Abstract
The development of pharmacological, genetic, and biochemical tools have allowed for detailed studies to determine the contribution of cytochrome P450 (CYP) metabolites of arachidonic acid to renal microvascular function. Renal microvessels can generate CYP hydroxylase metabolites including 20-hydroxyeicosatetraenoic acid (20-HETE) and CYP epoxygenase metabolites, epoxyeicosatrienoic acids (EETs). 20-HETE constricts afferent arterioles and contributes to renal blood flow autoregulation. EETs act as endothelium-dependent hyperpolarizing factors (EDHFs) on the renal microcirculation. 20-HETE inhibits whereas EETs activate renal microvascular smooth muscle cell large-conductance calcium-activated K+ channels (KCa). Likewise, 20-HETE renal microvascular actions are pro-hypertensive and EET actions are anti-hypertensive. These findings in the renal microvasculature and those of others have provided impetus for the development of enzymatic inhibitors, agonists, and antagonists for 20-HETE and EETs to determine their potential therapeutic value. Initial genetic studies and experimental studies with soluble epoxide hydrolase inhibitors to increase EETs, EET analogs, and 20-HETE inhibitors have demonstrated improved renal microvascular function in hypertension. These findings have demonstrated the important contributions that 20-HETE and EETs play in the regulation of renal microvascular function.
Introduction
The recognition that cytochrome P450 (CYP) enzymes had the capacity to metabolize arachidonic acid and generate epoxyeicosatrienoic acids (EETs) and hydroxysatetraenoic acids (HETEs) ignited curiosity to determine their biological actions [1,2]. As the identification of the CYP enzymes that catalyzed the reactions were being identified and further characterized in the 1980s, there was slower progress with the determination of the physiological actions for EETs and HETEs. Early studies demonstrated that kidneys had significant expression of CYP enzymes and that EETs and HETEs had actions on epithelial cells to alter sodium transport [3,4]. Vascular actions for EETs as dilators were first described towards the end of 1980s [5]. Around this same time period it was becoming evident that nitric oxide was an endothelial-derived relaxing factor [6,7]. It was also apparent that the endothelial cells released a hyperpolarizing factor (EDHF) that was speculated to be a non-cyclooxygenase arachidonic acid metabolite [6,7]. EETs became a candidate for being an EDHF and a number of laboratories pursued this idea during the 1990s [8–10]. On the other hand, 20-HETE was determined to be a vasoconstrictor in the early 1990s [11,12]. A point of contention was that the epithelial actions attributed to 20-HETE were anti-hypertensive whereas the vascular actions were pro-hypertensive [13]. Therefore, the 1990s were an era that took CYP generated EETs and HETEs from a biological curiosity to a metabolic pathway that could significantly impact physiological and pathophysiological states.
There were numerous hurdles to overcome to determine the physiological and pathophysiological importance of CYP arachidonic acid metabolites. Pharmacological, molecular biological, and analytical tools had to be developed to determine the biological actions attributed to CYP enzymes, EETs, and 20-HETE. The laboratories of Jorge Capdevila and John Falck developed many of the tools necessary for investigators to determine the biological importance of this pathway [13,14]. These tools led to a number of experimental studies in my laboratory to determine the impact of CYP enzymes, EETs, and 20-HETE on renal microvascular function (Figure 1). This review article will focus on findings demonstrating renal microvascular actions for EETs and 20-HETE and their contribution to hypertension.
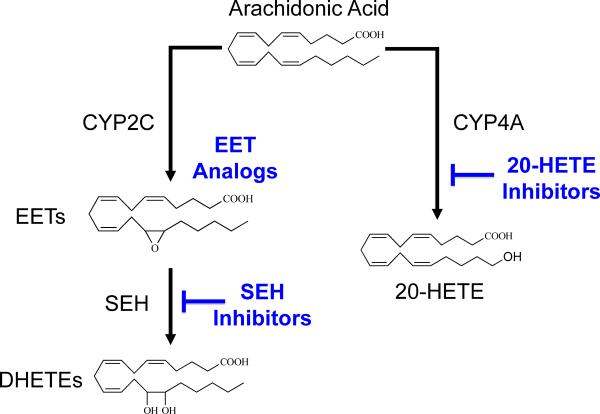
Figure 1.
Therapeutic targeting for the epoxygenase and hydroxylase pathways: Epoxyeicosatrienoic acids (EETs) are generated from arachidonic acid by cytochrome P450 (CYP2C) enzymes. EETs are converted to dihydroxyeicosatrienoic acids (DHETEs) by the soluble epoxide hydrolase (sEH) enzyme. 20-hydroxysatetraenoic acid (20-HETE) is generated by cytochrome P450 (CYP4A) enzymes. EET analogs, sEH inhibitors, and 20-HETE inhibitors are therapeutic targets for hypertension, renal, and cardiovascular diseases.
20-HETE & Afferent Arteriolar Autoregulatory Responses
Early experimental studies determined that renal arterioles, glomeruli, and vasa recta capillaries expressed CYP4A hydroxylase enzymes that are primarily responsible for generating 20-HETE [12,13]. Other experimental studies determined that 20-HETE levels were elevated in spontaneously hypertensive rats and 20-HETE constricted canine renal arteries [11,15,16]. 20-HETE afferent arteriolar constriction was determined to be due to inhibition of calcium-activated K+ (KCa) channels, membrane depolarization, activation of L-type calcium channels, and an increase in intracellular calcium [11,12,13] (Figure 2). Besides the direct action of 20-HETE to constrict afferent arterioles, a central role for 20-HETE is its contribution to renal blood flow autoregulation [17,18].
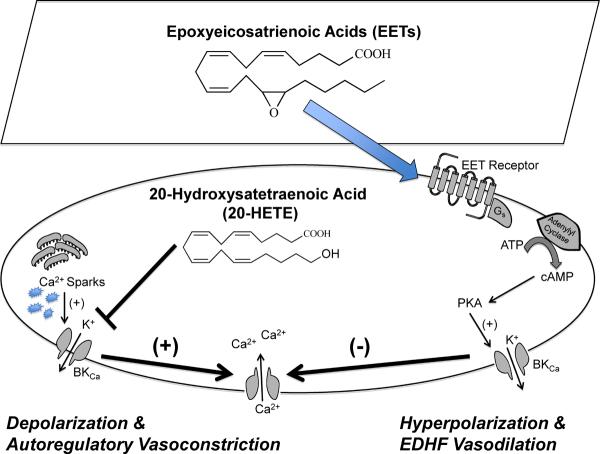
Figure 2.
