Abstract
Objectives/Hypothesis
Mutation of the TP53 gene occurs in over half the cases of head and neck squamous cell carcinoma (HNSCC). However, little is known about how specific TP53 mutations affect tumor progression. The objective of this study is to determine the gain of function of mutant p53 with a proline-to-serine substitution at codon 151.
Study Design
Laboratory based study.
Methods
A panel of HNSCC cell lines was determined with anoikis assays and orthotopic mouse experiments were performed. TP53 was sequenced. The shRNA knockdown and over-expression approaches were used for testing mutant p53 functions. The crystal structure of the p53 protein was analyzed using an in silico approach.
Results
An anoikis resistant cell line, Tu138, was found to have a proline-to-serine substitution at codon 151 of TP53, which results in loss of wild-type p53 transcriptional activity. Moreover, the mutant p53 was shown to promote anoikis resistance and soft agar growth. Using an in silico approach based on the crystal structure of wild type p53 protein, substitution of proline by serine at position 151 would create a cavity in a hydrophobic pocket, the loss of van der Waals contacts and the thermodynamically unfavorable placement of a polar group, the hydroxyl oxygen atom of the serine, within a hydrophobic region, all of which likely cause a locally altered structure.
Conclusions
Our data suggest that mutation at position 151 leads to a structural alteration, which results in significant functional changes in the p53 protein that impact tumor progression.
Keywords: anoikis, head and neck squamous cell carcinoma, p53, orthotopic
Introduction
Head and neck squamous cell carcinoma (HNSCC) ranks among the cancers most highly associated with p53 mutation, with a prevalence of over 50% of HNSCC specimens having p53 sequence alterations. 1, 2 The mutation pattern of p53 is unique in that there appears to be a strong selection bias for missense mutations, particularly involving amino acids in the DNA-binding domain.3–6 Wild type p53 maintains genomic integrity through the induction of cell cycle regulatory and apoptosis inducing genes in response to DNA damage. P53 mutation can result in loss of these wild type functions since the commonly mutated p53 proteins detected in human cancers lose DNA-binding activity of p53 responsive elements.7 Some p53 mutants also have a dominant negative effect as they can bind and inhibit the function of remaining wild-type p53.8 Moreover, some mutant p53 displays oncogenic properties, termed “gain of function” (GOF), which is independent of wild-type p53 function. 9 Accordingly, gain of function p53 mutants can enhance cell transformation, increase tumor formation in mice and confer cellular resistance to chemotherapy.10–14
Normal epithelial cells including those that comprise the mucosa of the upper aerodigestive tract are dependent upon cell survival signals that are mediated through adherence to the extracellular basement. When epithelial cells lose contact with their basement membrane, they undergo apoptosis, which is termed anoikis.15 Acquiring resistance to anoikis may be a general prerequisite for the development and progression of cancers of epithelial origin, or carcinoma, and previous studies reported have shown that acquisition of anoikis resistance is a critical step in the progression of oral tongue cancer.16
During our studies of anoikis resistance of HNSCC cells, a cell line, Tu138, was identified which is highly resistant to anoikis, and it was found that this cell line has a serine substitution of proline at codon 151 of TP53 (herein referred to p53P151S). We hypothesized that the P151S mutation confers a gain of function (GOF) to the p53 protein, which in turn promotes oral cancer progression. This manuscript describes the characterization of the Tu138 cell line as well as the subcloning and expressing of p53P151S in non-p53 expressing anoikis-sensitive oral cancer cells, UMSCC1 cells. Through these studies we demonstrate that TP53P151S, can lead to loss of p53 transcriptional activity and mediate anoikis-resistance and soft agar growth.
MATERIALS AND METHODS
Cell lines
The HNSCC cell lines, which were used in the study, are listed in Table 1 and have been authenticated by STR analysis (short tandem repeat). Tu138 cells were obtained from Dr. Gary Clayman at The University of Texas M.D. Anderson Cancer Center. FaDu and Detroit562 cell lines were purchased from American Type Culture Collection (ATCC). The PCI cell lines were provided by Dr. Jennifer Grandis, the University of Pittsburgh. JHU cells were provided by Dr. David Sidransky, Johns Hopkins University and the UM-SCC cell lines were developed and provided by Dr. Thomas Carey, University of Michigan. UMSCC1c2 cells were cloned and isolated from UMSCC1 cells. The HN4, HN30, and HN31 cells lines were prepared by Dr. John Ensley, Wayne State University. Dr. Peter Sacks, New York University, developed and provided the MDA series of cell lines. Adherent monolayer cultures were maintained and incubated at 37°C in 5% carbondioxide and 95% air. The cultures were free of Mycoplasma.
Table 1.
