Abstract
Unraveling the intricate interactions between Trypanosoma brucei, the protozoan parasite causing African trypanosomiasis, and the tsetse (Glossina) vector remains a challenge. Metacyclic trypanosomes, which inhabit the tsetse salivary glands, transmit the disease and are produced through a complex differentiation and unknown program. By overexpressing a single RNA-binding protein, TbRBP6, in cultured noninfectious trypanosomes, we recapitulated the developmental stages that have been observed in tsetse, including the generation of infective metacyclic forms expressing the variant surface glycoprotein. Thus, events leading to acquisition of infectivity in the insect vector are now accessible to laboratory investigation, providing an opening for new intervention strategies.
Reproducing the life cycle of infectious organisms in vitro offers unique opportunities for studying pathogenesis and for developing intervention strategies. The protozoan parasite Trypanosoma brucei is the causative agent of sleeping sickness in humans, and the related disease “nagana” in livestock, which remain a public health challenge in sub-Saharan Africa. Human African trypanosomiasis is fatal if left untreated, and the livestock disease profoundly impacts on the economic development of affected countries. The blood-feeding tsetse fly (Glossina subspecies) vector transmits the disease, and its range determines the distribution of the disease. Bloodstream forms (BSFs) are taken up with the bloodmeal, and in the fly midgut stumpy forms (SFs) differentiate into procyclic forms that are no longer infectious to mammals (1). Several morphological and metabolic changes take place during this developmental stage, including repositioning of the kinetoplast (the mitochondrial genome) midway between the posterior pole and the nucleus, elaboration of mitochondrial cisternae, silencing of variable surface glycoprotein (VSG) expression, VSG coat loss, and expression of procyclin on the cell surface (1). Procyclics then move from the midgut to the proventriculus, the terminal portion of the foregut, where they elongate and become epimastigotes, with the kinetoplast positioned next to the nucleus in the anterior position. Epimastigotes undergo asymmetric division to produce a long epimastigote, which probably degenerates, and a short epimastigote, which instead goes on to colonize the salivary glands where it attaches to the epithelium. Attached epimastigotes divide and ultimately differentiate into mature nondividing metacyclics that detach from the epithelium and are found in the lumen of the salivary glands. During metacylogenesis, the mitochondrion regresses to the single tubule characteristic of BSFs, indicating that mitochondrial function is inhibited; the VSG coat, synthesized from VSG genes in metacyclic-type transcription units (metacyclic expression sites), reappears; endocytosis increases; and the cells reacquire infectivity to mammals (1). Although the intricate nature of trypanosome development in the fly has been recognized formore than a century (2), the molecular mechanisms are still mysterious, due in part to experimental challenges of studying parasites in the fly.
RNA-binding proteins (RBPs) are important regulators of trypanosome gene expression as mediators of posttranscriptional control (3). A survey of the T. brucei transcriptome in infected tsetse tissues by high-throughput RNA sequencing (RNA-Seq) revealed that the mRNA abundance for RBP6 (Tb927.3.2930, containing a single RNA-recognition motif) is increased by a factor of 13 (relative to midgut-derived procyclics) in trypanosomes from the proventriculus (fig. S1). Experimentally induced RBP6 overexpression in cultured noninfectious procyclics resulted in the appearance of developmental stages that have been previously described in tsetse; namely, long and short epimastigotes and metacyclic trypomastigotes (Fig. 1A and figs. S2 to S4). The prevalence of metacyclics depended on RBP6 expression levels (fig. S5), and we also observed asymmetrically dividing long epimastigotes (fig. S6).
Fig. 1.
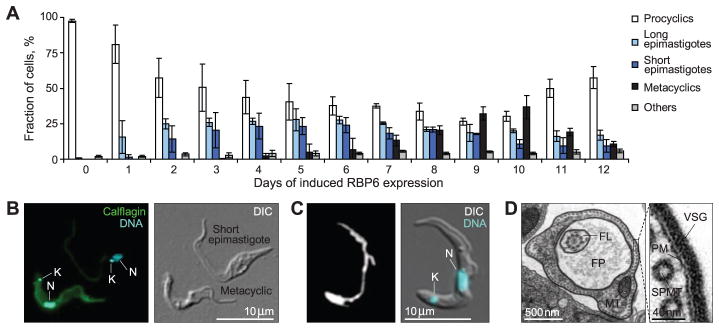
T. brucei RBP6 induces metacyclogenesis. (A) Timeline for the appearance of different cell types upon induction of RBP6 expression (three independent experiments; error bars, SD). (B) Immunofluorescent detection of calflagin (K, kinetoplast; N, nucleus) in a culture induced to express RBP6 for 10 days DIC, differential interference contrast. (C) Live-cell imaging of the mitochondrion in a metacyclic cell with Mitotracker Deep Red FM. (D) Transmission electron microscopy confirms the presence of a variant surface glycoprotein (VSG) coat. Shown is a transverse section across the flagellar pocket (FP), which, like blood-stream form trypomastigotes, contains fibrous material (MT, mitochondrion; FL, flagellum; PM, plasma membrane; SPMT, subpellicular microtubules).
