c-kit and the same blot was stripped and re-probed for the loading control β-actin. The intensity of c-kit bands was quantified relative to the loading control β-actin. The upper band detected by anti-c-kit antibody is variably detected in different samples and is most likely due to glycosylation. Data are representative of two independent experiments. (c) CT and the allergen HDM upregulate c-kit expression on BMDCs in a cAMP-dependent fashion. BMDCs were pre-treated with the cAMP antagonist Rp-cAMPS at indicated concentrations for 1 h and then stimulated with CT (1 μg/ml) or HDM (10 μg/ml) for 24 h. The cells were harvested and analyzed for c-kit expression by flow cytometry. The culture supernatants were analyzed for IL-6 production by ELISA and the data shown are mean values of triplicates± s.d. This experiment was performed twice with similar results. *, P<0.05. (d) BMDCs from IL-6+/+ (WT mice) and IL-6 −/− mice were incubated with or without CT for 24 h and the expression of c-kit was analyzed by flow cytometry. The shaded grey represents the isotype control and the black overlay represents staining with the anti-c-kit antibody. The numbers in the histograms represent the percentage of CD11c+c-kit +cells. This experiment was repeated twice with similar results.
c-kit positive BMDCs from CT-stimulation induce a Th2 response whereas c-kit negative BMDCs induce a Th1 response
To further understand the functional significance of c-kit upregulation on DCs, we sorted c-kit positive and negative cells after stimulation with CT. The sorted populations were then co-cultured with T cells from naïve DO11.10 mice in the presence of OVA peptide. The supernatants from the co-culture were assayed for IL-13, IL-17 and IFN-γ levels by ELISA. The CT-stimulated BMDCs that were c-kit positive skewed the T cells towards production of high levels of IL-13 and IL-17 and low levels of IFN-γ (Fig. 3a). This cytokine profile was different in culture supernatants that included the c-kit negative population showing higher IFN-γ and lower IL-17 and IL-13 levels (Fig. 3a). In contrast to generation of a higher frequency of c-kit+ cells in response to CT, BMDCs stimulated with CpG ODN, a Th1-skewing adjuvant, resulted in the majority of the cells being c-kit− and this population of DCs strongly promoted a Th1 response (Fig. 3b). These results strengthened our notion that the upregulation of c-kit on DCs elicits a strong Th2 and Th17 response while suppressing a Th1 response.
Figure 3. c-kit+ but not c-kit- BMDCs resulting from CT stimulation skew T cells predominantly towards a Th2 and Th17 response.
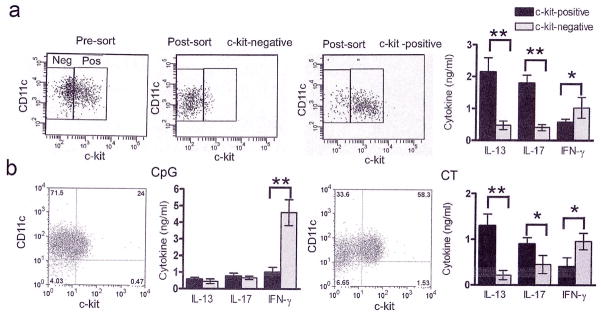
(a) BMDCs were stimulated with CT (1 μg/ml) and sorted based on the expression of c-kit. The purity of the c-kit+ and c-kit− populations after sorting was >93%. The supernatants from the co-cultures of either c-kit+ or c-kit− cells with CD4+ T cells were assayed for IL-13, IL-17 and IFN-γ by ELISA. (b) BMDCs were stimulated with either CpG ODN (1 μM) or CT (1 μg/ml) and sorted based on c-kit expression as in panel a resulting in similar purity of c-kit+ and c-kit− populations. The sorted cells from each treatment were co-cultured with CD4+ T cells and the culture supernatants were assayed for cytokine levels by ELISA. Data shown are means of triplicates ± s.d. and are representative of two independent experiments *, P<0.05, **, P<0.001.
CT- or HDM-mediated c-kit signaling regulates IL-6 production and the expression of Notch ligands
The ability of c-kit negative cells to trigger a strong Th1 response (Fig. 3) suggested that the lack of c-kit upregulation could be a potential mechanism to subvert a Th2 response. To test this hypothesis, we isolated lung DCs and stimulated them with Th2- or Th1-skewing agents and the expression of different molecules associated with DC maturation and T helper differentiation was examined. A significant increase in the percentage of c-kit-expressing DCs was noted upon treatment with CT or HDM, but not CpG ODN. In contrast, CpG ODN, but not HDM or CT, promoted expression of CD40, which is associated with increased IL-12 production in DCs and Th1 response 27(Fig. 4a).
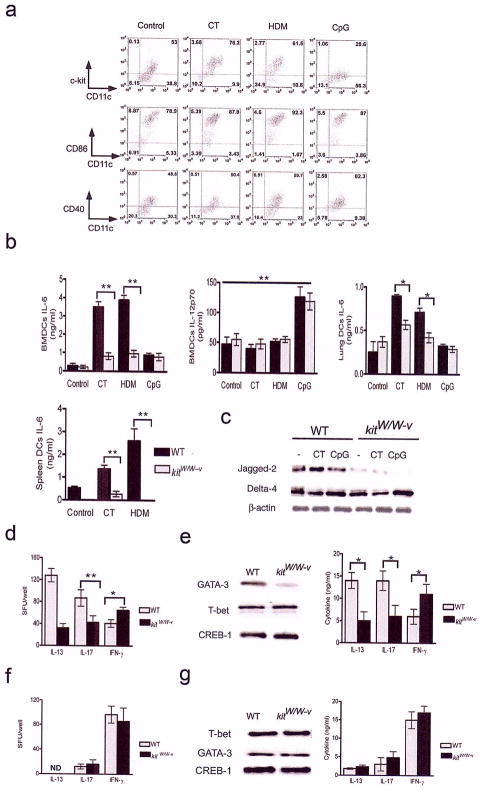
Figure 4. CT- or HDM-induced c-kit signaling regulates IL-6 production and the expression of Notch ligands.