Renal microvascular actions for 20-hydroxysatetraenoic acid (20-HETE) and epoxyeicosatrienoic acids (EETs): 20-HETE inhibits renal microvascular smooth muscle cell KCa channels resulting in membrane depolarization, calcium influx through L-type Ca2+ channels and autoregulatory vasoconstriction. Endothelial-derived EETs activate G-protein, cAMP, and PKA in renal microvascular smooth muscle cells resulting in activation of KCa channels, membrane hyperpolarization and endothelial-dependent hyperpolarizing factor (EDHF) mediated vasodilation.
Renal blood flow autoregulation is the ability to keep blood flow and glomerular filtration rate constant in the face of changes in perfusion pressure. The kidney is able to maintain a constant renal blood flow between 80 and 160 mmHg through two mechanisms, the myogenic response and tubuloglomerular feedback. The contribution of CYP metabolites to renal blood flow autoregulation was demonstrated by infusing the non-selective CYP inhibitor 17-ODYA into the renal artery [18]. Renal blood flow and cortical blood flow increased in response to increases in mean arterial pressure in the presence of CYP inhibition [18]. Experiments in the juxtamedullary nephron preparation determined that the afferent arteriolar constriction was attenuated and that glomerular capillary pressure increased as perfusion pressure increased from 80 to 160 mmHg [17]. Additional studies established a contribution for CYP metabolites to the afferent arteriolar myogenic and tubuloglomerular feedback responses [17–19]. Renal microvascular myogenic responses in the absence of tubuloglomerular feedback were attenuated by CYP inhibition [17]. Micopuncture studies demonstrated that CYP inhibition abolished the tubuloglomerular feedback response and the addition of 20-HETE to the tubular perfusate restored the feedback response [19]. One concern with these studies was that 17-ODYA is unable to determine the contribution of hydroxylase metabolite 20-HETE versus epoxygenase EET metabolites because 17-ODYA inhibits the renal generation of EETs as well as 20-HETE [17–19]. Relatively selective inhibition of the epoxygenase pathway with imidazole derivatives including miconazole and clotrimazole had no effect on renal blood flow autoregulation [18]. This finding provides support to the notion that EETs were not contributing to autoregulation and more clearly established a contribution of the hydroxylase metabolite 20-HETE to renal blood flow autoregulation.
The development of selective CYP epoxygenase and hydroxylase inhibitors, as well as, EET and 20-HETE antagonists allowed for more direct evaluation of the specific CYP metabolites that contributed to afferent arteriolar autoregulation. The development of selective CYP epoxygenase (PPOH and MS-PPOH) and hydroxylase (DDMS) inhibitors and EET (14,15-EEZE) and 20-HETE (20-HEDE) antagonists allowed for experimental studies to delineate the contributions of EETs and 20-HETE to renal microvascular function [20,21]. The selective hydroxylase inhibitor DDMS attenuated the decrease in afferent arteriolar diameter to increasing perfusion pressure [21]. Moreover, the selective epoxygenase inhibitors PPOH and MS-PPOH had the opposite effect and in response to elevations in perfusion pressure resulted in enhanced afferent arteriolar constriction [21]. These findings provided significant support that 20-HETE is a critical component of the afferent arteriolar response to increases in perfusion pressure and that dilator EETs attenuates this vasoconstriction.
A parallel concept that purines adenosine and ATP and their receptors contributed to renal blood flow autoregulation and afferent arteriolar autoregulatory responses was gaining substantial experimental support [22,23]. This led to the idea that interactions between purinergic receptors and CYP metabolites may exist at a level that controls afferent arteriolar autoregulation. Similar to CYP hydroxylase inhibition, inactivation of ATP P2X receptors on preglomerular microvessels abolishes autoregulatory behavior [22,23]. Interestingly, hydroxylase inhibition or 20-HETE antagonism attenuated the initial afferent arteriolar constriction and abolished the sustained constriction to the P2 receptor agonist ATP [24]. The afferent arteriolar constriction to the P2X receptor agonist !, ! -methylene ATP was also greatly attenuated by DDMS or 20-HEDE [24]. Additionally, the increase in renal microvascular cell calcium evoked by ATP and the P2X receptor agonist, !, ! -methylene ATP, but not the P2Y agonist, UTP, were markedly reduced by 20-HETE inhibition [25]. These studies provided compelling evidence that endogenous 20-HETE contributes to the P2X receptor-mediated afferent arteriolar autoregulatory response by influencing vascular smooth muscle cell calcium influx.
Overall, 20-HETE is an important autocrine factor that contributes to the regulation of renal blood flow and afferent arteriolar function. There is significant evidence that there is a connection between ATP P2X receptor activation, 20-HETE, and afferent arteriolar autoregulatory responses. Future studies are required to fully establish the contribution and cell signaling mechanisms by which 20-HETE regulates afferent arteriolar function.
EETs, EDHF, & Afferent Arteriolar Responses
The kidney has a high capacity to generate EETs and these metabolites have actions on epithelial cell transport and renal hemodynamics [1,3,26]. CYP2C enzymes are primarily responsible for the renal eptihelial and endothelial cell generation of EETs [26]. In regards to renal hemodynamics, there was initial controversy as to whether or not EETs were vasodilators or vasoconstrictors. These experimental studies determined that cyclooxygenase (COX) enzymes could metabolize 5,6-EET and 8,9-EET to vasoconstrictors that act on thromboxane receptors and that 11,12-EET and 14,15-EET act as vasodilators [26,27]. EETs can be metabolized by sEH to their corresponding diols, dihydroxyeicosatrienoic acids (DHETEs). The diol of 11,12-EET, 11,12-DHETE, at micromolar concentrations had no effect on afferent arteriolar diameters [27}. This finding suggested that inhibitors of sEH could block degradation of EETs to DHETEs and enhance their vascular actions. There has also been a significant amount of interest in the renal microvascular actions attributed to EETs since they have been identified as an EDHF.
