Abstract
Purpose
Previous studies have shown that the onset of high-contrast, fast reversing patterned stimuli induces rapid blood flow increase in retinal vessels in association with slow changes of the steady-state PERG signal. We tested the hypothesis that adaptive PERG changes of normal controls (NC) differed from those of glaucoma suspects (GS) and patients with early manifest glaucoma (EMG).
Methods
Subjects were 42 GS (SAP MD −0.89 ±1.8 dB), 22 EMG (MD −2.12 ±2.4 dB) with visual acuity of ≥20/20 and 16 age-matched NC from a previous study. The PERG signal was sampled every ~15 s over 4 minutes in response to gratings (1.6 cyc/deg, 100% contrast) reversing 16.28 times/s. Amplitude/phase values of successive PERG samples were fitted with a non-parametric LOWESS smoothing function to retrieve the initial and final values and calculate their difference (delta) and the residual standard deviation around the fitted function (SDr). The magnitude of PERG adaptive change compared to random variability was calculated as log10 of percentage coefficient of variation CoV=100*SDr ÷ |delta|. Grand-average PERGs were also obtained by averaging all samples of the same series.
Results
The grand-average PERG amplitude (ANOVA, p=0.02), but not phase (ANOVA, p=0.63), decreased with increasing severity of disease. Adaptive changes (log10 (CoV) of PERG amplitude were not significantly associated with disease severity (ANOVA, p=0.27), but adaptive changes (log10 (CoV) of PERG phase were (ANOVA, p=0.037; linear trend, p=0.011).
Conclusions
The steady-state PERG signal displayed slow adaptive changes over time that could be isolated from random variability. PERG adaptive changes differed from those of grand-average PERGs (corresponding the standard steady-state PERG), thus representing a new source of biological information about retinal ganglion cell function that may have potential in the study of glaucoma and optic nerve diseases.
Keywords: retinal ganglion cell function, pattern electroretinogram, glaucoma, retinal metabolism
Introduction
A well established notion is that changes of neural activity in the brain are associated with corresponding changes of blood flow and metabolism.1, 2 In the retina, presentation of flickering light induces changes of blood flow in retinal and optic nerve vessels.3, 4 Pattern-reversing gratings or checkerboards without changes in mean luminance are also very effective stimuli for eliciting a neurovascular response in retinal and optic nerve vessels. 5
An interesting question is whether the pattern electroretinogram (PERG), a signal associated with retinal ganglion cell (RGC) function, is altered by conditions known to elicit a neurovascular response. Recently, it has been shown that the steady-state pattern electroretinogram (PERG) recorded during continuous presentation of fast-reversing, high-contrast, fine gratings displays slow adaptive changes of the response amplitude that have been interpreted as metabolic adaptation.6, 7 In contrast, the flicker ERG — a measure of outer retina function — remains unchanged during continuous presentation of a luminance-modulated uniform field.8 Altogether, these results imply that fast contrast-reversing patterns induce adaptive functional changes in the inner, but not outer, retina in response to changed metabolic requirements. Adaptive changes can be measured by PERG. While the precise mechanisms underlying this neurovascular/neurometabolic coupling remain to be elucidated, 5 it is possible that they are impaired in early glaucoma, which affects the RGC and the optic nerve9 as well as their vascular supply.10
Here we tested the hypothesis that adaptive changes of the steady-state PERG differ between normal controls (NC) and patients with either suspicion of glaucoma (GS) or early manifest glaucoma (EMG).
Materials and Methods
Subjects
Subjects of this study were 81 individuals of both sexes ranging in age between 30 and 80 years, and no systemic or retinal disease diseases as assessed by routine ophthalmologic examination. Forty-two subjects were suspected of having glaucoma (GS, n=42, mean age 54.9±10.7 years); twenty-two had early manifest glaucoma (EMG, n=22, mean age 60.3 ±10.2 years), and sixteen were normal controls (NC, n=16; mean age 52.7 ±12.8 years) from a previous study.7 No control subject had family history of glaucoma, suspicious glaucomatous optic disk, or IOP> 16 mm Hg. The mean ages of the groups were not significantly different (ANOVA, P=0.082). Patients were part of a larger longitudinal cohort of patients enrolled as glaucoma suspects at their initial visit based on a detailed medical and ocular history and a comprehensive eye examination as previously described.11 Inclusion criteria were: best corrected Snellen visual acuity equal to 20/20 or better (refractive errors within −5 to +3 diopters, ± 3 cyl diopters), normal Standard Automated Perimetry (SAP) according to the OHTS criteria 12 (reliability <15% on all indices, normality >5% on all global indices in two consecutive sessions 6 months apart), and glaucomatous optic disc appearance (C/D ≥0.5, C/D asymmetry ≥0.2, localized thinning of the disc, splinter hemorrhage) or increased IOP (> 21 mm Hg). Twenty out of 22 EMG patients (91 %) and 8 out of 42 GS patients (19%) were taking IOP-lowering medications at the time of testing. Topical treatment included Beta-Blockers (BB), Prostaglandin analogs (PA), Alpha-Agonists (AA), Carbonic Anhydrase Inhibitors (CAI) either alone or in combination. Patients’ characteristics are summarized in Table 1.