(a) Lung DCs were stimulated with different stimuli and phenotyped for the markers shown. Numbers denote percentage of cells in that quadrant. (b) Cytokine production by BMDCs, lung DCs and spleen DCs was assayed by ELISA. Data shown are means of triplicates ± s.d. and are representative of two independent experiments. * P <0.05, **, P<0.01. (c) Total cell lysates of variously treated BMDCs containing equal amounts of protein were sequentially analyzed by immunoblotting technique for expression of the Notch ligands Jagged-2 and Delta-4. The blot was stripped and reprobed for β-actin levels to ensure similar protein loading. (d) Cells from lung-draining lymph nodes of mice after OVA/CT immunization were stimulated with PMA (25 ng/ml) and ionomycin (500 ng/ml) for 24 h to assess T cell priming by ELISPOT methods. 1×105 lymph node cells from the individual mice were plated in each well and the spot forming units (sfu) for each cytokine shown were identified using ELISPOT techniques. *, P<0.05, **, P<0.005. (e) Lung-draining lymph nodes were harvested after intranasal administration of OVA/CT into kitW/W-v mice and WT mice. The harvested lymph nodes cells were cultured for 5 days in the presence of OVA. Nuclear extracts prepared from the cultures were analyzed sequentially by immunoblotting for GATA-3 and T-bet expression. The blot was last stripped and probed for CREB-1 as a marker for protein loading. This experiment was performed three times. The supernatants from the co-cultures were analyzed for the cytokines IL-13, IL-17 and IFN-γ by ELISA and data shown are means of triplicates ± s.e.m. *, P<0.05. Data shown are representative of three independent experiments. (f) Cells from lung-draining lymph nodes following OVA/CpG immunization were stimulated for 24 h with PMA+ionomycin and the sfu were assayed by ELISPOT. ND: not detected. (g) The recall response was assayed by stimulating the lymph node cells with OVA for three days. The transcription factor profile was assayed by immunoblotting technique and the cytokine levels in the culture supernatants were assayed by ELISA.
Since CT and HDM upregulated c-kit expression in BMDCs, and the expression of this molecule was critical for IL-6 production, we next investigated whether c-kit signaling was an important component of IL-6 production in BMDCs and, in turn, in the regulation of T cell responses. BMDCs were cultured from WT or kit-mutant (kitW/W-v) mice. There was no difference in BMDCs between WT and kitW/W-v mice with respect to purity, yield and expression of MHC class II and the co-stimulatory molecules CD40 and CD86 (Supplementary Fig. 2 online). The DCs were incubated with or without CT or HDM and the cytokine profile was analyzed by ELISA. DCs from kitW/W-v mice secreted significantly lower levels of IL-6 upon CT or HDM stimulation indicating that c-kit signaling in BMDCs was important for IL-6 production in response to these agents. The level of IL-12p70 in the culture supernatants showed no significant difference between the mouse groups, however CpG ODN upregulated the production of this cytokine (Fig. 4b). These observations were essentially similar when DCs were isolated from the spleen or the lung (Fig. 4b) clearly showing that mutations that impair c-kit signaling affect IL-6 secretion from BMDCs as well as tissue DCs.
Antigen presenting cells have been shown to upregulate the Notch ligands Delta-4 and Jagged-2 in response to Th1- and Th2-inducing stimuli respectively19. We, therefore, investigated whether the expression pattern of these ligands was altered in DCs from WT and kitW/W-v mice. As shown in Fig. 4, panel c, at the basal level, BMDCs from kitW/W-v mice showed markedly reduced levels of Jagged-2 compared to that in DCs from WT mice suggesting a developmental link between c-kit and Jagged-2. Also, CT stimulated Jagged-2 expression in BMDCs from kit+/+ mice (Fig. 4c). The expression of Delta-4, on the other hand, was not compromised by c-kit defect and was slightly elevated upon CpG ODN treatment (Fig. 4c).
To confirm that impaired c-kit signaling has physiological relevance in T cell responses, we used an in vivo model of airway inflammation in which OVA in combination with CT was administered via the intranasal route 28 to WT and kitW/W-v mice. After 4 days of rest, the animals were sacrificed and lung-draining lymph nodes were harvested. The lymph node cells from the kitW/W-v mice showed reduced IL-13 and IL-17 spot forming units (sfu) and a slight increase in IFN-γ sfu as determined by ELISPOT assay (Fig. 4d). When analyzed in a recall response to antigen, lower expression of the Th2-specific factor GATA-3 4,5 was detected in nuclear extracts derived from kitW/W-vmice, which was not apparent for the Th1-specific factor T-bet 9 (Fig. 4e). Correspondingly, a decreased level of IL-13 was noted in cultures containing lymph node cells from kitW/W-v mice as compared to those containing cells from WT mice and the converse was noted for IFN-γ levels (Fig. 4e). The increase in IFN-γ levels when using cells from the kitW/W-v mice suggested a decrease in IL-17 production due to known cross-regulation by IFN-γ which was confirmed by ELISA. As expected, cultures established from lymph nodes of kitW/W-v mice generated less IL-6 (Supplementary Fig. 3 online).
As shown in Fig. 4f, the combination of OVA and CpG ODN elicited a potent Th1 response which was not different in the kit+/+ or kitW/W-v mice (Fig. 4g). Taken together, c-kit signaling was found to be important for the elicitation of a Th2 but not a Th1 response.
