Abstract
Osteopontin (OPN) is a secreted glycoprotein implicated to function in cancer development and metastasis. Although elevated expression of OPN are observed in cancer cells of various types, in some cases, only the cells in the stromal region surrounding the tumor express OPN, suggesting distinct functional roles for this protein derived from host cells and from cancer cells. To provide a model for addressing the functions and mechanisms of host-derived OPN in cancer progression and metastasis, a cutaneous squamous cell carcinoma cell line (ONSC) that lacks the OPN gene, Spp1, was established. This line of cells was derived from a squamous cell carcinoma that developed in a female, OPN-null mouse subjected to two-stage skin carcinogenesis. Morphologically, ONSC cells resemble epithelial cells, and they express the epithelial markers, K1, K14, and p63, as confirmed by immunohistochemical analyses. Genomic analyses indicate the presence of mutated H-Ras and p53 genes. ONSC cells form colonies in soft agar and, subcutaneously injected into athymic nude mice, develop into squamous cell carcinomas that metastasize to the lungs. Lacking OPN expression, these SCC cells provide a model to address the function of host OPN in the context of cancer progression and metastasis.
Keywords: Osteopontin, cutaneous squamous cell carcinoma, cell line, tumor progression, metastasis, nude mice
Squamous cell carcinoma (SCC) is one of the most prevalent cancers, and can be deadly because of its tendency to metastasize. Patients diagnosed with SCC of the head and neck have a poor survival rate (Menzin et al., 2007). Elderly and immune-suppressed individuals are at higher risk of developing cutaneous SCC with the propensity to metastasize (Weinberg et al., 2007). An understanding of the mechanisms of SCC progression and metastasis would enhance the design of therapy to prevent these processes. To elucidate the mechanisms of SCC progression and metastasis, appropriate cancer cell models that can be studied in an immune-competent mouse are necessary. Here we report the establishment and preliminary characterization of a murine cutaneous SCC cell line that has the capacity to develop tumors subcutaneously and to metastasize, not only in nude mice, but also in immune-competent mice (Hsieh et al., manuscripts in preparation). This SCC cell line lacks the Spp1 gene, which codes for the matricellular protein, osteopontin (OPN), a protein implicated to function in tumorigenesis (Rittling & Chambers, 2004; Hsieh et al., 2006), tumor progression, and metastasis (Crawford et al., 1998; Wai & Kuo, 2008).
OPN is an acidic glycoprotein found mainly in the extracellular environment. Its elevated expression during tumorigenesis, tumor progression, and metastasis is consistent with its function in enhancing cell survival, cell proliferation, and migration (Rittling & Chambers, 2004; Hsieh et al., 2006; Wai & Kuo, 2008). Immunohistochemical analyses indicate that, in various cancer types, OPN is expressed by cancer cells, but, in some cases, it is expressed mainly by cells in the stromal surrounding the cancer (Brown et al., 1994; Crawford et al., 1998; Tunio et al., 1998; Hoshi et al., 2005), suggesting that the functional roles of OPN-derived from the host cells maybe distinct from those derived from cancer cells. Thus, an SSC cell line lacking OPN expression would allow assessment on the function of host-derived OPN in the tumor microenvironment and at the systemic level. The establishment and characterization of a cutaneous, OPN-null squamous cell carcinoma cell line, named ONSC, are described below.
Mice used in these experiments were housed under germ-free conditions in the Animal Resource Program facility (AAALAC approved) at the University of Alabama at Birmingham (UAB). Procedures for animal studies were approved by the UAB IACUC.