Anoikis sensitivity in head and neck squamous carcinoma cells after detached culture for 24 and 48hrs
| Cell lines | Apoptotic cells (%) | |
|---|---|---|
| 24h (%) | 48h | |
| Detroit 562 | 1.260 | 2.700 |
| HN5 | 2.600 | 5.500 |
| FADU | 3.250 | 6.123 |
| MDA1986LN | 3.810 | 48.263 |
| PCI-15B | 4.600 | 30.973 |
| UMSCC22A | 5.081 | 23.541 |
| UMSCC2 | 6.273 | 14.619 |
| TR146 | 6.768 | 22.646 |
| UMSCC25 | 6.573 | 67.721 |
| UMSCC 17A | 9.283 | 22.967 |
| MDA1386LN | 10.667 | 62.661 |
| PE/CA-PJ-34 | 16.579 | 18.729 |
| Tu138 | 15.326 | 24.256 |
| Ca9-22 | 16.242 | 43.814 |
| MDA1686 | 19.424 | 51.020 |
| OSC-19 | 20.621 | 43.783 |
| MDA686LN | 20.248 | 67.773 |
| HN30 | 26.262 | 58.978 |
| UMSCC17B | 29.977 | 43.188 |
| UMSCC47 | 30.431 | 58.585 |
| PIC-13 | 33.024 | 68.137 |
| 138 | 35.261 | 63.302 |
| JHU012 | 38.702 | 55.705 |
| PIC-15A | 40.465 | 67.179 |
| MDA 1386Tu | 41.358 | 60.774 |
| UMSCC22B | 42.992 | 66.057 |
| HN31 | 46.333 | 47.250 |
| MDA1168 | 53.030 | 66.375 |
| HN4 | 53.250 | 67.619 |
| Sqccy1 | 54.645 | 80.560 |
| MDA1586 | 59.994 | 86.008 |
| UMSCC11A | 59.966 | 90.038 |
| 584A2 | 64.568 | 91.753 |
| JHU011 | 67.339 | 95.254 |
| Tu167 | 80.846 | 96.258 |
| Tu167c2 | 82.232 | 97.464 |
| UMSCC1 | 82.132 | 98.125 |
Anoikis assay
Anoikis experiments were performed as previously reported.16 Briefly, 2×106 cells were grown in a 15-ml conical tube (Falcon, Becton-Dickinson, Franklin Lakes, New Jersey) with a vented cap (Biocoat, Becton-Dickinson, Bedford, MA) that was placed on a rotating wheel for different time periods in a humidified incubator at 37 °C with 5% CO2. Trypan-blue was used to identify dead cells. In addition, the DNA fragmentation assay was used to confirm the cell death by apoptosis. For this assay, the cells were suspended in 1 ml of lysis buffer (20 mM Tris HCl [pH 8], 10 mM ethylenediaminetetraacetic acid [pH 8], and 0.5% Triton X-100). The DNA in the supernatant was extracted with ammonium acetate (2 M) and two volumes of ethanol and was analyzed using 1.5% agarose gel, which was visualized by ethidium bromide staining of the gel.
The orthotopic nude mouse model of HNSCC
To determine the tumorigenicity of the 8 most anoikis-resistant and 8 most anoikis sensitive HNSCC cell lines, 5 × 104 cells suspended in 30 μL of serum-free media were injected into the mouse tongue as described previously.17 Ten to 12 mice were prepared for each cell line.
Analysis of TP53 mutation
The genomic DNA from UMSCC1 and Tu138 cells was isolated with Puregene Core Kit 13 (GIAGENE) according to the manufacturer’s instructions and TP53 sequence was analyzed in the DNA Sequencing Core Facility at Baylor College of Medicine.
Modeling the proline-to-serine substitution at position 151 of p53
The coordinates of the human p53 DNA binding domain bound to DNA (RCSB, Accession Code 2AC0, tetrameric p53 binding to DNA) were used in a commercially available structural analysis program, PyMol ™ (Copyright 2008 DeLano Scientific LLC), to model possible local structural effects that result from the substitution of the proline at position 151 of p53 with serine.
Plasmids and cell infection
To make a construct expressing mutant p53P151S, total RNA was isolated from Tu138cells with TRIzol reagent (Invitrogen) and Reverse transcription PCR was performed to amplifying p53 cDNA with IscriptTM cDNA Synthesis kit (Bio-Rad) using the primers: 5′-BamH I: 5′ CGGGATCCAAGTCTAGAGCCACCGTCCA and 3′-EcoR I, 5′-GGAATTCTCAGTCTGAGTCAGGCCCTTCTGTC. The PCR products were inserted into BamH I and EcoR I of pBaBe-puro retroviral vector (Addgene). After verification of the mutant p53 by sequencing, the pBaBe-p53P151S construct was transfected into Phoenix cells with two helpers, pCGP and pVSVG, to generate viruses. The cell culture supernatant was filtered and used to infect UMSCC1 cells. The infected cells were selected with puromycin and pooled cells were used in the study. To knockdown the mutant p53 in Tu138 cells, Lenti-viral vector pLVUHshp53 containing shRNA that targets p53 and its helper plasmids, pMD2.G and pCMV-dR8.2 dvpr, were purchased from Addgene and were transfected into 293T cells. The supernatant was filtered and used to infect Tu138 cells. The positive infected cells were sorted with flow cytometry since this lenti-viral vector contains GFP selection marker. The positive cells were pooled for next experiments.