DCs with defective c-kit signaling elicit a poor Th2 response and allergic airway inflammation upon adoptive transfer to wt recipients
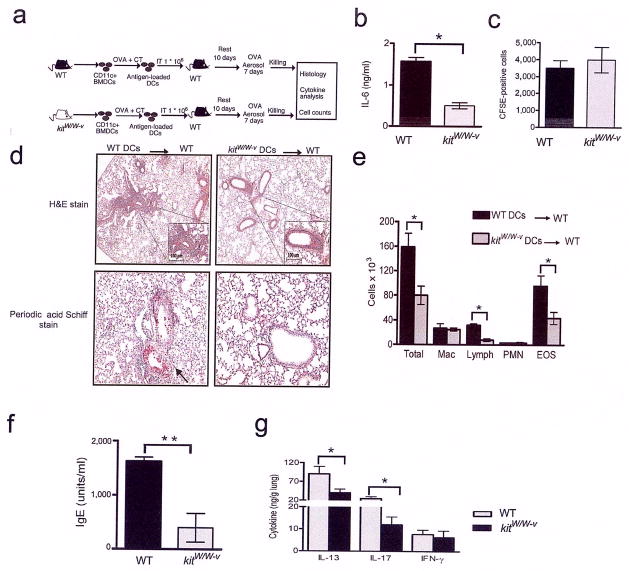
Given that kitW/W-v mice also have a defect in mast cells which are associated with allergic responses, we tested whether c-kit deficiency in DCs specifically explained the reduced Th2- and Th17-priming in kitW/W-v mice. To address this, we adoptively transferred either WT or kitW/W-v DCs into WT recipients with a complete repertoire of all cell types including mast cells and examined development of allergic airway inflammation. The cartoon in Fig. 5a depicts our experimental strategy. Fig. 5b shows that BMDCs generated from kitW/W-v mice produced less IL-6 compared to those from WT mice after incubation with OVA+C although both migrated similarly to lung draining lymph nodes subsequent to intratracheal transfer as confirmed using CFSE-labeled DCs (Fig. 5c). In mice subjected to repeated antigen challenge after DC transfer, assessment of lung histology (Fig. 5d), differential cell counts in bronchoaleveolar lavage (BAL) fluid (Fig. 5e ), antigen specific IgE levels (Fig. 5f ) and cytokine production (Fig. 5g), showed that BMDCs from kitW/W-v mice induce reduced allergic airway inflammation.
Figure 5. Impaired Th2 and Th17 response in vivo when BMDCs containing mutant c-kit, and consequently secreting less IL-6, were adoptively transferred into WT mice.
(a) Cartoon depicting experimental scheme. (b) Supernatants from BMDCs of WT and kitW/W-v mice stimulated with OVA/CT overnight were assayed for IL-6 production by ELISA. Data shown are means of triplicates± s.e.m. *, P<0.05. Data are representative two independent experiments. (c) The presence of CFSE-labeled CD11c+ BMDCs in lung-draining lymph nodes subsequent to intratracheal transfer was assessed 24 h following transfer. This experiment was performed twice and data shown are mean ± s.d. (d) Histological examination of lung sections of recipient mice stained with hematoxylin and eosin (for assessment of inflammation) or PAS (to assess mucus production). Magnification 100X with the inset at higher magnification (200X). (e) Total and differential counts of cells recovered in the BAL fluid are shown. *, P<0.05. The statistical analysis was performed over 4–5 mice in each group. (f) Antigen-specific IgE levels were measured by ELISA. The results represent the means of triplicates ±s.d. *, P<0.01. (g) The cytokines IL-13, IL-17 and IFN-γ in lung homogenates were measured by ELISA and shown are mean values of triplicates ±s.d. *, P<0.05. Shown is a representative experiment of two.
We also addressed the role of c-kit in a different model associated with a Th17 response, which is the Klebsiella pneumoniae model of lung infection 29. WT and kitW/W-v mice were infected with the bacteria and the mice were sacrificed on day 4 following the infection. Analysis of cfu in the mice showed that the c-kit mutants had significantly lower cfu in the spleen, liver and lungs. Interestingly, the c-kit mutants also had a higher IFN-γ response in the lung-draining lymph nodes as assayed by ELISPOT technique (Supplementary Fig. 4 online). This was similar to increased IFN-γ secretion upon neutralization of IL-6 (Fig. 1) and in response to OVA/CT in c-kit mutant mice (Fig. 4d). Thus, in the Klebsiella infection model too, compromised c-kit signaling in the c-kit mutant mice impaired Th17 response, but increased IFN-γ response and the mice showed less bacterial burden in different organs.
Differential expression of the c-kit ligand, stem cell factor (SCF), in CT or HDM-stimulated BMDCs
We next asked how c-kit was stimulated in DCs to induce IL-6 production. Prior studies have shown that c-kit is internalized upon binding its ligand, stem cell factor (SCF), indicating an important role for SCF in regulating cell surface expression of c-kit30. Analysis of SCF levels in culture supernatants of control versus CT-stimulated BMDCs showed lower levels in the latter (Fig. 6a). and the expression of c-kit was reduced upon treatment with soluble SCF (Fig. 6b).
Figure 6. Decreased expression of soluble SCF but increased expression of the membrane-bound form in CT-stimulated DCs.
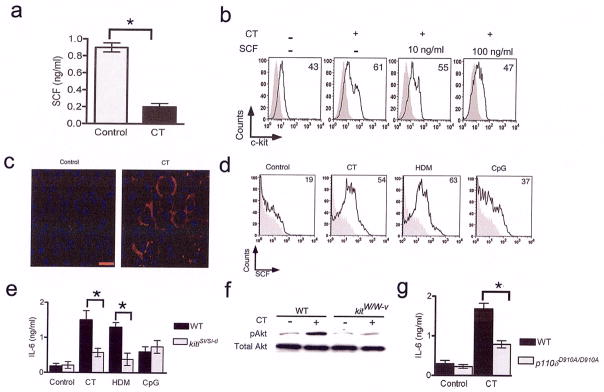
(a) Supernatants collected 24 h after incubation of BMDCs with or without CT were assayed for soluble SCF by ELISA. The results shown are representative of four independent experiments. The error bars indicate means of triplicates ± s.e.m. *, P<0.001. Data are representative of four independent experiments. (b) Soluble SCF reduces c-kit expression in CT-stimulated BMDCs. BMDCs were incubated with or without CT for 24 h in the presence or absence of soluble SCF as indicated. The cells were stained for c-kit expression by flow cytometry. (c) CT promotes expression of membrane-bound SCF in BMDCs. BMDCs were incubated with or without CT for 24 h. Cytospins prepared from the cultures were immunostained with anti-SCF antibody and the nuclei were identified with DAPI. Red bar represents 50 μm. (d) The BMDCs from the different stimulations were assessed for expression of membrane-bound SCF. The numbers represent the percentage of cells expressing SCF on the cell surface. The shaded grey represents staining with the isotype control. (e) BMDCs generated from WT and SCF mutant mice were stimulated for 24h as indicated and IL-6 production was assayed by ELISA. Data shown are means of triplicates ±s.e.m. and are representative of two independent experiments. *, P<0.01. (f) Total cell lysates prepared from BMDCs of kitW/W-v mice and kit+/+ mice incubated with or without CT for 24h were immunoblotted for pAkt. The same blot was stripped and re-probed for Akt. (g) BMDCs from p110δD910A/D910A and WT mice were incubated with or without CT for 24 h. IL-6 levels in the culture supernatants were assayed and data shown are means of triplicates ± s.e.m. The experiments were repeated twice with similar results. *, P<0.05.