The ONSC line was derived from SCCs, which arose in mice subjected to two-stage skin chemical carcinogenesis (Hsieh et al., 2006). In this procedure, the dorsal regions of 129S6/SvEv female OPN-/- mice were shaved and naired (< 1 min) two days prior to topical application of 7,12-dimethyl-benz[a]anthracene (43.7 nmol) and followed a week later by application of 4 μg of 12-O-tetradecanoylphorbol-13-acetate (TPA) twice weekly for 27 weeks. To monitor for SCC development, the mice were kept for an additional 10 weeks or more following termination of TPA treatment. SCCs were excised and incubated with collagenase to disperse the cells, which were rinsed prior to plating on a dish coated with collagen type I (Sigma, St. Louis, MO). After the ONSC cells began to proliferate and to be passaged, they were continuously subcultured. About passage 21, the cells grew on uncoated culture plates.
The ONSC cells were maintained in high-glucose, low-calcium DMEM containing 10% fetal bovine serum (FBS) (Hyclone, Logan, UT), 2 mM of L-glutamine (Irvine Scientific, Santa Ana, CA), and 100 μg/ml of penicillin/streptomycin (Fisher Scientific, Pittsburgh, PA). Cells were routinely tested for mycoplasma by a previously described procedure (McGarrity et al., 1983). In characterizing ONSC cells in vitro and in vivo, cell passages of 35-45 were used.
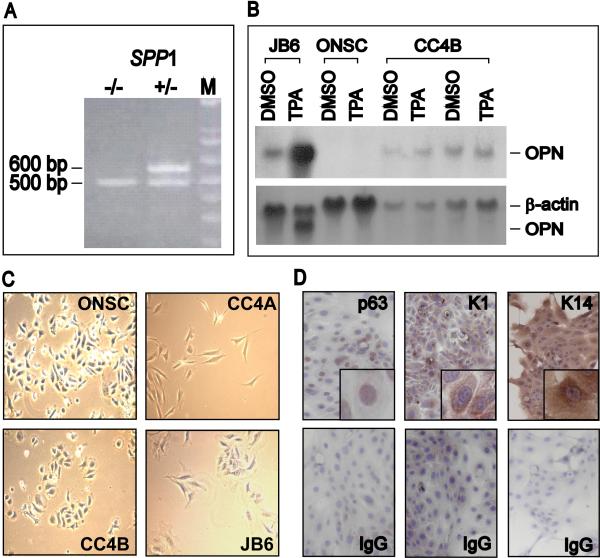
To confirm that the OPN gene (Spp1) is not present in ONSC cells, their genomic DNA was analyzed by PCR by use of specific primers (Liaw et al., 1998). DNA analysis of OPN heterozygous (OPN+/-) control mice yielded an OPN band of 600 bp and a disrupted OPN band of 500 bp (Fig. 1A). In contrast, PCR analysis of DNA from ONSC cells resulted in a single band corresponding with the disrupted Spp1 gene. Lack of the Spp1 gene in ONSC cells resulted in a lack of expression of OPN mRNA. Control cells, CC4B, a cutaneous SCC derived from wild-type (WT) mice subjected to a chemical carcinogenesis procedure (Ruggeri et al., 1994), and JB6 Cl41.5a, a promotable murine epidermal-like cell line (Colburn, 1980), along with ONSC cells, were exposed to 0.001% DMSO as a vehicle or to 10 ng/ml of TPA. Total RNA isolation and Northern blot analyses were accomplished as previously described (Chang et al., 2002). JB6 cells expressed minimal basal OPN mRNA, but the amount was elevated in the presence of TPA (Fig. 1B). CC4B cells expressed OPN mRNA in the presence of the solvent, but this mRNA was not induced by TPA. In contrast, no OPN mRNA was detected in ONSC cells even after TPA stimulation (Fig. 1B).
Figure 1.