Soft agar assay
For soft agar assay, 1 ml of 0.6% agarose in medium containing 10% FBS was plated in 6-well plate as bottom gel. The cells, 1×104, were seeded in 0.3%, 0.6% or 0.9% agarose, 20% FBS, on the top of solidified bottom layer agar. The cells were maintained in an incubator at 37 °C in 5% CO2 for 2–3 weeks supplemented with 100 μl media every 3 days.
Western blot
Western blot was performed as described.18 The first antibodies used were: p53 and MDM2 from Santa Cruz Biotechnology and p21Waf1/Cip1 from BD Transduction Laboratories. The horseradish peroxidase conjugated secondary antibodies were from Santa Cruz Biotechnology.
Statistical analysis
Pearson’s correlation coefficients were computed for the relationship between anoikis resistance and tumorigenicity. Other data were analyzed with Student’s t test. For all comparisons, P < 0.05 was considered statistically significant.
Results
HNSCC cell lines have a wide range of sensitivity to anoikis, cell death induced by detachment from the extra-cellular matrix (ECM)
Our previous study has shown that normal oral epithelial cells undergo apoptosis in suspension culture or anoikis, and that the few HNSCC cell lines that tested had a varied capacity to survive in culture after detachment from the ECM.16 To further explore this observation, a panel of HNSCC cell lines was acquired and authenticated by STR (short tandem repeat) analysis and the percent of cells surviving after 24 and 48 hours growth in suspension cultures was assessed. As shown in Table 1, a wide range of sensitivity and resistance to detachment (anoikis resistance) can be seen across these cell lines ranging from 2.7–98.1% apoptotic cells after 48 hrs in suspension culture.
Anoikis resistance is associated with enhanced local tumor growth of HNSCC in an orthotopic nude mouse model of oral tongue cancer
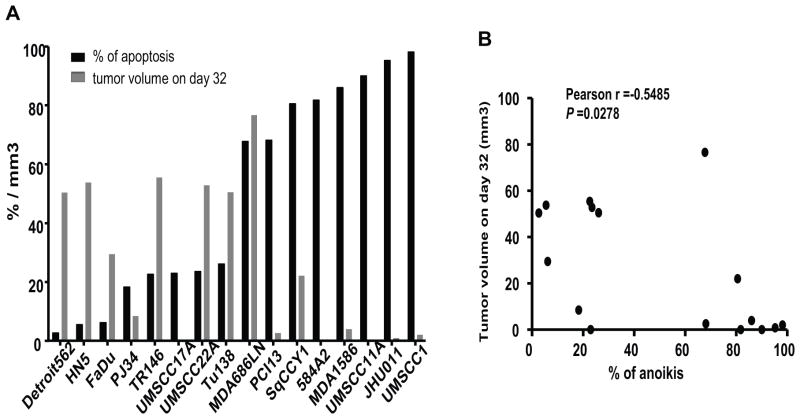
Following up on our previous studies, which had demonstrated an association of anoikis resistance with aggressive tumor growth in an orthotopic nude mouse model of oral tongue cancer, the 8 most anoikis-resistant and 8 most anoikis sensitive cell lines were injected into the tongues of nude mice. Anoikis resistance was found to be positively associated with increased tumor size (Figure 1).
Figure 1.
The top 8 anoikis-resistant and 8 anoikis sensitive cell lines were selected and injected into the tongues of nude mice. The association of anoikis resistance (detached for 48 hrs) with tumor size at 32 days after injection was analyzed.
P53P151S mutation displays anoikis resistance
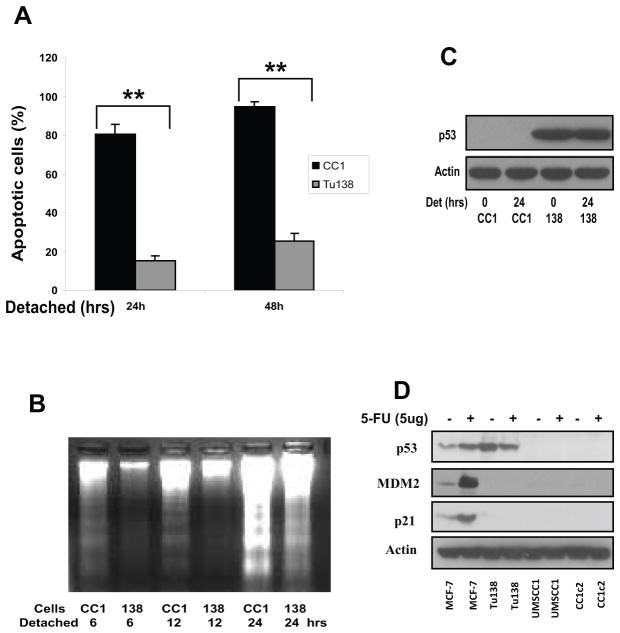
The relatively anoikis-resistant cell line, Tu138 as well as an anoikis-sensitive line, UMSCC1 were selected for further characterization and confirmed their relative sensitivities to anchorage independent growth using several different methods. As shown in Fig. 2A, Tu138 cells are very resistant to anoikis in suspension culture, while UMACC1 cells are very sensitive as assessed for cell death by exclusion of Trypan blue (Fig. 2A). Apoptotic cell death upon detachment was also determined with a DNA fragmentation ladder assay shown in Fig. 2B, which confirms the results found in Fig. 2A. It was hypothesized that alterations in p53 structure may be contributing to differences in anoikis sensitivity between these two lines, and therefore the p53 gene was sequenced. It was found that Tu138 cells harbor a proline to serine substitution at position 151 of p53, while UMSCC1 and UMSCC1c2 (a subclone cell of UMSCC1) cells carry a mutation in intron 5 at the splicing site from G to G/A, thereby destroying the splicing site and leading to complete loss of p53 expression at the protein level. Western blotting shows that p53 is stably expressed in Tu138 cells at baseline and under detached conditions at 24 hrs, and cannot be detected in UMSCC1 cells (Fig. 2C). To assess the transcriptional activity of these the two p53 mutant isoforms, both the Tu138 and UMSCC1 cell lines were treated with the DNA damaging drug 5-fluorouracil(5-FU), which leads to the induction of p53, mdm-2 and p21 expression in wild type p53 MCF-7 cells, a breast cancer cell line (Fig 2D). While UMSCC1 has no evidence of p53 expression or functional activity, the mutant p53 in Tu138 shows no apparent activity in inducing expression of either mdm-2 or p21 (Fig. 2D).