Table 1.
Summary of patients’ characteristics
| GS, n=42; mean (SD) | EMG, n= 22; mean (SD) | |
|---|---|---|
| IOP OD (mm Hg) | 15.16 (3.53) | 15.6 (3.24) |
| IOP OS (mm Hg) | 15.92 (4.2) | 15.4 (2.7) |
| Vertical C/D OD | 0.53 (0.14) | 0.56 (0.17) |
| Vertical C/D OS | 0.53 (0.15) | 0.56 (0.20) |
| CCT OD (μm) | 542.17 (39.4) | 530.80 (46.19) |
| CCT OS (μm) | 541.93 (38.72) | 529.70 (50.74) |
| MD OD (dB) | −0.83 (1.82) | −2.34 (2.47) |
| MD OS (dB) | −0.96 (1.79) | −1.90 (2.26) |
| Treatment | PA, n=6; BB, n=2 |
PA, n=11 BB, n=2 AA, n=1 BB+ PA, n=4 PA+ CAI, n=1 AA+CAI, n=1 |
Legend. PA, prostaglandin analogs; BB, beta-blockers; AA, alpha-agonists; CAI, carbonic anhydrase inhibitors
The methods applied in the study adhered to the tenets of the Declaration of Helsinki for the use of human subjects in biomedical research. Institutional Review Board/Ethics Committee approval was obtained for this study, and informed consent was obtained from each subject before recording.
The Pattern Electroretinogram
The physiological activity of RGC may be assessed by means of the electroretinogram in response to contrast-modulated patterns (Pattern ERG, PERG).13–16 As the PERG amplitude in response to reversing gratings depends on the spatio-temporal parameters of the visual stimulus,6, 17–19 it can be maximized by choosing the appropriate conditions (maximum contrast, 1–2 cycles/degree spatial frequency, ~ 8 Hz temporal frequency).17, 18 It has been previously shown that stimulus conditions that maximize PERG response also elicit maximal, sustained increase of blood flow (~ 40% compared to baseline) at the neuro-retinal rim of the optic disk,5, 20, 21 which remains stable over ~ 1 hour of measurements. 5 Stimulus conditions that maximize PERG amplitude also display the largest amplitude losses in OHT and glaucoma compared to non-maximizing conditions. 22–24
Recording PERG adaptive changes
The PERG was recorded simultaneously from both eyes with standard skin surface electrodes (Grass gold, 10 mm diameter) taped on the lower eyelids, on the ipsilateral temples (references) and central forehead (ground) using a commercially available system, (Lace Elettronica, Rome, Italy) similar to the procedure previously described as the PERGLA paradigm.11, 25–28 The pattern stimulus consisted of black-white horizontal gratings with a square-wave profile (1.7 cycles/degree, 25 degree diameter circular field, 99% contrast, 40 cd/m2 mean luminance), temporally modulated (square-wave) in counterphase at 8.14 Hz (16.28 contrast reversals/s) and displayed on a TV monitor placed within a Ganzfeld dome at the viewing distance of 30 cm. Signals were band pass filtered (1–30 Hz), amplified (100,000 fold), and averaged in synchrony with the stimulus frequency (epochs of 122.8 ms period, corresponding to 2 contrast reversals) and analyzed in the frequency domain (DFT) to isolate the amplitude (in μV) and phase (in π radians) of the sinusoidal component at 16.28 Hz.6, 7 Epochs contaminated by eye blinks or gross eye movements were automatically rejected over threshold voltage of 25 μV. In the clinical procedure previously described as the PERGLA paradigm,11, 25–28 the PERG signal was processed by robust averaging (600 epochs) over several minutes.25 While this robust averaging drastically improved the signal-to-noise ratio, it precluded the investigation of possible PERG changes over time. In order to assess the temporal dynamics, the PERG signal was averaged in successive samples of 50 epochs each over 5 minutes. We have previously shown 6, 7 that even with averaging limited to 50 epochs, amplitude and phase of successive samples can be reliably measured to analyze their progressive trends (examples in Fig. 1). DFT was computed over one full stimulus period (122.8 ms). Due to intrinsic limitations of the commercial system used, every other epoch was lost in the average as previously discussed.7 Therefore, the acquisition time for averaging 50 epochs was longer than theoretically needed by a factor of two (2 × 122.8 ms × 50 = 12.28 s). Including occasional rejections of epochs contaminated by eye blinks, the acquisition time for averaging 50 artifact-free epochs could be longer than 12.28 s but never longer than 15 s. To simplify description of results and analysis, each sample was assumed to be 15 s. This had no effect on the analysis, as rejections occurred to a similar extent in controls and patients. Before presentation of the pattern stimulus, subjects were exposed to a uniform grey stimulus equiluminant with the pattern stimulus for duration of 1 minute, during which a “noise” response was recorded. Subjects wore the appropriate correction for the viewing distance to achieve J1+ Jager acuity, and none of them reported visual strain or problems in maintaining fixation. We have previously checked in 6 subjects by simultaneously recording the electrooculogram from the temporal electrodes that the amplitude/frequency of horizontal eye movements did not change with time over 20 minutes of continuous recording.7