Since SCF is also expressed in a membrane-bound form due to alternative splicing 31, we analyzed expression of membrane-bound SCF by different techniques. The results of immunostaining showed that CT-treatment of BMDCs promoted expression of membrane bound-SCF rather than the soluble form which was favored in control cells (Fig. 6c). When analyzed by flow cytometry, CT or HDM stimulation induced a greater frequency of SCF-expressing BMDCs than stimulation by CpG ODN (Fig. 6d). To further elucidate the functional significance of this differential expression, we used DCs from kitlsl/sl-d mice in which expression of the membrane-bound form of SCF is selectively compromised31,32. As shown in Fig. 6e, IL-6 expression was significantly compromised upon CT- or HDM-stimulation of DCs generated from kitlsl/sl-d mice (Fig. 6e). DCs generated from kitlsl/sl-d mice showed negligible cell surface staining for SCF (Supplementary Fig. 5 online).
Among signaling pathways induced by c-kit, PI3K plays an important role 33. The sustained expression of c-kit after 24 h coupled with the high level of expression of membrane-bound SCF suggested persistent signaling through c-kit via PI3 kinase in the DCs. As shown in Fig. 6f, phosphoAkt, triggered by activated PI3K 34, was readily detectable in cell extracts prepared from CT-stimulated BMDCs derived from but not kitW/W-v mice. We next investigated whether CT-stimulated IL-6 production was compromised in PI3 kinase 110δ mutant mice (p110δD910A/D910A). These mice lack the 110delta subunit of PI3 kinase and have impaired B and T cell signaling and mounting of allergic airways disease 35,36. CT-stimulated BMDCs from p110δD910A/D910A mice secreted less IL-6 as compared to cells from WT mice showing the involvement of the PI3 kinase pathway in IL-6 production (Fig. 6g). Thus, c-kit expressed by BMDCs in conjunction with membrane-bound SCF causes prolonged activation of the PI3 kinase/Akt pathway in DCs that promotes a higher IL-6 and lower IL-12 expression profile in DCs, which in turn, promotes T cell differentiation towards the Th2 and Th17 lineage.
DISCUSSION
The discovery of DCs has enhanced our knowledge of the divergent pathways of Th1, Th2 and the recently discovered Th17 responses. In this study, we show that dual upregulation of c-kit and membrane-bound SCF on DCs causing sustained activation of the c-kit/PI3 kinase signaling axis is important for promoting IL-6 production. Defective c-kit signaling, associated with reduced IL-6 production, compromised the ability of DCs to mount a robust Th2 and Th17 response and allergic airway inflammation in mice.
Our results demonstrate the specificity of c-kit and its ligand SCF for IL-6 production since the Th1-inducing adjuvant, CpG ODN, failed to upregulate c-kit expression. IL-12 production in DCs by microbial stimuli has been tightly linked to CD40 ligation 27 and IL-12 promotes Th1 differentiation. While IL-6 production by DCs has been associated with Th2 and Th17 differentiation 10,11,20, no cell surface event in DCs has been identified heretofore that promotes IL-6 and limits IL-12 production. Interestingly, in mice lacking RABGEF1 (Rab guanine nucleotide exchange factor 1), which is a negative regulator of c-kit, the levels of IL-6 were found to be elevated 37. Collectively, our data show that activation of the c-kit-PI3 kinase axis in DCs stimulating IL-6 production is an important regulatory mechanism that promotes Th2/Th17 but limits a Th1 response.
Not only did this study unravel a link between c-kit and IL-6 production in DCs, but the expression of a Notch ligand, Jagged-2, specifically aligned with Th2 response 19, was also found to be dependent on functional c-kit. Important regulatory roles for Notch have been recently identified in the immune system. For example, Notch has been implicated in T cell polarization, which has been associated with upregulation of specific Notch ligands in response to Th2 or Th1 stimuli on APCs 19. We have recently demonstrated cross-talk between Notch- and TGF-β-induced pathways in antigen-induced tolerance in the airways 38. Here, we show that DCs expressing functionally inactive c-kit express low levels of Jagged-2 compared to those from WT mice identifying a potentially important role for c-kit during development in the expression of a specific Notch ligand. The relationship between Jagged-2 and c-kit is also in line with previous observations showing that the Notch-Jagged 2 axis promotes IL-6 secretion in different cell types 39. While Notch activation in CD4+ T cells is important for Th2 differentiation 19,40,41, bidirectional Notch-Notch ligand signaling has been documented in Th:DC conjugates with evidence of nuclear translocation of Hes1 and STAT3 in both cell types 42. Thus, the Notch pathway may be important in promoting IL-6 gene expression in DCs with c-kit playing an important regulatory role in Jagged-2 expression.