Afferent arteriolar responses to EETs in particular 11,12-EET and 14,15-EET provided initial evidence for being EDHFs [27]. These studies determined that 11,12-EET and 14,15-EET dilated afferent arterioles through direct actions on vascular smooth muscle and activated renal microvascular smooth muscle cell KCa channels [28]. EETs also were found to contribute to the nitric oxide- and COX-independent renal vasodilation that provided additional evidence that EETs were an EDHF. Epoxygenase inhibition with MS-PPOH was determined to attenuate the afferent arteriolar dilations to acetylcholine and bradykinin [29,30]. These studies found that EETs accounted for the nitric oxide- and COX-independent afferent arteriolar dilation that accounted for approximately one-third the total response to bradykinin [29]. EETs have also been suggested to contribute to bradykinin-mediated portion of the renal microvascular dilation in response to angiotensin converting enzyme inhibition [31,32]. Furthermore, isolated renal microvessels increase EET production by 50% upon stimulation with bradykinin [29]. Like 20-HETE, EETs appear to participate in purine receptor responses. Adenosine increases renal microvascular EET generation and EETs contribute to adenosine A2A receptor mediated vasodilation [33]. Overall, these findings provided a stimulus for studies to determine the structure-activity relationship for EETs and determine cell-signaling mechanisms responsible for afferent arteriolar dilation.
EET analogs / mimetics were designed and synthesized to increase stability and to determine the structure activity relationship for EETs [34]. The overall stability of EETs and degradation by sEH to DHETEs and ! -oxidation made it very difficult to conduct experimental studies. This led to the initial 11,12-EET and 14,15-EET sulfonimide analogs that prevented ! -oxidation at the carboxylic acid [35,36]. Subsequently, Dr. Falck generated numerous EET analogs that were tested for dilator activity primarily in coronary, mesenteric, and renal microvessels. These studies led to a similar structure activity relationship for 11,12-EET and 14,15-EET [34,37]. Three main components were required for full EET activity, an acidic carboxyl group, !8 olefin bond, and a cis-epoxide. This information led to the development of EET analogs for cell-signaling studies and the development of EET antagonists.
11,12-EET analogs have allowed for cell signaling studies in renal microvessels and determined a contribution for cAMP and protein kinase A (PKA) to activation of KCa channels [35,38]. Initial studies with analog 11,12-EET-SI determined that PKA inhibition attenuated the afferent arteriolar dilation [35]. A major drawback for the 11,12-EET-SI analog was that it contained an epoxide at the 11,12 position that was susceptible to conversion to the diol by the sEH enzyme. Therefore, 11,12-EET analogs were designed and synthesized to resists degradation by sEH. This series of 11,12-EET analogs was extensively studied in mesenteric resistance arteries and afferent arterioles [38,39]. These 11,12-EET analogs activated renal microvascular smooth muscle cell KCa channels when evaluated in the cell-attached patch mode [38]. Afferent arteriolar dilation to this series of 11,12-EET analogs were greatly attenuated by the large-conductance KCa inhibitor iberiotoxin but not by the intermediate- or small-conductance KCa inhibitors charybdotoxin, apamin, or TRAM-34 [38]. Additional experiments in renal microvascular smooth muscle cell cultures determined that 11,12-EET analogs increased cAMP but not cGMP levels [38]. These findings support the notion that 11,12-EET stimulates the cAMP/PKA pathway resulting in activation of large conductance KCa channels and afferent arteriolar dilation (Figure 2).
The cell-signaling pathways responsible for afferent arteriolar dilation could be more complex based on findings in other arterioles and arteries. Evidence in vascular smooth muscle cells has implicated vallinoid type 4 TRP (TRPV4) channels in the EET activation of large-conductance KCa channels [40,41]. EETs can activate small- and intermediate KCa channels in endothelial cells via TRPC3 and TRPC6 channel activation [42,43]. These findings coupled with findings in the coronary arteries that EETs activate vascular smooth muscle cell KCa channels through G Protein (Gs)-dependent mechanism support the notion that EETs act through receptors [35,37,38,39,44]. Other evidence in coronary arteries provides evidence that ADP ribose is an important signaling molecule for 11,12-EET activation of KCa channels [45,46]. One could speculate that ADP ribose is an early event required for initial channel opening and that subsequent activation of the cAMP/PKA pathway sustains KCa activation. More direct evidence for EET receptors and cAMP/PKA signaling has been provided by radioligand binding studies in U937 macrophage cells [47]. These experimental studies demonstrated that 11,12-EET was more potent than 14,15-EET in increasing cAMP and had a Ki that was 3 times lower than 14,15-EET [47]. The identity of EET receptors remains elusive; however, evidence in afferent arterioles suggests that 11,12-EET acts through a receptor that increases cAMP levels and activates smooth muscle cell large-conductance KCa channels resulting in vasodilation.
Information from the EET analogs and structure activity relationship resulted in development of EET antagonists [48]. The primary EET antagonists used to date is 14,15-EEZE that is very similar in structure to 14,15-EET but lacks 8–9, and 11,12- carbon double bonds [48]. 14,15-EEZE is a non-selective EET antagonists and inhibits the vasodilation to all EETs while 14,15-EE5ZE-SI was found to be a selective antagonist for 14,15-EET [49]. Additionally, 14,15-DHE5ZE, the product of 14,15-EE5ZE hydrolysis, inhibited coronary arteriolar relaxations to 14,15-EET but was without effect on the relaxations to other regioisomers [49]. Thus, 14,15-DHE5ZE is a selective 14,15-EET antagonist. More recently, 11,12,20-THE8ZE was synthesized and evaluated for its antagonist activity against EET-induced relaxation in mesenteric resistance arteries and bovine coronary arteries [50]. 11,12,20-THE8ZE inhibits 11,12-EET-induced relaxation but not the relaxations to other EET regioisomers [50]. Thus, EET antagonists have now been developed that can separate the vascular actions of 11,12-EET and 14,15-EET. Future studies using these 11,12-EET and 14,15-EET selective antagonists can be used to more precisely determine the contribution of each of these regioisomers to afferent arteriolar function.