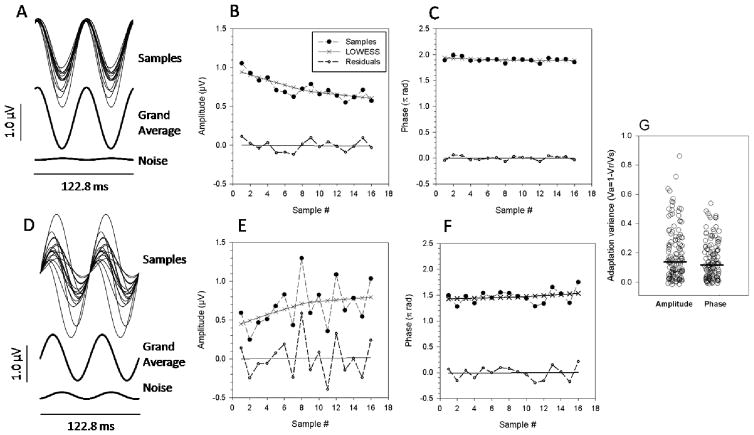
Figure 1.
Summary of adaptive PERG changes occurring during continuous presentation over 4 minutes of a pattern stimulus reversing in contrast 16.28 times/s. A, D:Representative examples of sequential PERG samples. SuperimposedPERG waveforms are the time-domain representations of the 16.28 HzPERG component evaluated by DFTanalysisofsuccessive samples of 50 epochs each(~ 15 s). Panels A, D also show the grand-average waveform of all samples recorded during pattern presentation, as well as a noise waveform obtained by averaging 4 samples during presentation of a uniform grey field. Panels B, C, E, F show how the amplitude and phase of successive samples (filled symbols) change over time together with corresponding LOWESS regression functions (continuous lines with x symbols), from which the initial and final amplitude or phase were calculated. Panels B, C, E, F also show the amplitude and phase of residuals (open symbols)and corresponding regression functions(continuous lines). Note that the magnitude and sign of adaptive changes, as well as the residual variability of PERG amplitude, is different in the two subjects. Also note that the residuals do not show any trend. G: Proportion of variance due to adaptive changes of the PERG signal relative to the total variance for all eyes of subjects. Variance due to adaptation(Va) was calculated as 1 minus the ratio between the variance of residuals (Vr) and the variance of samples (Vs). The horizontal thick lines represent the median Va for amplitude and phase.
Evaluating the temporal dynamics of PERG in response to steady-state stimuli
In order to have an objective evaluation of the dynamic changes of PERG amplitude, amplitude/phase measurements corresponding to samples sequentially recorded during stimulus presentation were fitted with a LOWESS(locally weighted polynomial regression, Sigmaplot 11.2, Systat Software inc.) smoothing function. LOWESS is considered a robust non-parametric regression that minimizes the noise while avoiding assumptions about the relationship among variables.29, 30 LOWESS was used with maximum smoothing parameter (1.0) in order to achieve a smooth fitting. Differences (residuals) between raw amplitude or phase of sample and the LOWESS estimates were calculated and their variance (Vr) measured and compared with the variance of samples (Vs). The variance due to adaptive changes (Va) was defined as Va= 1-Vr/Vs, which is equivalent to R2. On average, LOWESS- fitted functions were monotonic, and Va was higher than that obtained using fixed parametric functions such as polynomials or exponentials.6, 7 However, the form of LOWESS fit could potentially be non-monotonic, resulting in a spuriously high R2 that would invalidate the interpretation of Va as an index of adaptive change. Therefore, all LOWESS fits were examined and were found to be monotone when R2 was higher than 0.3 for both amplitude and phase. The first point (at time ~15 s) and last point (at time ~4 min) of the function fitting amplitude or phase data acquired after pattern stimulus onset were considered as Initial Amplitude/Phase and Final Amplitude or phase, respectively, and their differences (deltas) calculated. Differences between raw and fitted amplitude or phases of samples were also calculated to retrieve the residual standard deviation around the fitted function (SDr). The percentage magnitude of PERG adaptive change compared to random variability was calculated as log10 of the percentage coefficient of variation CoV=100×SDr ÷ |delta|. Grand-average PERGs were also obtained by averaging all samples of the same series. The grand-average PERG corresponds to the ordinary steady-state PERG. The grand-average of samples acquired during the 1 minute presentation of uniform grey stimulus was considered as noise. Examples are shown in Figure 1.