Figure 2.
A. Tu138 and UMSCC1 cells were cultured in detached conditions for 24 and 48 hrs and dead cells were determined using Trypan-blue (** p<0.01, n=3). B. Tu138 and UMSCC1 cells were detached for 6, 12 and 24 hrs and fragmented DNA was isolated and subjected to agarose gel electrophoresis. C. The p53 expression in Tu138 and UMSCC1 cells was determined with Western blot after normal and detached culture for 24 hrs. D. Tu138, UMSCC1 and UMSCC1c2 cells were treated with 5ug/ml 5-FU for 20 hrs and the wild-type p53 MCF-7 cells were used as control. The p53 target genes p21 and MDM2 were examined using Western blotting.
The P151S substitution suggests structural changes
In order to determine whether the substitution of proline at position of 151 of p53 had been previously reported in human cancers, the p53 database of the International Association for Research on Cancer of the World Health Organization (www-p53.iarc.fr) was searched, and it was found that this mutation has been reported in 91 cancer cases including 5 cases of laryngeal cancer, 2 cases of tongue cancer, 3 oral cancer cases, 2 cases of oropharyngeal cancer and 4 cases at non-specified head and neck cancer sites. This mutation was seen in the germline in 3 different reported tumors in one Li-Fraumeni family. Functional studies in yeast, LnCap, Sao2-2, and H1299 cells summarized in the database have shown mixed results.
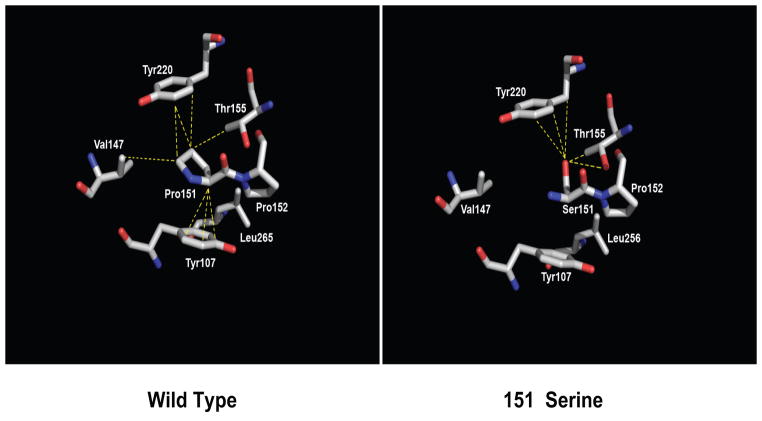
To investigate any possible impact on p53 structure that the substitution of the proline at position 151 with a serine residue might cause, the coordinates of the human p53 DNA binding domain bound to DNA (RCSB, Accession Code 2AC0) were used in the structural analysis. It was noted that residue P151 is tens of ängstroms from the DNA binding face of p53 and hence its substitution would not interfere directly with the DNA binding function of the protein. Second, proline is a larger amino acid than serine with its side chain covalently bonded to the peptide backbone, thus generating a closed ring system that restricts the peptide backbone to a limited area of conformational space. By contrast the smaller serine residue, with just a two-atom side chain, allows greater peptide backbone conformational accessibility. In silico modeling reveals that the P151S substitution results in a cavity in a hydrophobic/aromatic pocket consisting of residues Y107, V147, L265 and Y220 and also leads to the loss of multiple van der Waals contacts, including all to residue Y107 (Fig. 3). The thermodynamic consequences of the loss of these contacts is clearly unfavorable and is exacerbated by the placement of a polar atom, the hydroxyl oxygen of Ser151, within this mostly hydrophobic region (Fig. 3). Although the hydroxyl group of Ser151 could hydrogen bond to the hydroxyl group of nearby residue Thr150, albeit with poor geometry, this interaction is unlikely to compensate completely the multiple lost interactions that the P151S substitution would cause. Hence, the replacement of the proline at position 151 by serine likely results in at the least a local conformational change about this residue that is deleterious to the function of the protein.