Besides EET analogs and antagonists, genetic approaches have been utilized to determine the contribution of EETs to renal vascular function. Cyp2j5 was the first epoxygenase enzyme to be knocked out in mice [51]. Unfortunately, the sex-specific phenotype in these mice and changes in afferent arteriolar function were not associated with EET generation but rather due to estrogen regulation in female mice [51]. The next genetic manipulation was to overexpress human CYP epoxygenases in endothelial cells [52]. Endothelial expression of the human CYP2J2 or CYP2C8 enhanced the afferent arteriolar dilator response to acetylcholine and attenuated the constrictor response to endothelin-1 [52]. The EET antagonist 14,15-EEZE reversed the changes afferent arteriolar responses in the transgenic mice [52]. Previous studies using pharmacological epoxygenase inhibitors demonstrated that EETs contribute to renal vascular dilation to acetylcholine and oppose endothelin-1 constriction [32,53]. Intriguingly, these CYP epoxygenase transgenic mice also had decreased blood pressures in response to chronic nitric oxide inhibition or angiotensin salt-sensitive hypertension [52]. Similar vascular and hypertension findings have been reported in Ephx2 gene deficient mice that result in increased EET levels [54,55]. Thus, there are consistent findings in terms of the contribution of EETs to afferent arteriolar function with pharmacological and genetic manipulation.
Development of sEH, EET, & 20-HETE Therapeutic Applications
The renal vascular and tubular EET actions to vasodilate and increase sodium excretion are consistent with the idea that EETs are anti-hypertensive. This led to the hypothesis that decrease kidney and vascular EET levels contribute to hypertension, endothelial dysfunction, and renal injury. Experimental studies provided significant evidence that decreased renal epoxygenase activity resulted in salt-sensitive hypertension [56]. This concept was most recently demonstrated in Cyp4a10 −/− mice. Although 20-HETE biosynthetic activity was unchanged in the Cyp4a10 −/− mice, urinary EET levels were decreased [57]. Consequently, Cyp4a10 −/− mice developed salt-sensitive hypertension that was demonstrated to be due to reduced epoxygenase activity [57]. Other studies demonstrated that increased EET degradation by sEH in the kidney was associated with renal microvascular dysfunction in hypertension [58,59]. Renal microvascular sEH but not CYP epoxygenase protein expression is increased in angiotensin hypertension [59,60]. Interestingly, the EET analog, 11,12-EET-SI, given in vitro acutely, ameliorated enhanced afferent arteriolar reactivity to angiotensin in hypertension [60]. These findings led to the development of sEH inhibitors and EET analogs and their testing in hypertension.
The development of sEH inhibitors occurred at a very rapid pace and reached the point of human clinical trials [61]. Carbamate urea sEH inhibitors were developed and demonstrated to lower blood pressure in many animal models of hypertension [54,58,59,61]. Like 11,12-EET-SI, administration of the sEH inhibitor, CDU improved the afferent arteriolar response to angiotensin in hypertension [58]. Chronic administration of sEH inhibitors to hypertensive animals lowered blood pressure, improved glomerular barrier function, and decreased renal injury [54,58,59,61]. These studies also demonstrated that the plasma and urinary EET to DHETE ratio increases in rodents treated with sEH inhibitors [58,59]. 14,15-EET appears to be the main EET regioisomer that increases and sodium excretion is increased in angiotensin hypertension in response to sEH inhibition [61,62]. Follow up studies determined that sEH inhibitor treatment could decrease glomerular and renal injury without lowering blood pressure [63]. Although these findings with sEH inhibitors are promising, major drawbacks are that they result in a generalized increase in EETs and that their effectiveness depends on EET generation by CYP epoxygenases. These drawbacks have led to development of EET analogs that can circumvent these problems.
EET analogs have been used extensively for in vitro studies and in 2009 and 2010 the first published reports that EET analogs could be used in vivo were published [64,65]. Afferent arteriolar and mesenteric resistance artery dilator responses were used as an initial screen for EET analogs [38,39,65]. These EET analogs were designed to have better solubility and resist ! -oxidation and sEH metabolism [34,66]. The 11,12-EET agonist which consisted of amidation of the carboxylic group on 11-nonyloxyl-undec-8(Z)-enoic acid structure with aspartic acid provided the EET analog NUDSA [65]. NUDSA and similar analogs were determined to dilate afferent arterioles and lower blood pressure in SHR and angiotensin hypertension [65]. Additionally, NUDSA reversed the metabolic syndrome phenotype in heme-oxygenase 2 (HO-2) gene-deficient mice [64]. Chronic administration of NUDSA lowered blood pressure, decreased body weight, lowered blood glucose, and improved endothelial-dependent vasodilation [64]. Newer generations of EET agonists have been developed and initial in vivo studies appear promising.
An increase in renal microvascular 20-HETE has been demonstrated to contribute to hypertension and human hypertensive patients demonstrate increased plasma levels of 20-HETE [13,67]. Evidence in genetic mice also demonstrated increased 20-HETE as a contributor to hypertension. Targeted disruption of the Cyp4a14 gene in mice resulted in androgen-sensitive hypertension that was a consequence of increased Cyp4a12 hydroxylase expression and increased 20-HETE generation [68]. The Cyp4a14 −/− mice had decreased afferent arteriolar diameters resulting in increased vascular resistance [68]. Additional evidence suggests that increased vascular 20-HETE levels can result in angiotensin dependent hypertension through activation of angiotensin converting enzyme and angiotensin type 1 receptors [69]. Inhibition of 20-HETE has been demonstrated to lower blood pressure in animal models of hypertension where vascular CYP4A expression or 20-HETE levels are elevated [13,69]. These 20-HETE pro-hypertensive actions contrast to the anti-hypertensive kidney tubular actions of 20-HETE to decrease sodium excretion [13]. This has made it difficult to therapeutically target 20-HETE for hypertension. Interestingly, combined sEH inhibition and 20-HETE attenuates hypertension and renal damage in Ren-2 transgenic rats [70]. Therefore combining the vascular anti-hypertensive actions of 20-HETE with the anti-hypertensive tubular transport actions of EETs could be a conceivable approach for treating this renal and cardiovascular disease.