Results
Figure 1 shows examples of successive PERG samples recorded from the right eyes of two representative subjects over 4 minutes of continuous stimulus presentation. The left panels display DFT-isolated time-domain representations of successive PERG samples together with the corresponding grand average and noise. PERG samples were superimposed to provide a general idea of the variability among samples. The right panels display how amplitude and phase of PERG samples changed over time, and how data were fitted with a LOWESS regression function. It is apparent in the waveforms shown in Fig. 1 that there was a substantial variability among samples. The scattergrams in Fig. 1 suggested that in both subjects there were progressive changes in amplitude and phase over time, in addition to random variability among samples. In order to isolate the variance due to progressive changes from the total variance, raw data were fit with a LOWESS regression function. Amplitudes and phases calculated from the LOWESS regression function were subtracted from corresponding raw amplitudes and phases of samples. As differences (residuals) did not show any progressive trend, the proportion of variance fitted with the LOWESS regression seemed to be completely associated with progressive (adaptive) changes. In the example of Fig. 1B, the ratio between the variance of residuals (Vr) and the variance of samples (Vs) was 0.28, meaning that the variance associated with adaptive changes (Va = 1−Vr/Vs = 0.72) accounted for 72% of the total variance. In the same eye, adaptive changes (Va) of PERG phase (Fig. 1C) were relatively smaller (Va=0.19). In the second example (Fig. 1E), adaptive changes were: amplitude Va =0.2; phase Va= 0.1. Adaptation variances (Va) of amplitudes and phases for all eyes (n=162) of all subjects (n=81) are shown (Figure 1G). There was a large spread of Va values for both amplitude and phase. The median Va of amplitude (0.138) was not significantly different from the median Va of phase (0.117). Overall, the data shown in Fig. 1 indicate that during continuous presentation of a high contrast, fast reversing patterned stimulus there may be adaptive changes of the PERG signal in addition to random variability; adaptive changes may account for a substantial portion of the total variance of the PERG signal over time.
In order to quantify the magnitude of adaptation compared to the variability over the period of adaptation, we calculated the coefficient of variation (CoV).31 First, amplitude and phase changes (deltas) were calculated as absolute differences between the final and initial amplitude/phase values measured with the LOWESS regression. Residuals were calculated as described above by subtracting fitted time points from corresponding raw amplitudes/phases points and their standard deviation (SDr) computed. Both the deltas and the SDrs had between-eye intraclass correlations too high (ICC ranged from 0.46–0.52) to justify independent analysis of the two eyes. Thus, deltas of the two eyes were averaged and the corresponding SDrs were Root-Mean-Squared. Finally, the percentage coefficient of variation (CoV) was calculated as: CoV=100×SDr ÷ |delta|. Large CoV values mean that adaptive changes are small relative to the signal random variability; the reverse is true for small CoV value. As CoVs were heteroscedastic and dispersed over approximately two log10 units, we used for analysis the log10 (CoV).
Figure 2 summarizes the distribution of log10 (CoV) for amplitude and phase for different subgroups. The log10(CoV) for amplitude was not different among groups (ANOVA, p=0.27), whereas the log10 (CoV) for phase was significantly different (ANOVA, p=0.037, 0.011 linear trend). Accounting for age with analysis of covariance did not affect the log10 (CoV) phase difference. We correlated all PERG variables (Deltas, log10 (CoV) of both amplitude and phase) with SAP-MD and IOP in patients (GS+EMG). For all variables, the average of the two eyes was used. The Pearson correlation ranged between 0.179 and 0.001 and never reached statistical significance of P<0.05 (2-tailed).
Figure 2.