As suggested by our data, the differential SCF expression seen in CT- or HDM-stimulated DCs could be an important mechanism by which prolonged activation of c-kit and its downstream targets is achieved. It should be noted that soluble SCF can transiently cause Akt phosphorylation, and phosphorylated Akt can no longer be detected after 15 min of addition of SCF 37. Our data showing persistent phosphoAkt levels in CT-stimulated DCs and the results obtained using cells from kitlsl/sl-d mice reiterate the fact that absence of membrane-bound SCF significantly reduces signaling via c-kit impairing IL-6 production. Thus, dual upregulation of c-kit receptor and membrane-bound form of SCF contributes to increased IL-6 production from allergen-stimulated DCs.
While c-kit has been best studied in the context of mast cells, administration of SCF in vivo was shown to have mast cell-independent effects 43. c-kit-expressiing DCs were shown to modulate NK cell function by increasing the cytolytic and IFN-γ-secreting ability of NK cells, thereby defining the importance of this receptor in one aspect of DC function15. Another important aspect of this study to note is that the authors clearly demonstrated that DCs were the pharmacological target of Gleevec administration in vivo, indicating mast cell-independent effects of Gleevec. While mast cells are known to fine tune T cell responses due to their ability to secrete a variety of cytokines, being professional antigen-presenting cells, DCs play a quintessential role in the orchestration of the adaptive immune response. The experiment involving adoptive transfer of DCs demonstrated that crippling c-kit signaling in DCs with intact mast cell c-kit signaling in recipients blunts the Th2 response, which prevents induction of full-blown allergic airway inflammation. Thus, although mast cells can modulate T cell responses, they are unable to supplant DCs in eliciting a strong Th2 response. Based on these results, it seems likely that defective DC functions may be responsible for the reported lower susceptibility of kitW/W-v mice to allergic airways disease 44.
The importance of c-kit signaling via PI3 kinase has also been explored in our study. Interestingly, p110δD910A/D910A mice have been shown to be resistant to allergic responses indicating a positive role for PI3 kinase in mediating Th2 responses35. A prior study has shown a negative role for PI3 kinase signaling in IL-12p70 production from DCs 45. Our study for the first time shows a positive role for this pathway in IL-6 production from DCs and our data suggest that sustained phosphorylation of Akt in DCs is required for the expression of specific cytokines such as IL-6 which can influence the outcome of an immune response. Prolonged c-kit expression coupled with expression of membrane-bound SCF ensures sustained Akt activation as is evident from our study and from previous studies in which soluble SCF was shown to impede signaling via c-kit 30 while membrane-bound SCF prolonged c-kit signaling 46.
The cytokine balance in the microenvironment is a key regulator of the net immune response. Our study highlights the importance of this balance in regulating T cell responses by demonstrating a central role for c-kit expressed by DCs in the fine-tuning of the IL-6/IL-12p70 expression profile. Our findings also have important ramifications in DC-based vaccines in cancer therapy where inhibition of c-kit may promote the efficacy of the vaccine by augmenting a Th1 response.
METHODS
Mice
Balbc/ByJ, C57BL/6, IL-6−/−(Stock number 002254), KitlSl/Sl-d (Stock number 100401), c-kit mutant WBB6F1W/W-v (kitW/W-v; Stock number 100410) and the wild type control (kit +/+) were all purchased from The Jackson Laboratory. DO11.10 transgenic mice were originally provided by Dr. Kenneth Murphy at Washington University, St. Louis and were bred at the animal facility at the University of Pittsburgh. All animals were housed under pathogen-free conditions and were used between 6 and 12 weeks of age. Within experiments, the animals were age and sex matched. All studies with animals were approved by the Animal Care and Use Committee at the University of Pittsburgh.
Cell culture
The media used for cell culture was RPMI 1640 (Gibco, Cat# 22400-089) supplemented with 10% heat inactivated fetal bovine serum (Gemini), penicillin/streptomycin, 1mM Na-pyruvate (Gibco) and 50 μM 2-mercaptoethanol (Sigma). All reagents used had <0.6 EU/ml of endotoxin. For DC cultures, the femur and tibia of mice were removed and the bone marrow flushed using a 27gauge needle. The cells were washed and cultured with 10ng/ml recombinant murine GM-CSF (Peprotech) at 1×106/ml. On the third day, the cells were refed with fresh medium and cytokine. On day 6, the loosely adherent cells were harvested. Prior to harvest, the flask was gently shaken at 7 hertz per second for 5 min on a MicroMix shaker (DPC instruments). The cells were then subjected to purification using magnetically labeled anti mouse CD11c+ beads (Miltenyi Biotech). The labeled cells were passed through the column twice and CD11c expression was >98%. The contaminating population of CD3+ and CD19+ was negligible. These cells were considered immature DCs as ascertained by low expression of CD40, CD86, and MHC class II (data not shown). The immature DCs were plated at 1×106/ml in 12 well plates in medium containing 10ng/ml GM-CSF with or without Cholera toxin (List Biologicals Labs) or HDM (Greer Labs) for 24 h. CT at 1μg/ml and HDM at 10 μg/ml, both dissolved in PBS, were used for stimulating the DCs unless specified otherwise. Both OVA and HDM preparations in PBS were stripped of LPS using EndoTrap column (Profos AG, Germany). The residual LPS in the solutions was estimated by the Limulus Amebocyte Lysate assay (Cambrex). The level of LPS in the cell cultures containing OVA or HDM was less than 0.1ng/ml. CT as purchased contained undetectable endotoxin. The lung and spleen DCs were isolated as described previously47. The lung DCs protocol was slightly modified. A high volume lavage was performed to isolate alveolar macrophages. The lung cells after CD11c separation was sorted on FACS ARIA based on the auto fluorescence. The low autofluorescent cells considered to be dendritic cells were sorted from the high autofluorescent cells, the macrophages and used in the experiments. For the inhibition of cyclic AMP, the BMDCs were pre-incubated with the cyclic AMP antagonist Rp-cAMPS, triethylammonium salt (Calbiochem) for 1 h before stimulation of cells. The cells were harvested and used in various experiments. The CD4+ T cells were obtained from splenocytes as previously described 28.