Figure 3.
p53P151S makes fewer inter residue contacts than wild type p53. (Left) Structure of wild type p53 in the vicinity of residue P151, Residue P151 and nearby interacting amino acid residues are shown as sticks and colored according to atom type whereby oxygen is red, nitrogen, blue and carbon, white. Dashed yellow lines indicate contacts between residue P151 and nearby residues (d ≤ 4.2 Å). (Right) The structure of the modeled p53P151S mutant, colored and demarked as in the left panel. The closest approach of the side chains of S151 and V147 is 5.8 Å.
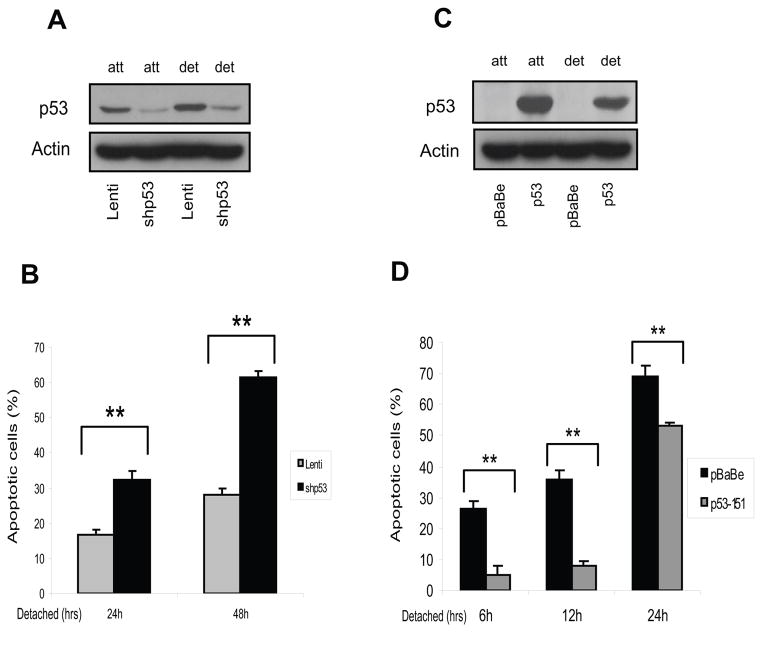
Mutant p53 P151S is critical for anoikis resistance
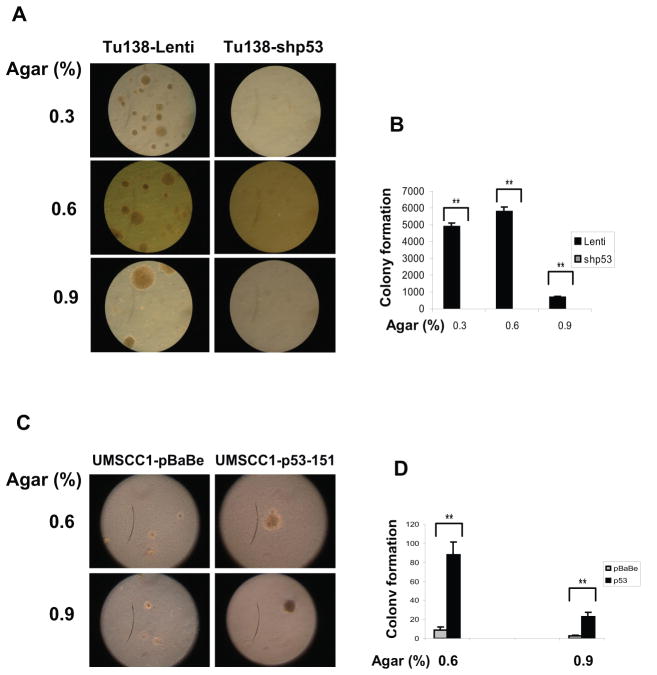
To determine the potential role of mutant p53P151S in mediating anoikis resistance, the mutant p53 was knocked-down in Tu138 cells with lentiviral shRNA for p53. As shown in Figure 4A, the p53 shRNA construct significantly down-regulated p53 protein expression in the Tu138 cells as compared to vector control cells. An anoikis assay showed that many more Tu138 cells undergo anoikis after p53 knock-down as compared to controls (Fig. 4B). Meanwhile, anoikis sensitive UMSCC1 cells were infected with retroviral pBaBe vector containing mutant p53P151S cloned from Tu138 cells (Fig. 4C). Consistently, expression of the mutant p53 led the anoikis sensitive UMSCCD1 cells to be markedly resistant to anoikis, suggesting that the mutant p53 plays an important role in mediating anoikis resistance. This observation was further explored using a soft agar growth assay with the two pairs of stable cell lines. Both of these cells were plated in 0.3%, 0.6% and 0.9% soft agar and cultured for 14 days (for Tu138 cells) and 21 days (for UMSCC1 cells). As shown in Figure 5A and B, the number and size of colonies in soft agar was dramatically reduced in Tu138-shp53 cells compared to the lenti vector controls, while greatly increased in UMSSc1 cells expressing p53P151S compared to their controls (Fig. 5C and D).