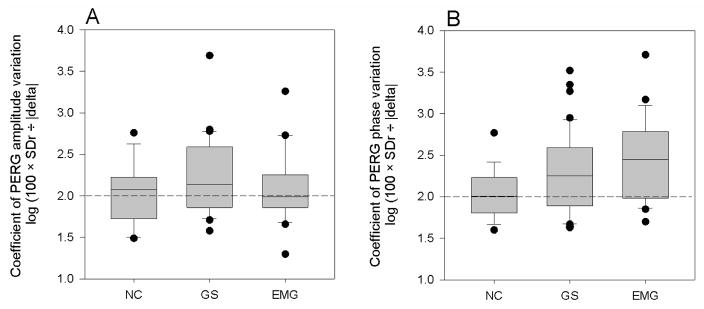
Box plots of the coefficient of variation (log10 (CoV))of the PERG amplitude and phase in different subjects groups (NC, normal controls, n=16; GS, glaucoma suspects, n=42; EMG, early manifest glaucoma, n=22). A: magnitude of adaptive PERG amplitude changes; B: magnitude of adaptive PERG phase changes. For each group, measurements represent the average of the two eyes. In both panels, the horizontal dashed lines identify the level of log10 (CoV) at which delta=SDr.
We investigated whether adapted (final) amplitudes/phases depended on corresponding nonadapted (initial) values in different subject groups. Amplitudes/phases of the two eyes were averaged and used as a single entry in a multiple regression model. Final amplitudes/phases were the dependent variables, whereas initial amplitudes/phases were the independent variables. Disease level (NC=0; GS=1; EMG=2) was used in the model as an independent categorical variable. The level of disease did not significantly contribute to the model, whereas both initial amplitude and phase did: Final amplitude (μV)= 0.219 + (0.619 * Initial amplitude, μV), P <0.001, R2 =0.68; Final phase (π rad)= 0.219 + (0.619 * Initial phase, π rad), P <0.001, R2 =0.42. Comparable results (R2) were obtained using log10 transformation of amplitude and phase. Results are summarized in Figure 3.
Figure 3.
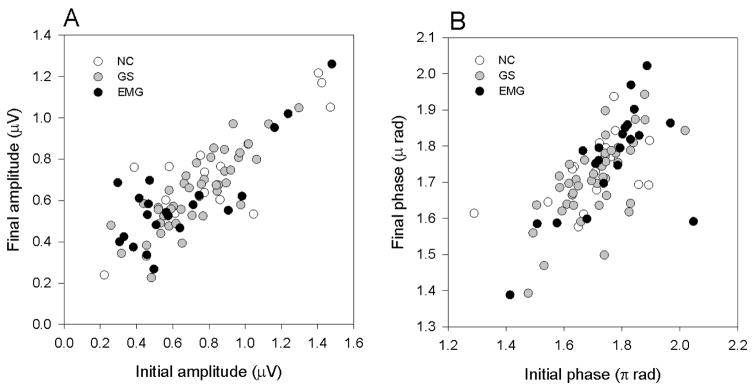
Relationship between initial and final amplitude(A) and initial and final phase (B)of the steady-state PERG in response to high contrast, pattern reversal stimuli presented over 4 minutes. Different symbols refer to different subjects groups (NC, normal controls, n=16; GS, glaucoma suspects, n=42; EMG, early manifest glaucoma, n=22). For all subjects, amplitudes/phases of the two eyes were averaged.
As shown in Fig. 1, averaging successive PERG samples resulted in a grand-average waveform, which corresponds to what is commonly known as steady-state PERG 32 and is widely used for applications in glaucoma.33, 34 Grand-average PERGs were compared among different subject groups (Figure 4). The grand-average median PERG amplitude decreased with increasing severity of disease (ANOVA, p = 0.025), whereas phase did not significantly change (ANOVA, p=0.63).
Figure 4.
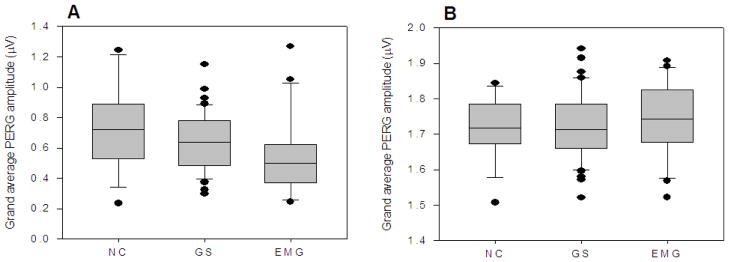
Box plots of grand average PERG amplitude (A) and phase (B) in different subject groups (NC, normal controls, n=16; GS, glaucoma suspects, n=42; EMG, early manifest glaucoma, n=22). For each group, measurements of the two eyes were averaged.