Antibodies
Antibodies for either flow cytometry or blocking experiments were purchased from BD Pharmingen unless otherwise stated. Anti-CD11c (APC; clone HL3), anti-CD40 (PE; clone 3/23), anti-CD86 (PE; clone GL1), anti-CD117 (c-kit) (PE; clone 2B8), anti-MHC class II (Southern Biotech; NIMR-4) were used along with the appropriate isotype controls for flow cytometry. Anti GATA-3, anti T-bet, anti-Jagged-2, anti-Delta-4, anti CREB-1 (Santa Cruz Biotechnology), anti-c-kit, anti-pAkt (Thr-308), and anti-Akt (Cell Signaling Technology) were used for western blot analysis. Anti-SCF from Chemicon was used for immunofluorescence studies and for detection of SCF by flow cytometry, indirect staining was carried out using anti-SCF from Abcam together with PE-conjugated goat-anti-rat IgG (Serotec). Anti IL-6 (clone MP5-20F3) and the isotype control were purchased from BD Pharmingen.
Intranasal antigen instillation
Intranasal administration of OVA/CT or OVA/CpG and ex vivo stimulation of single cell suspension of cells from lung-draining lymph nodes with OVA were performed as described previously28,47. The experiments with HDM (Greer labs) were performed as described 23 with minor modifications. Mice were anesthetized with isoflurane before intranasal administration of any antigen. In the experiments involving immunization of mice, 25μl of the instilled solution contained 10ng of residual LPS. Intranasal administration of 25μg of HDM was carried out for 7 consecutive days and the animals were rested for 3 days. Single cell suspensions of the lung-draining lymph nodes were stimulated ex vivo with HDM (10 μg/ml). Nuclear extracts were prepared from OVA- or HDM-stimulated cells after 5 days. The CpG ODN 1826 (5′-TCCATGACGTTCCTGACGTT-3′), which is known to be optimal for stimulation of murine cells, and the control ODN 1911 (5′-TCCAGGACTTTCCTCAGGTT-3′) were used at a concentration of 1 μM to stimulate DCs. The control ODN did not influence cytokine production or expression of any molecules in DCs in initial experiments as previously reported by us and was not used in all experiments47. The LPS level in the ODN preparations was low (<0.1 ng/mg DNA). The oligonucleotides were purchased from Oligos Etc.
Adoptive transfer of DCs into mice and induction of allergic airway inflammation
CD11c+ BMDCs from kit+/+ and kitW/W-v were isolated and incubated overnight with OVA (100μg/ml) and CT (1μg/ml). The cells were harvested, washed and 1×106 cells were adoptively transferred via the intratracheal route into C57BL/6 recipients. After transfer, the mice were rested for 10 days and then challenged by exposure to aerosolized 1 % OVA for 7 consecutive days using an ultrasonic nebulizer (Omron Healthcare). 24 h after the last aerosol, the mice were sacrificed and the lungs were examined for inflammation. The cell count in the BAL fluid and lung histology were performed as previously described 6,28,48 and PAS staining was performed according to manufacturer’s instructions (Richard Allan Scientific)48. For tracking the cells in vivo, the cells were labeled with CFSE dye after stimulation with OVA+CT. 10 X106 BMDCs were labeled with CFSE dye (1 μM, Invitrogen) for 15 min at 37°C. The labeling was stopped by washing the cells twice with PBS containing 2%FBS. The cells were re-counted and suspended at a concentration of 1 X106 BMDCs in 50 μl of PBS.
Flow cytometry
The staining procedures were essentially as described previously28. The live cells were gated using propidium iodide staining and at least 10,000 events within the live gate were collected using a FACSCalibur flow cytometer. (BD) Staining for SCF was performed by first adding the unconjugated primary antibody(1μg) and incubating on ice for 1h. The tubes were washed and the secondary antibody (PE conjugated goat-anti-rat IgG) was added at the same concentration and incubated for 0.5h. The data were analyzed using FlowJo software (Tree Star corporation).
Cell extracts and immunoblotting
Nuclear extracts and total cell lysates were prepared as stated previously24,49. Total cell lysates were prepared from DCs at 24 h post- stimulation using 1X cell lysis buffer (Cell signaling).
Microarray analysis
The isolation of RNA and microarray analysis was performed essentially as described previously24. RNA was isolated from BMDCs incubated with or without cholera toxin (1 μg/ml) for 24h. Total RNA quality was assessed prior to cRNA target preparation and labeling. cRNA was synthesized from the total RNA samples by using the CodeLink Expression Assay Reagent Kit (GE Healthcare). The labeled cRNA was fragmented at and hybridized to CodeLink UniSet Mouse I Bioarrays (GE Healthcare) that identifies 10012 unique murine genes. The hybridized bioarrays were scanned using the GenePix 4000B Array Scanner (Axon Instruments, Union City, CA). The Normalized Intensities of all slides were then imported into Spotfire to create one file, which included annotations supplied by CodeLink. Only the Discovery genes and genes with Good or Low quality flags were selected with the Spotfire query devices. These selected genes were then exported into Excel. Then the data was formatted into the acceptable form for the DOS based data analysis program, Scoregene. Scoregene globally normalized the data by calculating the geometric mean across the controls and experimentals and then log transformed the data to base 2. T-test p values were also calculated by Scoregene. Genes with T test p-values < 0.05 and a fold change of 1.5 (SLR signal log ratio of 0.5849625) were determined to be statistically significant and selected for hierarchical clustering in Spotfire. The clustering method used was UPGMA (Unweighted Pair-Group Method Average) with a Euclidean distance similarity measurement.Genes were considered to be significantly upregulated or down regulated if the fold change was greater than +1.5 or less than −1.5. RNA was isolated in three independent experiments from control and CT-treated BMDCs and bone marrows were pooled from at least 4 mice in each experiment.