Characterization of ONSC cells as OPN-null SCCs. (A) ONSC cells lack the Spp1 gene. PCR analyses were performed with DNA purified from ONSC cells and from OPN heterozygous (+/-) mice. Lane M, DNA molecular marker; 600 bp indicates OPN+ alleles; 500 bp indicates OPN- alleles; Spp1, OPN gene. (B) Northern blot analysis of OPN mRNA. Total RNA extracted from JB6 Cl41.5a, CC4B, and ONSC cells was probed with radiolabeled OPN or β-actin cDNA, used as a loading control (lower panel). The observed OPN in the lower panel is due to non-stripped radiolabeled OPN probe. (C) Comparison of cell morphology with epidermal cell lines. ONSC, an OPN-null cutaneous SCC line; CC4A, an undifferentiated spindle cell carcinoma cell line; CC4B, a well-differentiated cutaneous SCC cell line; JB6 Cl41.5a, a preneoplastic mouse epidermal-like cell line. Magnification, ×200. (D) Immunohistochemical analyses for the expression of p63, K1 and K14 in ONSC cells. ONSC cells were immunostained with anti-p63, K1, and K14 antibodies or their isotype controls (IgG). Magnification, ×200; ×400 (inserted panel).
Examined by phase-contrast microscopy (Nikon, Japan), ONSC cells resembled epithelial cells and were similar to cells of the SCC line CC4B, but they were morphologically distinct from cells of CC4A, a poorly differentiated spindle cell carcinoma (Fig. 1C). To verify that ONSC cells are of cutaneous epithelial origin, immunohistochemical analyses were performed to determine their expression of the epithelial markers, K1, K14 and p63 (Reis-Filho et al., 2002). ONSC cells were seeded in two-well chamber slides and cultured until 85-95% confluence. Cells were fixed in methanol, treated with 3% hydrogen peroxide, and blocked. Primary antibodies to p63 (Ab-1, mouse monoclonal antibody, 1 μg/ml, NeoMarkers, Fremont, CA), K14 (NCL-LL002, mouse monoclonal antibody 1:20, Novocastra Laboratories Ltd, UK), and K1 (AF 109, polyclonal antibody, 1 μg/ml, Covance Research Products, Berkeley, CA) or their respective isotype-matched immunoglobulins at the same concentration were added to the cells for 2 hours. This was followed by the addition of secondary antibody conjugated to biotin. To detect positive staining, cells were incubated with streptavidin conjugated to horseradish peroxidase followed by the addition of the substrate, 3′,3′-diaminobenzidine tetrahydrochloride (BioGenex, San Ramon, CA). Slides were counterstained with hematoxylin and coverslipped. Photos were taken with a Nikon Eclipse TS100 microscope.
ONSC cells were positive for K1and K14 in their cytoplasm and for nuclear p63 (Fig. 1D). These cells exhibited only minimal background staining with the corresponding isotype controls for K1, K14, and p63.
Additionally, ONSC cells were assessed for H-Ras and p53 mutations, which are characteristics of SCC derived from cancers induced in the two-stage skin carcinogenesis procedure (Frame et al., 1998). To detect mutated H-Ras and p53 genes, purified DNA from ONSC cells was analyzed with PCR primers, as previously reported (Nelson et al., 1992; Melnikova et al., 2005). The PCR products, sequenced to verify the mutation sites, revealed that the H-ras and p53 genes of ONSC cells exhibited an H-Ras mutation at the 61st codon (A → T) and p53 mutations at the 270th and 275th codons (C → T), respectively (Table 1). In contrast, the non-tumorigenic JB6 cells did not exhibit mutated H-Ras or p53 (Sun et al., 1993). CC4B cells are known to have p53 mutations (Ruggeri et al., 1994).
Table 1.