Conclusions
The past 15 years have resulted in great advances in our understanding of the renal microvascular actions and functions for CYP metabolites of arachidonic acid. The initial descriptions of 20-HETE as a constrictor and EETs as dilators of afferent arterioles gave rise to studies to determine cell-signaling mechanisms and their contribution to autoregulatory and hormonal responses. 20-HETE influences on calcium influx and participation in afferent autoregulatory responses were determined. Meanwhile, experimental evidences established that EETs activated KCa channels to hyperpolarize renal microvascular smooth muscle cells and determined to be an EDHF. This resulted in development of genetic and pharmacological tools to further define the physiological and pathological importance of 20-HETE and EETs to renal microvascular function. The results of these experimental studies have now reached a point where novel therapeutics that target 20-HETE and EETs are being tested (Figure 1). Future advances will require studies to better define the cellular mechanisms by which CYP metabolites control renal microvascular function and determining their significance in renal diseases.
Highlights
-
! !
20-HETE constricts afferent arterioles and contributes to autoregulation.
-
! !
EETs dilate afferent arterioles and act as an EDHF.
-
! !
20-HETE and EETs contribute to purinergic receptor afferent arteriolar responses.
-
! !
Soluble epoxide hydrolase, EETs, and 20-HETE are therapeutic targets.
Acknowledgements
This work was supported by NIH grants HL59699 and DK38226. I especially thank Dr. Jorge Capdevila for his leadership, guidance, and support that made these studies and the NIH Program Project Grant a success.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Capdevila JH, Falck JR. The CYP P450 arachidonic acid monooxygenases: from cell signaling to blood pressure regulation. Biochemical and Biophysical Research Communications. 2001;285:571–576. doi: 10.1006/bbrc.2001.5167. [DOI] [PubMed] [Google Scholar]
- 2.Spector AA, Fang X, Snyder GD, Weintraub NL. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog Lipid Res. 2004;43:55–90. doi: 10.1016/s0163-7827(03)00049-3. [DOI] [PubMed] [Google Scholar]
- 3.Jacobson HR, Corona S, Capdevila JH, Chacos N, Manna S, Womack A, Falck JR. Effects of epoxyeicosatrienoic acids on ion transport in the rabbit cortical collecting tubule. In: Braquet P, Garay RP, Frohlich JC, Nicosia S, editors. Prostaglandins, and Membrane Ion Transport. Raven Press; New York: 1985. pp. 311–318. [Google Scholar]
- 4.Schwartzman M, Ferreri NR, Carroll MA, Songu-Mize E, McGiff JC. Renal cytochrome P450-related arachidonate metabolite inhibits (Na+ + K+)ATPase. Nature. 1985;314:620–622. doi: 10.1038/314620a0. [DOI] [PubMed] [Google Scholar]
- 5.Proctor KG, Falck JR, Capdevila J. Intestinal vasodilation by epoxyeicosatrienoic acids: arachidonic acid metabolites produced by a cytochrome P450 monooxygenase. Circ Res. 1987;60:50–59. doi: 10.1161/01.res.60.1.50. [DOI] [PubMed] [Google Scholar]
- 6.Furchgott RF, Vanhoutte PM. Endothelium-derived relaxing and contracting factors. FASEB J. 1989;3:2007–2018. [PubMed] [Google Scholar]
- 7.Busse R, Edwards G, Félétou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. TRENDS in Pharmacological Sciences. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- 8.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- 9.Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature. 1999;401:493–497. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- 10.Archer SL, Gragasin FS, Wu X, Wang S, McMurtry S, Kim DH, Platonov M, Koshal A, Hashimoto K, Campbell WB, Falck JR, Michelakis ED. Endothelium-derived hyperpolarizing factor in human internal mammary artery is 11,12-epoxyeicosatrienoic acid and causes relaxation by activating smooth muscle BK(Ca) channels. Circulation. 2003;107:769–776. doi: 10.1161/01.cir.0000047278.28407.c2. [DOI] [PubMed] [Google Scholar]
- 11.Ma YH, Gebremedhin D, Schwartzman ML, Falck JR, Clark JE, Masters BS, Harder DR, Roman RJ. 20-Hydroxyeicosatertraenoic acid is an endogenous vasoconstrictor of canine renal arcuate arteries. Circ Res. 1993;72:126–136. doi: 10.1161/01.res.72.1.126. [DOI] [PubMed] [Google Scholar]
- 12.Imig JD, Zou A-P, Stec DE, Harder DR, Falck JR, Roman RJ. Formation and actions of 20-hydroxyeicosatetraenoic acid in the renal microcirculation. Am J Physiol Regul Integr Comp Physiol. 1996;270:R217–R227. doi: 10.1152/ajpregu.1996.270.1.R217. [DOI] [PubMed] [Google Scholar]
- 13.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 14.Capdevila JH, Falck JR, Imig JD. Roles of the cytochrome P450 arachidonic acid monooxygenases in the control of systemic blood pressure and experimental hypertension. Kidney Int. 2007;72:683–689. doi: 10.1038/sj.ki.5002394. [DOI] [PubMed] [Google Scholar]
- 15.Schwartzman ML, da Silva JL, Lin F, Nishimura M, Abraham NG. Cytochrome P450 4A expression and arachidonic acid omega-hydrolxylation in the kidney of the spontaneously hypertensive rat. Nephron. 1996;73:652–663. doi: 10.1159/000189154. [DOI] [PubMed] [Google Scholar]