Immunofluorescence
1×105 cells were cytospun on slides and fixed with 95% ethanol and 5% glacial acetic acid. The slides were blocked with 2% goat serum diluted in PBS and incubated overnight at 4°C with the primary anti-SCF antibody. The slides were washed with PBS and incubated with the secondary antibody tagged with Cy3 for visualization. The slides were mounted with mounting medium Vectashield containing DAPI stain (Vector labs) to identify the nuclei. The specificity of staining was ensured using control slides in which the incubation with the primary antibody was skipped.
ELISA
ELISAs for IL-12p70, IL-6, IL-4, IL-13 and IFN-γ were performed with kits from R&D Systems per manufacturer’s instructions. ELISA for soluble SCF was performed using a kit from Peprotech. Unless otherwise indicated, all statistical analyses were carried out using mean values of triplicate wells and results shown are representative of two to four independent experiments. The OVA-specific IgE levels were measured as previously described 28. The concentration of IgE was derived from standard curves generated using pooled sera from animals immunized with OVA/alum and repeatedly challenged with aerosolized OVA and the standard was assigned an arbitrary unit of 10,000 U/ml.
ELISPOT
The IL-13, IL-17 and IFN-γ ELISPOT assays were perfomed using kits from eBioscience per the manufacturer’s specifications. Briefly, ELISPOT plates (Millipore 96-well MultiScreen HTS) were pre-coated with the primary antibody at 4° C overnight. Lymph nodes cells were plated at a concentration of 1×105 or 1 ×104 per well and stimulated with PMA (25ng/ml) and ionomycin (500 ng/ml) for 24 h. Biotin-conjugated secondary antibody was used to detect the secreted cytokine. The plates were developed with Avidin-HRP and peroxidase substrate (Vectastatin). The spot forming units (sfu) were quantified using an automated ELISPOT plate reader (ImmunoSpot; Cellular Technology).
Statistical analysis
Student’s unpaired two-tailed t-test was used for all statistical analyses. Differences between groups were considered significant if P<0.05. All statistical analyses (except that performed for microarray data) were performed using GraphPad Prism software.
Accession codes
Gene Expression Omnibus microarray accession code, GSE10815.
Supplementary Material
Acknowledgments
We dedicate this study to the memory of Professor Ray Wu who inspired us to tread the untrodden path. We would like to thank A. Henry and B. Dixon-McCarthy for technical assistance with the adoptive transfer experiment and assessment of airway inflammation, M. Zheng and J.K. Kolls for help with the Klebsiella infection model, S. Plevy for providing us with breeding pairs of p110δD910A/D910A mice and S. Shapiro for critically reading the manuscript. This work was supported by U.S. National Institutes of Health grants HL 060207 and HL 069810 (to P.R.), HL 077430 and AI 048927 (to A.R.) and HL 084932 (to P.R. and A.R.).
Footnotes
AUTHOR CONTRIBUTIONS
N.K., T.B.O. and M.F. performed in vitro and in vivo experiments. M.P. and M.V. performed microarray and PCR experiments. B.V. provided PI3 kinase mutant mice and critically read the manuscript. N.K. and T.B.O. also participated in study design and N.K. prepared the manuscript. P.R. and A.R. conceived the study, analyzed the data and wrote the manuscript.
Competing interests statement: The authors declare no competing financial interests.
References
- 1.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 2.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 4.Zhang DH, Cohn L, Ray P, Bottomly K, Ray A. Transcription factor GATA-3 is differentially expressed in murine Th1 and Th2 cells and controls Th2-specific expression of the interleukin-5 gene. J Biol Chem. 1997;272:21597–21603. doi: 10.1074/jbc.272.34.21597. [DOI] [PubMed] [Google Scholar]
- 5.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 6.Das J, et al. A critical role for NF-kappa B in GATA3 expression and TH2 differentiation in allergic airway inflammation. Nat Immunol. 2001;2:45–50. doi: 10.1038/83158. [DOI] [PubMed] [Google Scholar]
- 7.Zhu J, et al. Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nat Immunol. 2004;5:1157–1165. doi: 10.1038/ni1128. [DOI] [PubMed] [Google Scholar]
- 8.Ivanov II, et al. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 9.Szabo SJ, et al. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 10.Rincon M, Anguita J, Nakamura T, Fikrig E, Flavell RA. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J Exp Med. 1997;185:461–469. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dodge IL, Carr MW, Cernadas M, Brenner MB. IL-6 production by pulmonary dendritic cells impedes Th1 immune responses. J Immunol. 2003;170:4457–4464. doi: 10.4049/jimmunol.170.9.4457. [DOI] [PubMed] [Google Scholar]
- 12.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 13.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 14.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 15.Borg C, et al. Novel mode of action of c-kit tyrosine kinase inhibitors leading to NK cell-dependent antitumor effects. J Clin Invest. 2004;114:379–388. doi: 10.1172/JCI21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chabot B, Stephenson DA, Chapman VM, Besmer P, Bernstein A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988;335:88–89. doi: 10.1038/335088a0. [DOI] [PubMed] [Google Scholar]
- 17.Geissler EN, Ryan MA, Housman DE. The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell. 1988;55:185–192. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- 18.Qiu FH, et al. Primary structure of c-kit: relationship with the CSF-1/PDGF receptor kinase family--oncogenic activation of v-kit involves deletion of extracellular domain and C terminus. Embo J. 1988;7:1003–1011. doi: 10.1002/j.1460-2075.1988.tb02907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amsen D, et al. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117:515–526. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 20.Braun MC, He J, Wu CY, Kelsall BL. Cholera toxin suppresses interleukin (IL)-12 production and IL-12 receptor beta1 and beta2 chain expression. J Exp Med. 1999;189:541–552. doi: 10.1084/jem.189.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Illi S, et al. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368:763–770. doi: 10.1016/S0140-6736(06)69286-6. [DOI] [PubMed] [Google Scholar]