Characterization of tumor properties of ONSC cells
| Properties | JB6 C141.5a | CC4B | ONSC |
|---|---|---|---|
| p53 mutation | No | Yes | Yes |
| H-Ras mutation | No | No | Yes |
| Anchorage-independent growth | Minimal | Minimal | Yes |
| Tumor growth in athymic nude mice | ND | Yesa | Yesb |
| Metastasis in athymic nude mice | ND | ND | Yesc |
Female athymic nude mice, n=8
Observed in all 8 mice post 10 wk injection s.c. with 2× 106 ONSC cells/mouse
S.C., subcutaneous; ND, not determined
To assess the tumorigenicity of ONSC cells in vitro, anchorage-independent growth studies were performed. ONSC, JB6 Cl41.5a, or CC4B cells (10,000) were embedded in 0.3% agar and layered on top of 0.5% agar for 14 days as previously described (Chang & Prince, 1991). ONSC cells grew independent of anchorage (Table 1), a property that correlates with tumorigenesis in vivo (Shin et al., 1975). CC4B and JB6 cells showed minimal colony formation.
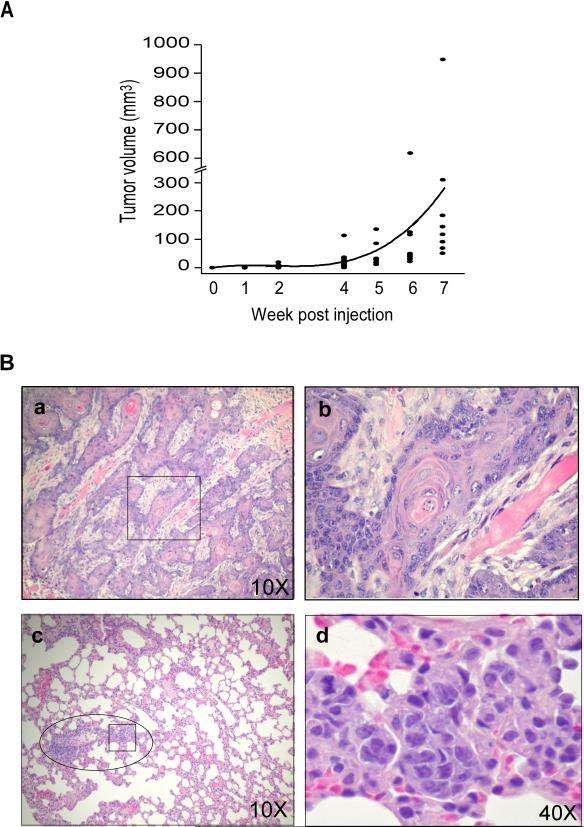
To test ONSC cells for tumorigenicity in intact animals, 2 × 106 cells were injected subcutaneously (s.c.) into the lower dorsal regions of 10 athymic NCr-nu/nu nude mice (Frederick Cancer Research Center, Frederick, MD). Tumor growth was monitored daily, and tumor volume was measured weekly by caliper for 7 wk, except on wk 3. At the 1st and 2nd weeks of tumor growth, one mouse was sacrificed. Tumors and all internal organs were harvested, fixed in 10% buffered formaldehyde, and embedded in paraffin blocks. Tumor and tissue sections were stained with hematoxylin and eosin (H&E) for histopathological analyses. Photographs were taken with a digital Nikon camera (coolpix 4500) attached to Olympus CX31 microscope. The remaining mice were sacrificed at 10 wk after injection of ONSC cells. As shown in Fig. 2A, ONSC cells developed into SCCs, the size of which increased with time.
Figure 2.
Time course of tumor development and tumor size and histology of SCCs from athymic nude mice injected s.c. with ONSC cells. (A) Time course of ONSC tumor development. ONSC cells (2×106) were injected into the lower dorsal regions of female nude mice. Tumors were measured each week (except wk 3) for 7 wk. The curved line represents mean tumor volumes at each week (n=8/group/wk). (B) Histology of SCC in athymic nude mice injected with ONSC cells. Athymic nude mice were injected s.c. with ONSC cells. (a and b) Well differentiated SCC at 1 wk after injection of ONSC cells. (c and d) SCC metastasized to the lung of athymic nude mice. (a and c), magnification 10x; (b d), magnification 40x. □, magnified regions in b and d; ○, region with SCC in the lung.