Discussion
This study shows that in the steady-state PERG in response to continuous presentation of a high contrast, fast reversing patterned stimulus there may be adaptive changes over time embedded in random fluctuations of amplitude/phase between successive samples. Adaptive changes may account for a substantial portion of within-test variance. Adaptive changes could be isolated from non-adaptive (random) variability, thus providing a novel source of biological information that is not available using standard averaging methods. Adaptive PERG changes likely reflect changes of neural activity in the inner retina, as the flicker ERG (originating from outer retinal activity) does not show adaptive changes.8
While adaptive PERG changes have been described before in normal subjects,6–8 this study focused on possible differences between normal controls, patients with suspicion of glaucoma, and patients with earliest stages of manifest open-angle glaucoma. Results show that the magnitude of adaptive PERG phase changes significantly decreased (log10 (CoV) increased) with increasing severity of disease, whereas adaptive PERG amplitude changes were similar in the three groups of subjects. In contrast to adaptive PERG amplitude changes, the grand average PERG amplitude (equivalent to the typical steady-stated PERG) decreased with severity of disease, in agreement with a number of previous studies,33, 34 whereas the grand average PERG phase was not different among groups. As the different magnitude of adaptive changes of PERG amplitude or phase in different patient groups could not be predicted from corresponding magnitudes of the grand-average PERG, adaptive changes represent a novel functional parameter distinct from the standard PERG.
We exclude the possibility that the measured adaptive changes were merely the results of “regression to the mean” of random signal fluctuation, whereby an initially higher or lower than normal signal will regress to a level in the opposite direction in successive samples. Previous studies6–8 have shown that PERG adaptation occurs within 2 minutes; the adapted signal remains stable at a level different from the non-adapted signal (initial amplitude/phase). This implies that adaptive changes cannot be the result of random fluctuations.
We can only speculate on the mechanisms underlying PERG adaptation. The slow time course of PERG changes is inconsistent with mechanisms of neural contrast gain control shown to occur in a time range of milliseconds to seconds (e.g., 35). Several VEP studies have shown that significant adaptation due to neural contrast gain control can occur at quite low contrasts (e.g.36). However, the PERG does not saturate with increasing contrast (e.g., 17). In addition, adaptation of steady state PERG no longer occurs at low contrast.6 The results summarized in Fig. 3 indicate that strong determinants of PERG adaptation are the initial levels of amplitude or phase. Thus, the slow PERG adaptation investigated here appears to be response-driven, rather than driven by mechanisms of contrast gain control. Response-driven adaptation had similar characteristics in NC, GS and EMG, although ranges of initial and final amplitude/phase did not fully overlap in different groups. It is worth noting that dependence of adaptation on initial conditions has been reported before for VEP37 and for single cortical neurons.38
An attractive hypothesis would be that adaptive changes reflect changes in RCG responsiveness associated with changes of inner retina metabolism. According to this hypothesis, the onset and continuous presentation of patterned visual stimulus shown to maximize PERG amplitude would trigger a series of neuro-vascular-metabolic events leading to a new metabolic equilibrium, eventually resulting in a different electrical activity compared to baseline. We have previously proposed an energy budget model that accounts for slow PERG adaptation in normal subjects. 7 A model of that kind might also account for O2 and other metabolism-related components. According to this view, our finding of impaired PERG phase CoV would suggest that in early glaucoma there would be a progressive loss of the ability of RGC (and their corresponding neuro-vascular network) to adapt in response to the increased energy demand associated with the high-contrast PERG stimulus. Loss of adaptive ability of PERG phase was associated with loss of the grand average PERG amplitude but not grand average PERG phase. Thus, adaptive PERG changes may represent a novel functional parameter to consider in the effort to predict development of glaucoma.
A limitation of this study is that pattern-evoked changes of blood flow have not been measured. There is some evidence that optic nerve blood flow responds to pattern stimuli. 20 However, the association between optic nerve blood flow and PERG dynamics has yet to be established. It is possible that IOP-lowering treatment in patients may have restored, at least in part, RGC function including their metabolic activity, thereby altering the magnitude of both grand average PERG and PERG adaptation. This study was not designed to compare PERG adaptive changes before and after IOP-lowering treatment. We plan to address this problem in future studies.
In conclusion, this study emphasizes a little known property of the steady state PERG –that is, adaptation – which may be related to metabolic changes occurring in the inner retina and seems to be altered in early glaucoma. Adaptive PERG changes are embedded in the within-test variability of the steady-state PERG and can be easily isolated from random variability. New protocols for PERG testing might take advantage of this PERG property to provide a new source of biological information of potential clinical relevance, and simultaneously control for the within-test variability. The potential of this tool must be further investigated also using more refined instrumentation than that used in the present study. Whether adaptive PERG changes are altered at stages of glaucoma more advanced than those tested in the present study, or in other diseases involving the inner retina/optic nerve remains to be established.
Acknowledgments
Financial support: NIH-NEI RO1 EY014957, NIH center grant P30-EY014801, unrestricted grant to Bascom Palmer Eye Institute from Research to Prevent Blindness, Inc.