Figure 4.
P53P151S can confer anoikis resistance. Western blot was performed to detect p53 knockdown effect in stable Tu138 cells after normal and detached culture for 24 hrs. B. Tu138 p53 knockdown cells and vector control cells were detached for 24 and 48 hrs and dead cells were determined with Trypan Blue (**, p<0.01, n=3). C. UMSCC1 cells were infected with retroviral vector pBaBe containing p53P151S cloned from Tu138 cells and empty vector infected cells as control. The stable cells were cultured in attached and detached conditions for 24 hrs. D. Anoikis assay was performed in UMSCC1 cells expressing p53P151S and their control cells (**, p<0.01, n=3).
Figure 5.
Mutant p53P151S promotes cell growth in soft agar. P53-knockdown Tu138 cells and UMSCC1 cells overexpressing mutant p53 and their control cells were cultured in soft agar for 14 days (for Tu138 cells, A and B. **, p<0.01, n=3) and 21 days (for UMSCC1cells, 5C and D. **, p<0.01, n=3).
Discussion
Since the term mutant p53 gain of function was formally suggested, increased evidence supports that mutant p53 enhances tumor growth and metastasis.14 However, most of the results have come from studies of hotspot mutant p53 forms. Different mutations may behave differently depending on the cell type and genetic background of the cell,14 but little is known about how specific p53 mutations affect tumor progression. The fact that about 50% HNSCC harbor p53 mutations indicates that mutation of p53 may be critical for the progression of HNSCC. Anoikis resistance may be a general prerequisite for the development and progression of HNSCC. Therefore, the sensitivity to anoikis in a panel of HNSCC cell lines was determined and the tumorigenicity of the 8 most anoikis-resistant and 8 most anoikis sensitive cell lines was assessed in an orthotopic nude mouse model of oral tongue cancer. The results show that anoikis resistance is closely associated with the increased tumor size, indicating again that anoikis resistance may be a critical step in the progression of oral tongue cancer.16
Based on the above results and the finding that a cell line expressing the mutant p53P151S is anoikis resistant, it was hypothesized that this mutation has gain of function properties and promotes anoikis resistance. To determine the role of p53P151S in anoikis resistance, two cell models were used in the study. First, p53-P151S was cloned from Tu138 cells and introduced into anoikis sensitive UMSCC1 cells, which is a non-p53 expressing cell line. Considering the concerns that the effects seen with overexpression of the mutant p53 could be artifactual and that most tumor cells carrying no p53 may have already been transformed and do not require mutant p53 for maintaining their transformed phenotypes, a second cell model used the knockdown of mutant p53 in Tu138 by shRNA. The anoikis assay with the two cell models shows that p53P151S is very important in mediating anoikis resistance and this was confirmed by the soft agar experiments. In terms of p53 function in anoikis, wildtype p53 as an anoikis inducer has been intensively studied. For example, a recent paper shows that wildtype p53 induces anoikis via upregulation of Bax and PUMA and that SIK1 links LKB1 to p53-dependent anoikis and suppresses metastasis.19 Another study demonstrates that if FAK or the correct ECM is absent, a p53-dependent pathway is activated leading to anoikis, which is suppressible by dominant-negative p53.20 However, p53 gain of function mutations regulating resistance to anoikis have not been previously reported.
As resistance to anoikis contributes to malignant transformation, tumor progression and development of metastasis,16, 21 consistent with the above observations, our previous data derived from animal experiments demonstrate the p53P151S gains oncogenic function that leads to increased tumor growth and metastases, resulting in shorter time of survival.22 The function of p53P151S has been previously studied and found to exhibit loss of function in Yeast and Saos-2 cells.23–26 Consistently, our data also indicate that p53P151S lose its transcriptional activity for its target genes p21/Waf1 and MDM2 after treatment with 5-Fu. In contrast to the result of p53P151S gain of function, two studies published by another group have demonstrated that while four gain-of-function p53mutants (G245S, R248W, R273H, and R273C) are able to mediate androgen-independent growth of LNCaP prostate cancer cells, p53 (P151S) is not.27, 28 The reason may be that p53P151S is not involved in the androgen regulated signal pathway. Meanwhile, their studies also showed that p53(R173H) can enhance androgen-independent growth by upregulation of H2 relaxin, however, other p53 mutants (G245S, R248W, and R273C) fail to increase expression of relaxin protein, meaning that different mutant forms may play roles in different signaling pathways. Moreover, the reason that in the current study the p53P151S gain of function was identified may be that this mutation displays oncogenic activity in HNSCC cell lines, but not in prostate cancer cells, and that the phenotype of mutant p53 gain of function sometime is cell type-dependent. In support of the current results is that p53P151S gain of function is identified in Saos-1 cells exhibiting transcription of MDR-1 and in Yeast cells showing p73beta interference.23, 25 The signaling pathways that underlie the p53P151S enhanced resistance to anoikis in HNSCC are under investigation.