Metacyclics generated in vitro featured a kinetoplast at the cell’s posterior pole (Fig. 1B and fig. S4), an undulating membrane (Fig. 1C), and BSF-like motility and, like BSFs, they did not adhere to glass. Metacyclic-like cells also displayed a range of mitochondrial architectures from multibranched to tubular (Fig. 1C and fig. S7), an indication of mitochondrial regression (4), which is distinctive of BSFs. Relative to procyclics and epimastigotes, metacyclics generated in vitro had higher levels of calflagin (Fig. 1B and fig. S8), mirroring results from salivary-gland metacyclics (5). Compared to procyclics and epimastigotes, metacyclics also showed higher internalization of the fluid-phase marker dextran 10,000 (fig. S9)—an indication of active endocytosis, which is a characteristic feature of BSFs (4). Up to 20% of the metacyclic-like cells could be purified by DEAE-cellulose chromatography, which combined with the gradation in mitochondrial regression (fig. S7) and calflagin expression (fig. S8), suggested that the cultures contained metacyclics at different stages of differentiation.
The hallmark of metacyclogenesis is the re-acquisition of a VSG coat (1, 4). We detected the VSG coat by transmission electron microscopy (Fig. 1D) and the mRNA for several VSGs by reverse transcriptase–polymerase chain reaction (RT-PCR) and RNA-Seq (Fig. 2A and table S1). The VSG transcripts with highest expression were derived from monocistronic expression sites (Fig. 2B and fig. S10) with metacyclic-type RNA polymerase I (Pol I) VSG promoters (Fig. 2C) (6), underscoring the identity of the metacyclics generated in vitro. Cultures containing these cells produced T. brucei infections in BALB/c (table S2) and c57BL/6 mice (table S3), and trypanosomes were detected in the bloodstream as early as day 3 after inoculation. Bloodstream stumpy forms, which initiate colonization of the fly midgut (4), were also present (fig. S11). In contrast, no trypanosomes were detected in mice inoculated with uninduced RBP6 cells grown in parallel or in tet-induced parental Lister 427 (29–13) cells.
Fig. 2.

T. brucei RBP6 induces the expression of monocistronic VSG genes. (A) RT-PCR detection of expressed VSG genes in cultures containing metacyclic cells. Ethidium bromide–stained agarose gel for samples processed with (+RT) or without (−RT) reverse transcriptase are shown. The identity of amplified products was confirmed by cloning and sequencing. (B) Diagrams of the genomic context of the VSG genes with metacyclic-type Pol I promoters. Red rectangles, VSG genes; purple triangles, subtelomeric or telomeric repeats; yellow rectangles, ribosomal mobile element (RIME) sequences; gray rectangles, 70–base pair repeats; bent arrows, transcription start sites; red circles, Pol I promoters. (C) Alignment of the metacyclic Pol I promoters of expressed VSG genes to a previously characterized metacyclic promoter sequence of the MVAT4 gene (6). Bent arrow indicates the position of the putative transcription start site. Red and purple indicate ≥60% and <60% sequence conservation, respectively.
Our results underscore the critical role for RBPs in the regulation of gene expression in trypanosomes and provide a robust in vitro system for recapitulating trypanosome developmental stages in the insect vector, including the production of infectious metacyclics.
Supplementary Material
Acknowledgments
We thank A. Savage and S. Aksoy for assistance with the infectivity assays, J. Bangs for providing the immunofluorescence protocol and advice, D. Engman for the antibody against calflagin, and M. Chaudhuri for the antibody against alternative oxidase. Supported by grants AI028798 (E.U.), AI043594 (C.T.), AI021729 (G.A.M.C.), and AI076879 (S. Aksoy) from the National Institute of Allergy and Infectious Diseases (NIAID) of the U.S. National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID or the NIH. The sequence data from this study have been submitted to the National Center for Biotechnology Information Sequence Read Archive under accession nos. SRP002243 and SRA059559.
Footnotes
References and Notes
- 1.Sharma R, et al. Trends Parasitol. 2009;25:517. doi: 10.1016/j.pt.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruce D, Hamerton AE, Bateman HR, Mackie FP. Proc R Soc Lond B. 1909;81:405. [Google Scholar]
- 3.Wurst M, et al. Mol Microbiol. 2012;83:1048. doi: 10.1111/j.1365-2958.2012.07988.x. [DOI] [PubMed] [Google Scholar]
- 4.Vickerman K. Br Med Bull. 1985;41:105. doi: 10.1093/oxfordjournals.bmb.a072036. [DOI] [PubMed] [Google Scholar]
- 5.Rotureau B, Subota I, Buisson J, Bastin P. Development. 2012;139:1842. doi: 10.1242/dev.072611. [DOI] [PubMed] [Google Scholar]
- 6.Ginger ML, et al. Eukaryot Cell. 2002;1:1000. doi: 10.1128/EC.1.6.1000-1009.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