- 16.Imig JD, Falck JR, Gebremedhin D, Harder DR, Roman RJ. Elevated renovascular tone in young spontaneously hypertensive rats: Role of cytochrome P450. Hypertension. 1993;22:357–364. doi: 10.1161/01.hyp.22.3.357. [DOI] [PubMed] [Google Scholar]
- 17.Imig JD, Zou A-P, Ortiz de Montellano PR, Sui Z, Roman RJ. Cytochrome P450 inhibitors alter the afferent arteriolar response to elevations in perfusion pressure. Am J Physiol Heart Circ Physiol. 1994;266:H1879–H1885. doi: 10.1152/ajpheart.1994.266.5.H1879. [DOI] [PubMed] [Google Scholar]
- 18.Zou A-P, Imig JD, Ortiz de Montellano PR, Sui Z, Roman RJ. Inhibition of renal vascular 20-HETE impairs autoregulation of renal blood flow. Am J Physiol Renal Physiol. 1994;266:F275–F282. doi: 10.1152/ajprenal.1994.266.2.F275. [DOI] [PubMed] [Google Scholar]
- 19.Zou A-P, Imig JD, Ortiz de Montellano PR, Sui Z, Falck JR, Roman RJ. Effect of P-450 ! -hydroxylase metabolites of arachidonic acid on tubuloglomerular feedback. Am J Physiol Renal Physiol. 1994;266:F934–F941. doi: 10.1152/ajprenal.1994.266.6.F934. [DOI] [PubMed] [Google Scholar]
- 20.Wang MH, Brand-Schieber E, Zand BA, Nguyen X, Falck JR, Balu N, Schwartzman ML. Cytochrome P450-derived arachidonic acid metabolism in the rat kidney: characterization of selective inhibitors. J Pharmacol Exp Ther. 1998;284:966–973. [PubMed] [Google Scholar]
- 21.Imig JD, Falck JR, Inscho EW. Contribution of cytochrome P450 epoxygenase and hydroxylase pathways to afferent arteriolar autoregulatory responsiveness. British Journal of Pharmacology. 1999;127:1399–1405. doi: 10.1038/sj.bjp.0702662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inscho EW, Cook AK, Navar LG. Pressure-mediated vasoconstriction of juxtamedullary afferent arterioles involves P2-purinoceptor activation. Am J Physiol Renal Fluid Electrolyte Physiol. 1996;271:F1077–F1085. doi: 10.1152/ajprenal.1996.271.5.F1077. [DOI] [PubMed] [Google Scholar]
- 23.Inscho EW, Cook AK, Imig JD, Vial C, Evans RJ. Physiological role for P2×1 receptors in renal microvascular autoregulatory behavior. J Clin Invest. 2003;112:1895–1905. doi: 10.1172/JCI18499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao X, Falck JR, Gopal VR, Inscho EW, Imig JD. P2× receptor stimulated calcium responses in preglomerular smooth muscle cells involves 20-hydroxyeicosatetraenoic acid. J Pharmacol Exp Ther. 2004;311:1211–1217. doi: 10.1124/jpet.104.070797. [DOI] [PubMed] [Google Scholar]
- 25.Zhao X, Inscho EW, Bondlela M, Falck JR, Imig JD. The CYP450 hydroxylase pathway contributes to P2× receptor-mediated afferent arteriolar vasoconstriction. Am J Physiol Heart Circ Physiol. 2001;281:H2089–H2096. doi: 10.1152/ajpheart.2001.281.5.H2089. [DOI] [PubMed] [Google Scholar]
- 26.Imig JD. Epoxide hydrolase and epoxygenase metabolites as therapeutic targets for renal diseases. Am J Physiol Renal Physiol. 2005;289:F496–503. doi: 10.1152/ajprenal.00350.2004. [DOI] [PubMed] [Google Scholar]
- 27.Imig JD, Navar LG, Roman RJ, Reddy KK, Falck JR. Actions of epoxygenase metabolites on the preglomerular vasculature. Journal of American Society of Nephrology. 1996;7:2364–2370. doi: 10.1681/ASN.V7112364. [DOI] [PubMed] [Google Scholar]
- 28.Zou AP, Fleming JT, Falck JR, Jacobs ER, Gebremedhin D, Harder DR, Roman RJ. Stereospecific effects of epoxyeicosatrienoic acids on renal vascular tone and K+-channel activity. Am J Physiol Renal Physiol. 1996;270:F822–F832. doi: 10.1152/ajprenal.1996.270.5.F822. [DOI] [PubMed] [Google Scholar]
- 29.Imig JD, Falck JR, Wei S, Capdevila JH. Epoxygenase metabolites contribute to the nitric oxide-independent afferent arteriolar vasodilation to bradykinin. Journal of Vascular Research. 2001;38:247–255. doi: 10.1159/000051053. [DOI] [PubMed] [Google Scholar]
- 30.Bussemaker E, Popp R, Fisslthaler B, Larson CM, Fleming I, Busse R, Brandes RP. Aged spontaneously hypertensive rats exhibit a selective loss of EDHF-mediated relaxation in the renal artery. Hypertension. 2003;42:562–568. doi: 10.1161/01.HYP.0000088852.28814.E2. [DOI] [PubMed] [Google Scholar]
- 31.Mombouli JV, Vanhoutte PM. Endothelium-derived hyperpolarizing factor(s) and the potentiation of kinins by converting enzyme. Am J Hypertens. 1995;8:19S–27S. doi: 10.1016/0895-7061(95)00029-o. [DOI] [PubMed] [Google Scholar]
- 32.Matsuda H, Hayashi K, Wakino S, Kubota E, Honda M, Tokuyama H, Takamatsu I, Tatematsu S, Saruta T. Role of endothelium-derived hyperpolarizing factor in ACE inhibitor-induced renal vasodilation in vivo. Hypertension. 2004;43:603–609. doi: 10.1161/01.HYP.0000118053.42262.71. [DOI] [PubMed] [Google Scholar]
- 33.Cheng MK, Doumad AB, Jiang H, Falck JR, McGiff JC, Carroll MA. Epoxyeicosatrienoic acids mediate adenosine-induced vasodilation in rat preglomerular microvessels (PGMV) via A2A receptors. Br J Pharmacol. 2004;141:441–448. doi: 10.1038/sj.bjp.0705640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sudhahar V, Shaw S, Imig JD. Epoxyeicosatrienoic Acid Analogs and Vascular Function. Curr Med Chem. 2010;17:1181–1190. doi: 10.2174/092986710790827843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imig JD, Inscho EW, Deichmann PC, Reddy KM, Falck JR. Afferent arteriolar vasodilation to the sulfonimide analog of 11, 12-epoxyeicosatrienoic acid involves protein kinase A. Hypertension. 1999;33:408–413. doi: 10.1161/01.hyp.33.1.408. [DOI] [PubMed] [Google Scholar]