Footnotes
Financial disclosure: VP, none, BB, none, PKP, none, OS, none, WJF, none, LMV, none
References
- 1.Roy CS, Sherrington CS. On the regulation of the blood supply of the brain. J Physiol. 1890;11:85–108. doi: 10.1113/jphysiol.1890.sp000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villringer A, Dirnagl U. Coupling of brain activity and cerebral blood flow: Basis of functional neuroimaging. Cerebrovascular and Brain Metabolism Reviews. 1995;7:240–276. [PubMed] [Google Scholar]
- 3.Bill A, Sperber GO. Control of retinal and choroidal blood flow. Eye. 1990;4:319–325. doi: 10.1038/eye.1990.43. [DOI] [PubMed] [Google Scholar]
- 4.Riva CE, Harino S, Shonat RD, Petrig BL. Flicker evoked increase in optic nerve head blood flow in anesthetized cats. Neuroscience Letters. 1991;128:291–296. doi: 10.1016/0304-3940(91)90282-x. [DOI] [PubMed] [Google Scholar]
- 5.Riva CE, Logean E, Falsini B. Visually evoked hemodynamical response and assessment of neurovascular coupling in the optic nerve and retina. Prog Retin Eye Res. 2005;24:183–215. doi: 10.1016/j.preteyeres.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Porciatti V, Sorokac N, Buchser W. Habituation of retinal ganglion cell activity in response to steady state pattern visual stimuli in normal subjects. Invest Ophthalmol Vis Sci. 2005;46:1296–1302. doi: 10.1167/iovs.04-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porciatti V, Ventura LM. Adaptive changes of inner retina function in response to sustained pattern stimulation. Vision Res. 2009;49:505–513. doi: 10.1016/j.visres.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fadda A, Di Renzo A, Parisi V, et al. Lack of habituation in the light adapted flicker electroretinogram of normal subjects: A comparison with pattern electroretinogram. Clin Neurophysiol. 2009 doi: 10.1016/j.clinph.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Quigley HA. Neuronal death in glaucoma. Prog Retin Eye Res. 1999;18:39–57. doi: 10.1016/s1350-9462(98)00014-7. [DOI] [PubMed] [Google Scholar]
- 10.Flammer J, Orgul S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21:359–393. doi: 10.1016/s1350-9462(02)00008-3. [DOI] [PubMed] [Google Scholar]
- 11.Ventura LM, Porciatti V, Ishida K, Feuer WJ, Parrish RK., 2nd Pattern electroretinogram abnormality and glaucoma. Ophthalmology. 2005;112:10–19. doi: 10.1016/j.ophtha.2004.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon MO, Kass MA. The Ocular Hypertension Treatment Study: design and baseline description of the participants. Arch Ophthalmol. 1999;117:573–583. doi: 10.1001/archopht.117.5.573. [DOI] [PubMed] [Google Scholar]
- 13.Maffei L, Fiorentini A. Electroretinographic responses to alternating gratings before and after section of the optic nerve. Science. 1981;211:953–955. doi: 10.1126/science.7466369. [DOI] [PubMed] [Google Scholar]
- 14.Fiorentini A, Maffei L, Pirchio M, Spinelli D, Porciatti V. The ERG in response to alternating gratings in patients with diseases of the peripheral visual pathway. Invest Ophthalmol Vis Sci. 1981;21:490–493. [PubMed] [Google Scholar]
- 15.Zrenner E. The physiological basis of the pattern electroretinogram. In: Osborne N, Chader G, editors. Progress in Retinal Research. Oxford: Pergamon Press; 1990. pp. 427–464. [Google Scholar]
- 16.Porciatti V. The mouse pattern electroretinogram. Doc Ophthalmol. 2007;115:145–153. doi: 10.1007/s10633-007-9059-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hess RF, Baker CL., Jr Human pattern-evoked electroretinogram. J Neurophysiol. 1984;51:939–951. doi: 10.1152/jn.1984.51.5.939. [DOI] [PubMed] [Google Scholar]
- 18.Porciatti V, Burr DC, Morrone MC, Fiorentini A. The effects of aging on the pattern electroretinogram and visual evoked potential in humans. Vision Res. 1992;32:1199–1209. doi: 10.1016/0042-6989(92)90214-4. [DOI] [PubMed] [Google Scholar]
- 19.Zapf HR, Bach M. The contrast characteristic of the pattern electroretinogram depends on temporal frequency. Graefes Arch Clin Exp Ophthalmol. 1999;237:93–99. doi: 10.1007/s004170050201. [DOI] [PubMed] [Google Scholar]