Histopathological examination of sections of tumors collected at wk 1 showed the development of well differentiated SCC with keratin pearls (Fig. 2B, a and b). Additionally, examination of H&E sections of tissues harvested at 10 wk showed that all 8 mice had developed well-differentiated SCC with metastasis to the lung (Fig. 2B, c and d). One of the athymic mice also showed multiple organ metastases. In addition to the capacity of ONSC cells to develop SCC and metastasize in nude mice, these cells also develop into tumors and metastasize in syngeneic WT and OPN-null mice (Hsieh et al., manuscripts in preparation).
In summary, we have established a cutaneous SCC cell line that lacks the Spp1 gene. To our knowledge, this cutaneous SCC is the only murine line of this type that develops tumors in immune-competent mice. One SCC line lacking the OPN gene has been reported; however, this line does not develop into tumors in syngeneic mice (Crawford et al., 1998). The capacity of ONSC cells to develop into tumors in immune-competent mice and their lack of inducible OPN expression provide an appropriate model to address the function of host-derived OPN in the context of SCC tumor progression and metastasis.
Acknowledgments
We thank Patricia Hicks for her technical assistance and Dr. Michael Ruppert for shared reagents. We greatly appreciate Dr. Don Hill for reviewing the manuscript. This work was partially supported by U.S. National Cancer Institute grant R01 CA90920 (P.-L. C.), and the Pi-Ling Chang Research Support.
References
- Brown LF, Papadopoulos-Sergiou A, Berse B, Manseau EJ, Tognazzi K, Perruzzi CA, Dvorak HF, Senger DR. Osteopontin expression and distribution in human carcinomas. Am. J. Pathol. 1994;145:610–623. [PMC free article] [PubMed] [Google Scholar]
- Chang PL, Prince CW. 1alpha,25-dihydroxyvitamin D3 stimulates synthesis and secretion of nonphosphorylated osteopontin (secreted phosphoprotein 1) in mouse JB6 epidermal cells. Cancer Res. 1991;51:2144–2150. [PubMed] [Google Scholar]
- Chang PL, Tucker MA, Hicks PH, Prince CW. Novel protein kinase C isoforms and mitogen-activated kinase kinase mediate phorbol ester-induced osteopontin expression. Intl. J. Biochem. Cell Biol. 2002;34:1142–1151. doi: 10.1016/s1357-2725(02)00035-3. [DOI] [PubMed] [Google Scholar]
- Colburn NH. Tumor promoter produces anchorage independence in mouse epidermal cells by an induction mechanism. Carcinogenesis. 1980;1:951–954. doi: 10.1093/carcin/1.11.951. [DOI] [PubMed] [Google Scholar]
- Crawford HC, Matrisian LM, Liaw L. Distinct roles of osteopontin in host defense activity and tumor survival during squamous cell carcinoma progression in vivo. Cancer Res. 1998;58:5206–5215. [PubMed] [Google Scholar]
- Frame S, Crombie R, Liddell J, Stuart D, Linardopoulos S, Nagase H, Portella G, Brown K, Street A, Akhurst R, Balmain A. Epithelial carcinogenesis in the mouse: correlating the genetics and the biology. Philos. Trans. R. Soc. Lond. B, Biol. Sci. 1998;353:839–845. doi: 10.1098/rstb.1998.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi N, Sugino T, Suzuki T. Regular expression of osteopontin in granular cell tumor: distinctive feature among Schwannian cell tumors. Pathol. Int. 2005;55:484–490. doi: 10.1111/j.1440-1827.2005.01857.x. [DOI] [PubMed] [Google Scholar]