- 23.Cates EC, et al. Intranasal exposure of mice to house dust mite elicits allergic airway inflammation via a GM-CSF-mediated mechanism. J Immunol. 2004;173:6384–6392. doi: 10.4049/jimmunol.173.10.6384. [DOI] [PubMed] [Google Scholar]
- 24.Arora M, et al. Simvastatin promotes Th2-type responses through the induction of the chitinase family member Ym1 in dendritic cells. Proc Natl Acad Sci U S A. 2006;103:7777–7782. doi: 10.1073/pnas.0508492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bagley KC, Abdelwahab SF, Tuskan RG, Fouts TR, Lewis GK. Cholera toxin and heat-labile enterotoxin activate human monocyte-derived dendritic cells and dominantly inhibit cytokine production through a cyclic AMP-dependent pathway. Infect Immun. 2002;70:5533–5539. doi: 10.1128/IAI.70.10.5533-5539.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crackower MA, et al. Regulation of myocardial contractility and cell size by distinct PI3K-PTEN signaling pathways. Cell. 2002;110:737–749. doi: 10.1016/s0092-8674(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 27.Schulz O, et al. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 2000;13:453–462. doi: 10.1016/s1074-7613(00)00045-5. [DOI] [PubMed] [Google Scholar]
- 28.Oriss TB, et al. Dynamics of dendritic cell phenotype and interactions with CD4+ T cells in airway inflammation and tolerance. J Immunol. 2005;174:854–863. doi: 10.4049/jimmunol.174.2.854. [DOI] [PubMed] [Google Scholar]
- 29.Happel KI, et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202:761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yee NS, Langen H, Besmer P. Mechanism of kit ligand, phorbol ester, and calcium-induced down-regulation of c-kit receptors in mast cells. J Biol Chem. 1993;268:14189–14201. [PubMed] [Google Scholar]
- 31.Flanagan JG, Chan DC, Leder P. Transmembrane form of the kit ligand growth factor is determined by alternative splicing and is missing in the Sld mutant. Cell. 1991;64:1025–1035. doi: 10.1016/0092-8674(91)90326-t. [DOI] [PubMed] [Google Scholar]
- 32.Brannan CI, et al. Steel-Dickie mutation encodes a c-kit ligand lacking transmembrane and cytoplasmic domains. Proc Natl Acad Sci U S A. 1991;88:4671–4674. doi: 10.1073/pnas.88.11.4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serve H, et al. Differential roles of PI3-kinase and Kit tyrosine 821 in Kit receptor-mediated proliferation, survival and cell adhesion in mast cells. Embo J. 1995;14:473–483. doi: 10.1002/j.1460-2075.1995.tb07023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franke TF, et al. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 35.Ali K, et al. Essential role for the p110delta phosphoinositide 3-kinase in the allergic response. Nature. 2004;431:1007–1011. doi: 10.1038/nature02991. [DOI] [PubMed] [Google Scholar]
- 36.Okkenhaug K, et al. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002;297:1031–1034. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- 37.Kalesnikoff J, et al. RabGEF1 regulates stem cell factor/c-Kit-mediated signaling events and biological responses in mast cells. Proc Natl Acad Sci U S A. 2006;103:2659–2664. doi: 10.1073/pnas.0511191103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ostroukhova M, et al. Treg-mediated immunosuppression involves activation of the Notch-HES1 axis by membrane-bound TGF-beta. J Clin Invest. 2006;116:996–1004. doi: 10.1172/JCI26490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Houde C, et al. Overexpression of the NOTCH ligand JAG2 in malignant plasma cells from multiple myeloma patients and cell lines. Blood. 2004;104:3697–3704. doi: 10.1182/blood-2003-12-4114. [DOI] [PubMed] [Google Scholar]
- 40.Amsen D, et al. Direct regulation of Gata3 expression determines the T helper differentiation potential of Notch. Immunity. 2007;27:89–99. doi: 10.1016/j.immuni.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fang TC, et al. Notch directly regulates Gata3 expression during T helper 2 cell differentiation. Immunity. 2007;27:100–110. doi: 10.1016/j.immuni.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luty WH, Rodeberg D, Parness J, Vyas YM. Antiparallel segregation of notch components in the immunological synapse directs reciprocal signaling in allogeneic Th:DC conjugates. J Immunol. 2007;179:819–829. doi: 10.4049/jimmunol.179.2.819. [DOI] [PubMed] [Google Scholar]
- 43.Chatterjea D, et al. Adoptive transfer of mast cells does not enhance the impaired survival of Kit(W)/Kit(W-v) mice in a model of low dose intraperitoneal infection with bioluminescent Salmonella typhimurium. Immunol Lett. 2005;99:122–129. doi: 10.1016/j.imlet.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 44.Lukacs NW, et al. Stem cell factor (c-kit ligand) influences eosinophil recruitment and histamine levels in allergic airway inflammation. J Immunol. 1996;156:3945–3951. [PubMed] [Google Scholar]
- 45.Fukao T, et al. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat Immunol. 2002;3:875–881. doi: 10.1038/ni825. [DOI] [PubMed] [Google Scholar]
- 46.Miyazawa K, et al. Membrane-bound Steel factor induces more persistent tyrosine kinase activation and longer life span of c-kit gene-encoded protein than its soluble form. Blood. 1995;85:641–649. [PubMed] [Google Scholar]
- 47.Chen L, et al. Distinct responses of lung and spleen dendritic cells to the TLR9 agonist CpG oligodeoxynucleotide. J Immunol. 2006;177:2373–2383. doi: 10.4049/jimmunol.177.4.2373. [DOI] [PubMed] [Google Scholar]
- 48.Zhang DH, et al. Inhibition of allergic inflammation in a murine model of asthma by expression of a dominant-negative mutant of GATA-3. Immunity. 1999;11:473–482. doi: 10.1016/s1074-7613(00)80122-3. [DOI] [PubMed] [Google Scholar]
- 49.Ostroukhova M, et al. Tolerance induced by inhaled antigen involves CD4(+) T cells expressing membrane-bound TGF-beta and FOXP3. J Clin Invest. 2004;114:28–38. doi: 10.1172/JCI20509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.