- 36.Chen JK, Falck JR, Reddy KM, Capdevila J, Harris RC. Epoxyeicosatrienoic acids and their sulfonimide derivatives stimulate tyrosine phosphorylation and induce mitogenesis in renal epithelial cells. J Biol Chem. 1998;273:29254–29261. doi: 10.1074/jbc.273.44.29254. [DOI] [PubMed] [Google Scholar]
- 37.Falck JR, Krishna UM, Reddy YK, Kumar PS, Reddy KM, Hittner SB, Deeter C, Sharma KK, Gauthier KM, Campbell WB. Comparison of vasodilatory properties of 14,15-EET analogs: structural requirements for dilation. Am J Physiol Heart Circ Physiol. 2003;284:H337–H349. doi: 10.1152/ajpheart.00831.2001. [DOI] [PubMed] [Google Scholar]
- 38.Imig JD, Dimitropoulou C, Reddy DS, White RE, Falck JR. Afferent arteriolar dilation to 11,12-EET analogs involves PP2A activity and Ca2+-activated K+ Channels. Microcirculation. 2008;15:137–150. doi: 10.1080/10739680701456960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dimitropoulou C, West L, Field MB, White RE, Reddy LM, Falck JR, Imig JD. Protein phosphatase 2A and Ca2+-activated K+ channels contribute to 11,12-epoxyeicosatrienoic acid analog mediated mesenteric arterial relaxation. Prostaglandins Other Lipid Mediat. 2007;83:50–61. doi: 10.1016/j.prostaglandins.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 40.Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 41.Earley S, Heppner TJ, Nelson MT, Brayden JE. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res. 2006;97:1270–1279. doi: 10.1161/01.RES.0000194321.60300.d6. [DOI] [PubMed] [Google Scholar]
- 42.Fleming I, Rueben A, Popp R, Fisslthaler B, Schrodt S, Sander A, Haendeler J, Falck JR, Morisseau C, Hammock BD, Busse R. Epoxyeicosatrienoic acids regulate Trp channel dependent Ca2+ signaling and hyperpolarization in endothelial cells. Arterioscler Thromb Vasc Biol. 2007;27:2612–2618. doi: 10.1161/ATVBAHA.107.152074. [DOI] [PubMed] [Google Scholar]
- 43.Loot AE, Popp R, Fisslthaler B, Vriens J, Nilius B, Fleming I. Role of cytochrome P450-dependent transient receptor potential V4 activation in flow-induced vasodilatation. Cardiovasc Res. 2008;80:445–452. doi: 10.1093/cvr/cvn207. [DOI] [PubMed] [Google Scholar]
- 44.Li PL, Campbell WB. Epoxyeicosatrienoic acids activate K+ channels in coronary smooth muscle through a guanine nucleotide binding protein. Circ Res. 1997;80:877–884. doi: 10.1161/01.res.80.6.877. [DOI] [PubMed] [Google Scholar]
- 45.Li PL, Chen CL, Bortell R, Campbell WB. 11,12-Epoxyeicosatrienoic acid stimulates endogenous mono-ADP-ribosylation in bovine coronary arterial smooth muscle. Circ Res. 1999;85:349–356. doi: 10.1161/01.res.85.4.349. [DOI] [PubMed] [Google Scholar]
- 46.Li PL, Zhang DX, Ge ZD, Campbell WB. Role of ADP-ribose in 11,12-EET-induced activation of K(Ca) channels in coronary arterial smooth muscle cells. Am J Physiol Heart Circ Physiol. 2002;282:H1229–1236. doi: 10.1152/ajpheart.00736.2001. [DOI] [PubMed] [Google Scholar]
- 47.Yang W, Tuniki VR, Anjaiah S, Falck JR, Hillard CJ, Campbell WB. Characterization of epoxyeicosatrienoic acid binding site in U937 membranes using a novel radiolabeled agonist, 20-125i-14,15-epoxyeicosa-8(Z)-enoic acid. J Pharmacol Exp Ther. 2008;324:1019–1027. doi: 10.1124/jpet.107.129577. [DOI] [PubMed] [Google Scholar]
- 48.Gauthier KM, Falck JR, Reddy LM, Campbell WB. 14,15-EET analogs: characterization of structural requirements for agonist and antagonist activity in bovine coronary arteries. Pharmacol Res. 2004;49:515–524. doi: 10.1016/j.phrs.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 49.Gauthier KM, Deeter C, Krishna UM, Reddy YK, Bondlela M, Falck JR, Campbell WB. 14,15-Epoxyeicosa-5(Z)-enoic acid: a selective epoxyeicosatrienoic acid antagonist that inhibits endothelium-dependent hyperpolarization and relaxation in coronary arteries. Circ Res. 2002;90:1028–1036. doi: 10.1161/01.res.0000018162.87285.f8. [DOI] [PubMed] [Google Scholar]
- 50.Bukhari IA, Shah AJ, Gauthier KM, Walsh KA, Konduru SR, Imig JD, Falck JR, Campbell WB. 11,12,20-Trihydroxy-eicosa-8(Z)-enoic acid: a selective inhibitor of 11,12-EET induced relaxations of bovine coronary and rat mesenteric arteries. Am J Physiol Heart Circ Physiol. 2012;302:H1574–H1583. doi: 10.1152/ajpheart.01122.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Athirakul K, Bradbury JA, Graves JP, DeGraff LM, Ma J, Zhao Y, Couse JF, Quigley R, Harder DR, Zhao X, Imig JD, Pedersen TL, Newman JW, Hammock BD, Conley AJ, Korach KS, Coffman TM, Zeldin DC. Increased blood pressure in mice lacking cytochrome P450 2J5. FASEB J. 2008;22:4096–4108. doi: 10.1096/fj.08-114413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee CR, Imig JD, Edin ML, Foley J, DeGraff LM, Bradbury JA, Graves JP, Lih FB, Clark J, Myers P, Perrow L, Lepp AN, Kannon A, Ronnekleiv OK, Alkayed NJ, Falck JR, Tomer KB, Zeldin DC. Endothelial expression of human cytochrome P450 epoxygenases lowers blood pressure and attenuates hypertension-induced renal injury in mice. FASEB J. 2010;24:3770–3781. doi: 10.1096/fj.10-160119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Imig JD, Pham BT, LeBlanc EA, Falck JR, Inscho EW. Cytochrome P450 and cyclooxygenase metabolites contribute to the endothelin-1 afferent arteriolar vasoconstrictor and calcium responses. Hypertension. 2000;35:307–312. doi: 10.1161/01.hyp.35.1.307. [DOI] [PubMed] [Google Scholar]