- Hsieh YH, Juliana MM, Hicks PH, Feng G, Elmets C, Liaw L, Chang PL. Papilloma development is delayed in osteopontin-null mice: implicating an antiapoptosis role for osteopontin. Cancer Res. 2006;66:7119–7127. doi: 10.1158/0008-5472.CAN-06-1002. [DOI] [PubMed] [Google Scholar]
- Liaw L, Birk DE, Ballas CB, Whitsitt JS, Davidson JM, Hogan BL. Altered wound healing in mice lacking a functional osteopontin gene (spp1). J. Clin. Invest. 1998;101:1468–1478. doi: 10.1172/JCI1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarrity GJ, Steiner T, Vanaman V. Detection of mycoplasma infection of cell cultures by DNA fluorochrome staining. In: Tully JG, Razin E, editors. Methods in Mycoplasmology. Academic Press; New York: 1983. pp. 155–208. [Google Scholar]
- Melnikova VO, Pacifico A, Chimenti S, Peris K, Ananthaswamy HN. Fate of UVB-induced p53 mutations in SKH-hr1 mouse skin after discontinuation of irradiation: relationship to skin cancer development. Oncogene. 2005;24:7055–7063. doi: 10.1038/sj.onc.1208863. [DOI] [PubMed] [Google Scholar]
- Menzin J, Lines LM, Manning LN. The economics of squamous cell carcinoma of the head and neck. Curr. Opin. Otolaryngol. Head Neck Surg. 2007;15:68–73. doi: 10.1097/MOO.0b013e328017f669. [DOI] [PubMed] [Google Scholar]
- Nelson MA, Futscher BW, Kinsella T, Wymer J, Bowden GT. Detection of mutant Ha-ras genes in chemically initiated mouse skin epidermis before the development of benign tumors. Proc. Natl. Acad. Sci. U S A. 1992;89:6398–6402. doi: 10.1073/pnas.89.14.6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis-Filho JS, Torio B, Albergaria A, Schmitt FC. p63 expression in normal skin and usual cutaneous carcinomas. J. Cutan. Pathol. 2002;29:517–523. doi: 10.1034/j.1600-0560.2002.290902.x. [DOI] [PubMed] [Google Scholar]
- Rittling SR, Chambers AF. Role of osteopontin in tumour progression. Br. J. Cancer. 2004;90:1877–1881. doi: 10.1038/sj.bjc.6601839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri BA, Bauer B, Zhang SY, Klein-Szanto AJ. Murine squamous cell carcinoma cell lines produced by a complete carcinogenesis protocol with benzo[a]pyrene exhibit characteristic p53 mutations and the absence of H-ras and cyl 1/cyclin D1 abnormalities. Carcinogenesis. 1994;15:1613–1619. doi: 10.1093/carcin/15.8.1613. [DOI] [PubMed] [Google Scholar]
- Shin SI, Freedman VH, Risser R, Pollack R. Tumorigenicity of virus-transformed cells in nude mice is correlated specifically with anchorage independent growth in vitro. Proc. Natl. Acad. Sci. U S A. 1975;72:4435–4439. doi: 10.1073/pnas.72.11.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Nakamura K, Hegamyer G, Dong Z, Colburn N. No point mutation of Ha-ras or p53 genes expressed in preneoplastic-to-neoplastic progression as modeled in mouse JB6 cell variants. Mol. Carcinog. 1993;8:49–57. doi: 10.1002/mc.2940080111. [DOI] [PubMed] [Google Scholar]
- Tunio GM, Hirota S, Nomura S, Kitamura Y. Possible relation of osteopontin to development of psammoma bodies in human papillary thyroid cancer. Arch. Pathol. Lab. Med. 1998;122:1087–1090. [PubMed] [Google Scholar]
- Wai PY, Kuo PC. Osteopontin: regulation in tumor metastasis. Cancer Metastasis Rev. 2008;27:103–118. doi: 10.1007/s10555-007-9104-9. [DOI] [PubMed] [Google Scholar]
- Weinberg AS, Ogle CA, Shim EK. Metastatic cutaneous squamous cell carcinoma: an update. Dermatol. Surg. 2007;33:885–899. doi: 10.1111/j.1524-4725.2007.33190.x. [DOI] [PubMed] [Google Scholar]