- 20.Logean E, Falsini B, Riva CE. Optic nerve head blood flow responses elicited using pattern contrast reversal checkerboard stimuli. IOVS. 2002;43:3314. [Google Scholar]
- 21.Logean E, Geiser MH, Riva CE. Laser Doppler instrument to investigate retinal neural activity-induced changes in optic nerve head blood flow. Optics and Lasers in Engineering. 2005;43:591–602. [Google Scholar]
- 22.Porciatti V, Falsini B, Brunori S, Colotto A, Moretti G. Pattern electroretinogram as a function of spatial frequency in ocular hypertension and early glaucoma. Doc Ophthalmol. 1987;65:349–355. doi: 10.1007/BF00149941. [DOI] [PubMed] [Google Scholar]
- 23.Bach M, Hiss P, Rover J. Check-size specific changes of pattern electroretinogram in patients with early open-angle glaucoma. Doc Ophthalmol. 1988;69:315–322. doi: 10.1007/BF00154412. [DOI] [PubMed] [Google Scholar]
- 24.Colotto A, Salgarello T, Giudiceandrea A, et al. Pattern electroretinogram in treated ocular hypertension: a cross- sectional study after timolol maleate therapy. Ophthalmic Res. 1995;27:168–177. doi: 10.1159/000267663. [DOI] [PubMed] [Google Scholar]
- 25.Porciatti V, Ventura LM. Normative data for a user-friendly paradigm for pattern electroretinogram recording. Ophthalmology. 2004;111:161–168. doi: 10.1016/j.ophtha.2003.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bowd C, Vizzeri G, Tafreshi A, Zangwill LM, Sample PA, Weinreb RN. Diagnostic accuracy of pattern electroretinogram optimized for glaucoma detection. Ophthalmology. 2009;116:437–443. doi: 10.1016/j.ophtha.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tafreshi A, Racette L, Weinreb RN, et al. Pattern Electroretinogram and Psychophysical Tests of Visual Function for Discriminating Between Healthy and Glaucoma Eyes. American journal of ophthalmology. 2010;149:488–495. doi: 10.1016/j.ajo.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sehi M, Grewal DS, Goodkin ML, Greenfield DS. Reversal of retinal ganglion cell dysfunction after surgical reduction of intraocular pressure. Ophthalmology. 2010;117:2329–2336. doi: 10.1016/j.ophtha.2010.08.049. [DOI] [PubMed] [Google Scholar]
- 29.Cleveland WS, Devlin SJ. Locally Weighted Regression: An Approach to Regression Analysis by Local Fitting. Journal of the American Statistical Association. 1988;83:596–610. [Google Scholar]
- 30.Jacoby WG. Loess: a nonparametric, graphical tool for depicting relationships between variables. Electoral Studies. 2000;19:517–613. [Google Scholar]
- 31.Mwanza JC, Oakley JD, Budenz DL, Chang RT, Knight OJ, Feuer WJ. Macular ganglion cell-inner plexiform layer: automated detection and thickness reproducibility with spectral domain-optical coherence tomography in glaucoma. Invest Ophthalmol Vis Sci. 2011;52:8323–8329. doi: 10.1167/iovs.11-7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bach M, Hawlina M, Holder GE, et al. Standard for pattern electroretinography. International Society for Clinical Electrophysiology of Vision Doc Ophthalmol. 2000;101:11–18. doi: 10.1023/a:1002732114721. [DOI] [PubMed] [Google Scholar]
- 33.Bach M, Hoffmann MB. Update on the pattern electroretinogram in glaucoma. Optom Vis Sci. 2008;85:386–395. doi: 10.1097/OPX.0b013e318177ebf3. [DOI] [PubMed] [Google Scholar]
- 34.Ventura LM, Porciatti V. Pattern electroretinogram in glaucoma. Curr Opin Ophthalmol. 2006;17:196–202. doi: 10.1097/01.icu.0000193082.44938.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demb JB. Functional circuitry of visual adaptation in the retina. J Physiol. 2008;586:4377–4384. doi: 10.1113/jphysiol.2008.156638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porciatti V, Bonanni P, Fiorentini A, Guerrini R. Lack of cortical contrast gain control in human photosensitive epilepsy. Nat Neurosci. 2000;3:259–263. doi: 10.1038/72972. [DOI] [PubMed] [Google Scholar]
- 37.Victor JD, Conte MM, Purpura KP. Dynamic shifts of the contrast-response function. Visual Neuroscience. 1997;14:577–587. doi: 10.1017/s0952523800012232. [DOI] [PubMed] [Google Scholar]
- 38.Maddess T, McCourt ME, Blakeslee B, Cunningham RB. Factors governing the adaptation of cells in area-17 of the cat visual cortex. Biol Cybern. 1988;59:229–236. doi: 10.1007/BF00332911. [DOI] [PubMed] [Google Scholar]