As for the impact of missense mutations on p53 structure, two classes of p53 mutations have been proposed based on the three-dimensional structure of the protein:29 the first class is referred to as contact mutations including those in residues directly involved in DNA binding, such as R248Q and R273H. The second class is termed as conformational mutations comprising substitution of residues that are involved in conformational changes and include R249S, G245S, R175H and R282W.29, 30 This led us to investigate the effects of the substitution of the proline at position 151 with serine on p53 structure. Subsequent modeling shows that the substitution of serine for proline creates a cavity in a hydrophobic pocket, causes the loss of multiple van der Waals interactions and places a polar side chain within a hydrophobic region, all of which are thermodynamically unfavorable. To compensate, it was predicted that the P151S substitution results in at least a local conformational change that alters the function of the protein. The current analysis is consistent with the reported observations demonstrating that proline 151 is important for the structure 29 and its mutation likely alters backbone structure and intra-and intermolecular side chain interactions.31
Conclusions
The P151S substitution likely results in tertiary structural alteration leading to significant functional changes in p53 protein with loss of p53 transcriptional activity, increased anoikis-resistance and soft agar growth. This highlights that specific p53 mutations in head and neck squamous cell carcinoma can have prognostic significance and could be targets for therapeutic strategies. Further investigations along these lines are currently in progress.
Acknowledgments
This work was supported by the NIH grants, RO1 Grant DE014613, Specialized Program of Research Excellence Grant P50CA097007, Cancer Center Support Grant CA016672 (CCSG), and the University of Texas Anderson Cancer Center PANTHEON program.
Footnotes
Level of Evidence: N/A
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1.Poeta ML, Manola J, Goldwasser MA, et al. TP53 mutations and survival in squamous-cell carcinoma of the head and neck. N Engl J Med. 2007;357:2552–2561. doi: 10.1056/NEJMoa073770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blons H, Laurent-Puig P. TP53 and head and neck neoplasms. Hum Mutat. 2003;21:252–257. doi: 10.1002/humu.10171. [DOI] [PubMed] [Google Scholar]
- 3.Hainaut P, Hollstein M. p53 and human cancer: the first ten thousand mutations. Adv Cancer Res. 2000;77:81–137. doi: 10.1016/s0065-230x(08)60785-x. [DOI] [PubMed] [Google Scholar]
- 4.Petitjean A, Mathe E, Kato S, et al. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Hum Mutat. 2007;28(6):622–629. doi: 10.1002/humu.20495. Version of the database (R14, November 2009). http://www-p53.iarc.fr/ [DOI] [PubMed] [Google Scholar]
- 5.Olivier M, Eeles R, Hollstein M, Khan MA, Harris CC, Hainaut P. The IARC TP53 database: new online mutation analysis and recommendations to users. Hum Mutat. 2002;19:607–614. doi: 10.1002/humu.10081. [DOI] [PubMed] [Google Scholar]
- 6.Olivier M, Petitjean A, Marcel V, et al. Recent advances in p53 research: an interdisciplinary perspective. Cancer Gene Ther. 2009;16:1–12. doi: 10.1038/cgt.2008.69. [DOI] [PubMed] [Google Scholar]
- 7.Kato S, et al. Understanding the function-structure and function-mutation relationships of p53 tumor suppressor protein by high-resolution missense mutation analysis. Proc Natl Acad Sci USA. 2003;100:8424–8429. doi: 10.1073/pnas.1431692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willis A, Jung EJ, Wakefield T, Chen X. Mutant p53 exerts a dominant negative effect by preventing wild-type p53 from binding to the promoter of its target genes. Oncogene. 2004;23:2330–2338. doi: 10.1038/sj.onc.1207396. [DOI] [PubMed] [Google Scholar]
- 9.Brosh R, Rotter V. When mutants gain new powers: news from the mutant p53 field. Nat Rev Cancer. 2009;9:701–713. doi: 10.1038/nrc2693. [DOI] [PubMed] [Google Scholar]
- 10.Wolf D, Harris N, Rotter V. Reconstitution of p53 expression in a nonproducer Ab-MuLV-transformed cell line by transfection of a functional p53 gene. Cell. 1984;38:119–126. doi: 10.1016/0092-8674(84)90532-4. [DOI] [PubMed] [Google Scholar]