- 54.Manhiani M, Quigley JE, Knight SF, Tasoobshirazi S, Moore T, Brands MW, Hammock BD, Imig JD. Soluble epoxide hydrolase gene deletion attenuates renal injury and inflammation with DOCA-salt hypertension. Am J Physiol Renal Physiol. 2009;297:F740–F448. doi: 10.1152/ajprenal.00098.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sinal CJ, Miyata M, Tohkin M, Nagata K, Bend JR, Gonzalez FJ. Targeted disruption of soluble epoxide hydrolase reveals a role in blood pressure regulation. J Biol Chem. 2000;275:40504–40510. doi: 10.1074/jbc.M008106200. [DOI] [PubMed] [Google Scholar]
- 56.Zhao X, Pollock DM, Inscho EW, Zeldin DC, Imig JD. Decreased renal cytochrome P450 2C enzymes and impaired vasodilation are associated with salt-sensitive hypertension. Hypertension. 2003;41:709–714. doi: 10.1161/01.HYP.0000047877.36743.FA. [DOI] [PubMed] [Google Scholar]
- 57.Nakagawa K, Holla VJ, Wei Y, Wang WH, Gatica A, Wei S, Mei S, Miller CM, Cha DR, Price E, Zent R, Pozzi A, Breyer MD, Guan Y, Falck JR, Waterman MR, Capdevila JH. Salt-sensitive hypertension is associated with dysfunctional Cyp4a10 gene and kidney epithelial sodium channel. J Clin Invest. 2006;116:1696–1702. doi: 10.1172/JCI27546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao X, Yamamoto T, Newman JW, Kim IH, Watanabe T, Hammock BD, Stewart J, Pollock JS, Pollock DM, Imig JD. Soluble epoxide hydrolase inhibition protects the kidney from hypertension-induced damage. J Am Soc Nephrol. 2004;15:1244–1253. [PubMed] [Google Scholar]
- 59.Imig JD, Zhao X, Capdevila JH, Morisseau C, Hammock BD. Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension. 2002;39:690–694. doi: 10.1161/hy0202.103788. [DOI] [PubMed] [Google Scholar]
- 60.Imig JD, Zhao X, Falck JR, Wei S, Capdevila JH. Enhanced renal microvascular reactivity to angiotensin II in hypertension is ameliorated by the sulfonimide analog of 11,12-epoxyeicosatrienoic acid. J Hypertens. 2001;19:983–992. doi: 10.1097/00004872-200105000-00020. [DOI] [PubMed] [Google Scholar]
- 61.Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov. 2009;8:794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jung O, Brandes RP, Kim IH, Schweda F, Schmidt R, Hammock BD, Busse R, Fleming I. Soluble epoxide hydrolase is a main effector of angiotensin II-induced hypertension. Hypertension. 2005;45:759–765. doi: 10.1161/01.HYP.0000153792.29478.1d. [DOI] [PubMed] [Google Scholar]
- 63.Olearczyk JJ, Quigley JE, Mitchell BC, Yamamoto T, Kim IH, Newman JW, Luria A, Hammock BD, Imig JD. Administration of a substituted adamantyl urea inhibitor of soluble epoxide hydrolase protects the kidney from damage in hypertensive Goto-Kakizaki rats. Clin Sci (Lond) 2009;116:61–70. doi: 10.1042/CS20080039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sodhi K, Inoue K, Gotlinger KH, Canestraro M, Vanella L, Kim DH, Manthati VL, Koduru SR, Falck JR, Schwartzman ML, Abraham NG. Epoxyeicosatrienoic acid agonist rescues the metabolic syndrome phenotype of HO-2-null mice. J Pharmacol Exp Ther. 2009;331:906–916. doi: 10.1124/jpet.109.157545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Imig JD, Elmarakby A, Nithipatikom K, Wei S, Capdevila JH, Tuniki VR, Sangra B, Anjaiah S, Manthati VL, Reddy DS, Falck JR. Development of epoxyeicosatrienoic acid analogs with in vivo anti-hypertensive actions. Frontiers in Vascular Physiology. 2010;1:157. doi: 10.3389/fphys.2010.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Imig JD. Targeting epoxides for organ damage in hypertension. J Cardiovasc Pharmacol. 2010;56:329–335. doi: 10.1097/FJC.0b013e3181e96e0c. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Minuz P, Jiang H, Fava C, Turolo L, Tacconelli S, Ricci M, Patrignani P, Morganti A, Lechi A, McGiff JC. Altered release of cytochrome P450 metabolites of arachidonic acid in renovascular disease. Hypertension. 2008;51:1379–1385. doi: 10.1161/HYPERTENSIONAHA.107.105395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holla VR, Adas F, Imig JD, Zhao X, Price E, Jr, Olsen N, Kovacs WJ, Magnuson MA, Keeney DS, Breyer MD, Falck JR, Waterman MR, Capdevila JH. Alterations in the regulation of androgen-sensitive Cyp 4a monooxygenases cause hypertension. Proc Natl Acad Sci U S A. 2001;98:5211–5216. doi: 10.1073/pnas.081627898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sodhi K, Wu CC, Cheng J, Gotlinger KH, Inoue K, Goli M, Falck JR, Abraham NG, Schwartzman ML. CYP4A2-induced hypertension is 20-HETE and angiotensin II-dependent. Hypertension. 2010;56:871–878. doi: 10.1161/HYPERTENSIONAHA.110.154559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Certikova Chabova V, Walkowska A, Kompanowska-Jezierska E, Sadowski J, Kujal P, Vernerova Z, Vanourkova Z, Kopkan L, Kramer HJ, Falck JR, Imig JD, Hammock BD, Vaneckova I, Cervenka L. Combined inhibition of 20-hydroxyeicosatetraenoic acid formation and of epoxyeicosatrienoic acids degradation attenuates hypertension and hypertension-induced end-organ damage in Ren-2 transgenic rats. Clin Sci (Lond) 2010;118:617–632. doi: 10.1042/CS20090459. [DOI] [PMC free article] [PubMed] [Google Scholar]