- 11.Shaulsky G, Goldfinger N, Rotter V. Alterations in tumor development in vivo mediated by expression of wild type or mutant p53 proteins. Cancer Res. 1991;51:5232–5237. [PubMed] [Google Scholar]
- 12.Dittmer D, Pati S, Zambetti G, et al. Gain of function mutations in p53. Nat Genet. 1993;4:42–46. doi: 10.1038/ng0593-42. [DOI] [PubMed] [Google Scholar]
- 13.Sigal A, Rotter V. Oncogenic mutations of the p53 tumor suppressor: the demons of the guardian of the genome. Cancer Res. 2002;60:6788–6793. [PubMed] [Google Scholar]
- 14.Oren M, Rotter V. Mutant p53 gain-of-function in cancer. Cold Spring Harb Perspect Biol. 2010;2:a001107. doi: 10.1101/cshperspect.a001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swan EA, Jasser SA, Holsinger FC, Doan D, Bucana C, Myers JN. Acquisition of anoikis resistance is a critical step in the progression of oral tongue cancer. Oral Oncol. 2003;39:648–655. doi: 10.1016/s1368-8375(03)00049-6. [DOI] [PubMed] [Google Scholar]
- 17.Myers JN, Holsinger FC, Jasser SA, Bekele BN, Fidler IJ. An orthotopic nude mouse model of oral tongue squamous cell carcinoma. Clin Cancer Res. 2002;8:293–298. [PubMed] [Google Scholar]
- 18.Xie TX, Huang FJ, Aldape KD, et al. Activation of stat3 in human melanoma promotes brain metastasis. Cancer Res. 2006;66:3188–3196. doi: 10.1158/0008-5472.CAN-05-2674. [DOI] [PubMed] [Google Scholar]
- 19.Cheng H, Liu P, Wang ZC, et al. SIK1 couples LKB1 to p53-dependent anoikis and suppresses metastasis. Sci Signal. 2009;2:ra35. doi: 10.1126/scisignal.2000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ilić D, Almeida EA, Schlaepfer DD, Dazin P, Aizawa S, Damsky CH. Extracellular matrix survival signals transduced by focal adhesion kinase suppress p53-mediated apoptosis. J Cell Biol. 1998;143:547–560. doi: 10.1083/jcb.143.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taddei ML, Giannoni E, Fiaschi T, Chiarugi P. Anoikis: an emerging hallmark in health and diseases. J Pathol. 2012;226:380–393. doi: 10.1002/path.3000. [DOI] [PubMed] [Google Scholar]
- 22.Sano D, Xie TX, Ow TJ, et al. Disruptive TP53 mutation is associated with aggressive disease characteristics in an orthotopic murine model of oral tongue cancer. Clin Cancer Res. 2011;17:6658–6670. doi: 10.1158/1078-0432.CCR-11-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi XB, Nesslinger NJ, Deitch AD, Gumerlock PH, deVere White RW. Complex functions of mutant p53 alleles from human prostate cancer. Prostate. 2002;51:59–72. doi: 10.1002/pros.10072. [DOI] [PubMed] [Google Scholar]
- 24.Campomenosi P, Monti P, Aprile A, et al. p53 mutants can often transactivate promoters containing a p21 but not Bax or PIG3 responsive elements. Oncogene. 2001;20:3573–3579. doi: 10.1038/sj.onc.1204468. [DOI] [PubMed] [Google Scholar]
- 25.Monti P, Campomenosi P, Ciribilli Y, et al. Characterization of the p53 mutants ability to inhibit p73 beta transactivation using a yeast-based functional assay. Oncogene. 2003;22:5252–5260. doi: 10.1038/sj.onc.1206511. [DOI] [PubMed] [Google Scholar]
- 26.Dearth LR, Qian H, Wang T, et al. Inactive full-length p53 mutants lacking dominant wild-type p53 inhibition highlight loss of heterozygosity as an important aspect of p53 status in human cancers. Carcinogenesis. 2007;28:289–298. doi: 10.1093/carcin/bgl132. [DOI] [PubMed] [Google Scholar]
- 27.Nesslinger NJ, Shi XB, deVere White RW. Androgen-independent growth of LNCaP prostate cancer cells is mediated by gain-of-function mutant p53. Cancer Res. 2003;63:2228–2233. [PubMed] [Google Scholar]
- 28.Vinall RL, Tepper CG, Shi XB, et al. The R273H p53 mutation can facilitate the androgen-independent growth of LNCaP by a mechanism that involves H2 relaxin and its cognate receptor LGR7. Oncogene. 2006;25:2082–2093. doi: 10.1038/sj.onc.1209246. [DOI] [PubMed] [Google Scholar]
- 29.Cho Y, Gorina S, Jeffrey PD, Pavletich NP. Crystal structure of a p53 tumor suppressor-DNA complex: understanding tumorigenic mutations. Science. 1994;265:346–355. doi: 10.1126/science.8023157. [DOI] [PubMed] [Google Scholar]
- 30.Bullock AN, Fersht AR. Rescuing the function of mutant p53. Nat Rev Cancer. 2001;1:68–76. doi: 10.1038/35094077. [DOI] [PubMed] [Google Scholar]
- 31.Walker DR, Bond JP, Tarone RE, et al. Evolutionary conservation and somatic mutation hotspot maps of p53: correlation with p53 protein structural and functional features. Oncogene. 1999;18:211–218. doi: 10.1038/sj.onc.1202298. [DOI] [PubMed] [Google Scholar]